Application of Flotation Tailings as a Substitute for Cement in Concrete Structures for Environmental Protection and Sustainable Development—Part I: Sulfide Neutralization
Abstract
1. Introduction
2. Theory
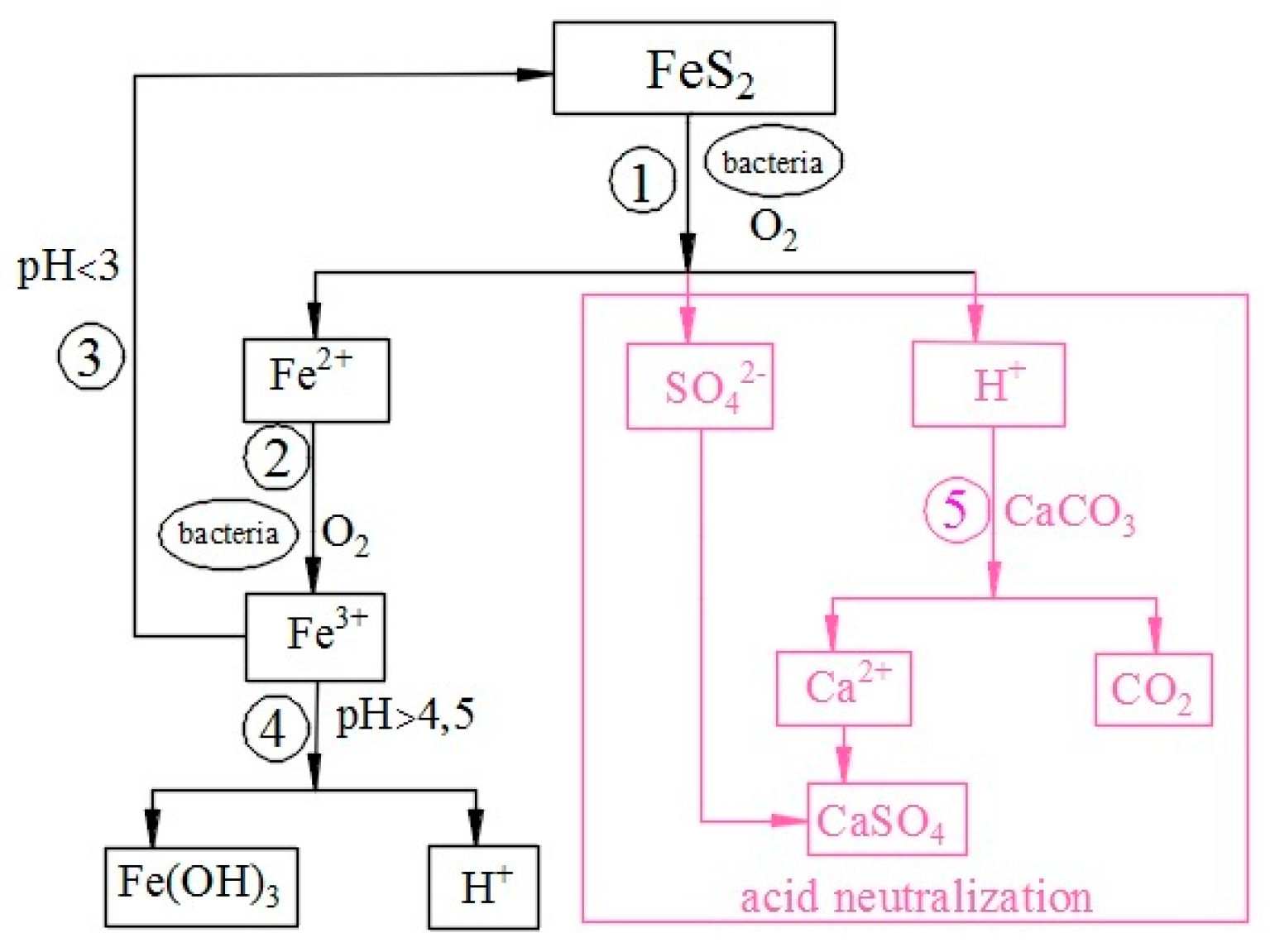
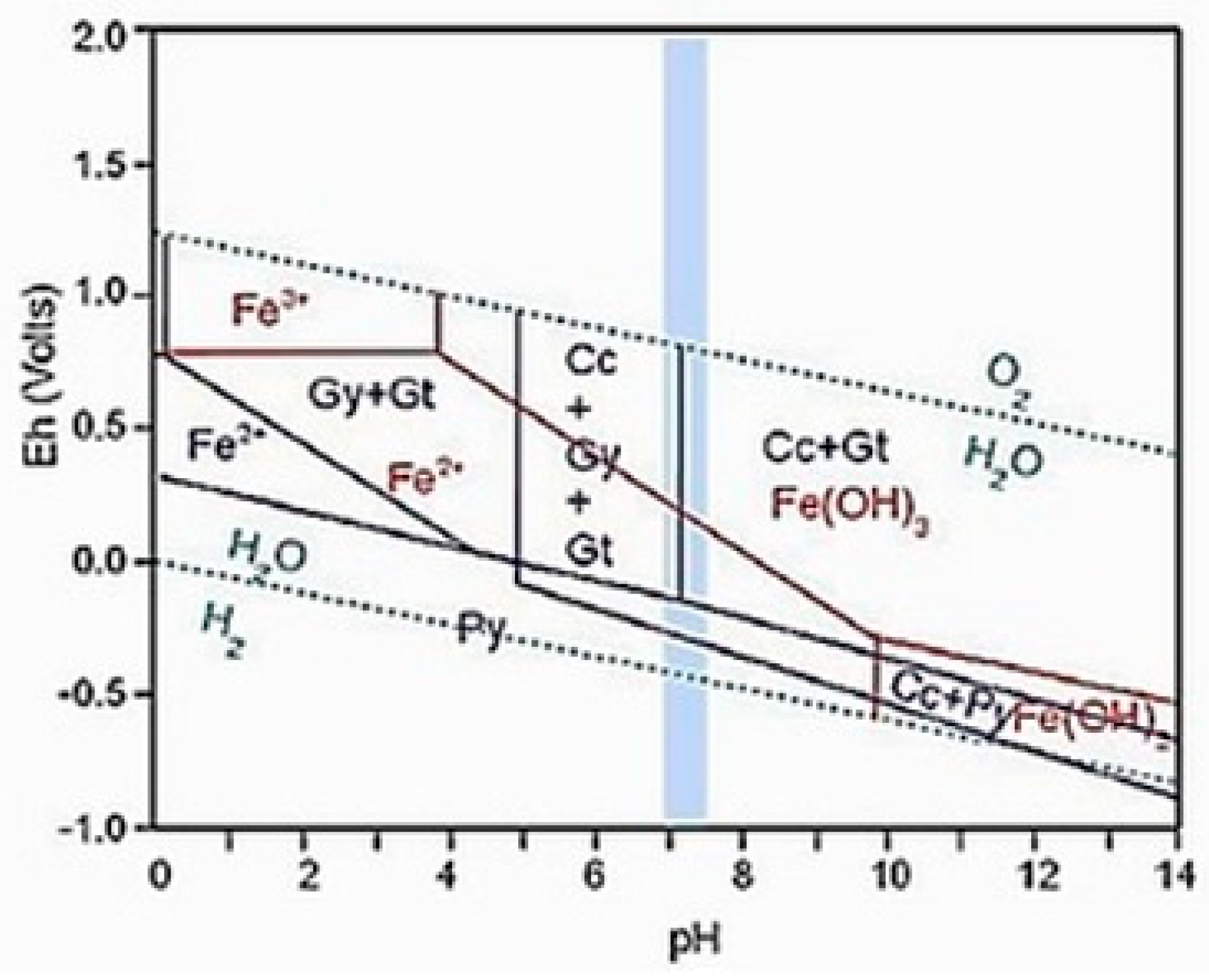
2.1. Theoretical Basis of Pyrite Oxidation
2.2. Mechanism of Oxidation of Fe2+ Ions by Bacteria
2.3. Prevention and Remediation of Acidic Mine Drainage Waters
3. Methods and Materials
3.1. Methods
3.2. Preparation of Concrete Samples and Their Mix Designs
3.3. Flotation Tailings and Aggregate
3.4. Examination Methodology
3.4.1. Assessment of the Potential of Creating Acidic Eluates
3.4.2. Assessment of Leaching of Heavy Metals from Monolithic Waste
3.4.3. Compressive Strength
3.4.4. Concrete Porosity
4. Results and Discussion
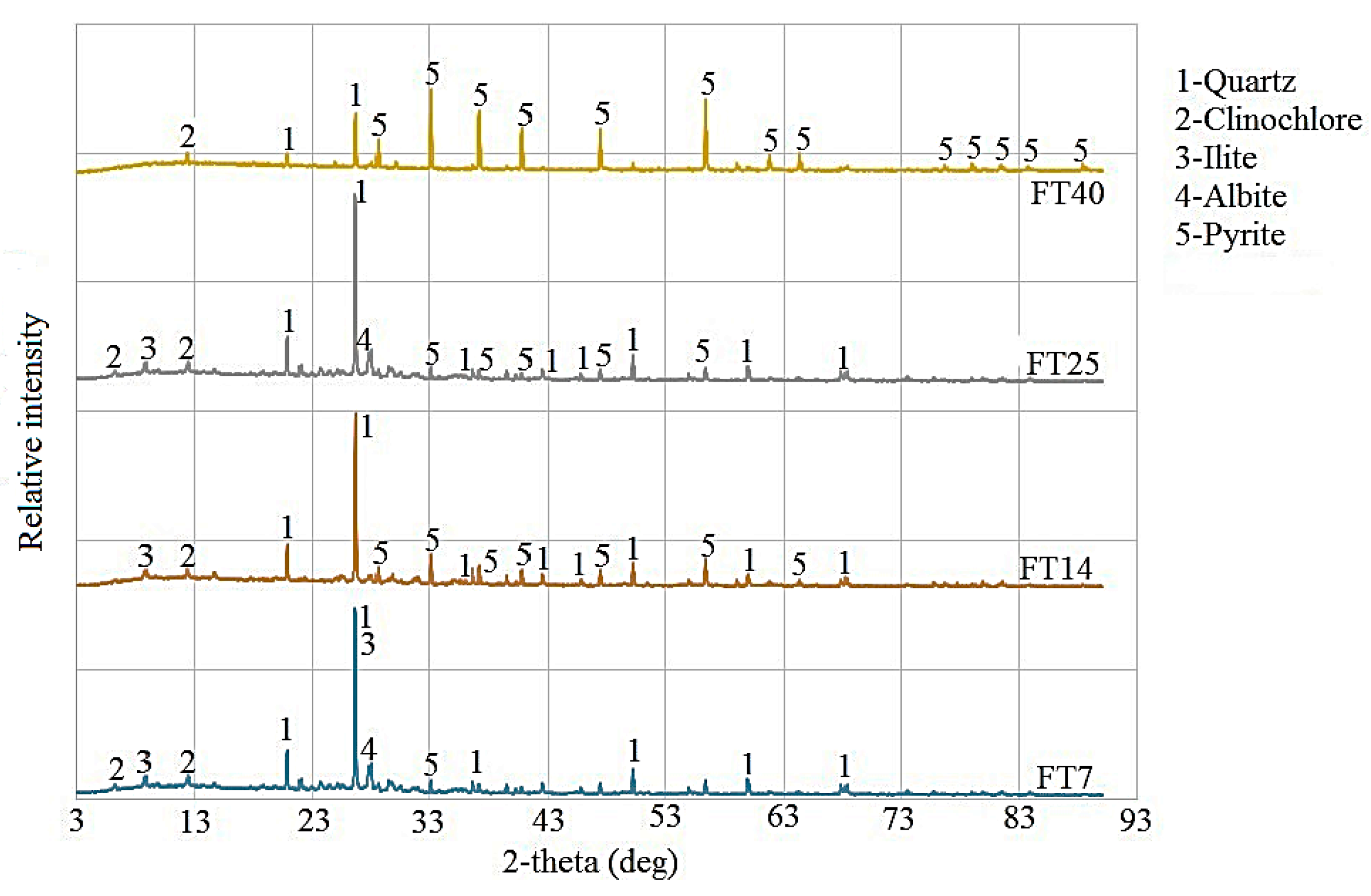
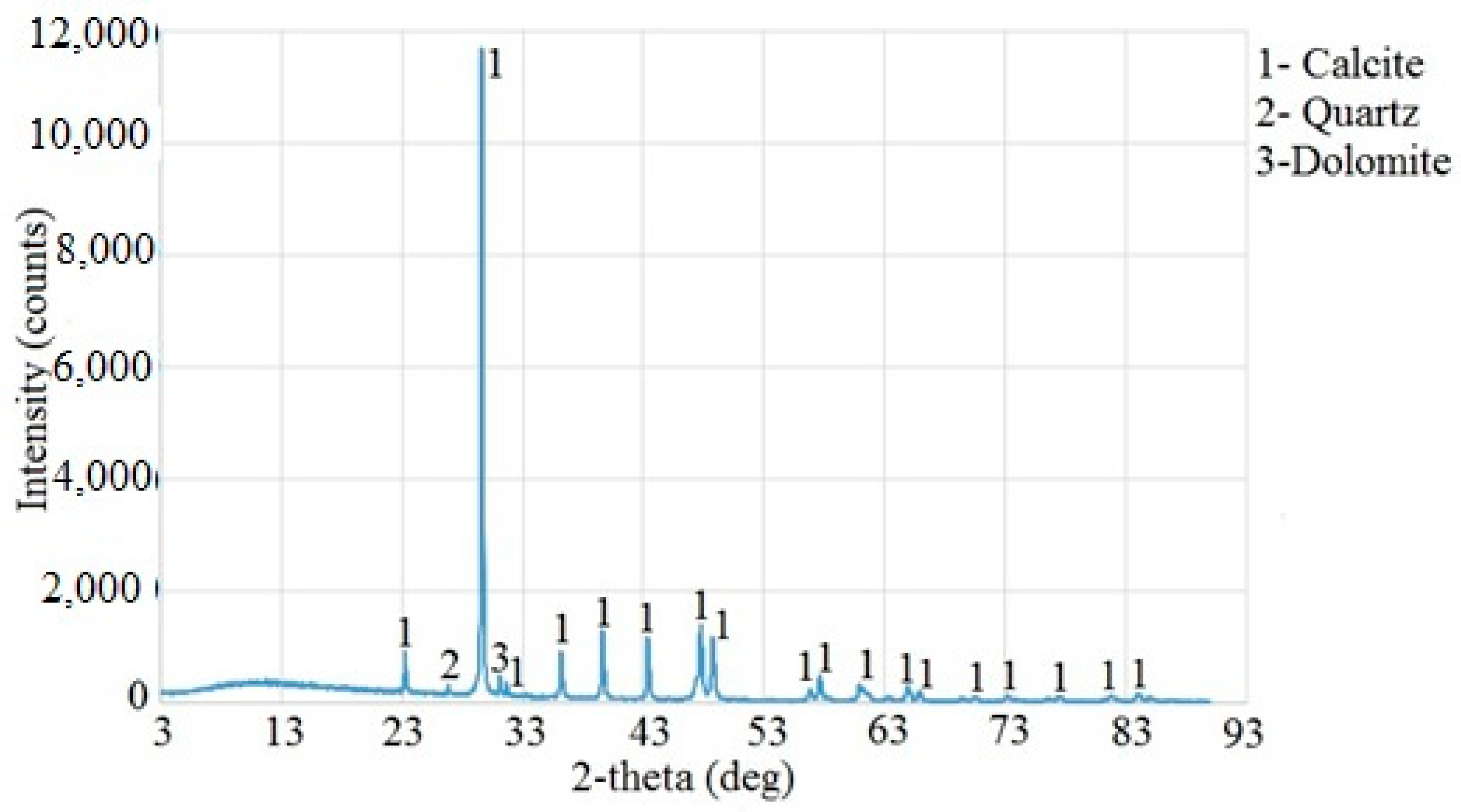
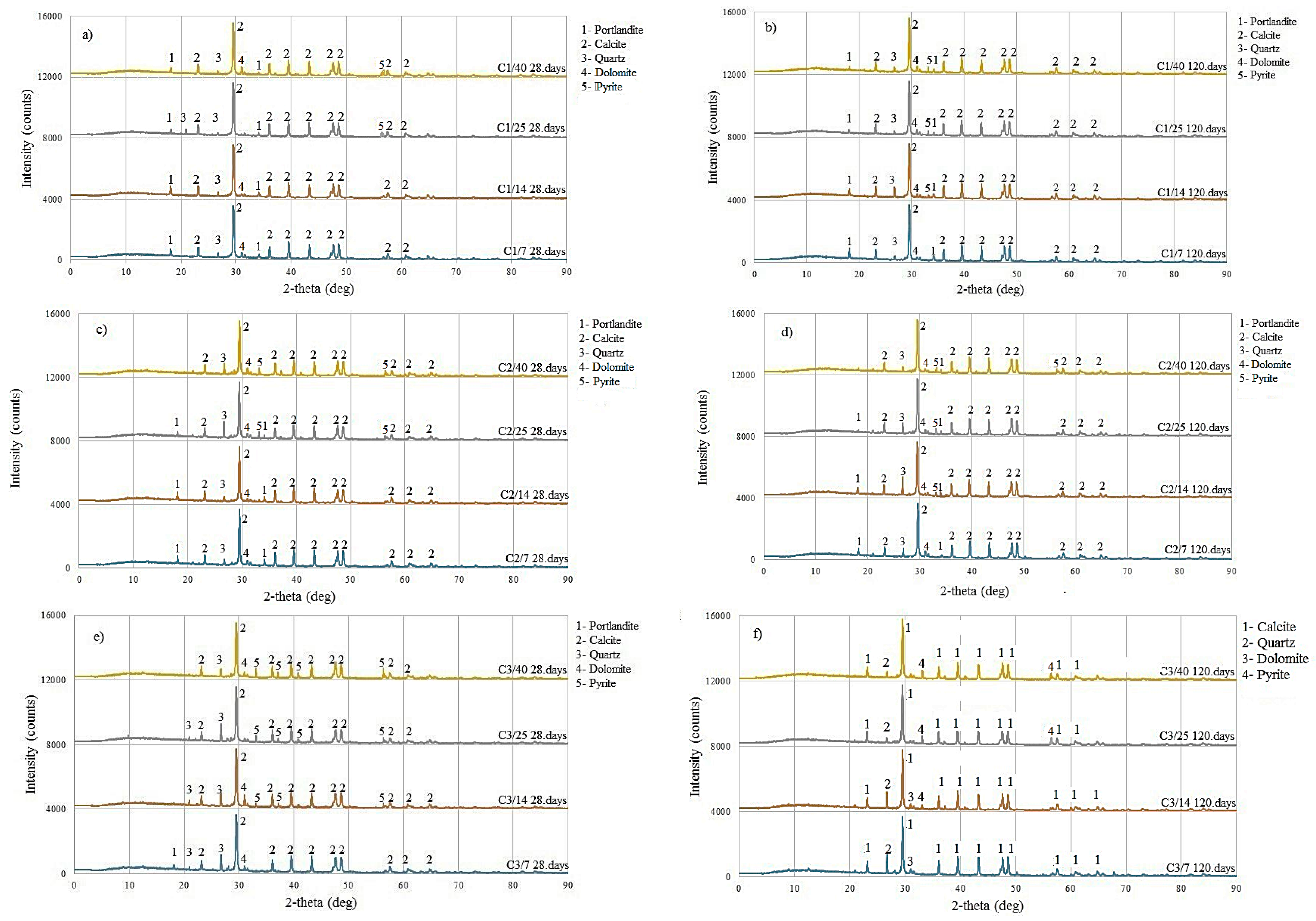
4.1. XRD Analysis
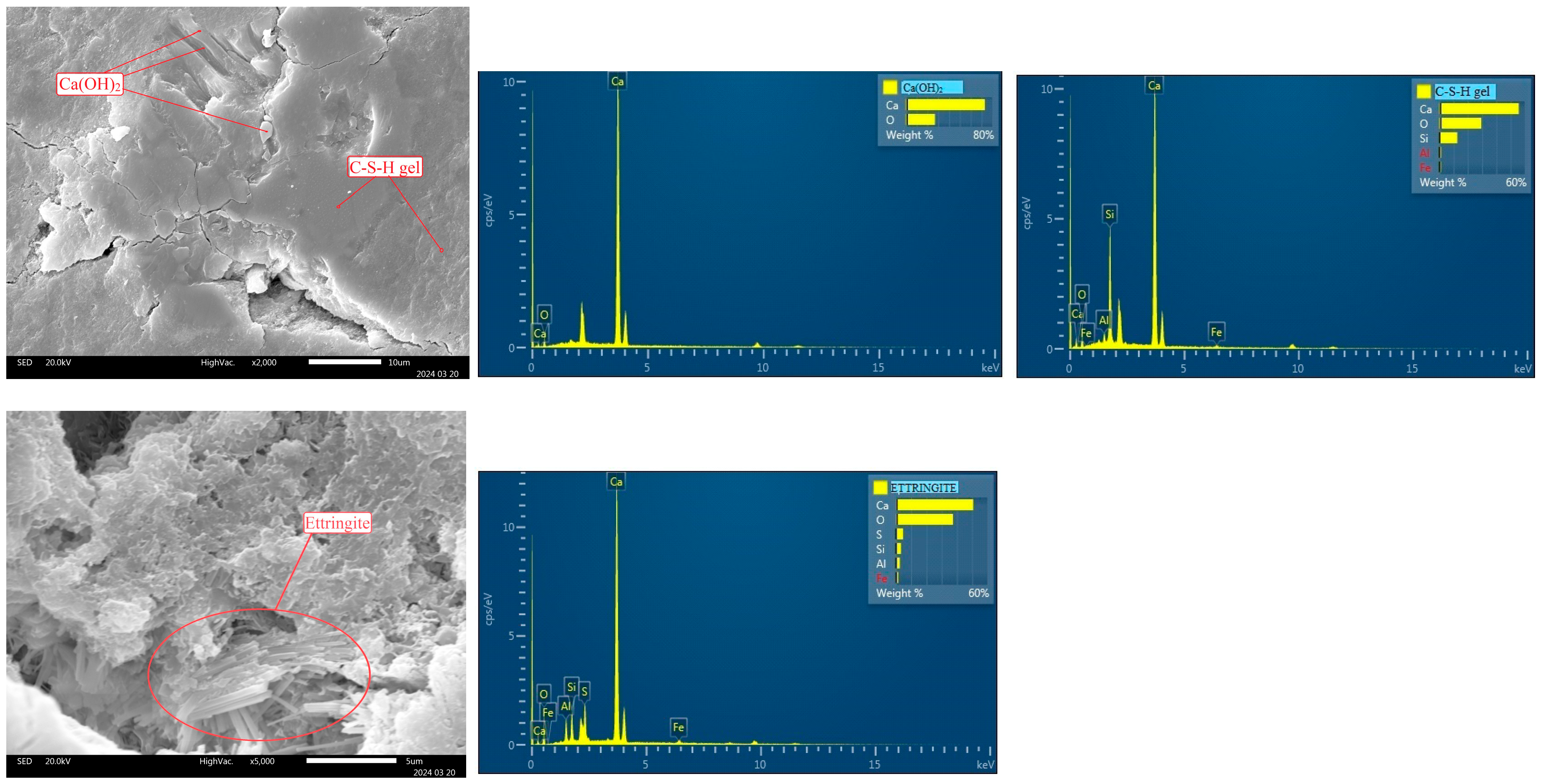
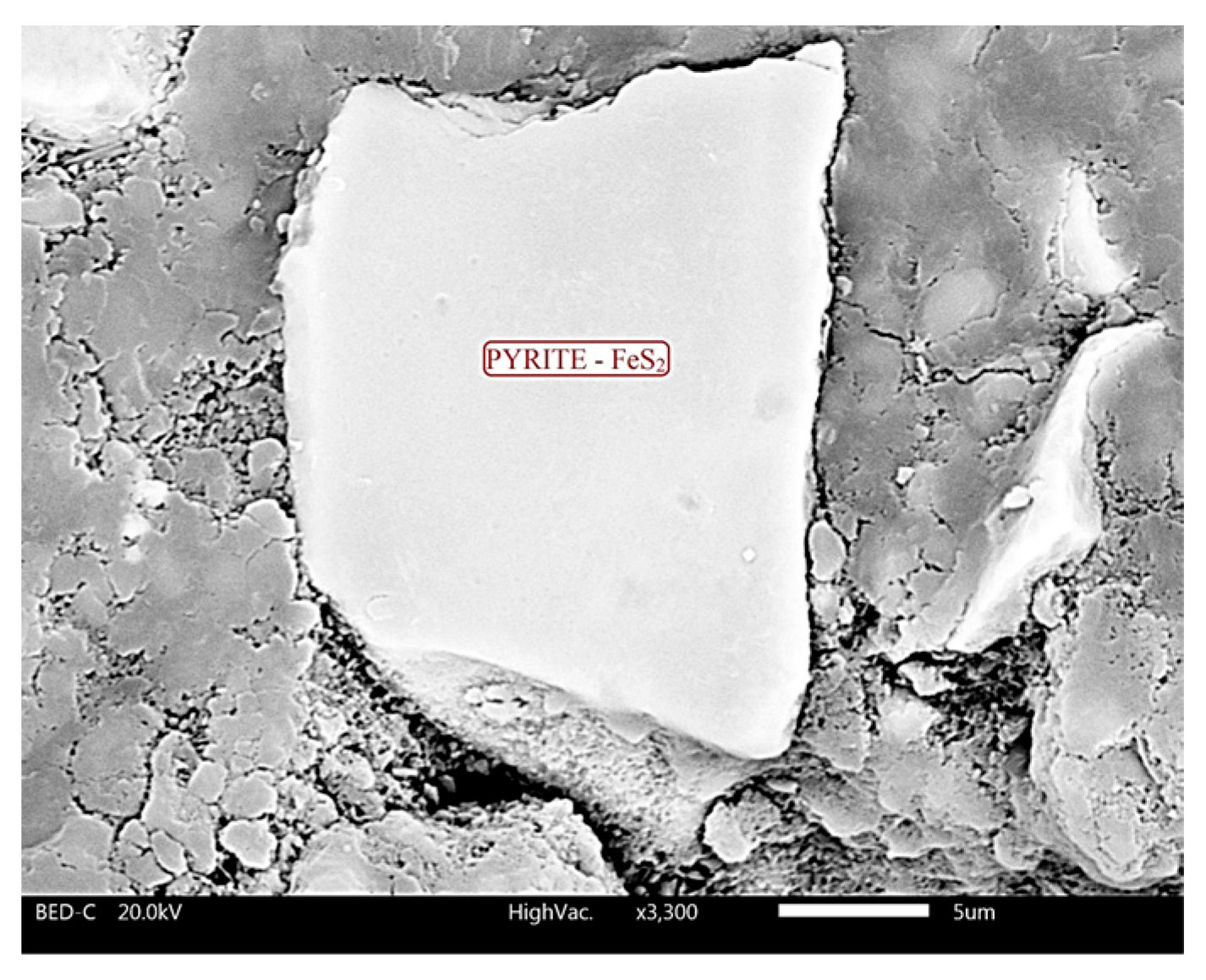
4.2. Scanning Electron Microscopy (SEM)
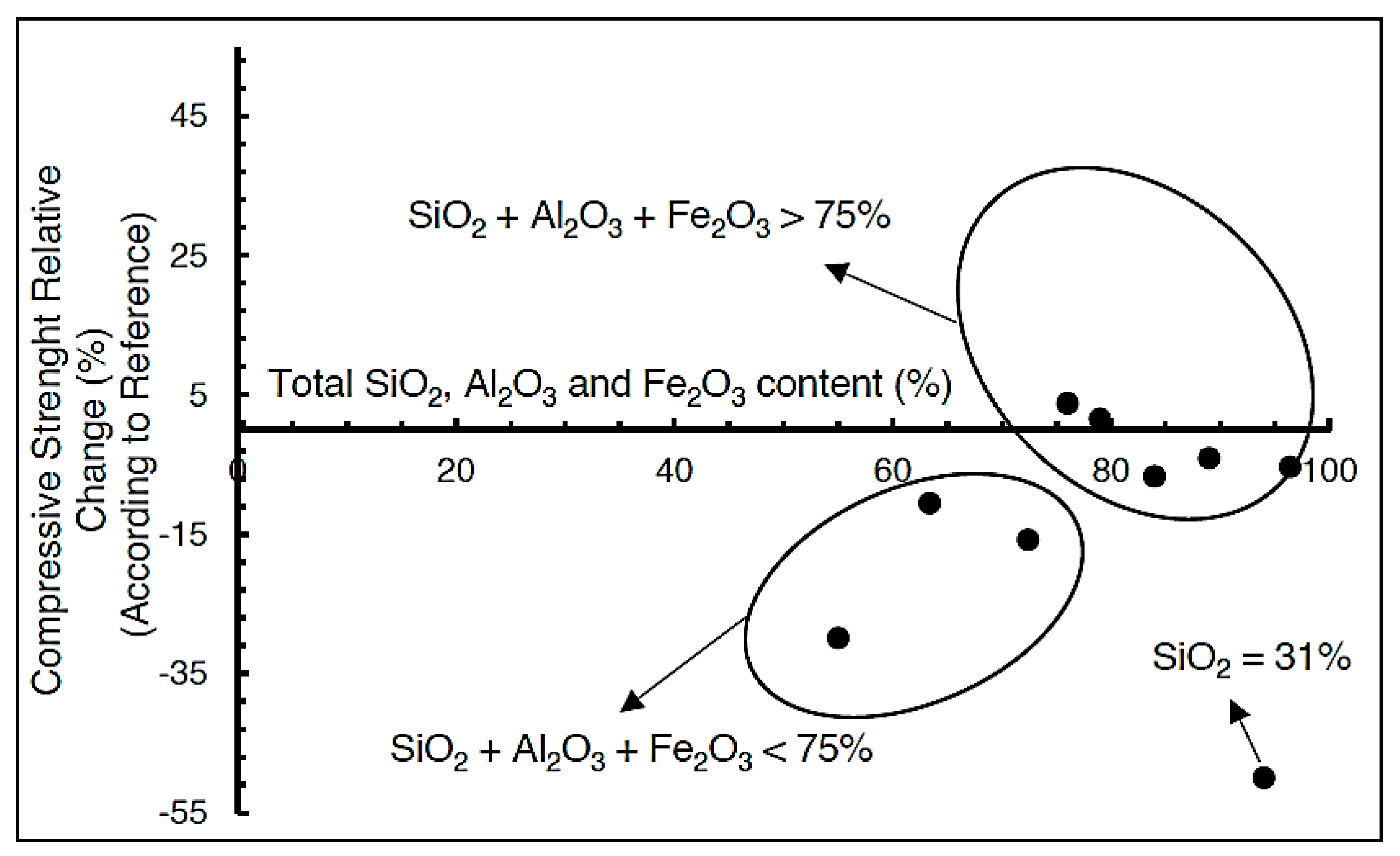
4.3. Uniaxial Compressive Strength
4.4. Assessment of Acid Formation Potential—Static Test
4.5. The Process of Leaching Toxic Elements
4.6. Assessment of Leaching of Heavy Metals from Monolithic Waste
5. Conclusions and Future Perspectives
- Acid generation potential analysis (static test) showed that the representative sample C3/40 (comprising 20% Portland cement (PC) and 80% FT, with a sulfur concentration in FT of 39.82%) has no acid generation potential (NAF). This confirms the effective neutralization of sulfides in the sample.
- Uniaxial compressive strength tests of samples C1, C2, and C3 after 360 days revealed values comparable to those recorded at 28 days. This indicates the absence of concrete degradation due to sulfide presence, further supporting the successful sulfide neutralization process.
- Microstructural and chemical analysis of sample C2/25, conducted using SEM-EDS, revealed a homogeneous structure without cracks or voids. The presence of hydration products—C-S-H gel, portlandite, ettringite, and tetracalcium aluminate hydrate—confirms that sulfides present in the concrete do not interfere with cement hydration or the formation of new compounds.
- Environmental impact assessments showed that all tested samples are environmentally benign. This was demonstrated using both the TCLP (Toxicity Characteristic Leaching Procedure) and heavy metal leaching tests on monolithic waste. TCLP analysis confirmed no leaching of toxic elements (Sb, As, Cu, Ba, Cd, Mo, Ni, Pb, Hg) from the samples. Furthermore, the leaching test for monolithic waste demonstrated that after 64 days, the eluates met the criteria for non-hazardous waste suitable for disposal at designated non-hazardous waste landfills.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nuorivaara, T.; Björkqvist, A.; Bacher, J.; Serna-Guerrero, R. Environmental remediation of sulfidic tailings with froth flotation: Reducing the consumption of additional resources by optimization of conditioning parameters and water recycling. J. Environ. Manag. 2019, 236, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Castillo Araiza, R.; Rodrigues, A.; Fournier, B.; Duchesne, J. A new accelerated testing scheme to evaluate the potential oxidation reaction of sulfide-bearing aggregates in concrete specimens. Cement Concr. Res. 2024, 180, 107507. [Google Scholar] [CrossRef]
- Mafra, C.; Bouzahzah, H.; Stamenov, L.; Gaydardzhiev, S. Insights on the effect of pyrite liberation degree upon the acid mine drainage potential of sulfide flotation tailings. Appl. Geochem. 2020, 123, 104774. [Google Scholar] [CrossRef]
- De Carvalho, A.L.C.B.; de Albuquerque, V.A.; Blannin, R.; Escobar, A.G.; Frenzel, M.; Rudolph, M.; Silva, A.C.; Goldmann, D. A study on the desulfurization of sulfidic mine tailings for the production of a sulfur-poor residue. Miner. Eng. 2023, 202, 108285. [Google Scholar] [CrossRef]
- Saedi, A.; Jamshidi-Zanjani, A.; Khodadadi Darban, A.; Mohseni, M.; Nejati, H. Utilization of lead–zinc mine tailings as cement substitutes in concrete construction: Effect of sulfide content. J. Build. Eng. 2022, 57, 104865. [Google Scholar] [CrossRef]
- Saedi, A.; Jamshidi-Zanjani, A.; Mohseni, M.; Khodadadi Darban, A. Effect of mechanochemical activation on hydration properties of lead-zinc sulfide tailings for concrete construction. Case Stud. Constr. Mater. 2023, 18, e01996. [Google Scholar] [CrossRef]
- Mends, E.A.; Tita, A.M.; Hussaini, S.; Thella, J.S.; Pan, L.; Chu, P. Investigation of leaching of nickel sulfide flotation tailings to recover valuable metals. Miner. Eng. 2024, 212, 108716. [Google Scholar] [CrossRef]
- Cacciuttolo, C.; Cano, D. Environmental impact assessment of mine tailings spill considering metallurgical processes of gold and copper mining: Case studies in the Andean countries of Chile and Peru. Water 2022, 14, 3057. [Google Scholar] [CrossRef]
- Đurđevac Ignjatović, L.; Krstić, V.; Radonjanin, V.; Jovanović, V.; Malešev, M.; Ignjatović, D.; Đurđevac, V. Application of cement paste in mining works, environmental protection and sustainable development goals in the mining industry. Sustainability 2022, 14, 7902. [Google Scholar] [CrossRef]
- Lu, H.J.; Qi, C.C.; Chen, Q.S.; Gan, D.Q.; Xue, Z.L.; Hu, Y.J. A new procedure for recycling waste tailings as cemented paste backfill to underground stopes and open pits. J. Clean. Prod. 2018, 188, 601–612. [Google Scholar] [CrossRef]
- Martins, N.P.; Helser, J.; Plötze, M.; Snellings, R.; Habert, G. Effect of sulfidic mine tailings used as mineral admixtures on the hydration of common and alternative cement. Mater. Struct. 2024, 57, 19. [Google Scholar] [CrossRef]
- Na, H.; Eom, Y.; Yoo, K.; Diaz Alorro, R. A review on the reprocessing of sulfide tailings for resource recovery and AMD prevention using mineral processing methods. Miner. Eng. 2024, 218, 109025. [Google Scholar] [CrossRef]
- Pomponi, F.; Moncaster, A. Circular economy for the built environment: A research framework. J. Clean. Prod. 2017, 143, 710–718. [Google Scholar] [CrossRef]
- Barbosa Júnior, I.D.O.; Macêdo, A.N.; Martins, V.W.B. Construction industry and its contributions to achieving the SDGs proposed by the UN: An analysis of sustainable practices. Buildings 2023, 13, 1168. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Dandautiya, R.; Singh, A.P. Utilization potential of fly ash and copper tailings in concrete as partial replacement of cement along with life cycle assessment. Waste Manag. 2019, 99, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Zunino, F.; Lopez, M. Decoupling the physical and chemical effects of supplementary cementitious materials on strength and permeability: A multi-level approach. Cem. Concr. Compos. 2016, 65, 19–28. [Google Scholar] [CrossRef]
- Gutteridge, W.A.; Dalziel, J.A. Filler cement: The effect of the secondary component on the hydration of Portland cement: Part, I. A fine non-hydraulic filler. Cem. Concr. Res. 1990, 20, 778–782. [Google Scholar] [CrossRef]
- Martins, N.P.; Srivastava, S.; Simão, F.V.; Niu, H.; Perumal, P.; Snellings, R.; Ilikainen, M.; Chambart, H.; Habert, G. Exploring the Potential for Utilization of Medium and Highly Sulfidic Mine Tailings in Construction Materials: A Review. Sustainability 2021, 13, 12150. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Aqueous pyrite oxidation and the consequent formation of secondary iron minerals. In Acid Sulfate Weathering; Kittrick, J.A., Fanning, D.S., Hossner, L.R., Eds.; Soil Science Society of America: Madison, WI, USA, 1982; pp. 37–56. [Google Scholar]
- Singer, P.C.; Stumm, W. Kinetics of the oxidation of ferrous iron. In Proceedings of the Second Symposium on Coal Mine Drainage Research, Pittsburgh, PA, USA, 14–15 May 1968; pp. 12–34. [Google Scholar]
- Smith, E.E.; Svanks, K.; Shumate, K.S. Sulfide to sulfate reaction studies. In Proceedings of the Second Symposium on Coal Mine Drainage Research, Pittsburgh, PA, USA, 14–15 May 1968; pp. 1–11. [Google Scholar]
- Garrels, R.M.; Thompson, M.E. Oxidation of pyrite in ferric sulfate solution. Am. J. Sci. 1960, 258, 57–67. [Google Scholar]
- Singer, P.C.; Stumm, W. Oxygenation of Ferrous Iron; Rep. 14010-06/69; FWQA: Lakeland, FL, USA, 1969. [Google Scholar]
- Moricz, F.; Madai, F.; Walder, I.F. Pyrite oxidation under circumneutral pH conditions. In Proceedings of the 23rd Conference on Earth Science and Environmental Protection, Miskolc, Hungary, 27–29 September 2012; Available online: https://www.researchgate.net/publication/328530759 (accessed on 4 March 2025).
- Trafford, B.D.; Bloomfield, C.; Kelso, W.I.; Pruden, G. Ochre formation in field drains in pyritic soils. J. Soil Sci. 1973, 24, 453–460. [Google Scholar] [CrossRef]
- Amaro, A.M.; Chamorro, D.; Seeger, M.; Arrendondo, R.; Perrano, I.; Jerez, C.A. Effect of external pH perturbations on in vivo protein synthesis by the acidophilic bacterium Thiobacillus ferrooxidans. J. Bacteriol. 1991, 173, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I. Mechanisms of inorganic oxidation and energy coupling. Annu. Rev. Microbiol. 1974, 28, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Temple, K.L.; Colmer, A.R. The autotrophic oxidation of iron by a new bacterium: Thiobacillus ferrooxidans. J. Bacteriol. 1951, 62, 605–611. [Google Scholar] [CrossRef]
- Lyalikova, N.N. A study of chemosynthesis in Thiobacillus ferrooxidans. Microbiologiya 1958, 27, 556–559. [Google Scholar]
- Hooper, A.B.; DiSpirito, A.A. In bacteria which grow in simple reductants, generation of proton gradient involves extra cytoplasmic oxidation of substrate. Microbial. Rev. 1985, 49, 140–157. [Google Scholar] [CrossRef]
- Ingledew, W.J.; Cobley, J.G. A potentiometric and kinetic study on the respiratory chain of ferrous iron-grown Thiobacillus ferrooxidans. Biochim. Biophys. Acta 1980, 590, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Ingledew, W.J. Thiobacillus ferrooxidans—The bioenergetics of an acidophilic chemolithotroph. Biochim. Biophys. Acta 1982, 683, 89–117. [Google Scholar]
- Skousen, J.; Foreman, J. Water management techniques for acid mine drainage control. Green Lands 2000, 30, 44–53. [Google Scholar]
- Singer, P.C.; Stumm, W. Acid mine drainage: The rate determining step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef]
- Kirby, D. Effective Treatment Options for Acid Mine Drainage in the Coal Region of West Virginia. Master’s Thesis, Marshall University, Huntington, WV, USA, 2014. [Google Scholar]
- SRPS EN 12457-2:2008; Characterization of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid-Solid Ratio of 10 l/kg for Materials with a High Content Solids and Particle Size of Less than 4 mm (with Decreasing Particle Size, or Without Decreasing). Institute for Standardization of Serbia: Belgrade, Serbia, 2008.
- SRPS EN ISO 11885:2007; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). Institute for Standardization of Serbia: Belgrade, Serbia, 2007.
- Regulation on Categories, Testing, and Classification of Waste. Official Gazette of the RS, No. 56/2010, 93/2019, 39/2021, 65/2024. (In Serbian). Available online: https://www.paragraf.rs/propisi/pravilnik-kategorijama-ispitivanju-klasifikaciji-otpada.html (accessed on 13 March 2025).
- U.S. EPA. Method 1311, Toxicity Characteristic Leaching Procedure; U.S. Environmental Protection Agency: Washington, DC, USA, 1992. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/1311.pdf (accessed on 13 March 2025).
- SRPS EN 206-1:2011; Concrete—Part 1: Specification Performance, Production and Conformity. Institute for Standardization of Serbia: Belgrade, Serbia, 2011.
- SRPS EN ISO 17892-4; Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 4: Determination of Particle Size Distribution. Institute for Standardization of Sebia: Belgrade, Serbia, 2016.
- SRPS EN 15875:2013; Characterization of Waste—Static Test for Determination of Acid Potential and Neutralization Potential of Sulfidic Waste. Institute for Standardization of Serbia: Belgrade, Serbia, 2013.
- Karlsson, T.; Räisänen, M.L.; Lehtonen, M.; Alakangas, L. Comparison of static and mineralogical ARD prediction methods in the Nordic environment. Environ. Monit. Assess. 2018, 190, 719. [Google Scholar] [CrossRef]
- NEN 7375; Leaching Characteristics of Moulded or Monolithic Building and Waste Materials—Determination of Leaching of Inorganic Components with the Diffusion Test—“The Tank Test”. Netherlands Normalisation Institute Standard: Amsterdam, The Netherlands, 2004.
- SRPS EN 12390-3:2019; Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens. Institute for Standardization of Serbia: Belgrade, Serbia, 2019.
- Xiong, B.; Wu, X.; Liu, W.; Lu, X.; Gao, H.; Lv, W. Influence of different aggregate characteristics on pervious concrete. Constr. Build. Mater. 2025, 460, 139789. [Google Scholar] [CrossRef]
- Grdić, Z. Concrete Technology; Printing House “SVEN”: Niš, Serbia, 2011; ISBN 978-86-80295-94-7. [Google Scholar]
- Koohestani, B.; Belem, T.; Koubaa, A.; Bussiere, B. Experimental investigation into the compressive strength development of cemented paste backfill containing Nano-silica. Cem. Concr. Compos. 2016, 72, 180–189. [Google Scholar] [CrossRef]
- Koohestani, B. Effect of saline admixtures on mechanical and microstructural properties of cementitious matrices containing tailings. Constr. Build. Mater. 2017, 156, 1019–1027. [Google Scholar] [CrossRef]
- Dong, Q.; Liang, B.; Jia, L.; Jiang, L. Effect of sulfide on the long-term strength of lead-zinc tailings cemented paste backfill. Constr. Build. Mater. 2019, 200, 436–446. [Google Scholar] [CrossRef]
- Wang, L.; Guo, F.; Lin, Y.; Yang, H.; Tang, S.W. Comparison between the effects of phosphorous slag and fly ash on the C-S-H structure, long-term hydration heat and volume deformation of cement-based materials. Constr. Build. Mater. 2020, 250, 118807. [Google Scholar] [CrossRef]
- Bušatlić, I.; Bušatlić, N.; Merdić, N.; Haračić, N. Basics of Portland Cement Chemistry and Technology; Printing House Fojnica d.d.: Fojnica, Bosnia and Herzegovina, 2020; pp. 171–176. Available online: https://www.researchgate.net/publication/343994073 (accessed on 20 April 2025)ISBN 978-9958-17-168-0.
- Geng, Z.; Tang, S.; Wang, Y.; A, H.; He, Z.; Wu, K.; Wang, L. Stress relaxation properties of calcium silicate hydrate: A molecular dynamics study. J. Zhejiang Univ.-Sci. A Appl. Phys. Eng. 2024, 25, 97–115. [Google Scholar] [CrossRef]
- Wang, L.; Jin, M.; Zhou, S.; Tang, S.; Lu, X. Investigation of microstructure of C-S-H and micro-mechanics of cement pastes under NH4NO3 dissolution by 29Si MAS NMR and microhardness. Measurement 2021, 185, 110019. [Google Scholar] [CrossRef]
- Mohamed, A.R.; Elsalamawy, M.; Ragab, M. Modeling the influence of limestone addition on cement hydration. Alex. Eng. J. 2015, 54, 1–5. [Google Scholar] [CrossRef]
- Sampson, M.I.; Phillips, C.V.; Ball, A.S. Investigation of the attachment of Thiobacillus ferrooxidans to mineral sulfides using scanning electron microscopy analysis. Miner. Eng. 2000, 13, 643–656. [Google Scholar] [CrossRef]
- Adiguzel, D.; Tuylu, S.; Eker, H. Utilization of tailings in concrete products: A review. Constr. Build. Mater. 2022, 360, 129574. [Google Scholar] [CrossRef]
- SRPS EN 450-1:2014; Fly Ash for Concrete—Part 1: Definition, Specifications and Conformity Criteria. Institute for Standardization of Serbia: Belgrade, Serbia, 2014.
- Sigvardsen, N.M.; Nielsen, M.R.; Potier, C.; Ottosen, L.M.; Jensen, P.E. Utilization of Mine Tailings As Partial Cement Replacement. Mod. Environ. Sci. Eng. 2018, 4, 365–374. [Google Scholar]
- Moosberg-Bustnes, H.; Lagerblad, B.; Forssberg, E. The function of fillers in concrete. Mater. Struct. 2004, 37, 74–81. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Xiong, Z.; Wang, Y.; Han, Y. Experimental study of high-flow and low-expansion backfill material. PLoS ONE 2020, 15, e0236718. [Google Scholar] [CrossRef]
- Ye, G.; Liu, X.; De Schutter, G.; Poppe, A.-M.; Taerwe, L. Influence of limestone powder used as filler in SCC on hydration and microstructure of cement pastes. Cem. Concr. Compos. 2007, 29, 94–102. [Google Scholar] [CrossRef]
- Deschner, F.; Winnefeld, F.; Lothenbach, B.; Seufert, S.; Schwesig, P.; Dittrich, S.; Goetz-Neunhoeffer, F.; Neubauer, J. Hydration of Portland cement with high replacement by siliceous fly ash. Cem. Concr. Res. 2012, 42, 1389–1400. [Google Scholar] [CrossRef]
- Nonat, A. Interactions between chemical evolution (hydration) and physical evolution (setting) in the case of tricalcium silicate. Mater. Struct. 1994, 27, 187–195. [Google Scholar] [CrossRef]
- Lawrence, P.; Cyr, M.; Ringot, E. Mineral admixtures in mortars effect of type, amount and fineness of fine constituents on compressive strength. Cem. Concr. Res. 2005, 35, 1092–1105. [Google Scholar] [CrossRef]
- Pervious Concrete: Guideline to Mixture Proportioning, NRMCA Engineering Division, NRMCA Publication Number: 2PE001, Version 1, February 2009. Available online: https://www.nrmca.org/wp-content/uploads/Pervious_Concrete_Guideline_Mixture_Proportioning.pdf (accessed on 13 March 2025).
- da Silva, R.G.; Couto, D.; Tavares, A.; Ribeiro, M.M.; Chaves, L.; Guimarães, L.; Fonseca, K.; Silva, A.C. Static tests to assess acid mine drainage potential of copper sulfide flotation tailings. Tecnol. Metal Mater. Min. 2023, 20, e2792. [Google Scholar] [CrossRef]
- Saedi, A.; Jamshidi-Zanjani, A.; Mohseni, M.; Darban, A.K. Mechanical activation for sulfidic tailings treatment by tailings: Environmental aspects and cement consumption reduction. Case Stud. Constr. Mater. 2023, 19, e02632. [Google Scholar] [CrossRef]


| Sample Abbreviation | Sample Composition | ||
|---|---|---|---|
| C100 | 100%PC | ||
| PC80-FT20 (C1) | PC80-FT20/7 | C1/7 | 80%PC, 20%FT and 7.56% S in FT |
| PC80-FT20/14 | C1/14 | 80%PC, 20%FT and 13.84% S in FT | |
| PC80-FT20/25 | C1/25 | 80%PC, 20%FT and 25.02% S in FT | |
| PC80-FT20/40 | C1/40 | 80%PC, 20%FT and 39.82% S in FT | |
| PC40-FT60 (C2) | PC40-FT60/7 | C2/7 | 40% PC, 60% FT and 7.56% S in FT |
| PC40-FT60/14 | C2/14 | 40% PC, 60% FT and 13.84% S in FT | |
| PC40-FT60/25 | C2/25 | 40% PC, 60% FT and 25.02% S in FT | |
| PC40-FT60/40 | C2/40 | 40% PC, 60% FT and 39.82% S in FT | |
| PC20-FT80 (C3) | PC20-FT80/7 | C3/7 | 20% PC, 80% FT and 7.56% S in FT |
| PC20-FT80/14 | C3/14 | 20% PC, 80% FT and 13.84% S in FT | |
| PC20-FT80/25 | C3/25 | 20% PC, 80% FT and 25.02% S in FT | |
| PC20-FT80/40 | C3/40 | 20% PC, 80% FT and 39.82% S in FT | |
| Component (wt.%) | PC | FT-7 | FT-14 | FT-25 | FT-40 |
|---|---|---|---|---|---|
| SiO2 | 24.56 | 60.21 | 56.31 | 52.14 | 40.21 |
| Al2O3 | 4.85 | 17.68 | 14.61 | 8.99 | 4.66 |
| Fe2O3 | 3.87 | 9.21 | 16.76 | 29.44 | 45.45 |
| CaO | 62.59 | 4.49 | 4.56 | 2.83 | 0.67 |
| K2O | 0.83 | 1.64 | 1.2 | 0.9 | 0.58 |
| Na2O | 0.45 | 1.2 | 1.07 | 0.95 | 0.85 |
| MgO | 2.01 | 1.66 | 0.78 | 0.96 | 0.22 |
| P2O5 | 0.027 | 0.12 | 0.078 | 0.089 | 0.05 |
| SO3 | 0.80 | 0.19 | 0.43 | 1.47 | 3.46 |
| Cl− | n.d. * | n.d. * | n.d. * | n.d. * | n.d. * |
| Total S | 0.32 | 7.56 | 13.84 | 25.02 | 39.82 |
| Loss on ignition | - | 7.99 | 12.75 | 17.29 | 25.71 |
| Sample Label | d10 (µm) | d50 (µm) | d90 (µm) | SG (g/cm3) |
|---|---|---|---|---|
| PC | 2.01 | 14.1 | 44.3 | 3.050 |
| FT-7 | 30.1 | 205.2 | 470.2 | 2.826 |
| FT-14 | 9.1 | 98.2 | 240.3 | 2.962 |
| FT-25 | 7.2 | 75.3 | 260.2 | 3.463 |
| FT-40 | 2.2 | 36.3 | 140.3 | 4.342 |
| FT with Different Sulfur Content | C1 (PC80-FT20) | ||||||
| Uniaxial Compressive Strength, [MPa] | Mean Value of Uniaxial Compressive Strength, [MPa] | StDEV | |||||
| 28 Day | 90 Day | 120 Day | 180 Day | 360 Day | |||
| C-100 | 38.97 | 40.68 | 41.88 | 42.87 | 43.21 | 41.52 | 2.151 |
| C1/7 | 42.33 | 42.44 | 43.08 | 43.57 | 43.91 | 43.07 | 0.855 |
| C1/14 | 42.15 | 42.36 | 43.68 | 43.91 | 44.01 | 43.22 | 1.109 |
| C1/25 | 29.88 | 30.61 | 30.85 | 31.02 | 31.35 | 30.74 | 0.685 |
| C1/40 | 28.38 | 28.74 | 29.95 | 31.39 | 31.97 | 30.09 | 1.961 |
| FT with Different Sulfur Content | C2 (PC40-FT60) | ||||||
| Uniaxial Compressive Strength, [MPa] | Mean Value of Uniaxial Compressive Strength, [MPa] | StDEV | |||||
| 28 Day | 90 Day | 120 Day | 180 Day | 360 Day | |||
| C-100 | 38.97 | 40.68 | 41.88 | 42.87 | 43.21 | 41.52 | 2.151 |
| C2/7 | 23.02 | 24.45 | 27.04 | 27.83 | 27.98 | 26.06 | 2.748 |
| C2/14 | 12.49 | 14.32 | 14.9 | 16.66 | 16.97 | 15.07 | 2.270 |
| C2/25 | 12.58 | 14.52 | 15.98 | 15.98 | 16.02 | 15.02 | 1.866 |
| C2/40 | 14.09 | 15.54 | 16.42 | 16.55 | 16.91 | 15.90 | 1.404 |
| FT with Different Sulfur Content | C3 (PC20-FT80) | ||||||
| Uniaxial Compressive Strength, [MPa] | Mean Value of Uniaxial Compressive Strength, [MPa] | StDEV | |||||
| 28 Day | 90 Day | 120 Day | 180 Day | 360 Day | |||
| C-100 | 38.97 | 40.68 | 41.88 | 42.87 | 43.21 | 41.52 | 2.151 |
| C3/7 | 3.30 | 4.40 | 6.46 | 7.48 | 7.89 | 5.91 | 2.465 |
| C3/14 | 3.45 | 5.11 | 6.74 | 7.22 | 7.92 | 6.09 | 2.235 |
| C3/25 | 3.15 | 4.91 | 6.61 | 7.16 | 7.93 | 5.95 | 2.382 |
| C3/40 | 4.01 | 6.03 | 6.23 | 6.60 | 7.77 | 6.13 | 1.691 |
| Sample Code | Porosity [%] | Porosity StDEV | Mean Value of Uniaxial Compressive Strength [MPa] | StDEV | |
|---|---|---|---|---|---|
| C1 (PC80-FT20) | T-7 | 3.24 | 1.331 | 43.07 | 2.151 |
| T-40 | 6.61 | 1.530 | 30.09 | 1.961 | |
| C2 (PC40-FT60) | T-7 | 6.82 | 1.594 | 26.06 | 2.748 |
| T-40 | 8.76 | 1.004 | 15.90 | 1.404 | |
| C3 (PC20-FT80) | T-7 | 9.08 | 1.783 | 5.91 | 2.151 |
| T-40 | 9.56 | 1.706 | 6.13 | 1.691 | |
| Static Test | FT-7 | FT-14 | FT-25 | FT-40 |
|---|---|---|---|---|
| S, [%] | 7.56 | 13.84 | 25.02 | 39.82 |
| AP, [CaCO3 kg/t] | 236.25 | 432.50 | 781.88 | 1244.38 |
| NP, [CaCO3 kg/t] | 13.13 | 5.25 | 4.25 | 11.75 |
| NNP, [CaCO3 kg/t] | −223.13 | −427.25 | −777.63 | −1256.13 |
| NP/AP | 0.06 | 0.01 | 0.01 | −0.01 |
| K2O+CaO+MgO, [%] | 7.79 | 6.54 | 4.69 | 1.47 |
| Sample characterization | PAF * | PAF * | PAF * | PAF * |
| Sample | S, [%] | AP, [CaCO3 kg/t] | NP, [CaCO3 kg/t] | NNP, [CaCO3 kg/t] | NP/AP | Sample Characterization |
|---|---|---|---|---|---|---|
| C3/40 | 5.62 | 175.63 | 734.85 | 559.23 | 4.18 | NAF * |
| Parameter | Unit | FT-7 | FT-14 | FT-25 | FT-40 | Reference Value for Non-Hazardous Waste * |
|---|---|---|---|---|---|---|
| Sb | mg/L | <0.006 | <0.006 | <0.006 | <0.006 | 15 |
| As | mg/L | <0.020 | <0.020 | <0.020 | <0.020 | 5 |
| Cu | mg/L | 4.9 | 16.9 | 2.9 | 1.2 | 25 |
| Ba | mg/L | 0.080 | 0.063 | 0.061 | 0.036 | 100 |
| Cd | mg/L | 0.004 | 0.041 | <0.004 | 0.007 | 1 |
| Mo | mg/L | <0.007 | <0.007 | <0.007 | <0.007 | 350 |
| Ni | mg/L | 0.024 | 0.030 | 0.008 | <0.007 | 20 |
| Pb | mg/L | 0.27 | 0.15 | 0.080 | 1.0 | 5 |
| Se | mg/L | <0.004 | <0.004 | <0.004 | <0.004 | 1 |
| Cr | mg/L | 0.045 | 0.034 | 0.010 | 0.015 | 5 |
| Zn | mg/L | 2.3 | 2.0 | 0.73 | 0.51 | 250 |
| Hg | mg/L | ˂0.0005 | ˂0.0005 | ˂0.0005 | ˂0.0005 | 0.2 |
| V | mg/L | <0.007 | <0.007 | <0.007 | <0.007 | 24 |
| Ag | mg/L | <0.005 | <0.005 | <0.005 | <0.005 | 5 |
| Parameter | Unit | FT-40 | C1/40 after 28 Days | C3/40 after 28 Days | C1/40 after 90 Days | C3/40 after 90 Days | Reference Value for Non-Hazardous Waste * |
|---|---|---|---|---|---|---|---|
| Sb | mg/L | <0.006 | 0.003 | 0.005 | 0.001 | 0.004 | 15 |
| As | mg/L | <0.020 | 0.0092 | 0.013 | 0.0088 | 0.010 | 5 |
| Cu | mg/L | 1.2 | 0.55 | 1.11 | 0.45 | 0.96 | 25 |
| Ba | mg/L | 0.036 | 0.021 | 0.033 | 0.018 | 0.03 | 100 |
| Cd | mg/L | 0.007 | 0.003 | 0.006 | 0.002 | 0.005 | 1 |
| Mo | mg/L | <0.007 | 0.003 | 0.005 | 0.001 | 0.005 | 350 |
| Ni | mg/L | <0.007 | 0.004 | 0.006 | 0.002 | 0.004 | 20 |
| Pb | mg/L | 1.0 | 0.23 | 0.88 | 0.12 | 0.64 | 5 |
| Se | mg/L | <0.004 | 0.002 | 0.003 | 0.001 | 0.002 | 1 |
| Cr | mg/L | 0.015 | 0.0082 | 0.011 | 0.0061 | 0.0098 | 5 |
| Zn | mg/L | 0.51 | 0.25 | 0.43 | 0.11 | 0.31 | 250 |
| Hg | mg/L | ˂0.0005 | 0.0002 | 0.0004 | 0.0001 | 0.0003 | 0.2 |
| V | mg/L | <0.007 | 0.002 | 0.006 | 0.001 | 0.004 | 24 |
| Ag | mg/L | <0.005 | 0.002 | 0.004 | 0.001 | 0.003 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đurđevac, V.; Staletović, N.; Ignjatović, L.Đ.; Jovanović, V.; Vuković, N.; Krstić, V. Application of Flotation Tailings as a Substitute for Cement in Concrete Structures for Environmental Protection and Sustainable Development—Part I: Sulfide Neutralization. Materials 2025, 18, 2804. https://doi.org/10.3390/ma18122804
Đurđevac V, Staletović N, Ignjatović LĐ, Jovanović V, Vuković N, Krstić V. Application of Flotation Tailings as a Substitute for Cement in Concrete Structures for Environmental Protection and Sustainable Development—Part I: Sulfide Neutralization. Materials. 2025; 18(12):2804. https://doi.org/10.3390/ma18122804
Chicago/Turabian StyleĐurđevac, Vanja, Novica Staletović, Lidija Đurđevac Ignjatović, Violeta Jovanović, Nikola Vuković, and Vesna Krstić. 2025. "Application of Flotation Tailings as a Substitute for Cement in Concrete Structures for Environmental Protection and Sustainable Development—Part I: Sulfide Neutralization" Materials 18, no. 12: 2804. https://doi.org/10.3390/ma18122804
APA StyleĐurđevac, V., Staletović, N., Ignjatović, L. Đ., Jovanović, V., Vuković, N., & Krstić, V. (2025). Application of Flotation Tailings as a Substitute for Cement in Concrete Structures for Environmental Protection and Sustainable Development—Part I: Sulfide Neutralization. Materials, 18(12), 2804. https://doi.org/10.3390/ma18122804






