Valorizing Organic Waste: Selenium Sulfide Production Mediated by Sulfate-Reducing Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
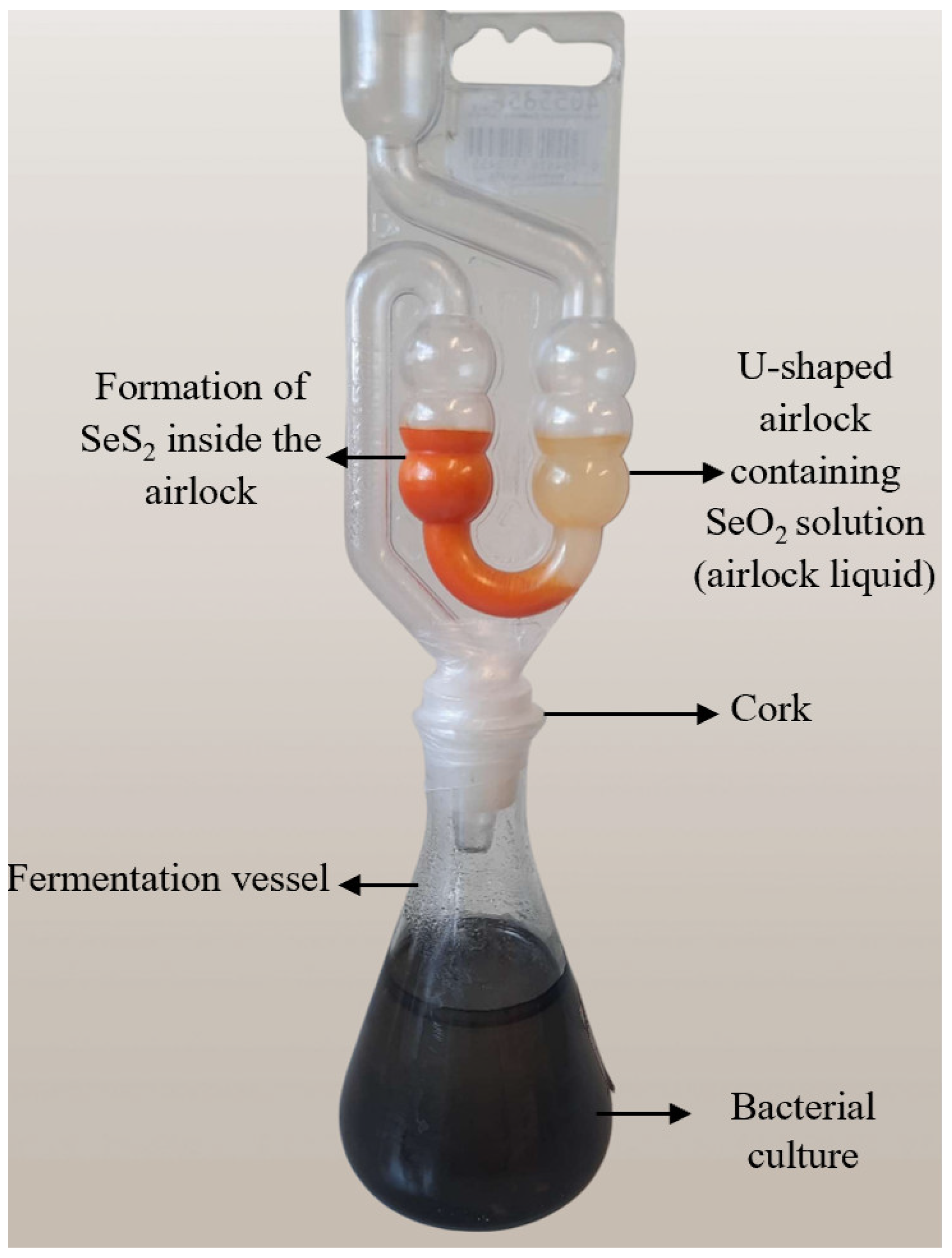
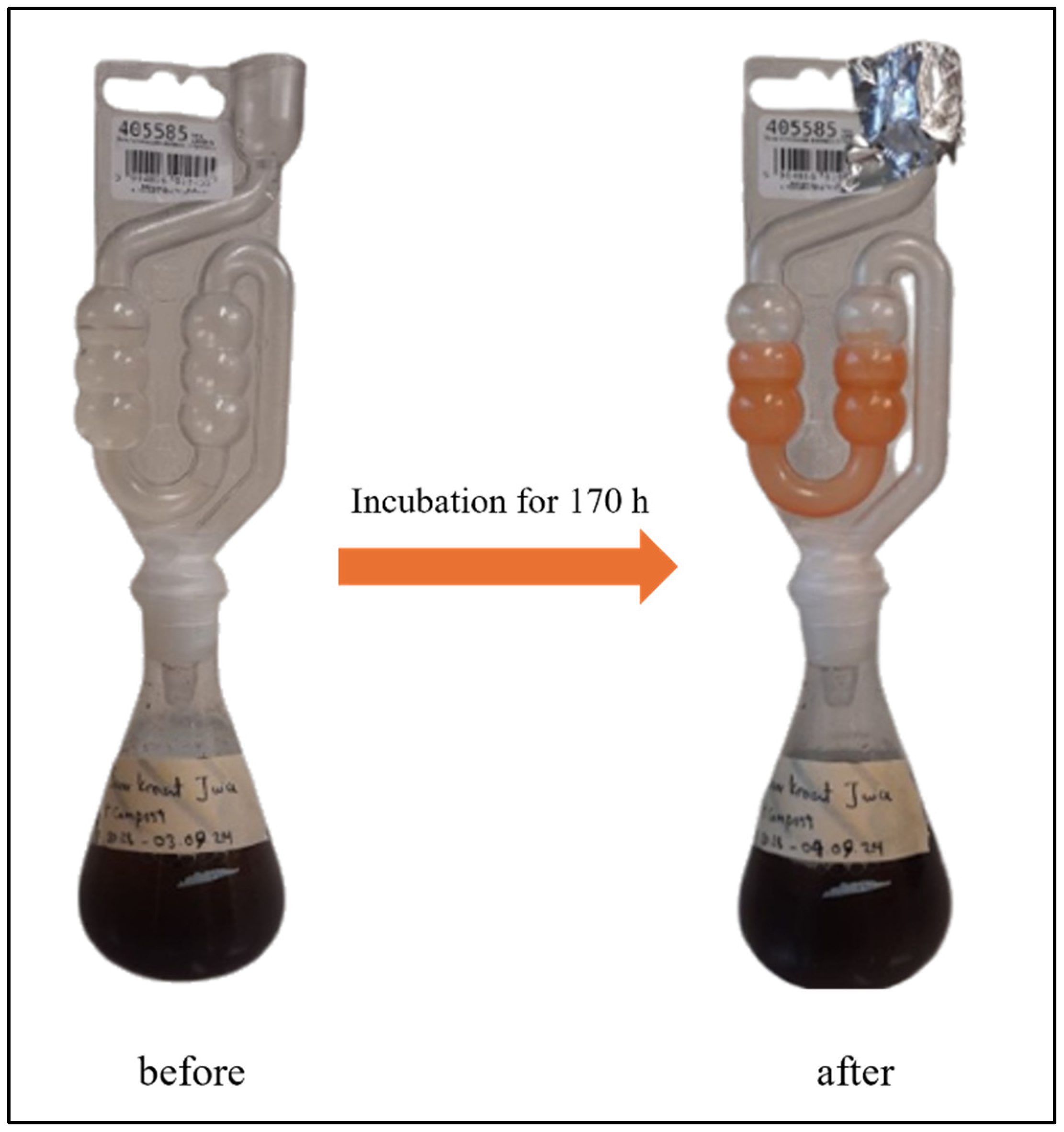
2.2. Design of Fermentation Equipment
2.3. Cultivation of SRB
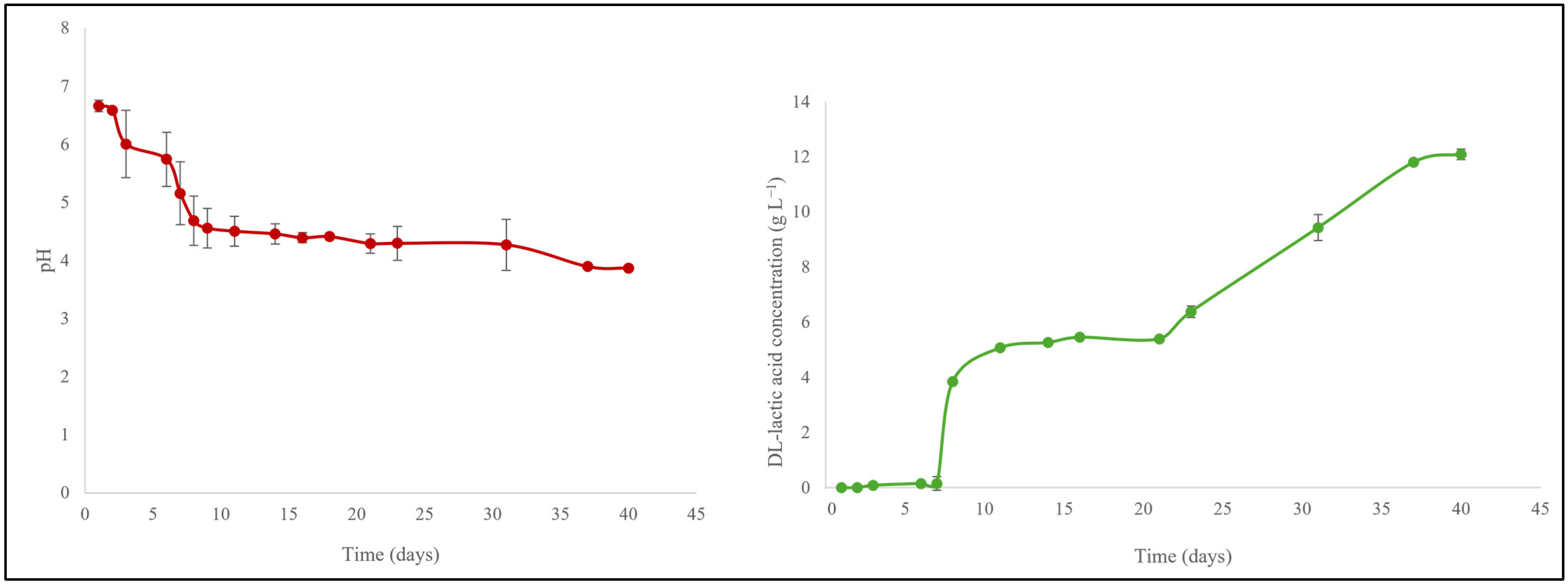
2.4. Quantification of DL-Lactic Acid
2.5. Quantification of H2S
2.6. Production of Selenium Sulfide
2.7. Characterization of Selenium Sulfide
3. Results
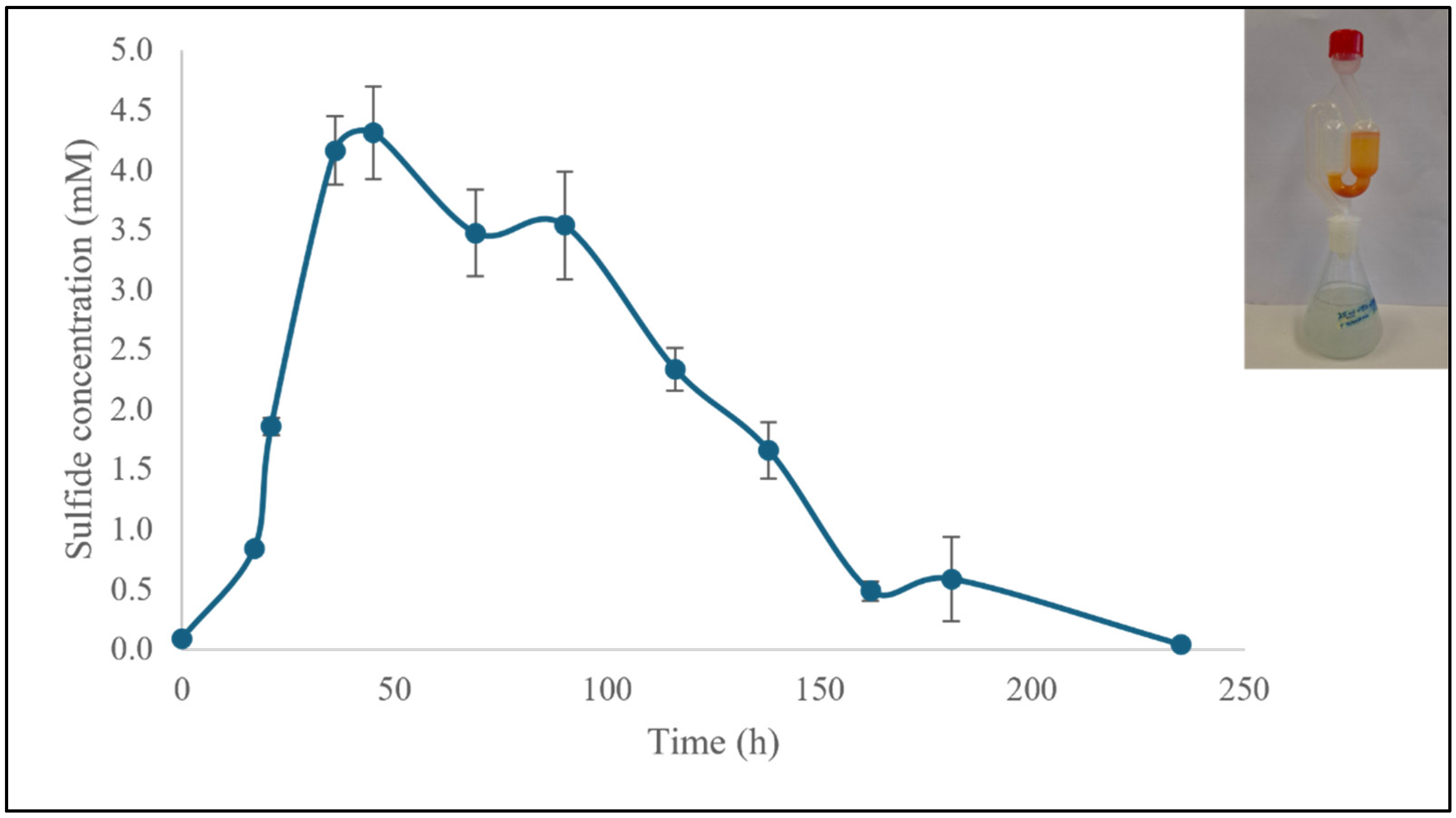
3.1. Fermentation Under Standard Conditions
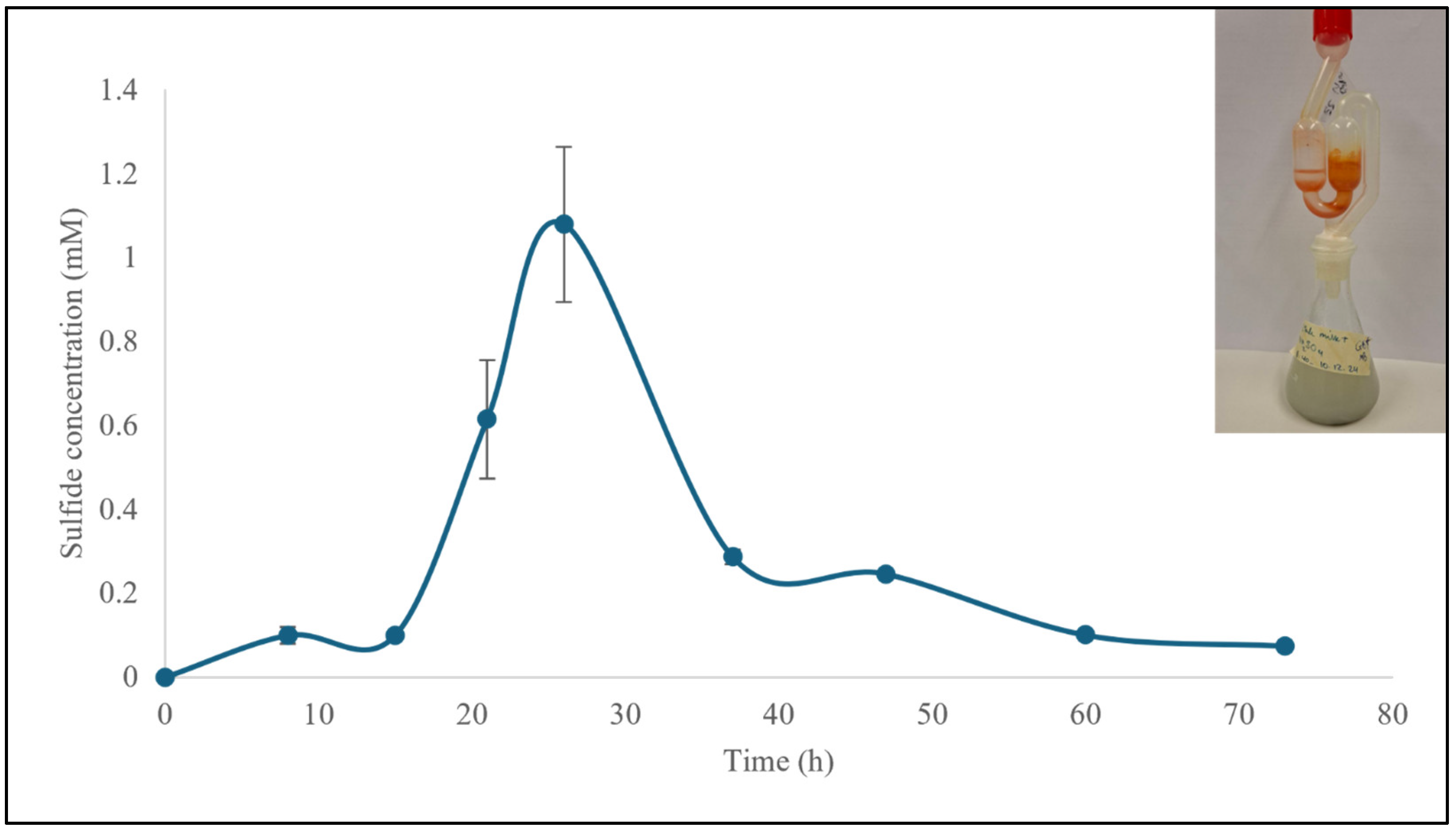
3.1.1. Production and Quantification of H2S
3.1.2. Selenium Sulfide Production
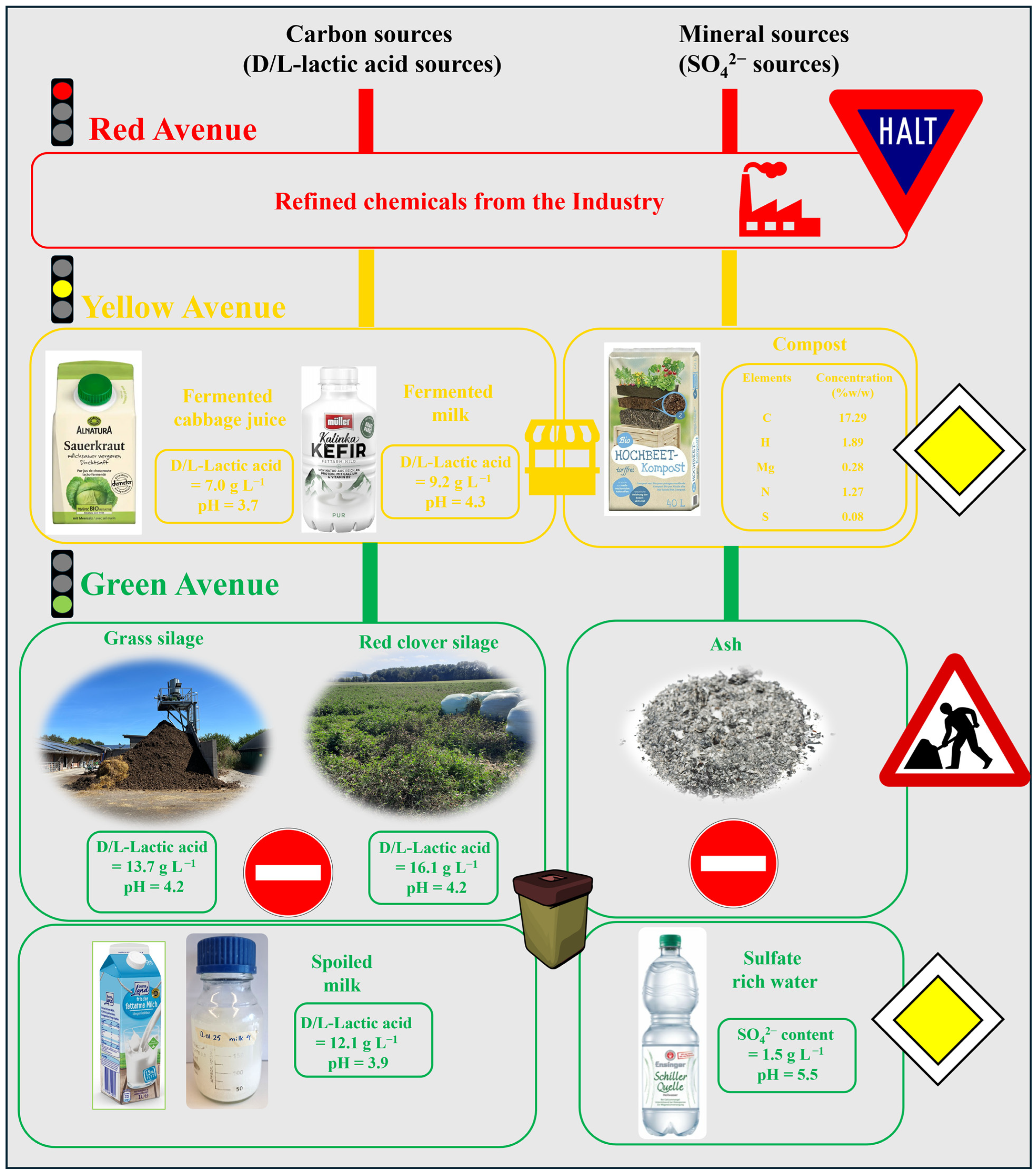
3.2. Variations on the Theme
3.2.1. The Sauerkraut Connection
3.2.2. The Silage Saga
3.2.3. The Milky Way
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heath, R.S.; Ruscoe, R.E.; Turner, N.J. The Beauty of Biocatalysis: Sustainable Synthesis of Ingredients in Cosmetics. Nat. Prod. Rep. 2022, 39, 335–388. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.N.; Zhang, D.; Miller, S.J.; Rossen, K.; Chirik, P.J.; Kozlowski, M.C.; Zimmerman, J.B.; Brooks, B.W.; Savage, P.E.; Allen, D.T.; et al. Green Chemistry: A Framework for a Sustainable Future. Environ. Sci. Technol. Lett. 2021, 8, 487–491. [Google Scholar] [CrossRef]
- Martinengo, B.; Diamanti, E.; Uliassi, E.; Bolognesi, M.L. Harnessing the 12 Green Chemistry Principles for Sustainable Antiparasitic Drugs: Toward the One Health Approach. ACS Infect. Dis. 2024, 10, 1856–1870. [Google Scholar] [CrossRef] [PubMed]
- Wynendaele, E.; Furman, C.; Wielgomas, B.; Larsson, P.; Hak, E.; Block, T.; Van Calenbergh, S.; Willand, N.; Markuszewski, M.; Odell, L.R.; et al. Sustainability in Drug Discovery. Med. Drug Discov. 2021, 12, 100107. [Google Scholar] [CrossRef]
- Razouk, A.; Tiganescu, E.; von Glahn, A.J.; Abdin, A.Y.; Nasim, M.J.; Jacob, C. The Future in the Litter Bin—Bioconversion of Food Waste as Driver of a Circular Bioeconomy. Front. Nutr. 2024, 11, 1325190. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Feng, K.; Liu, J. Oriented Fermentation of Food Waste towards High-Value Products: A Review. Energies 2020, 13, 5638. [Google Scholar] [CrossRef]
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and By-Products Through Fermentation Processes. Front. Microbiol. 2020, 11, 581997. [Google Scholar] [CrossRef]
- Zhu, S.H.; Zhu, S.M.; Zhu, L. Production Method of Selenium Sulfide. CN 201010571362, 25 November 2010. [Google Scholar]
- Steudel, R.; Laitinen, R. Cyclic Selenium Sulfides. In Inorganic Ring Systems; Springer: Berlin/Heidelberg, Germany, 1982; pp. 177–197. [Google Scholar]
- Laitinen, R.S. Selenium Sulfide Ring Molecules. Acta Chem. Scand. 1987, 41, 361–376. [Google Scholar] [CrossRef]
- Laitinen, R.S.; Pakkanen, T.A. Selenium-77 NMR Spectroscopic Characterization of Selenium Sulfide Ring Molecules SenS8−n. Inorg. Chem. 1987, 26, 2598–2603. [Google Scholar] [CrossRef]
- Ausma, T.; De Kok, L.J. Atmospheric H2S: Impact on Plant Functioning. Front. Plant Sci. 2019, 10, 743. [Google Scholar] [CrossRef]
- Aiuppa, A.; Moussallam, Y. Hydrogen and hydrogen sulphide in volcanic gases: Abundance, processes, and atmospheric fluxes. Comptes Rendus. Géoscience 2024, 356, 85–108. [Google Scholar] [CrossRef]
- Carbajo, J.M.; Maraver, F. Sulphurous Mineral Waters: New Applications for Health. Evid.-Based Complement. Altern. Med. 2017, 2017, 8034084. [Google Scholar] [CrossRef] [PubMed]
- Malone Rubright, S.L.; Pearce, L.L.; Peterson, J. Environmental Toxicology of Hydrogen Sulfide. Nitric Oxide 2017, 71, 1–13. [Google Scholar] [CrossRef]
- Tiganescu, E.; Safinazlou, S.; Abdin, A.Y.; Lilischkis, R.; Schäfer, K.-H.; Fink-Straube, C.; Nasim, M.J.; Jacob, C. Selenium Disulfide from Sustainable Resources: An Example of “Redneck” Chemistry with a Pinch of Salt. Materials 2024, 17, 5733. [Google Scholar] [CrossRef]
- Quellen. Available online: https://www.badnenndorf.de/gesundheit-wellness/quellen (accessed on 3 April 2025).
- Tian, G.; Yeung, M.; Xi, J. H2S Emission and Microbial Community of Chicken Manure and Vegetable Waste in Anaerobic Digestion: A Comparative Study. Fermentation 2023, 9, 169. [Google Scholar] [CrossRef]
- Austigard, Å.D.; Svendsen, K.; Heldal, K.K. Hydrogen Sulphide Exposure in Waste Water Treatment. J. Occup. Med. Toxicol. 2018, 13, 10. [Google Scholar] [CrossRef]
- Kushkevych, I.; Vítězová, M.; Vítěz, T.; Bartoš, M. Production of Biogas: Relationship between Methanogenic and Sulfate-Reducing Microorganisms. Open Life Sci. 2017, 12, 82–91. [Google Scholar] [CrossRef]
- Kushkevych, I.; Vítězová, M.; Vítěz, T.; Kováč, J.; Kaucká, P.; Jesionek, W.; Bartoš, M.; Barton, L. A New Combination of Substrates: Biogas Production and Diversity of the Methanogenic Microorganisms. Open Life Sci. 2018, 13, 119–128. [Google Scholar] [CrossRef]
- Kushkevych, I.; Cejnar, J.; Treml, J.; Dordević, D.; Kollar, P.; Vítězová, M. Recent Advances in Metabolic Pathways of Sulfate Reduction in Intestinal Bacteria. Cells 2020, 9, 698. [Google Scholar] [CrossRef]
- Kushkevych, I.V. Dissimilatory Sulfate Reduction in the Intestinal Sulfate-Reducing Bacteria. Біологічні студії/Stud. Biol. 2017, 10, 197–228. [Google Scholar] [CrossRef]
- Bagheri Novair, S.; Biglari, Z.; Asgari Lajayer, B.; Shu, W.; Price, G.W. The Role of Sulphate-Reducing Bacteria (SRB) in Bioremediation of Sulphate-Rich Wastewater: Focus on the Source of Electron Donors. Process Saf. Environ. Prot. 2024, 184, 190–207. [Google Scholar] [CrossRef]
- Gopi Kiran, M.; Pakshirajan, K.; Das, G. An Overview of Sulfidogenic Biological Reactors for the Simultaneous Treatment of Sulfate and Heavy Metal Rich Wastewater. Chem. Eng. Sci. 2017, 158, 606–620. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Guo, H.; Kumseranee, S.; Punpruk, S.; Mohamed, M.E.; Saleh, M.A.; Gu, T. Carbon Source Starvation of a Sulfate-Reducing Bacterium–Elevated MIC Deterioration of Tensile Strength and Strain of X80 Pipeline Steel. Front. Mater. 2021, 8, 794051. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R. Interpretation and Use of Silage Fermentation Analysis Reports. Focus Forage 2001, 3, 1–5. [Google Scholar]
- Bouteille, R.; Gaudet, M.; Lecanu, B.; This, H. Monitoring Lactic Acid Production during Milk Fermentation by in Situ Quantitative Proton Nuclear Magnetic Resonance Spectroscopy. J. Dairy Sci. 2013, 96, 2071–2080. [Google Scholar] [CrossRef]
- Leibniz Institute. DSMZ: Video Tutorials. Available online: https://www.dsmz.de/shop-1/faqs/video-tutorials (accessed on 3 April 2025).
- PlantApp—Botanist in Your Pocket. Available online: https://plantapp.app/ (accessed on 3 April 2025).
- Taylor, K.A.C.C. A Simple Colorimetric Assay for Muramic Acid and Lactic Acid. Appl. Biochem. Biotechnol. 1996, 56, 49–58. [Google Scholar] [CrossRef]
- Li, Z.-G. Chapter Six—Quantification of Hydrogen Sulfide Concentration Using Methylene Blue and 5,5′-Dithiobis(2-Nitrobenzoic Acid) Methods in Plants. In Methods in Enzymology; Cadenas, E., Packer, L., Eds.; Hydrogen Sulfide in Redox Biology, Part A; Academic Press: Cambridge, MA, USA, 2015; Volume 554, pp. 101–110. [Google Scholar]
- Tiganescu, E.; Abdin, A.Y.; Razouk, A.; Nasim, M.J.; Jacob, C. The Redox Riddle of Selenium Sulfide. Curr. Opin. Chem. Biol. 2023, 76, 102365. [Google Scholar] [CrossRef]
- Han, X.; Tong, Y. Vibrational Dynamics of SxSey Ring Clusterson AB Initio Ccalculation. Chalcogenide Lett. 2018, 15, 459–466. [Google Scholar]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Production of Probiotic Cabbage Juice by Lactic Acid Bacteria. Bioresour. Technol. 2006, 97, 1427–1430. [Google Scholar] [CrossRef]
- Alm, L. Effect of Fermentation on L(+) and D(−) Lactic Acid in Milk. J. Dairy Sci. 1982, 65, 515–520. [Google Scholar] [CrossRef]
- Farag, M.A.; Jomaa, S.A.; Abd El-Wahed, A.; R. El-Seedi, H. The Many Faces of Kefir Fermented Dairy Products: Quality Characteristics, Flavour Chemistry, Nutritional Value, Health Benefits, and Safety. Nutrients 2020, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P.; Lankaputhra, W.E.V.; Britz, M.L.; Kyle, W.S.A. Survival of Lactobacillus acidophilus and Bifidobacterium bifidum in Commercial Yoghurt during Refrigerated Storage. Int. Dairy J. 1995, 5, 515–521. [Google Scholar] [CrossRef]
- Settachaimongkon, S.; van Valenberg, H.J.F.; Gazi, I.; Nout, M.J.R.; van Hooijdonk, T.C.M.; Zwietering, M.H.; Smid, E.J. Influence of Lactobacillus plantarum WCFS1 on Post-Acidification, Metabolite Formation and Survival of Starter Bacteria in Set-Yoghurt. Food Microbiol. 2016, 59, 14–22. [Google Scholar] [CrossRef]
- Nugroho, A.D.W.; Kleerebezem, M.; Bachmann, H. A Novel Method for Long-Term Analysis of Lactic Acid and Ammonium Production in Non-Growing Lactococcus Lactis Reveals Pre-Culture and Strain Dependence. Front. Bioeng. Biotechnol. 2020, 8, 580090. [Google Scholar] [CrossRef]
- Shangguan, Y.; Yang, D.; Zhao, L.; Rao, L.; Liao, X. High-Pressure-Induced Viable but Non-Culturable Lactic Acid Bacteria Inhibit Its Post-Acidification. Bioresour. Technol. 2025, 422, 132221. [Google Scholar] [CrossRef] [PubMed]
- Ensinger Schiller Quelle—Ensinger. Available online: https://www.ensinger.de/produkte/ensinger-schiller-quelle (accessed on 3 April 2025).
- Asghari-Paskiabi, F.; Imani, M.; Rafii-Tabar, H.; Razzaghi-Abyaneh, M. Physicochemical Properties, Antifungal Activity and Cytotoxicity of Selenium Sulfide Nanoparticles Green Synthesized by Saccharomyces Cerevisiae. Biochem. Biophys. Res. Commun. 2019, 516, 1078–1084. [Google Scholar] [CrossRef]
- Wu, J.; He, R.; Cheng, S.; Han, L.; Hong, H.; Zhu, N. Simultaneous Immobilization of CO2 and H2S by Propargyl Amines under Mild Conditions: Efficient Synthesis of Thiazolidine-2-Ones. ACS Sustain. Chem. Eng. 2022, 10, 1214–1219. [Google Scholar] [CrossRef]
- Aguilar, L.; Zha, S.; Cheng, Z.; Winnick, J.; Liu, M. A Solid Oxide Fuel Cell Operating on Hydrogen Sulfide (H2S) and Sulfur-Containing Fuels. J. Power Sources 2004, 135, 17–24. [Google Scholar] [CrossRef]
- Jia, R.; Tan, J.L.; Jin, P.; Blackwood, D.J.; Xu, D.; Gu, T. Effects of Biogenic H2S on the Microbiologically Influenced Corrosion of C1018 Carbon Steel by Sulfate Reducing Desulfovibrio vulgaris Biofilm. Corros. Sci. 2018, 130, 1–11. [Google Scholar] [CrossRef]
- Kowalski, A.; Agati, G.; Grzegorzewska, M.; Kosson, R.; Kusznierewicz, B.; Chmiel, T.; Bartoszek, A.; Tuccio, L.; Grifoni, D.; Vågen, I.M.; et al. Valorization of Waste Cabbage Leaves by Postharvest Photochemical Treatments Monitored with a Non-Destructive Fluorescence-Based Sensor. J. Photochem. Photobiol. B Biol. 2021, 222, 112263. [Google Scholar] [CrossRef] [PubMed]
- IndexBox. Global Cabbage Market to Reach 80M Tonnes by 2025. Available online: https://www.globaltrademag.com/global-cabbage-market-to-reach-80m-tonnes-by-2025/ (accessed on 4 April 2025).
- Le Roux, B.; Van der Laan, M.; Vahrmeijer, T.; Annandale, J.G.; Bristow, K.L. Water Footprints of Vegetable Crop Wastage along the Supply Chain in Gauteng, South Africa. Water 2018, 10, 539. [Google Scholar] [CrossRef]
- World Cabbage Production by Country. Available online: https://www.atlasbig.com/en-gb/countries-by-cabbage-production (accessed on 3 April 2025).
- Juicernet. Average Juice Yields for Fruits & Vegetables; Juicernet: Jupiter, FL, USA, 2021. [Google Scholar]
- Holden, P. The Woe of Wasted Milk; Sustainable Food Trust: Bristol, UK, 2023. [Google Scholar]
- Campbell, C.G.; Feldpausch, G.L. Invited Review: The Consumer and Dairy Food Waste: An Individual plus Policy, Systems, and Environmental Perspective. J. Dairy Sci. 2022, 105, 3736–3745. [Google Scholar] [CrossRef] [PubMed]
- The Estimated Amount, Value, and Calories of Postharvest Food Losses at the Retail and Consumer Levels in the United States|Economic Research Service. Available online: https://www.ers.usda.gov/publications/pub-details?pubid=43836 (accessed on 3 April 2025).
- Wu, Y.-F.; Huang, H.; Zhang, J.; Hu, G.; Wang, J.; Peng, C.; Kappler, A.; Zhao, F.-J. Sulfate-Mediated Fe(III) Mineral Reduction Accelerates Arsenic Mobilization by a Desulfovibrio Strain Isolated from Paddy Soil. Sci. Total Environ. 2024, 954, 176529. [Google Scholar] [CrossRef]
- Kushkevych, I.; Hýžová, B.; Vítězová, M.; Rittmann, S.K.-M. Microscopic Methods for Identification of Sulfate-Reducing Bacteria from Various Habitats. Int. J. Mol. Sci. 2021, 22, 4007. [Google Scholar] [CrossRef]
- Jia, R.; Wang, D.; Jin, P.; Unsal, T.; Yang, D.; Yang, J.; Xu, D.; Gu, T. Effects of Ferrous Ion Concentration on Microbiologically Influenced Corrosion of Carbon Steel by Sulfate Reducing Bacterium Desulfovibrio vulgaris. Corros. Sci. 2019, 153, 127–137. [Google Scholar] [CrossRef]
- Hodgetts, R. Gold-Coated Steak Backlash Earns Franck Ribery ‘Heavy Fine’. Available online: https://www.cnn.com/2019/01/06/sport/franck-ribery-gold-steak-dubai-bayern-munich-fine-spt-intl/index.html (accessed on 3 April 2025).


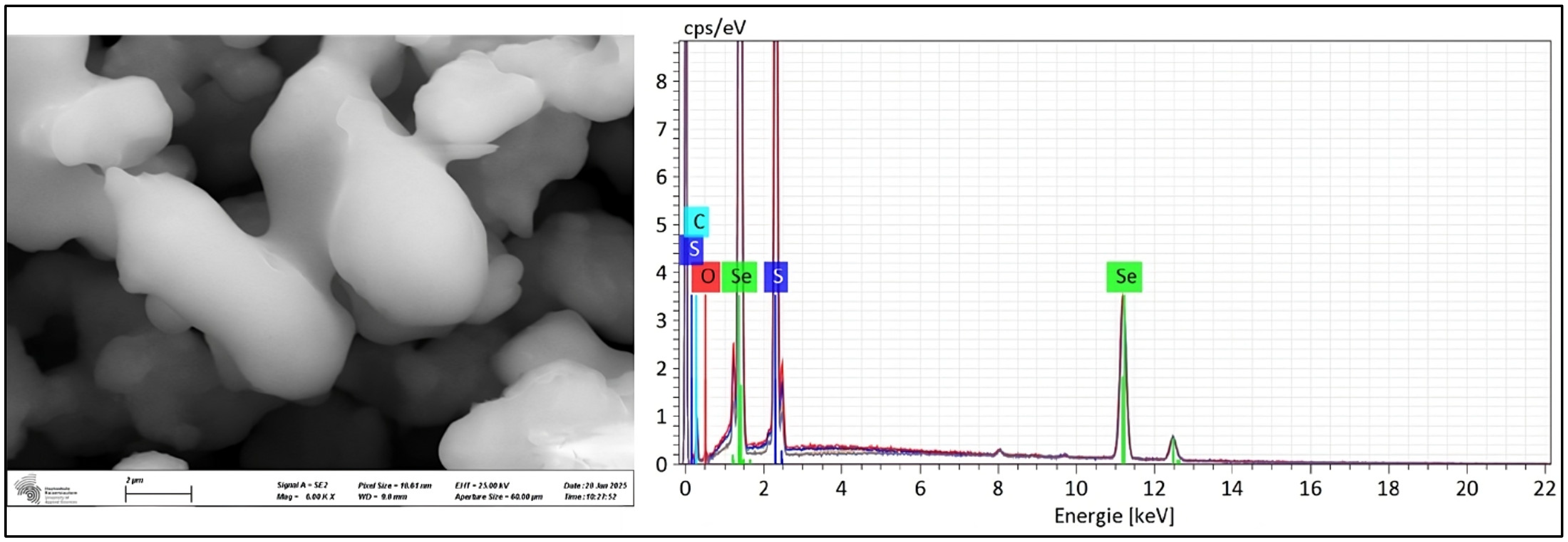
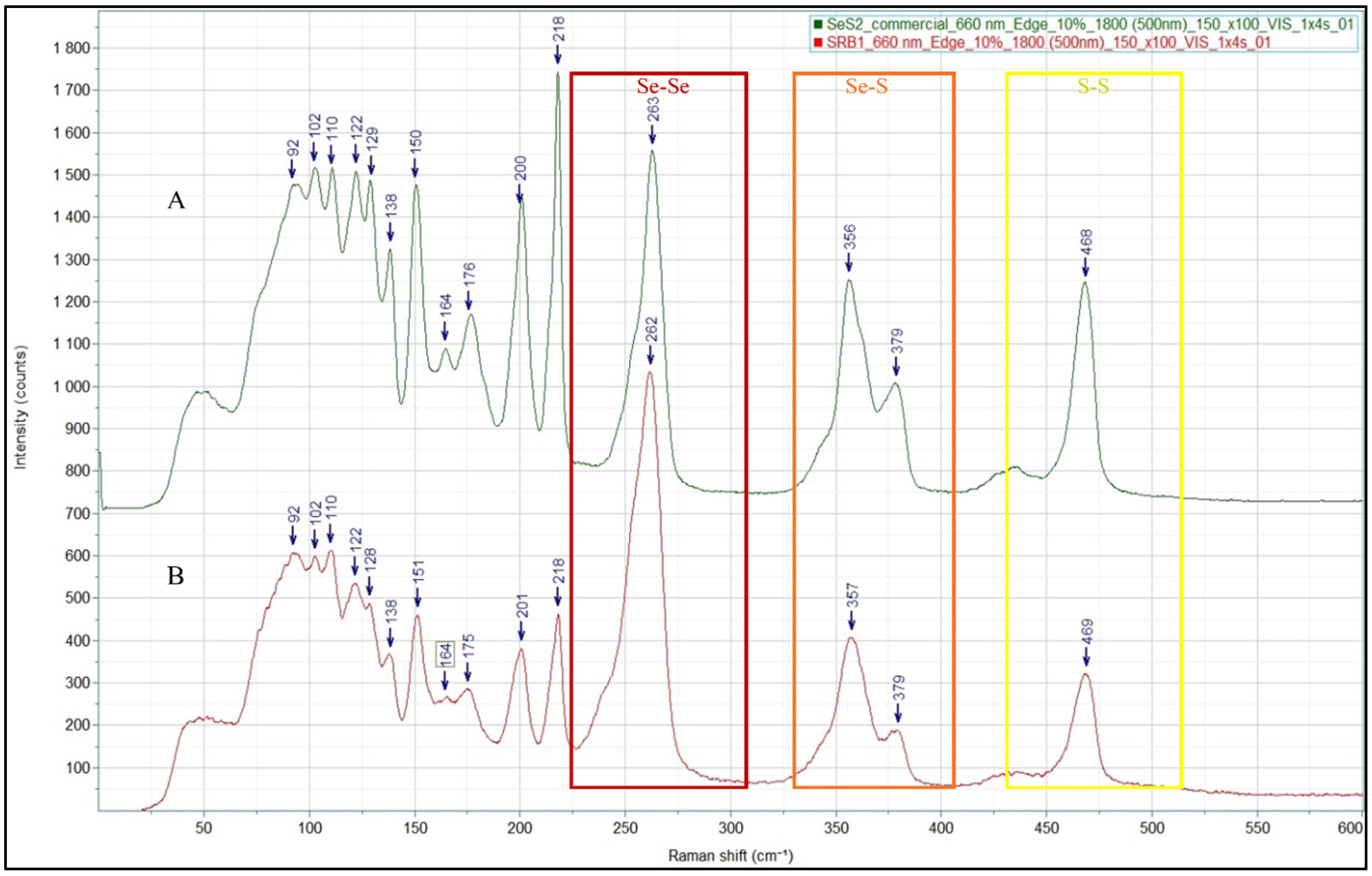

| Characterization Method | Biologically Produced Selenium Sulfide (wt%) | Commercial Selenium Sulfide (wt%) |
|---|---|---|
| CHNS | S: 35.02 | S: 47.98 |
| ICP-OES | S: 21.50 | S: 43.21 |
| Se: 78.50 | Se: 56.79 | |
| EDX | S: 24.05 | S: 45.02 |
| Se: 75.95 | Se: 54.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safinazlou, S.; Abdin, A.Y.; Tiganescu, E.; Lilischkis, R.; Schäfer, K.-H.; Fink-Straube, C.; Nasim, M.J.; Jacob, C. Valorizing Organic Waste: Selenium Sulfide Production Mediated by Sulfate-Reducing Bacteria. Materials 2025, 18, 2784. https://doi.org/10.3390/ma18122784
Safinazlou S, Abdin AY, Tiganescu E, Lilischkis R, Schäfer K-H, Fink-Straube C, Nasim MJ, Jacob C. Valorizing Organic Waste: Selenium Sulfide Production Mediated by Sulfate-Reducing Bacteria. Materials. 2025; 18(12):2784. https://doi.org/10.3390/ma18122784
Chicago/Turabian StyleSafinazlou, Shahrzad, Ahmad Yaman Abdin, Eduard Tiganescu, Rainer Lilischkis, Karl-Herbert Schäfer, Claudia Fink-Straube, Muhammad Jawad Nasim, and Claus Jacob. 2025. "Valorizing Organic Waste: Selenium Sulfide Production Mediated by Sulfate-Reducing Bacteria" Materials 18, no. 12: 2784. https://doi.org/10.3390/ma18122784
APA StyleSafinazlou, S., Abdin, A. Y., Tiganescu, E., Lilischkis, R., Schäfer, K.-H., Fink-Straube, C., Nasim, M. J., & Jacob, C. (2025). Valorizing Organic Waste: Selenium Sulfide Production Mediated by Sulfate-Reducing Bacteria. Materials, 18(12), 2784. https://doi.org/10.3390/ma18122784









