Phosphorus–Silicon Additive Increases the Mechanical and Fire Resistance of Epoxy Resins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
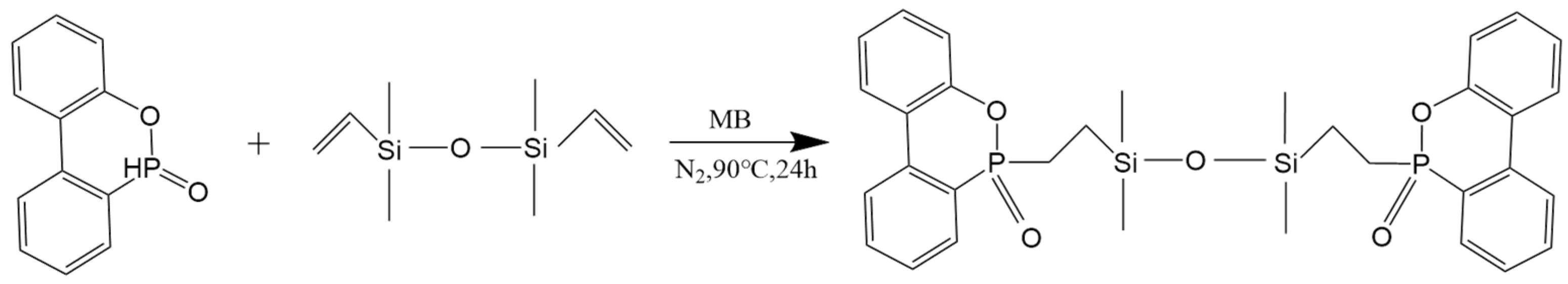
2.2. Synthesis of the Flame Retardant
2.3. Incorporation into Epoxy Resin
2.4. Spectroscopic Characterization
2.5. Thermal and Fire Resistance Tests
2.6. Analysis of Flame Retardancy Mechanism
2.7. Mechanical Property Tests
2.8. Transparency Test
3. Results and Discussion
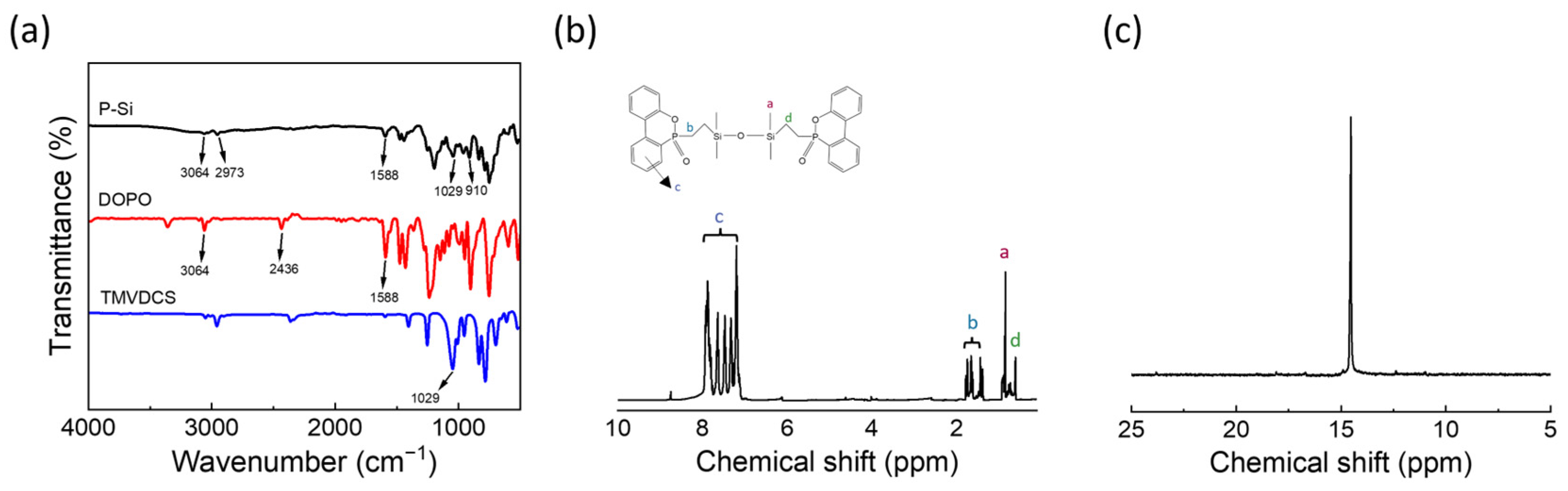
3.1. Structural Characterization of P–Si
3.2. Curing Behavior
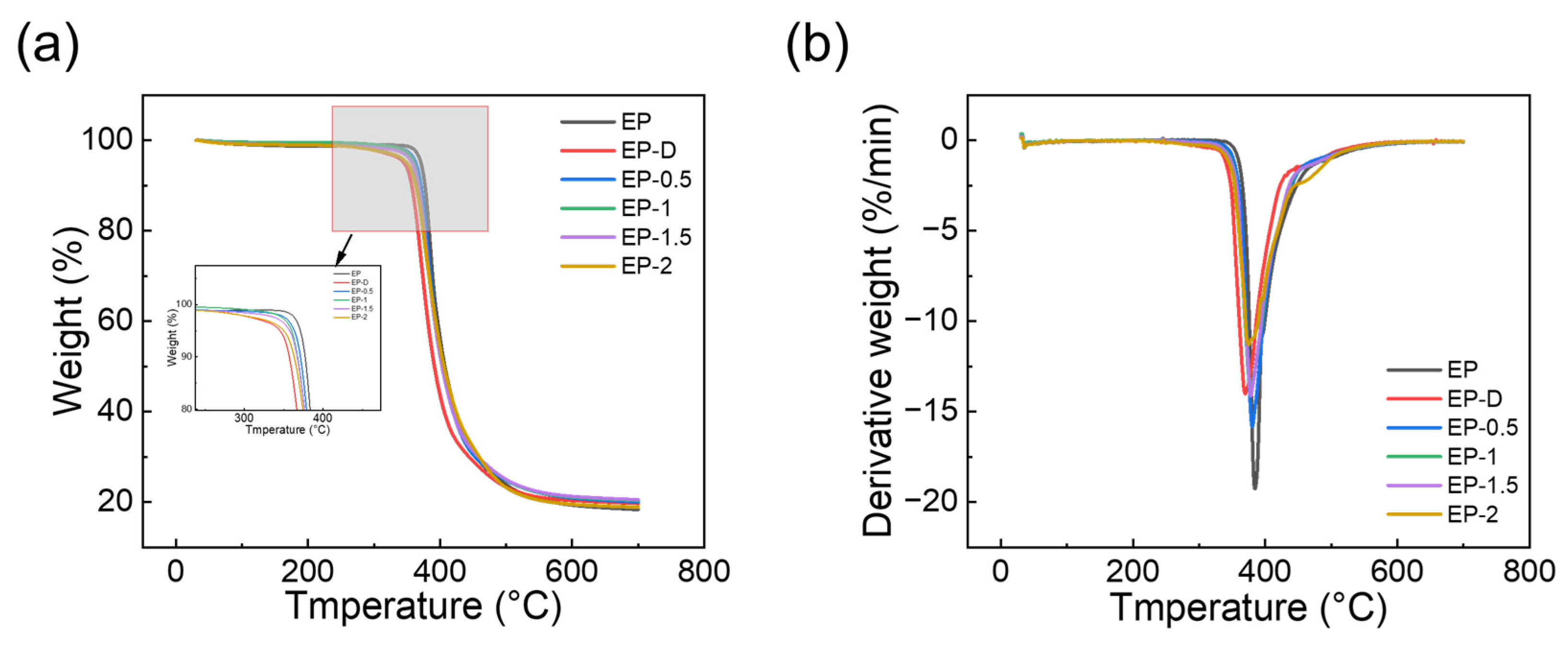
3.3. Thermal Stability
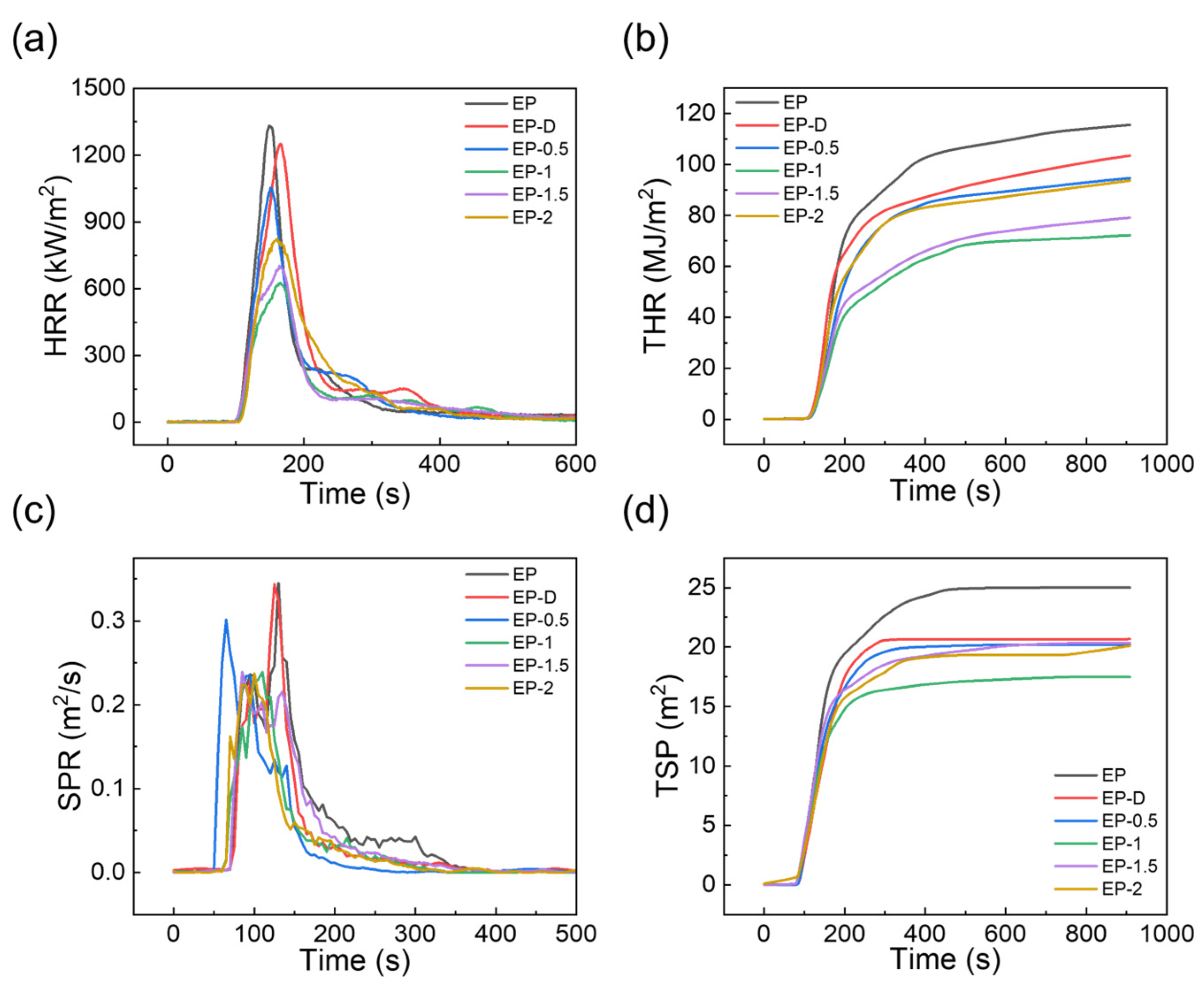
3.4. Flame-Retardant Properties
3.5. Gas Phase Analysis
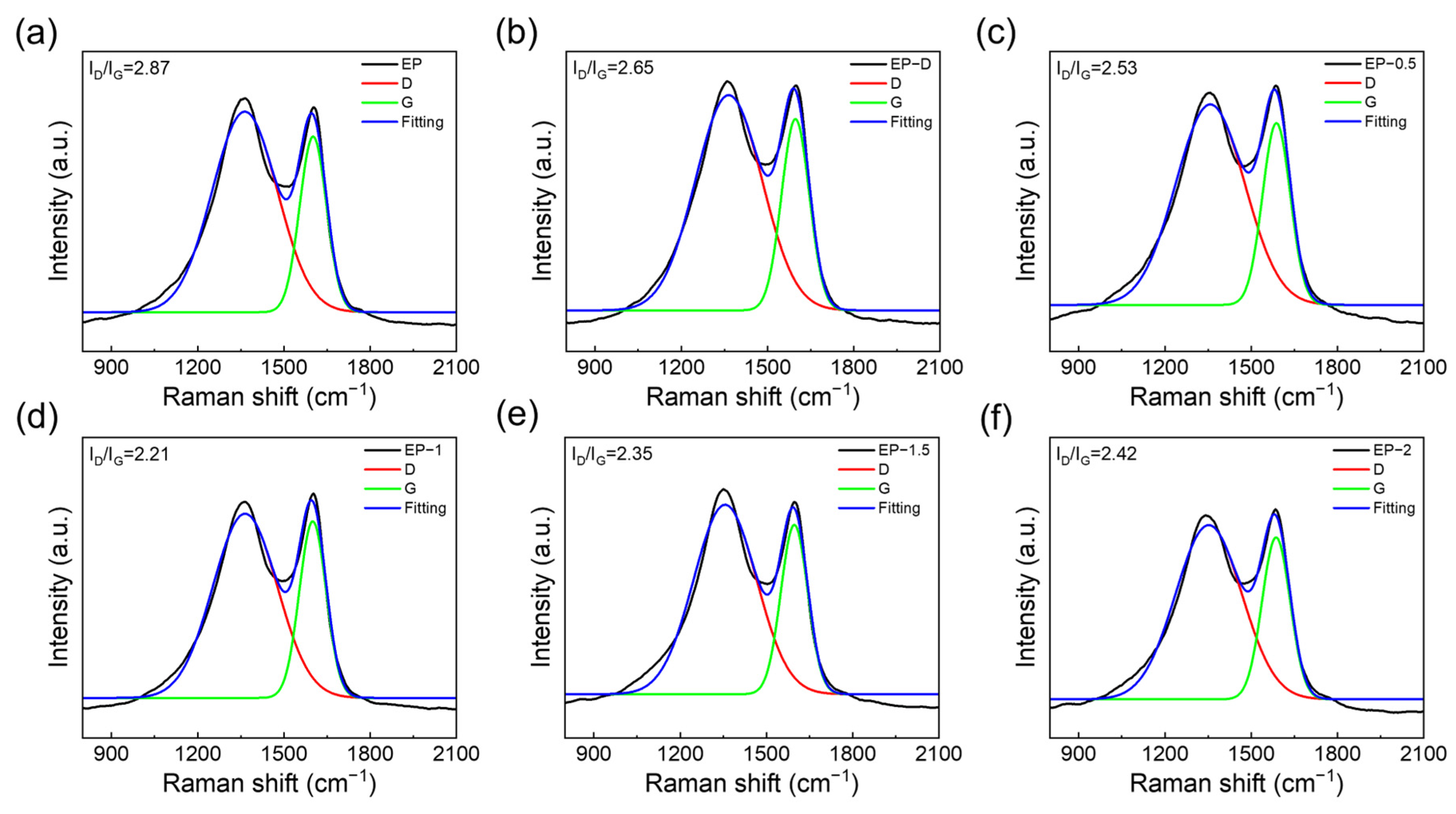
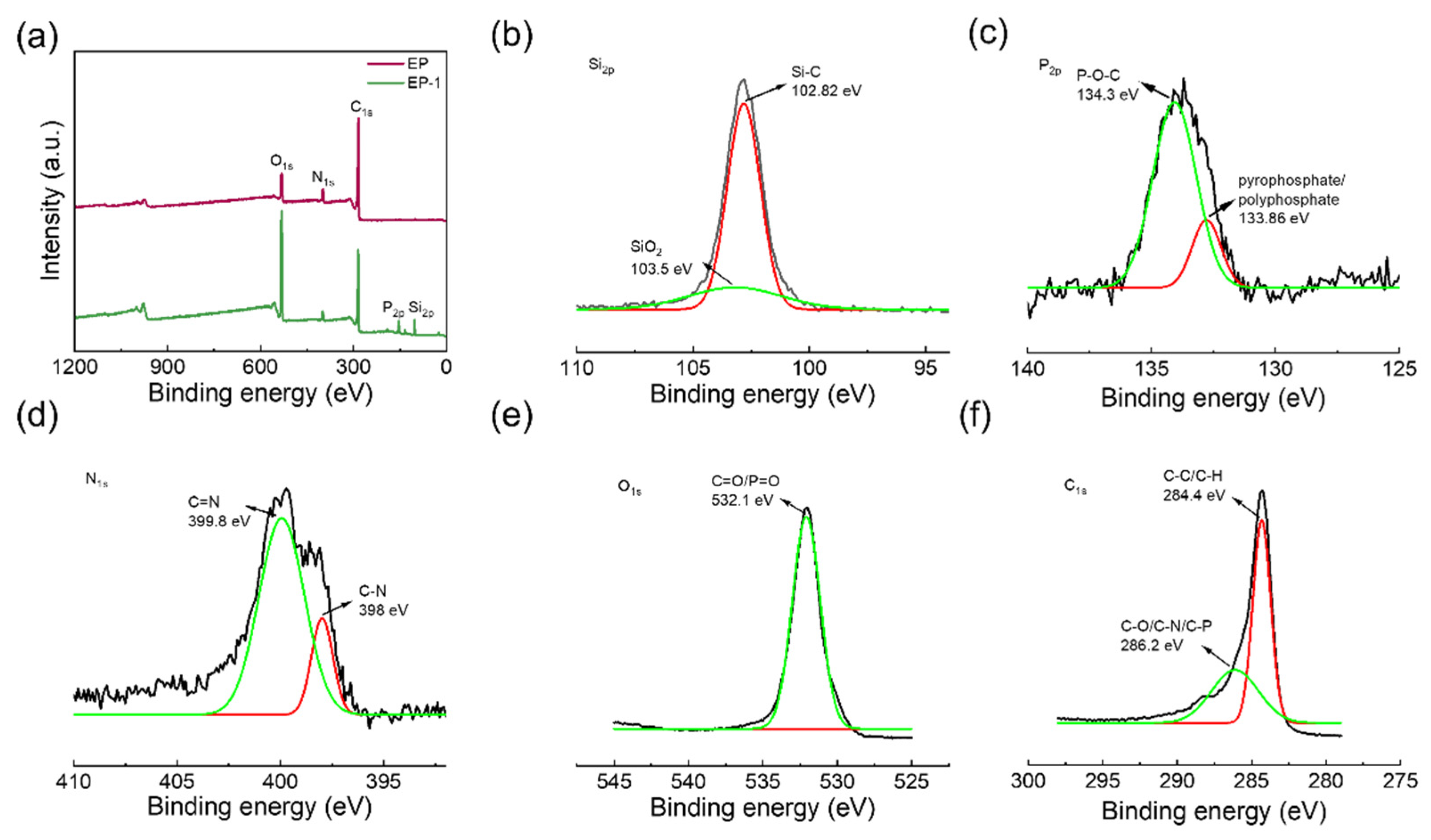
3.6. Char Morphology and Chemical Analysis
3.7. Mechanical Properties and Strengthening Mechanism
3.8. Transparency Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer composite materials: A comprehensive review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, Y.-L.; Zhang, Y.-J. A study on the synthesis, curing behavior and flame retardance of a novel flame retardant curing agent for epoxy resin. Polymers 2022, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-J.; Ding, H.; Liu, T.-X.; Ou, R.-X.; Lin, J.-Y.; Puglia, D.; Xu, P.; Wang, Q.-W.; Dong, W.-F. Design of intrinsically flame-retardant vanillin-based epoxy resin for thermal-conductive epoxy/graphene aerogel composites. ACS Appl. Mater. Interfaces 2021, 13, 59341–59351. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, J.-Z.; Yuan, Y.-H.; Gao, C.; Cui, Y.-G.; Li, S.-C.; Wang, H.-Y.; Peng, C.; Liu, X.; Wu, Z.-J.; et al. A new strategy to improve the toughness of epoxy thermosets by introducing the thermoplastic epoxy. Polymer 2022, 240, 124518. [Google Scholar] [CrossRef]
- Vanganici, C.-D.; Rosu, L.; Bifulco, A.; Rosu, D.; Muatata, F.; Gaan, S. Recent advances in flame retardant epoxy systems from reactive DOPO–based phosphorus additives. Polym. Degrad. Stabil. 2022, 202, 110020. [Google Scholar] [CrossRef]
- Li, S.-S.; Chen, M.-F.; Su, L.-P.; Lin, X.-H.; Liu, C.-P. Highly efficient multielement flame retardant for multifunctional epoxy resin with satisfactory thermal, flame-retardant, and mechanical properties. Polym. Adv. Technol. 2019, 31, 146–159. [Google Scholar] [CrossRef]
- Bifulco, A.; Varganici, C.; Rosu, L.; Mustata, F.; Rosu, D.; Gaan, S. Recent advances in flame retardant epoxy systems containing non-reactive DOPO based phosphorus additives. Polym. Degrad. Stabil. 2022, 200, 109962. [Google Scholar] [CrossRef]
- Matykiewicz, D. Modification of glass reinforced epoxy composites by ammonium polyphosphate (APP) and melamine polyphosphate (PNA) during the resin powder molding process. Compos. Part B Eng. 2017, 108, 224–231. [Google Scholar] [CrossRef]
- Van Der Veen, I.; De Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.; Guo, X. Preparation and mechanism of toughening and flame retardance of epoxy resin using novel silsesquioxane molecules. React. Funct. Polym. 2023, 190, 105645. [Google Scholar] [CrossRef]
- Wang, C.-H.; Zhang, Y.-Q.; Wang, X.-Y. Preparation and properties of epoxy resin modified with phosphorus and nitrogen flame retardants. Mater. Res. Express 2023, 10, 035303. [Google Scholar] [CrossRef]
- Jian, R.-K.; Ai, Y.-F.; Xia, L.; Zhao, L.-J.; Zhao, H.-B. Single component phosphamide-based intumescent flame retardant with potential reactivity towards low flammability and smoke epoxy resins. J. Hazard. Mater. 2019, 371, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Oh, S.-W.; Lee, Y.-H.; Kim, I.-J.; Lee, D.-J.; Lim, J.-C.; Park, C.-C. Preparation and properties of flame-retardant epoxy resins containing reactive phosphorus flame retardant. J. Eng. Fibers Fabr. 2020, 115, 1558925020901323. [Google Scholar] [CrossRef]
- Velencoso, M.-M.; Battig, A.; Markwart, J.-C.; Schartel, B.; Wurm, F.-R. Molecular Firefighting—How modern phosphorus chemistry can help solve the challenge of flame retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Huo, S.; Song, P.-G.; Yu, B.; Ran, S.-Y.; Chevali, V.-S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Han, Y.-C.; Jin, L.; Xu, T.-T.; Zhao, H.; Wang, X.-L.; Yuan, L.-L. A novel phosphorus compound acting as a substitute of DOPO for flame retard of epoxy resin. J. Appl. Polym. Sci. 2022, 139, e52426. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, X.; Fan, R.; Yue, S.; Zheng, L.; Liu, Q.; He, Y. A comprehensive review of reactive flame-retardant epoxy resin: Fundamentals, recent developments, and perspectives. Polym. Degrad. Stabil 2022, 201, 109976. [Google Scholar] [CrossRef]
- Zhang, W.-C.; He, X.-D.; Song, T.-L.; Jiao, Q.-J.; Yang, R.-J. The influence of the phosphorus-based flame retardant on the flame retardancy of the epoxy resins. Polym. Degrad. Stabil. 2014, 109, 209–217. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Yang, S.; Wang, J.; Cheng, J.-W.; Zhang, Q.-X.; Ding, G.-P.; Hu, Y.-F. A DOPO based reactive flame retardant constructed by multiple heteroaromatic groups and its application on epoxy resin: Curing behavior, thermal degradation and flame retardancy. Polym. Degrad. Stabil. 2019, 167, 10–20. [Google Scholar] [CrossRef]
- Chen, B.; Luo, W.; Lv, J.; Lin, S.; Zheng, B.; Zhang, H.; Chen, M. A universal strategy toward flame retardant epoxy resin with ultra-tough and transparent properties. Polym. Degrad. Stabil. 2022, 205, 110132. [Google Scholar] [CrossRef]
- Luo, Y.; Cai, J.-Y.; Li, L.; Lin, X.-C. Multi-DOPO-based derivative for enhancing flame retardancy and mechanical properties of epoxy resin. Prog. Org. Coat. 2023, 184, 107862. [Google Scholar] [CrossRef]
- Guo, Y.; Rong, H.; Chen, Z.-W.; Chen, T.-T. A novel DOPO derivative containing multifunctional groups aiming to improve fire safety, thermal stability and curing state towards epoxy resin. Polym. Degrad. Stabil. 2022, 205, 110142. [Google Scholar] [CrossRef]
- Li, P.-Y.; Wang, J.-H.; Wang, C.Z.; Xu, C.-X.; Ni, A.-Q. The flame retardant and mechanical properties of the epoxy modified by an efficient DOPO-based flame retardant. Polymers 2024, 16, 631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-C.; Li, X.-M.; Yang, R.-J. Pyrolysis and fire behaviour of epoxy resin composites based on a phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS). Polym. Degrad. Stabil. 2011, 96, 1821–1832. [Google Scholar] [CrossRef]
- Zhang, W.-C.; Fina, A.; Cuttica, F.; Camino, G.; Yang, R.-J. Blowing-out effect in flame retarding epoxy resins: Insight by temperature measurements during forced combustion. Polym. Degrad. Stabil. 2016, 131, 82–90. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, T.-T.; Li, J.; Tan, J.-H.; Zhu, X.-B. Enhancing toughness, flame retardant, hydrophobic and dielectric properties of epoxy resin by incorporating multifunctional additive containing phosphorus/silicon. Mater. Des. 2023, 225, 111529. [Google Scholar] [CrossRef]
- Zhang, W.-C.; Li, X.-M.; Fan, H.-B.; Yang, R.-J. Study on mechanism of phosphorus–silicon synergistic flame retardancy on epoxy resins. Polym. Degrad. Stabil. 2012, 97, 2241–2248. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Yang, R.-J. Study of the synergistic effect of polyhedral oligomeric octadiphenylsulfonylsilsesquioxane and 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide on flame-retarded epoxy resins. Polym. Degrad. Stabil. 2014, 109, 233–239. [Google Scholar] [CrossRef]
- Bu, M.-L.; Zhang, X.-Q.; Zhou, T.; Lei, C.-H. In-situ formation of polynanosiloxane by a multifunctional phosphaphenanthrene–silicone–epoxy soybean oil flame retardant improves the toughness and transparency of epoxy resin. ACS Appl. Polym. Mater. 2024, 6, 8056–8072. [Google Scholar] [CrossRef]
- Liu, C.; Huang, K.-X.; Liu, R.; Li, Y.-T.; Dai, L.-Z. A multi-element flame retardant on the basis of silicon-phosphorus-nitrogen for combustibility suppressing of epoxy. Polym. Test. 2022, 111, 107582. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Tang, G.; Chen, J.-M.; Huang, Z.-Q. Biobased polyelectrolyte multilayer-coated hollow mesoporous silica as a green flame retardant for epoxy resin. J. Hazard. Mater. 2018, 342, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-C.; Li, X.-M.; Li, L.-M.; Yang, R.-J. Study of the synergistic effect of silicon and phosphorus on the blowing-out effect of epoxy resin composites. Polym. Degrad. Stabil. 2012, 97, 1041–1048. [Google Scholar] [CrossRef]
- Gan, H.-L.; Seraji, S.; Zhang, J.; Swan, S.-R. Synthesis of a phosphorus-silicone modifier imparting excellent flame retardancy and improved mechanical properties to a rapid cure epoxy. React. Funct. Polym. 2020, 157, 104743. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Yan, H.-X.; Feng, G.-P.; Liu, R.; Yang, K.-M. Non-aromatic Si, P, N-containing hyperbranched flame retardant on reducing fire hazards of epoxy resin with desirable mechanical properties and lower curing temperature. Compos. Part B Eng. 2021, 222, 109043. [Google Scholar] [CrossRef]
- Guo, W.-W.; Liang, F.-W.; Chen, S.; Zhang, D.-T.; Li, W.-B.; Qian, K. Synthesis of magnolol-derived bisphosphate for fabrication of bismaleimide resins with intrinsic anti-flammability and smoke suppression. Polym. Degrad. Stabil. 2022, 202, 110002. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Akbari, V.; Paran, S.-M.-R. Super-crosslinked ionic liquid-intercalated montmorillonite/epoxy nanocomposites: Cure kinetics, viscoelastic behavior and thermal degradation mechanism. Polym. Eng. Sci. 2020, 60, 1940–1957. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Raran, S.-M.R.; Jannesari, A.; Saeb, M.-R. ‘Cure Index’ for thermoset composites. Prog. Org. Coat. 2019, 127, 429–434. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Xiao, H. Flame retardant effect and mechanism of a novel DOPO based tetrazole derivative on epoxy resin. J. Anal. Appl. Pyrol. 2019, 139, 104–113. [Google Scholar] [CrossRef]
- Elangovan, N.; Cangadharappa, B.; Thomas, R.; Irfan, A. Synthesis of a versatile Schiff base 4-((2-hydroxy-3,5-diiodobenzylidene)amino) benzenesulfonamide from 3,5-diiodosalicylaldehyde and sulfanilamide, structure, electronic properties, biological activity prediction and experimental antimicrobial properties. J. Mol. Struct. 2022, 1250, 131700. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, J.; Zhu, Z.-M.; Yin, X.-Z.; Weng, Y.-W.; Wang, Z.-J.; Yang, F.-H.; Zhan, J.-Y.; Wang, H. High performance epoxy resin composites modified with multifunctional thiophene/phosphaphenanthrene-based flame retardant: Excellent flame retardance, strong mechanical property and high transparency. Compos. Part B Eng. 2021, 277, 109392. [Google Scholar] [CrossRef]
- Huang, G.; Huo, S.; Xu, X.; Chen, W.; Jin, Y.; Li, R.; Song, P.; Wang, H. Realizing simultaneous improvements in mechanical strength, flame retardancy and smoke suppression of ABS nanocomposites from multifunctional graphene. Compos. Part B Eng. 2019, 177, 107377. [Google Scholar] [CrossRef]
- Miao, J.; Fang, Y.; Guo, Y.; Zhu, Y.; Hu, A.; Wang, G. Interpenetrating Polymer Networks of Porous Organic Polymers and Polyurethanes for Flame Resistance and High Mechanical Properties. ACS Appl. Energy Mater. 2019, 1, 2692–2702. [Google Scholar] [CrossRef]
- Tang, Y.; Lewin, M.; Rearce, E.-M. Effects of annealing on the migration behavior of PA6/clay nanocomposites. Macromol. Rapid Commun. 2006, 27, 1545–1549. [Google Scholar] [CrossRef]
- Qiu, C.; Luo, J.; Ling, Y.; Lu, Z.; Ni, L.; Chen, Y.; Zou, H.-W.; Heng, Z.-G.; Liang, M. Thermal degradation behavior and mechanism of organosilicon modified epoxy resin. Macromol. Chem. Phys. 2022, 223, 2200164. [Google Scholar] [CrossRef]
- Hamdani, S.; Lobguet, C.; Perrin, D.; Lopez-cuesta, J.-M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stabil. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Tang, S.; Wachtendorf, V.; Klack, P.; Qian, L.-J.; Dong, Y.-P. Enhanced flame-retardant effect of a montmorillonite/phosphaphenanthrene compound in an epoxy thermoset. RSC Adv. 2017, 7, 720–728. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Qian, L.-J.; Xu, B.; Xi, W. Synthesis and characterization of aluminum poly-hexamethylenephosphinate and its flame-retardant application in epoxy resin. Polym. Degrad. Stabil. 2015, 122, 8–17. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, D.-Y.; Jian, R.-K.; Liu, Z.; Huang, G. Chemical structure construction of DOPO-containing compounds for flame retardancy of epoxy resin: A review. Prog. Org. Coat. 2023, 175, 107316. [Google Scholar] [CrossRef]
- Škrbic, B.; Marinkovic, V.; Spaic, S.; Milanko, V. Profiles of polycyclic aromatic hydrocarbons in smoke from combustion and thermal decomposition of poplar wood pellets and sawdust. Microchem. J. 2018, 139, 9–17. [Google Scholar] [CrossRef]
- Peng, C.; Li, J.; Wu, Z.-J.; Peng, W.-B.; Zhou, D.-Y. Investigating into the liquid oxygen compatibility of a modified epoxy resin containing silicon/phosphorus and its mechanical behavior at cryogenic temperature. RSC Adv. 2016, 6, 38300–38309. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.-P.; Fox, B. Thermal and mechanical properties of a dendritic hydroxyl-functional hyperbranched polymer and tetrafunctional epoxy resin blends. J. Polym. Sci. 2010, 48, 417–424. [Google Scholar] [CrossRef]
- Fang, F.; Ran, S.; Fang, Z.; Song, P.; Wang, H. Improved flame resistance and thermo-mechanical properties of epoxy resin nanocomposites from functionalized graphene oxide via self-assembly in water. Compos. Part B Eng. 2019, 165, 406–416. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Effect of a triazine ring-containing charring agent on fire retardancy and thermal degradation of intumescent flame retardant epoxy resins. Polym. Adv. Technol. 2011, 22, 2480–2487. [Google Scholar] [CrossRef]
- Zhi, M.-Y.; Liu, Q.-Y.; Chen, H.; Chen, X.-T.; Feng, S.-H. Thermal stability and flame retardancy properties of epoxy resin modified with functionalized graphene oxide containing phosphorus and dilicon elements. ACS Omega 2019, 4, 10975–10984. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-Z.; He, C.-G.; Wem, Y.-F.; Ye, Y.-S.; Zhou, X.-P. Improving thermal and flame retardant properties of epoxy resin by functionalized graphene containing phosphorous, nitrogen and silicon elements. Compos. Part A Appl. Sci. Manuf. 2017, 103, 74–83. [Google Scholar] [CrossRef]
- Cai, H.-P.; Huang, W.-Y.; Li, B.-L.; Gao, L.-H.; Huang, X. A multi-element flame retardant for rail transit to improve the flame retardant performance of epoxy resin while reducing smoke density. J. Appl. Polym. Sci. 2024, 141, e54841. [Google Scholar] [CrossRef]
- Yang, B.; Song, N.; Chen, Z.; Yu, Y.; Chen, Z.; Rong, F.; Chen, T.; Chen, T.; Guo, Y.; Wang, K.; et al. Fabrication of a cinnamaldehyde-based bi-DOPO flame retardant with excellent glass transition temperature, fire safety and mechanical properties for epoxy resins. Colloids Surf. A Physicochem. Eng. Asp. 2024, 681, 132815. [Google Scholar] [CrossRef]
- Sharifi, M.; Jang, C.; Abrams, C.F.; Palmese, G.R. Epoxy polymer networks with improved thermal and mechanical properties via controlled dispersion of reactive toughening agents. Macromolecules 2015, 48, 7495–7502. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, T.; Li, J.; Tan, J.; Zhang, M.; Zhou, Y.; Zhu, X. Facile synthesis of eugenol-based phosphorus/silicon-containing flame retardant and its performance on fire retardancy of epoxy resin. ACS Appl. Polym. Materi. 2022, 4, 1794–1804. [Google Scholar] [CrossRef]
- Teng, N.; Dai, J.-Y.; Wang, S.-P.; Hu, J.-Y.; Liu, X.-Q. Hyperbranched flame retardant for epoxy resin modification: Simultaneously improved flame retardancy, toughness and strength as well as glass transition temperature. Chem. Eng. J. 2022, 428, 131226. [Google Scholar] [CrossRef]
- Huo, S.-Q.; Zhou, Z.-X.; Jiang, J.-W.; Sai, T.; Ran, S.-Y. Flame-retardant, transparent, mechanically-strong and tough epoxy resin enabled by high-efficiency multifunctional boron-based polyphosphonamide. Chem. Eng. J. 2022, 427, 131578. [Google Scholar] [CrossRef]
- Zhao, P.; Rao, W.-H.; Luo, H.-Q.; Wang, L.; Liu, Y.-L.; Yu, C.-B. Novel organophosphorus compound with amine groups towards self-extinguishing epoxy resins at low loading. Materi. Des. 2020, 193, 108838. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Min, X.-Q.; Chen, S.-Y.; Xu, Z.-J.; Zhang, D.-H.; Miao, M.-H.; Wang, J.-S. A bio-based hyperbranched flame retardant for epoxy resins. Chem. Eng. J. 2020, 381, 122719. [Google Scholar] [CrossRef]
- Shi, Y.-Q.; Fu, T.; Xu, Y.-J.; Li, D.-F.; Wang, X.-L.; Wang, Y.-Z. Novel phosphorus-containing halogen-free ionic liquid toward fire safety epoxy resin with well-balanced comprehensive performance. Chem. Eng. J. 2018, 354, 208–219. [Google Scholar] [CrossRef]
- Ma, C.; Qiu, S.-L.; Wang, J.-L.; Wang, C.-M.; Zeng, W.R. Economical and environment-friendly synthesis of a novel hyperbranched poly(aminomethylphosphine oxide-amine) as co-curing agent for simultaneous improvement of fire safety, glass transition temperature and toughness of epoxy resins. Chem. Eng. J. 2017, 322, 618–631. [Google Scholar] [CrossRef]
- Ma, C.; Qian, L.-J.; Li, J. Effect of functional groups of magnolol-based cyclic phosphonate on structure and properties of flame retardant epoxy resin. Polym. Degrad. Stabil. 2021, 190, 109630. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Naturally occurring acids as cross-linkers to yield VOC-free, high-performance, fully bio-based, degradable thermosets. Macromolecules 2015, 48, 7127–7137. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Yan, S.; Zhao, J.; Liu, S.; Zhang, M.; Zheng, X.; Jian, L. Multiply fully recyclable carbon fibre reinforced heat-resistant covalent thermosetting advanced composites. Nat. Commun. 2017, 8, 14657. [Google Scholar] [CrossRef]
- Zhou, D.-X.; Gu, A.-J.; Liang, G.-Z.; Hu, J.-T. Flame retardancy materials based on a novel fully end-capped hyperbranched polysiloxane and bismaleimide/diallylbisphenol a resin with simultaneously improved integrated performance. J. Mater. Chem. 2011, 21, 6584–6594. [Google Scholar] [CrossRef]
- Liu, D.-Y.; Jiang, F.; Zhang, T.-L.; Yu, C.-X.; Hu, Z.-Y.; Zhang, L.; Shi, S.-Y. A DOPO-based reactive flame retardant containing benzimidazole groups: Synthesis and its flame-retardation on epoxy resin. J. Appl. Polym. Sci. 2024, 141, e54890. [Google Scholar] [CrossRef]
- Cheng, C.; Yan, J.; Lu, Y.-L.; Ma, W.-N.; Du, S.-G. Effect of chitosan/lignosulfonate microencapsulated red phosphorus on fire performance of epoxy resin. Thermochim. Acta 2021, 700, 178931. [Google Scholar] [CrossRef]
- Chen, R.; Luo, Z.-J.; Yu, X.-J.; Tang, H.; Zhou, Y.; Zhou, H. Synthesis of chitosan-based flame retardant and its fire resistance in epoxy resin. Carbohydr. Polym. 2020, 245, 116530. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-X.; Guo, J.-H.; Ye, Y.; Zhao, T.-J. Molecular design and properties of intrinsic flame-retardant P-N synergistic epoxy resin. J. Appl. Polym. Sci. 2024, 141, e54885. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, X.-D.; Ding, H.; Xu, P.-W.; Zhang, C.-J.; Ren, Y.-Z.; Yang, W.-J. Synthesis of eugenol-based phosphorus-containing epoxy for enhancing the flame-retardancy and mechanical performance of DGEBA epoxy resin. React. Funct. Polym. 2022, 180, 105383. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.-J.; Su, Y.; Yu, C.; Han, J.; Liu, J.-P. Preparation of a polymeric phosphoramide flame-retardant and its effect on the flame-retardant properties of epoxy Resin. Polymers 2024, 16, 1224. [Google Scholar] [CrossRef]
- Yu, H.-H.; Xu, X.-H.; Xia, Y.-F.; Pan, M.-Z.; Zarshad, N.; Pang, B. Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin. e-Polymers 2020, 20, 303–316. [Google Scholar] [CrossRef]
- Yang, G.; Wu, W.-H.; Wang, Y.-H.; Jiao, Y.-H.; Lu, L.-Y.; Qu, H.-Q. Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in Reducing the fire Hazard of epoxy resin. J. Hazard. Mater. 2019, 366, 78–87. [Google Scholar] [CrossRef]
- Cao, Y.-F.; Sun, L.; Ding, L.; Yu, J.-K. A flexible silicon-oxygen chain segment-containing phosphonamidite toward modification of epoxy resin: Flame retardancy, toughness and transparency. Eur. Polym. J. 2025, 223, 113636. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, T.-T.; Li, J.; Tan, J.-H.; Zhu, X.-B. Synthesis of a multifunctional Phosphorus/Silicon flame retardant via an industrial feasible technology. ACS Sustain. Chem. Eng. 2023, 11, 11965–11977. [Google Scholar] [CrossRef]






| Sample | EP (g) | DOPO (g) | P–Si (g) | DDM (g) | P (wt%) |
|---|---|---|---|---|---|
| EP | 30.00 | / | / | 7.59 | / |
| EP-D | 30.00 | 2.25 | / | 7.59 | 1.00 |
| EP-0.5 | 30.00 | / | 1.50 | 7.59 | 0.50 |
| EP-1 | 30.00 | / | 3.33 | 7.59 | 1.00 |
| EP-1.5 | 30.00 | / | 5.00 | 7.59 | 1.50 |
| EP-2 | 30.00 | / | 7.00 | 7.59 | 2.00 |
| Sample | a Tonset (°C) | b TP (°C) | c Tend (°C) | ΔT (°C) | d ΔH ∞ (J/g) | ΔT * | ΔH * | eCI | f Cure State |
|---|---|---|---|---|---|---|---|---|---|
| EP | 137.50 | 160.68 | 180.99 | 43.49 | 294.56 | / | / | / | / |
| EP-D | 134.50 | 159.86 | 180.50 | 46.00 | 306.83 | 1.06 | 1.04 | 1.10 | Good |
| EP-0.5 | 132.49 | 159.20 | 174.69 | 42.20 | 329.45 | 0.97 | 1.12 | 1.10 | Excellent |
| EP-1 | 130.99 | 157.17 | 169.49 | 38.50 | 315.06 | 0.89 | 1.07 | 0.95 | Excellent |
| EP-1.5 | 128.49 | 154.91 | 165.90 | 37.41 | 311.16 | 0.86 | 1.06 | 0.91 | Excellent |
| EP-2 | 126.01 | 154.28 | 162.38 | 36.37 | 325.68 | 0.84 | 1.11 | 0.94 | Excellent |
| Sample | a T5% | b TMax | c RMax | d CY700 (%) |
|---|---|---|---|---|
| EP | 371.06 | 384.34 | 19.25 | 18.39 |
| EP-D | 345.42 | 369.67 | 13.99 | 19.73 |
| EP-0.5 | 363.53 | 380.37 | 15.83 | 20.02 |
| EP-1 | 359.84 | 378.33 | 13.89 | 21.58 |
| EP-1.5 | 357.33 | 378.01 | 14.11 | 21.32 |
| EP-2 | 349.85 | 374.67 | 11.28 | 20.87 |
| Sample | LOI (%) | a t1 (s) | b t2 (s) | Dripping or Not | UL-94 Rating |
|---|---|---|---|---|---|
| EP | 23 | None | None | Yes | c NR |
| EP-D | 31 | 6 | 5 | No | V-1 |
| EP-0.5 | 29 | 8.7 | 6 | No | V-1 |
| EP-1 | 33 | 1 | 1 | No | V-0 |
| EP-1.5 | 31 | 1 | 3.2 | No | V-0 |
| EP-2 | 30 | 3 | 4.4 | No | V-0 |
| Sample | a TTI (s) | b pHRR (kW/m2) | c THR (MJ/m2) | d av-COY (kg/kg) | e av-CO2Y (kg/kg) | f av-EHC (MJ/kg) | g TML (wt%) |
|---|---|---|---|---|---|---|---|
| EP | 82 ± 1 | 1322.55 ± 125.61 | 116.20 ± 3.17 | 0.520 ± 0.005 | 3.24 ± 0.17 | 27.5 ± 0.24 | 94.2 ± 1.1 |
| EP-D | 84 ± 2 | 1272.72 ± 93.43 | 103.11 ± 1.94 | 0.408 ± 0.004 | 3.16 ± 0.13 | 24.2 ±0.83 | 88.7 ± 0.3 |
| EP-0.5 | 87 ± 1 | 1032.60 ± 46.74 | 96.73 ± 3.34 | 0.314 ± 0.006 | 3.13 ± 0.09 | 23.3 ± 0.71 | 85.4 ± 0.3 |
| EP-1 | 93 ± 1 | 629.11 ± 28.03 | 74.72 ± 2.81 | 0.258 ± 0.003 | 2.33 ± 0.15 | 17.0 ± 0.32 | 80.1 ± 0.5 |
| EP-1.5 | 91 ± 1 | 697.45 ± 37.23 | 83.22 ± 3.49 | 0.320 ± 0.002 | 2.71 ± 0.24 | 20.7 ± 0.48 | 82.3 ±1.3 |
| EP-2 | 88 ± 2 | 827.23 ± 41.84 | 92.92 ± 1.21 | 0.327 ± 0.005 | 2.97 ± 0.31 | 21.7 ± 0.65 | 83.1 ±0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Guo, S.; Yu, W.; Liang, X. Phosphorus–Silicon Additive Increases the Mechanical and Fire Resistance of Epoxy Resins. Materials 2025, 18, 2753. https://doi.org/10.3390/ma18122753
Wang Z, Guo S, Yu W, Liang X. Phosphorus–Silicon Additive Increases the Mechanical and Fire Resistance of Epoxy Resins. Materials. 2025; 18(12):2753. https://doi.org/10.3390/ma18122753
Chicago/Turabian StyleWang, Zhe, Shuaijun Guo, Wenwen Yu, and Xiaohong Liang. 2025. "Phosphorus–Silicon Additive Increases the Mechanical and Fire Resistance of Epoxy Resins" Materials 18, no. 12: 2753. https://doi.org/10.3390/ma18122753
APA StyleWang, Z., Guo, S., Yu, W., & Liang, X. (2025). Phosphorus–Silicon Additive Increases the Mechanical and Fire Resistance of Epoxy Resins. Materials, 18(12), 2753. https://doi.org/10.3390/ma18122753





