Preparation of MgF2 Coatings on AZ31 Mg Alloy in Micro-Arc Oxidation Process Based on the Solubility Product Rule
Abstract
1. Introduction
2. Materials and Methods
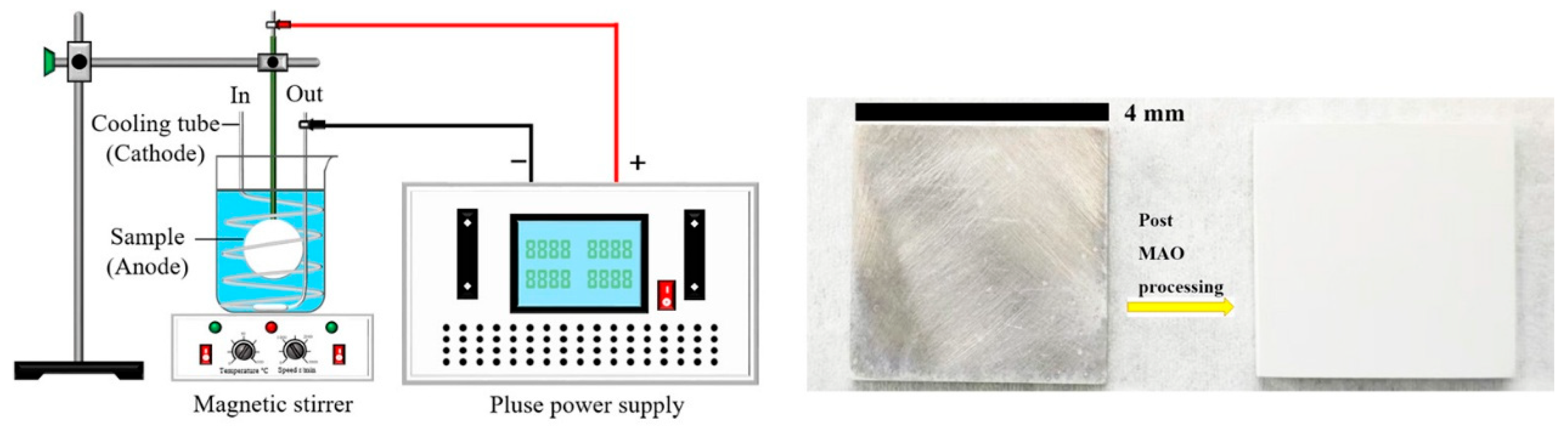
2.1. Coating Preparation
2.2. Coating Characterization
3. Results
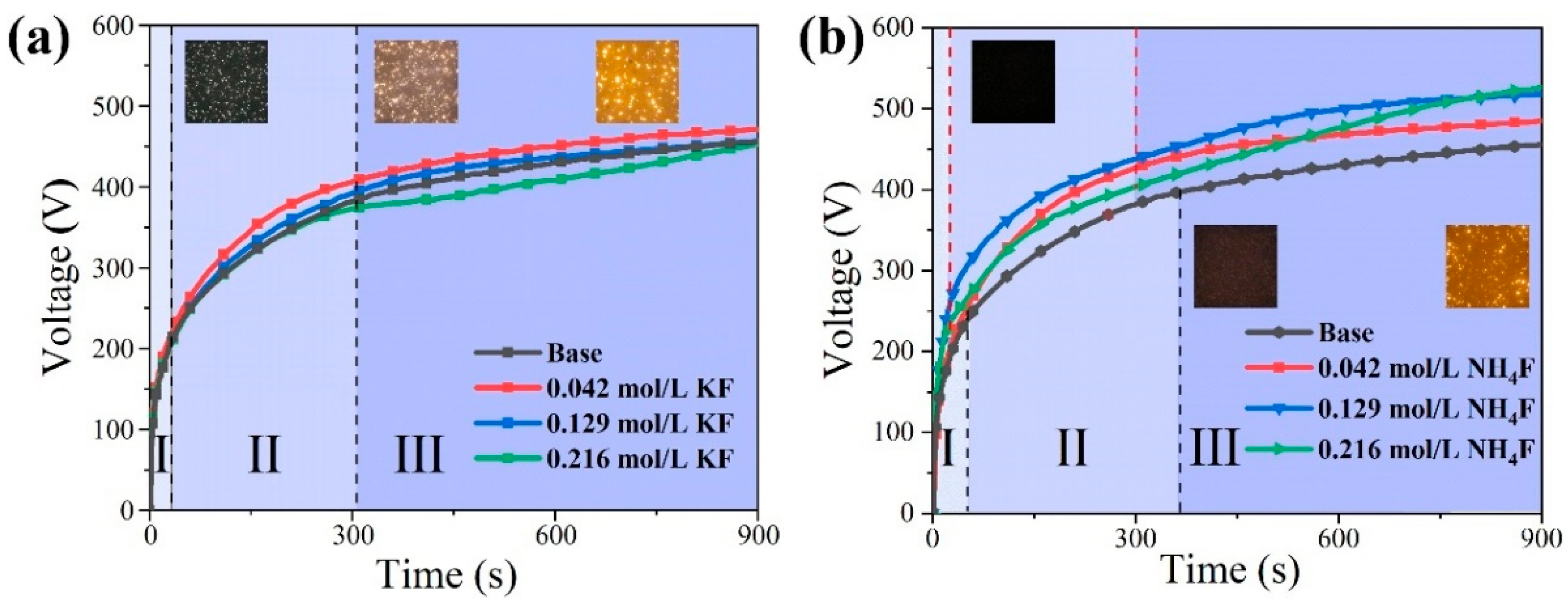
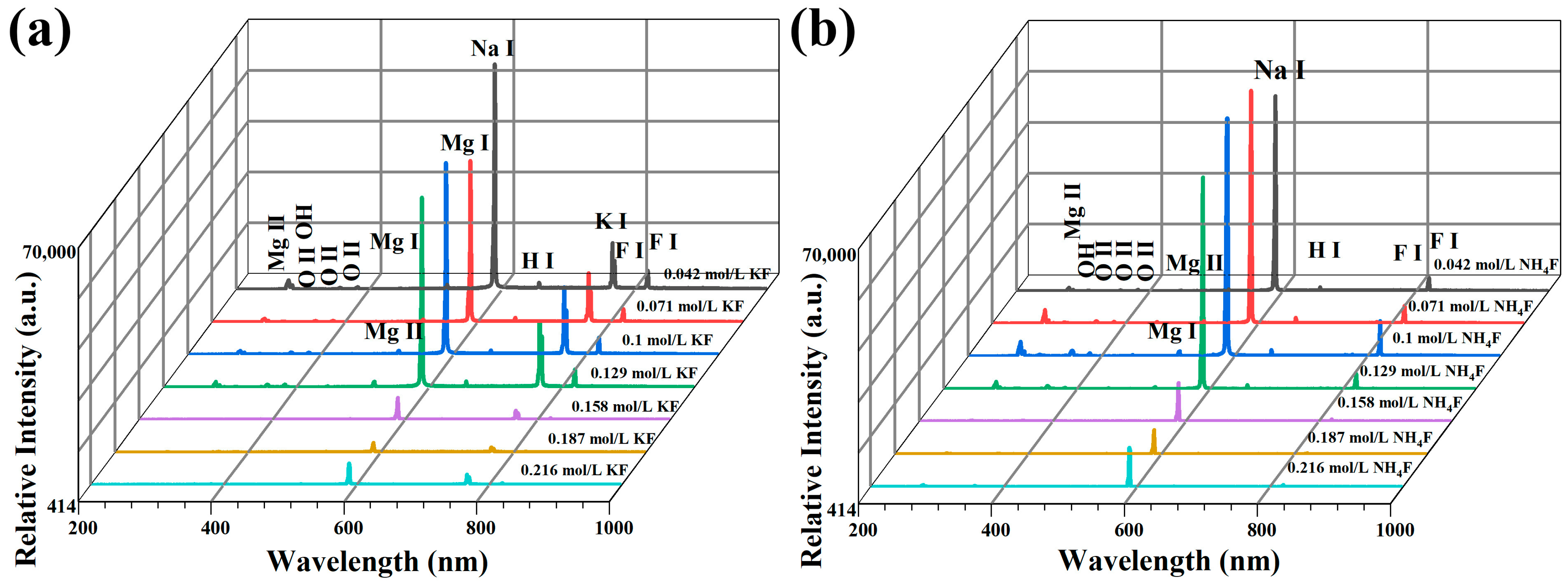
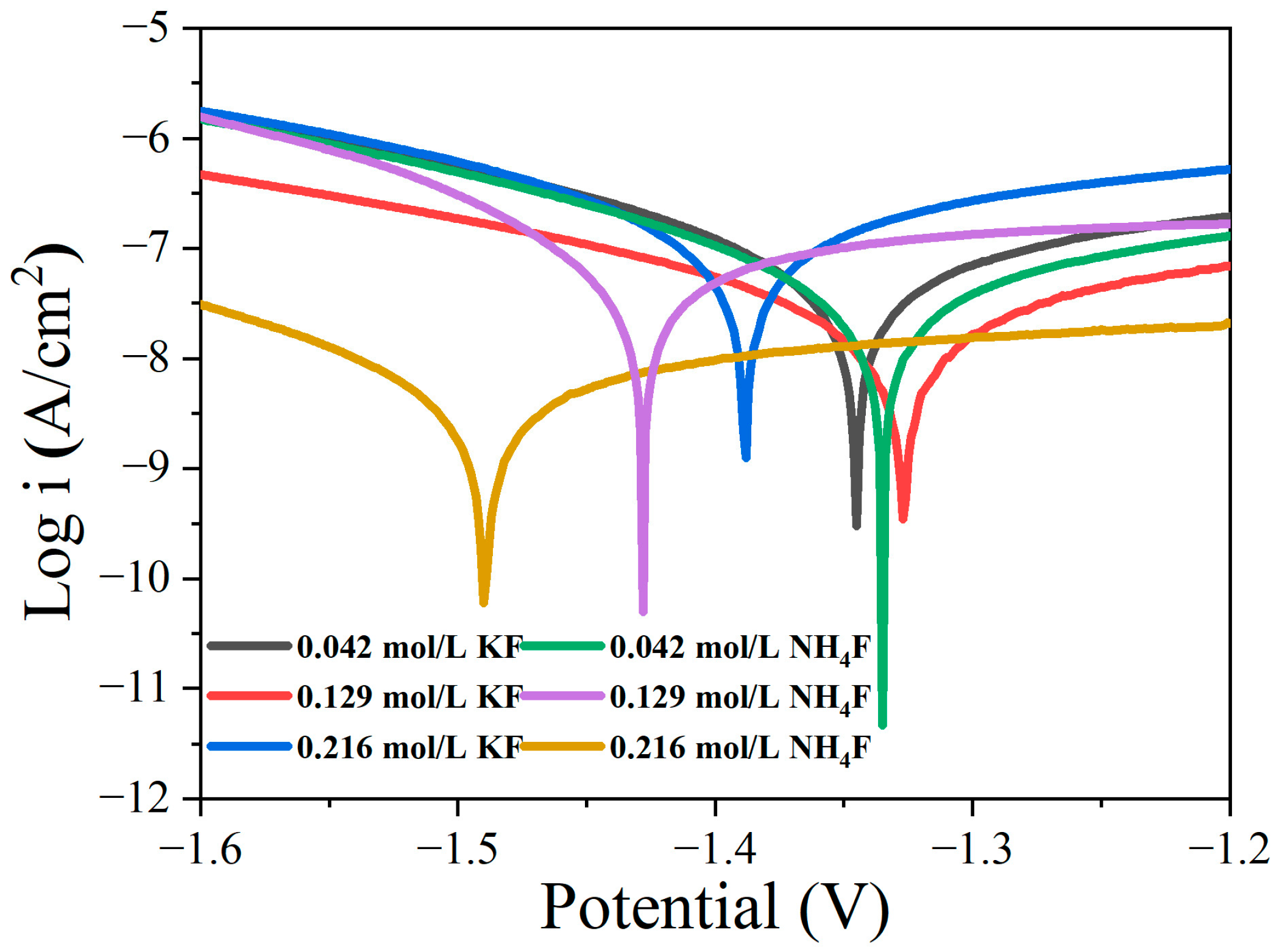
3.1. Discharge Behavior of MAO in Electrolytes with Different Fluorine Additives
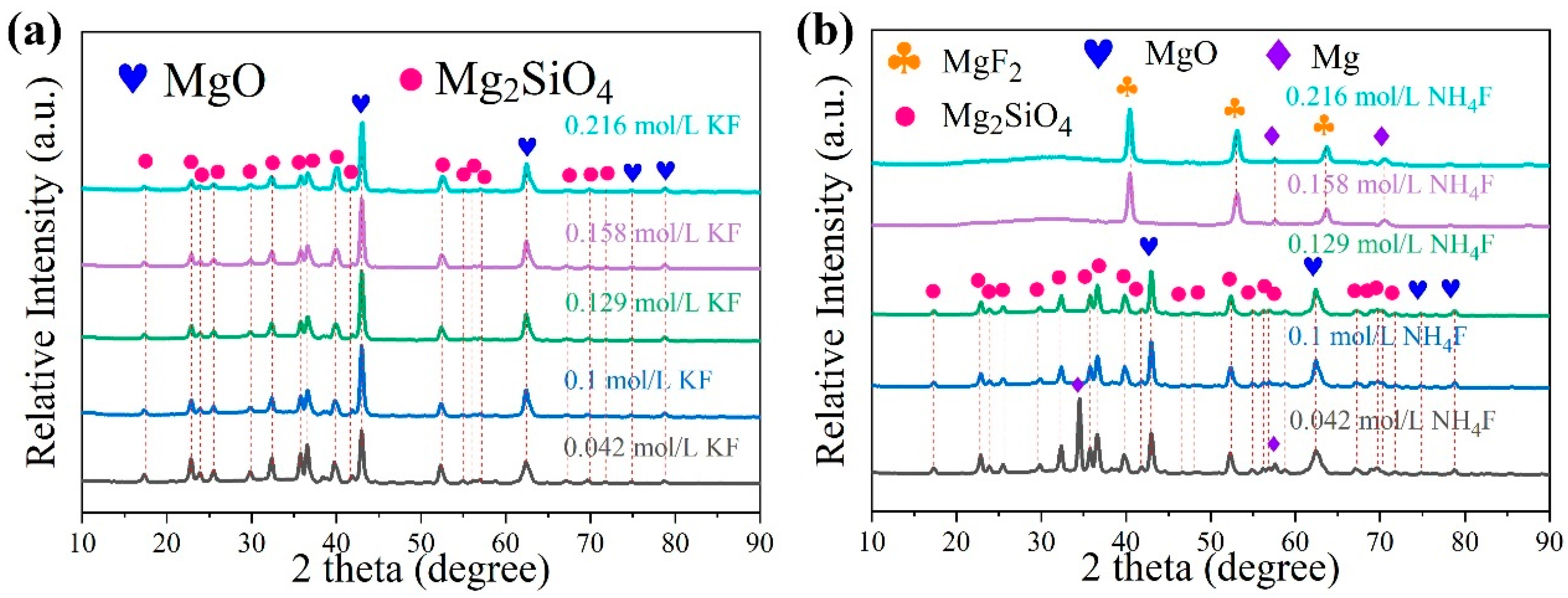
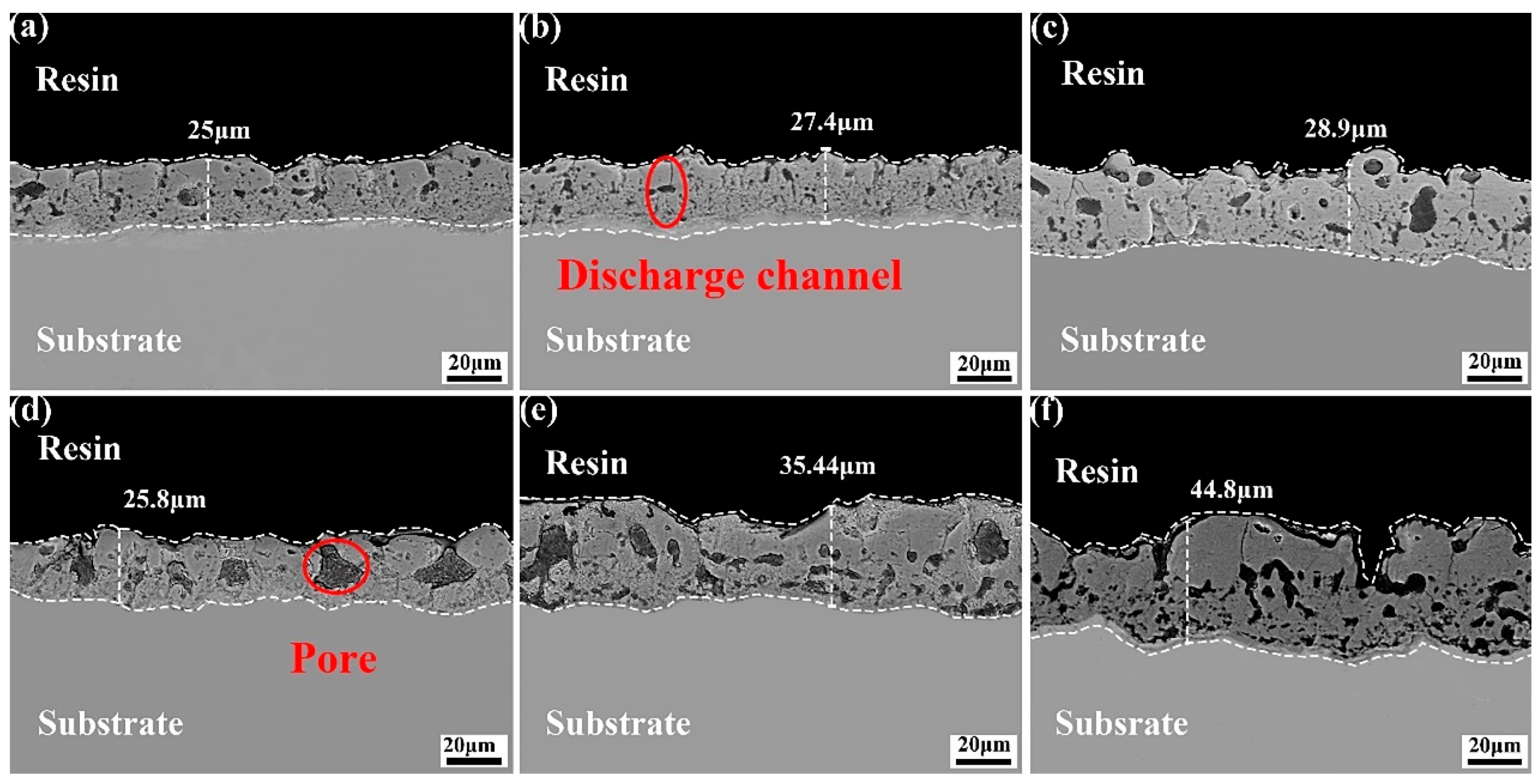
3.2. Microstructure of the Coating in Electrolyte with Different Fluorine Additives
3.3. Effect of Different Fluoride Additives on the Corrosion Resistance of Coatings
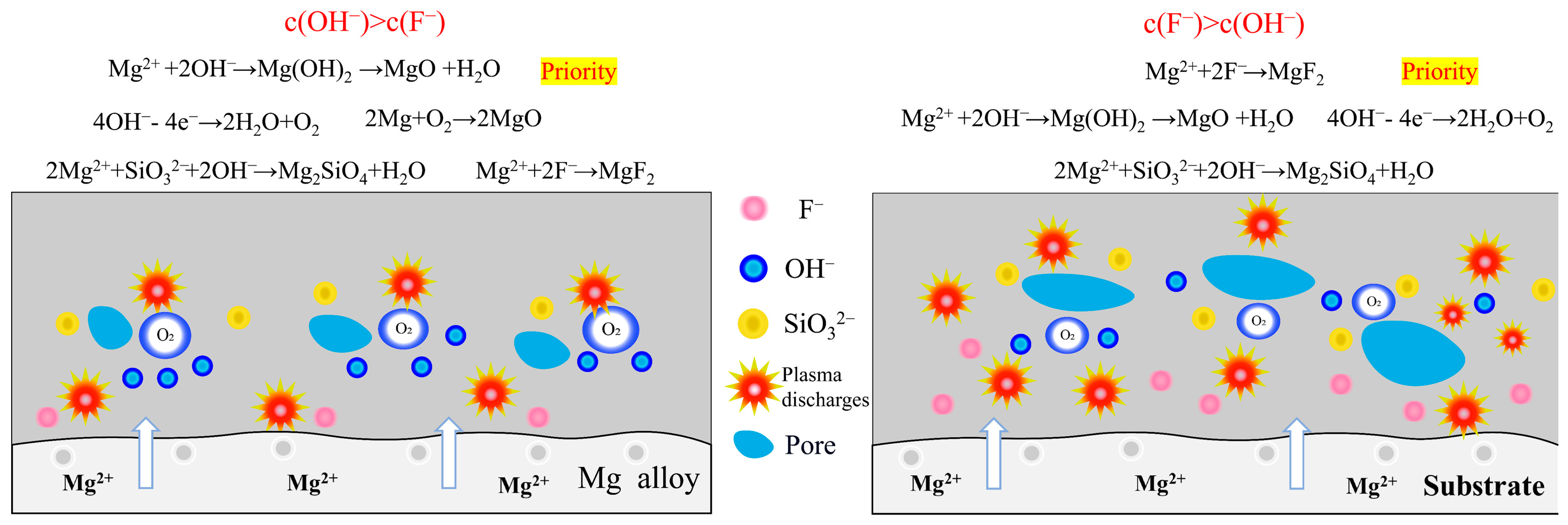
4. Discussion
5. Conclusions
- In silicate-based electrolyte system, regardless of the variations in KF content, the main component of the MAO coatings formed on Mg alloy remained MgO along with a minor presence of magnesium silicate Mg2SiO4. This was because the introduction of KF not only elevated the F− concentration in the electrolyte but also increased the OH− concentration as a result of F− hydrolysis. Based on the solubility product constants (Ksp) of MgO and MgF2, a relatively lower concentration of Mg2+ was sufficient for the formation of MgO. Hence, Mg2+ consistently exhibited preferential reactivity with OH−, leading to the formation of MgO. In the silicate-based electrolyte system, it was not feasible to prepare a MgF2 coating simply by increasing the KF concentration.
- On the contrary, in NH4F electrolyte with a high pH, the OH− preferred F− reacts with Mg2+, and the main coating-forming phases of the coating are MgO and Mg2SiO4. Conversely, in an electrolyte with increasing NH4F concentration, F−-preferred OH− reacts with Mg2+, and the main coating-forming phases of the coating are transformed from MgO and Mg2SiO4 to MgF2. However, MgF2 coatings have many defects, with large surface pores, which actually lead to decreased corrosion resistance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, K. Corrosion and protection of magnesium alloys: Recent advances and future perspectives. Coatings 2023, 13, 1533. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Yu, X.; Sun, X.; Yang, H. Functionalization treatment of micro-arc oxidation coatings on magnesium alloys: A review. J. Alloys Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- Muhaffel, F.; Cimenoglu, H. Development of corrosion and wear resistant micro-arc oxidation coating on a magnesium alloy. Surf. Coat. Technol. 2019, 357, 822–832. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Wang, J.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Bahrampour, S.; Bordbar-Khiabani, A.; Hossein Siadati, M.; Gasik, M.; Mozafari, M. Improving the inflammatory-associated corrosion behavior of magnesium alloys by Mn3O4 incorporated plasma electrolytic oxidation coatings. Chem. Eng. J. 2024, 483, 149016. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Lee, D.K.; Sohn, Y.-S. Electrochemical corrosion properties of plasma electrolytic oxidation coatings on az31 mg alloy in sodium silicate-based electrolyte containing naalo2. J. Sens. Sci. Technol. 2024, 33, 453–457. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Ma, Y.; An, S.J.; Wang, S.; An, L.Y. Effect of electrolytes on the formability and corrosion resistance of micro-arc oxidation coating of AZ91D magnesium alloy. Rare Met. Mat. Eng. 2022, 51, 3057–3069. [Google Scholar]
- Zhang, Y.; Liu, G.; Li, X.; Zhai, D.; Tang, Q.; Chen, S.; Li, H.; Shen, J. Effect of p–si binary system on the formation mechanism of az91d mao coating. Metall. Mater. Trans. A 2024, 55, 1229–1242. [Google Scholar] [CrossRef]
- Cheng, S.T.; Wang, R.C.; Peng, C.Q.; He, Y.Q.; Wang, X.F.; Cai, Z.Y.; Feng, Y. Effect of voltage on microstructure and corrosion resistance of micro-arc oxidation coating on Mg-Zn-Ca biodegradable alloy. J. Mater. Res. Technol-JMRT 2024, 30, 3021–3033. [Google Scholar] [CrossRef]
- Yang, S.K.; Wang, C.; Li, F.Z.; Liu, N.H.; Shi, P.T.; Wang, B.J.; Sun, R.X. One-step in situ growth of a simple and efficient pore-sealing coating on micro-arc oxidized AZ31B magnesium alloy. J. Alloys Compd. 2022, 909, 13. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Zhang, T.; Wang, F. Effect of adding K2TiF6 and K2ZrF6 to neutral electrolyte on the performance of plasma electrolytic oxidation coatings on AZ91 mg alloy. J. Mater. Res. Technol. 2024, 30, 7684–7696. [Google Scholar] [CrossRef]
- Ji, R.; Liu, L.; Yin, H.; Li, W.; Sun, J.; Zhu, P.; Cao, C. Synthesis of corundum rich coating on 7055 aluminum alloy by micro-arc oxidation and its corrosion resistant property in a saline medium. Surf. Coat. Technol. 2024, 476, 130182. [Google Scholar] [CrossRef]
- Yang, C.; Sheng, L.; Zhao, C.; Chen, P.; Ouyang, W.; Xu, D.; Zheng, Y.; Chu, P.K. Improved lubricating and corrosion resistance of mao coatings on ZK61 mg alloy by co-doping with graphite and nano-zirconia. J. Mater. Res. Technol. 2024, 33, 2275–2291. [Google Scholar] [CrossRef]
- Zhang, W.; Du, Y.; Zhang, P. Excellent plasma electrolytic oxidation coating on AZ61 magnesium alloy under ordinal discharge mode. J. Magnes. Alloys 2022, 10, 2460–2474. [Google Scholar] [CrossRef]
- Nagarajan, B.; Mathalai Sundaram, C. Evaluation of different electrolytic solution composition on the microstructure and corrosion study on az31 magnesium alloy using peo method. Phys. Scr. 2024, 99, 125003. [Google Scholar]
- Chen, W.H.; Huang, S.-Y.; Chu, Y.-R.; Yang, S.-H.; Cheng, I.C.; Jian, S.-Y.; Lee, Y.-L. Effect of TiO2 nanoparticles on the corrosion resistance, wear, and antibacterial properties of microarc oxidation coatings applied on AZ31 magnesium alloy. Surf. Coat. Technol. 2024, 476, 130238. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Wang, Q.; Zhao, X.; Zhang, T.; Wang, F. Enhancing wear and corrosion resistance of magnesium alloys through in-situ Al2O3 doping for plasma electrolytic oxidation coating. Mater. Today Commun. 2024, 38, 108234. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, W.; Yang, Y.; Sun, X.; Men, B.; Yu, S. Enhanced hydrophobic properties, wear and corrosion resistance of plasma electrolyte oxidation coatings on AZ31B magnesium alloys with addition of tio2 nanoparticles. Ceram. Int. 2024, 50, 52941–52956. [Google Scholar] [CrossRef]
- Giavaresi, G.; Bellavia, D.; De Luca, A.; Costa, V.; Raimondi, L.; Cordaro, A.; Sartori, M.; Terrando, S.; Toscano, A.; Pignatti, G.; et al. Magnesium alloys in orthopedics: A systematic review on approaches, coatings and strategies to improve biocompatibility, osteogenic properties and osteointegration capabilities. Int. J. Mol. Sci. 2024, 25, 41. [Google Scholar]
- Zhang, Y.; Guo, Y.R.; Zhou, P.; Zhang, T.; Wang, F.H.; Chen, L.Y. A plasma electrolytic oxidation coating on high-purity mg with excellent long-term degradation performance and biocompatibility. Surf. Coat. Technol. 2024, 493, 13. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, W.; Yu, F.; Cao, W.; Hu, S. Effect of sodium fluoride additive on microstructure and corrosion performance of micro-arc oxidation coatings on EK30 magnesium alloy. Surf. Coat. Technol. 2025, 496, 131628. [Google Scholar] [CrossRef]
- Liu, F.; Yu, J.; Song, Y.; Shan, D.; Han, E.-H. Effect of potassium fluoride on the in-situ sealing pores of plasma electrolytic oxidation film on AM50 mg alloy. Mater. Chem. Phys. 2015, 162, 452–460. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, Y.; Dong, S.; Chen, X.-B.; Dong, J. Effects of fluoride ions as electrolyte additives for a peo/ni-p composite coating onto mg alloy AZ31B. Surf. Coat. Technol. 2021, 417, 126883. [Google Scholar] [CrossRef]
- Lujun, Z.; Hongzhan, L.; Qingmei, M.; Jiangbo, L.; Zhengxian, L. The mechanism for tuning the corrosion resistance and pore density of plasma electrolytic oxidation (peo) coatings on mg alloy with fluoride addition. J. Magnes. Alloys 2023, 11, 2823–2832. [Google Scholar] [CrossRef]
- Rong, Z.; Bai, Y.; Tian, H.; Dou, Z.; Zhang, Y.; Liu, L.; Chen, F. Influence of sodium fluoride in acidic electrolytic solution on the structure and properties of plasma electrolytic oxidation coating on AZ91D magnesium alloy. J. Mater. Sci. 2023, 58, 8103–8117. [Google Scholar] [CrossRef]
- Thanaa, T.T.; Fattah-alhosseini, A.; Alkaseem, M.; Kaseem, M. Improving the surface properties of mg based-plasma electrolytic oxidation (peo) coatings under the fluoride electrolytes: A review. Inorg. Chem. Commun. 2024, 170, 113163. [Google Scholar] [CrossRef]
- Yang, C.; Sheng, L.; Zhao, C.; Wu, D.; Zheng, Y. Regulating the ablation of nanoparticle-doped mao coating on mg alloy by MgF2 passivation layer construction. Mater. Lett. 2024, 355, 135559. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z.; Fu, W. Optical emission spectroscopy studies of discharge mechanism and plasma characteristics during plasma electrolytic oxidation of magnesium in different electrolytes. Surf. Coat. Technol. 2010, 205, 1651–1658. [Google Scholar] [CrossRef]
- Matthew, J.A.D. Crc handbook of chemistry and physics-weast,rc. Nature 1988, 331, 127. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (peo) process. J. Phys. D-Appl. Phys. 2010, 43, 13. [Google Scholar] [CrossRef]


| KF | NH4F | |||
|---|---|---|---|---|
| F− (mol/L) | Conductivity (mS/cm) | pH | Conductivity (mS/cm) | pH |
| 0.042 | 42.7 | 12.9 | 34.6 | 12.6 |
| 0.071 | 45.8 | 13.0 | 29.4 | 12.6 |
| 0.1 | 47.6 | 13.0 | 26.3 | 12.2 |
| 0.129 | 50.9 | 13.1 | 23.2 | 12.1 |
| 0.158 | 54.1 | 13.2 | 20.7 | 11.9 |
| 0.187 | 56.6 | 13.3 | 18.7 | 11.6 |
| 0.216 | 57.9 | 13.3 | 19.2 | 10.3 |
| KF (mol/L) | Content of Elements (wt.%) | |||||
|---|---|---|---|---|---|---|
| Mg | F | Si | O | Na | K | |
| 0.042 | 44.23 | 0.94 | 20.39 | 32.94 | 1.19 | 0.31 |
| 0.129 | 43.72 | 3.15 | 17.82 | 31.92 | 1.98 | 1.41 |
| 0.216 | 42.67 | 5.43 | 15.53 | 31.05 | 2.85 | 2.47 |
| NH4F (mol/L) | Content of Elements (wt.%) | |||||
|---|---|---|---|---|---|---|
| Mg | F | Si | O | Na | N | |
| 0.042 | 41.95 | 1.39 | 18.91 | 35.25 | 1.22 | 1.28 |
| 0.129 | 39.01 | 6.73 | 17.89 | 32.62 | 2.45 | 1.30 |
| 0.216 | 35.81 | 20.91 | 14.53 | 23.51 | 3.72 | 1.52 |
| KF | NH4F | |||
|---|---|---|---|---|
| F− (mol/L) | Average Size (μm) | Porosity | Average Size (μm) | Porosity |
| 0.042 | 7.126 | 5.027 | 7.756 | 4.007 |
| 0.129 | 5.928 | 4.342 | 6.691 | 3.582 |
| 0.216 | 6.86 | 4.274 | 19.211 | 6.065 |
| Specimens | Ecorr (mV vs. Ag/AgCl) | icorr (A/cm2) |
|---|---|---|
| 0.042 mol/L KF | −1341 | 3.93 × 10−8 |
| 0.129 mol/L KF | −1327 | 8.91 × 10−9 |
| 0.216 mol/L KF | −1386 | 6.61 × 10−8 |
| 0.042 mol/L NH4F | −1332 | 2.08 × 10−8 |
| 0.129 mol/L NH4F | −1425 | 2.65 × 10−8 |
| 0.216 mol/L NH4F | −1486 | 2.45 × 10−9 |
| Specimens | OH− (mol/L) | Ksp [Mg(OH)2] c (Mg2+) (mol/L) | Ksp [MgF2] c (Mg2+) (mol/L) |
|---|---|---|---|
| 0.042 mol/L KF | 6.31 × 10−2 | 8.89 × 10−10 | 2.92 × 10−8 |
| 0.129 mol/L KF | 7.74 × 10−2 | 3.38 × 10−10 | 3.10 × 10−9 |
| 0.216 mol/L KF | 2.09 × 10−1 | 1.29 × 10−10 | 1.11 × 10−9 |
| 0.042 mol/L NH4F | 4.27 × 10−2 | 3.08 × 10−9 | 2.92 × 10−8 |
| 0.129 mol/L NH4F | 1.26 × 10−2 | 3.54 × 10−8 | 3.10 × 10−9 |
| 0.216 mol/L NH4F | 1.81 × 10−4 | 1.69 × 10−4 | 1.11 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yang, Y.; Liu, C.; Lu, X. Preparation of MgF2 Coatings on AZ31 Mg Alloy in Micro-Arc Oxidation Process Based on the Solubility Product Rule. Materials 2025, 18, 2717. https://doi.org/10.3390/ma18122717
Wang H, Yang Y, Liu C, Lu X. Preparation of MgF2 Coatings on AZ31 Mg Alloy in Micro-Arc Oxidation Process Based on the Solubility Product Rule. Materials. 2025; 18(12):2717. https://doi.org/10.3390/ma18122717
Chicago/Turabian StyleWang, Hao, Yifeng Yang, Cancan Liu, and Xuchen Lu. 2025. "Preparation of MgF2 Coatings on AZ31 Mg Alloy in Micro-Arc Oxidation Process Based on the Solubility Product Rule" Materials 18, no. 12: 2717. https://doi.org/10.3390/ma18122717
APA StyleWang, H., Yang, Y., Liu, C., & Lu, X. (2025). Preparation of MgF2 Coatings on AZ31 Mg Alloy in Micro-Arc Oxidation Process Based on the Solubility Product Rule. Materials, 18(12), 2717. https://doi.org/10.3390/ma18122717





