Coating Formulations Based on Carbon Black: An Alternative to Develop Environmentally Friendly Conductive Cellulose Paper
Abstract
1. Introduction
2. Materials and Methods
2.1. Conductive Coating
2.2. Paper Substrate
2.3. Coating Technique
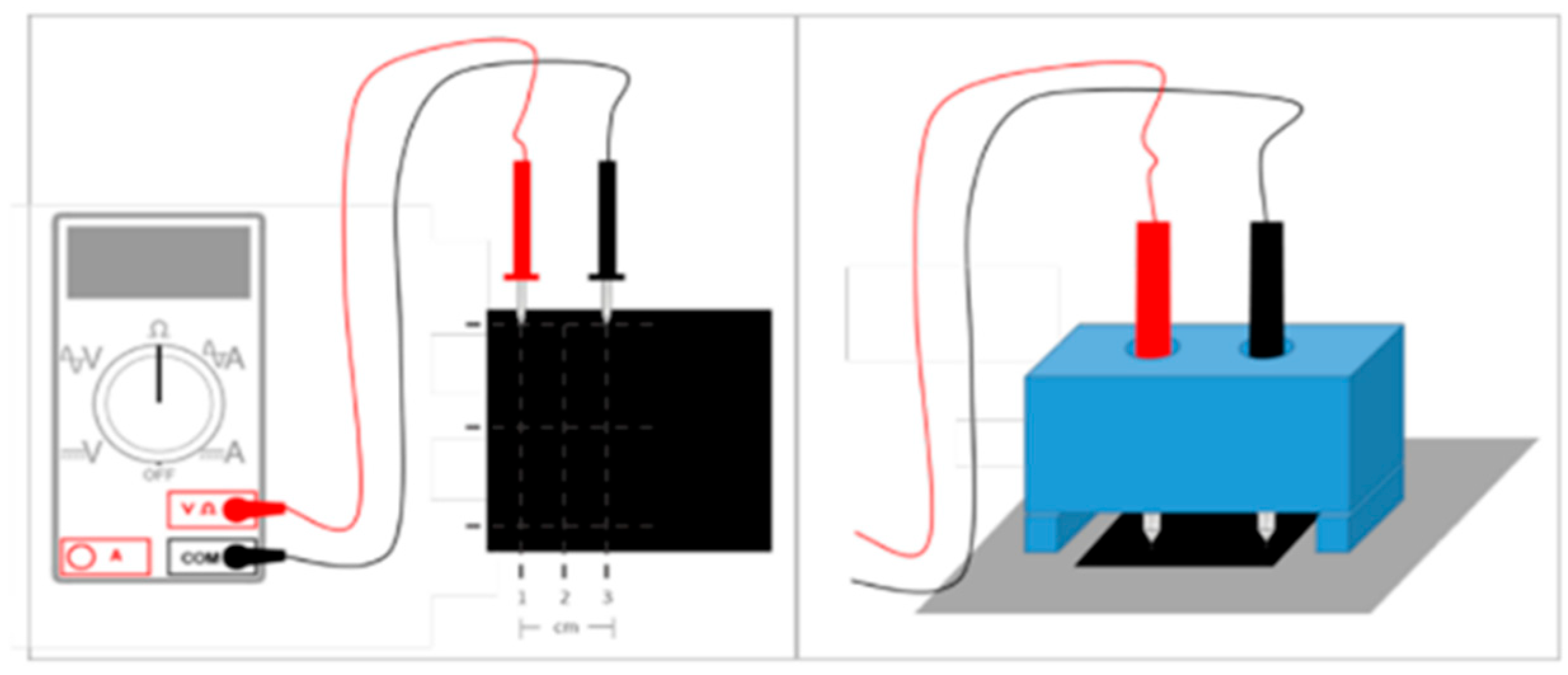
2.4. Coating Evaluation
- ksheet: absorption coefficient of the sheet sample at 950 nm;
- kink: absorption coefficient of the ink at 950 nm.
3. Results and Discussion
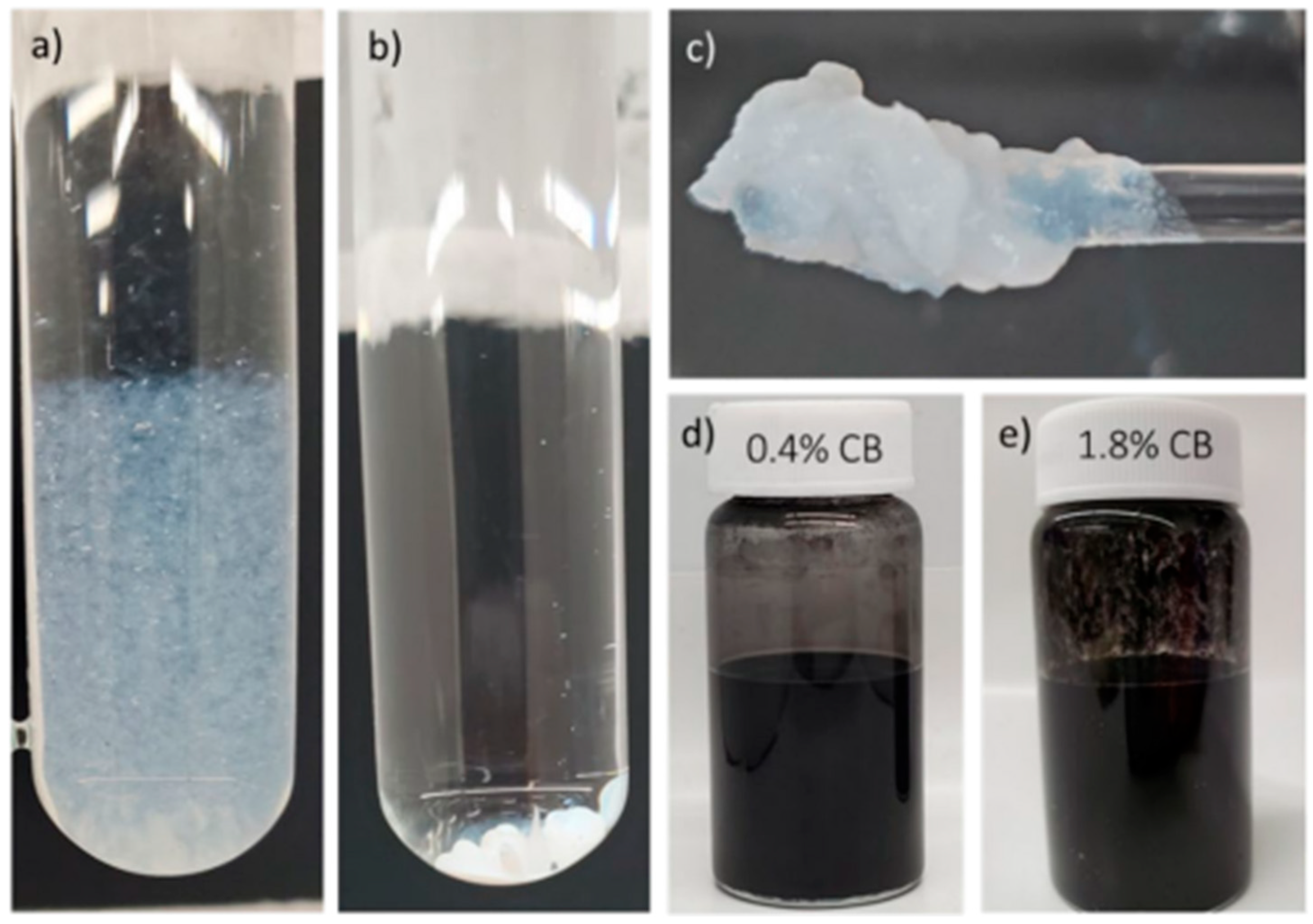
3.1. Coating Formulation Stability
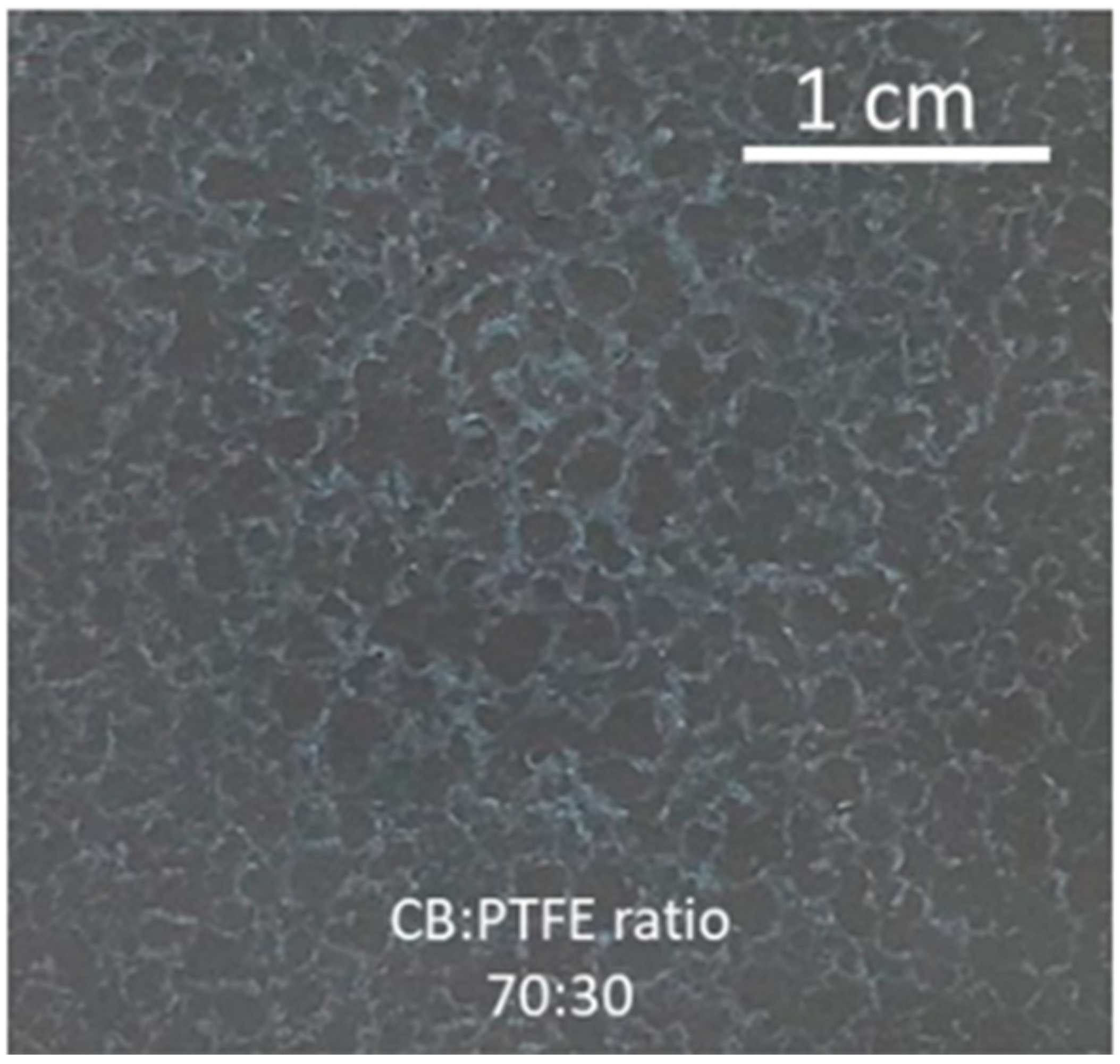
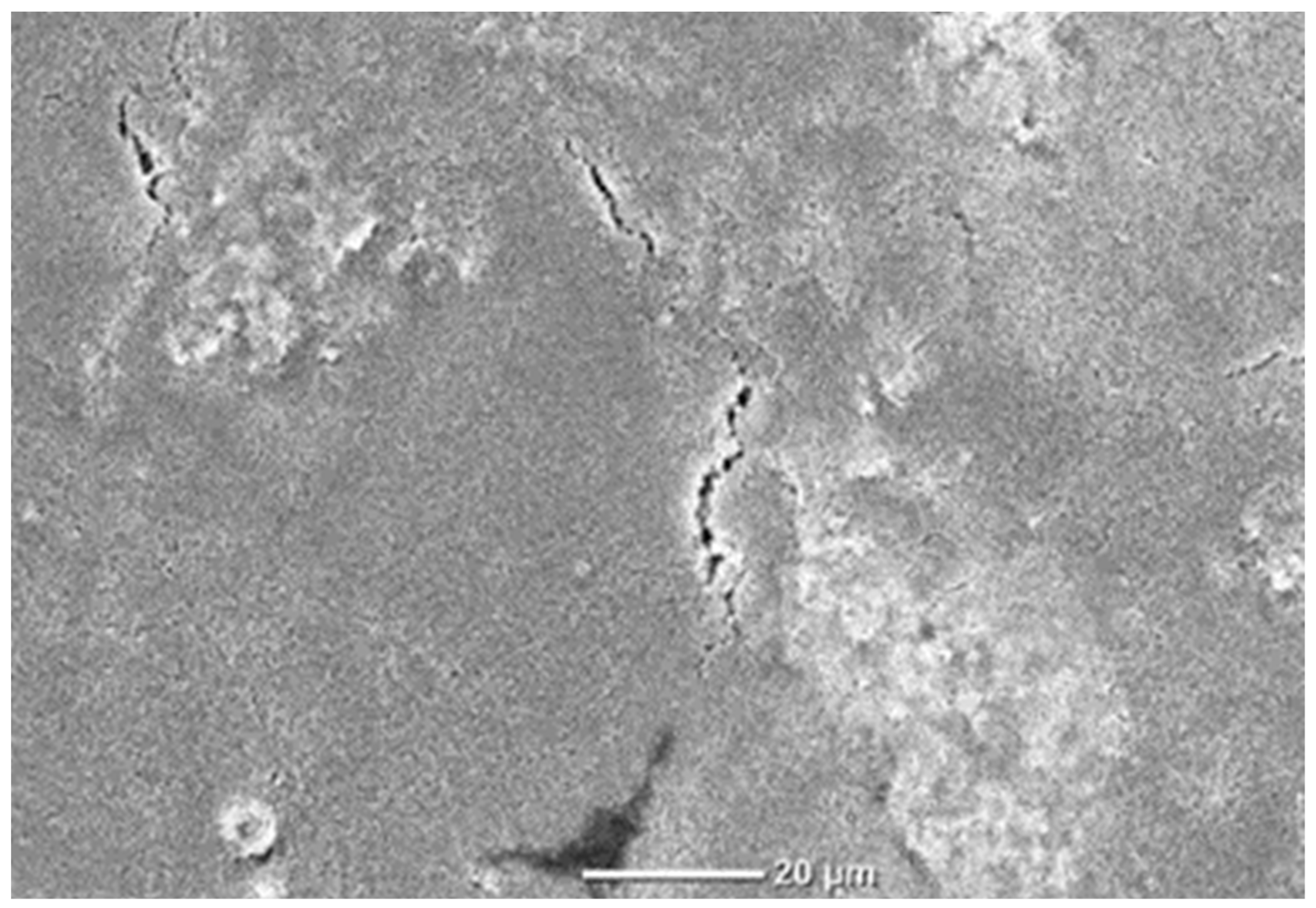
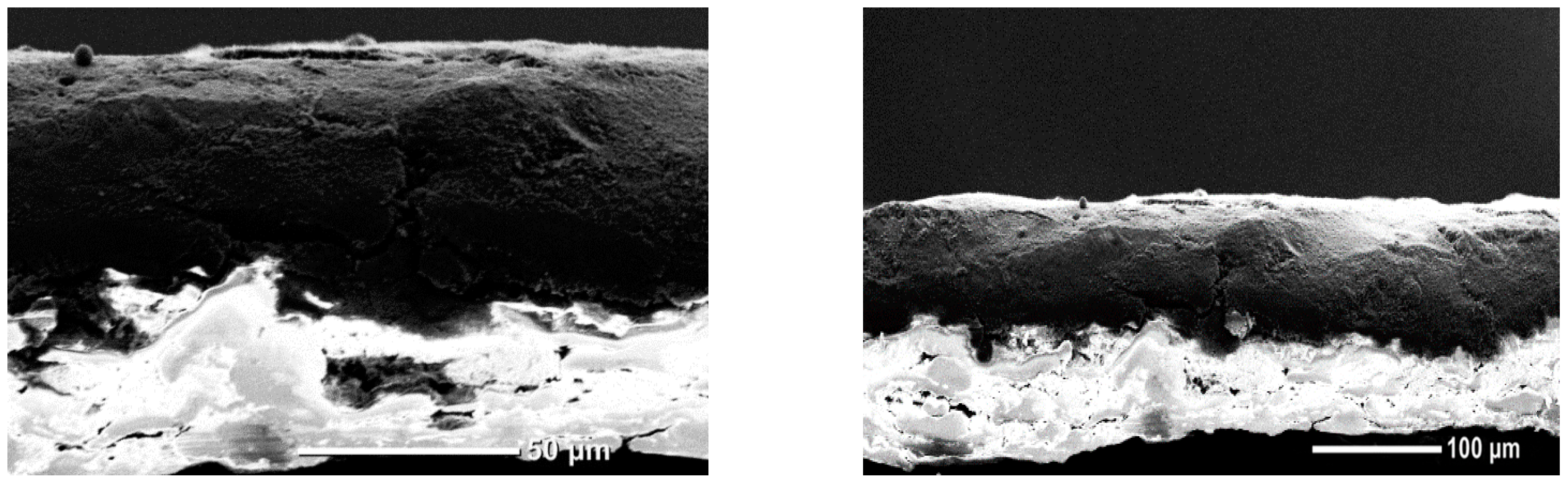
3.2. Effects of Formulation on Resistance and Coating Integrity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Tang, Z.; Liu, Z.; Zhi, C. Evaluating Flexibility and Wearability of Flexible Energy Storage Devices. Joule 2019, 3, 613–619. [Google Scholar] [CrossRef]
- Tai, Y.L.; Yang, Z.G. Fabrication of paper-based conductive patterns for flexible electronics by direct-writing. J. Mater. Chem 2011, 21, 5938–5943. [Google Scholar] [CrossRef]
- Wang, D.; Han, C.; Mo, F.; Yang, Q.; Zhao, Y.; Li, Q.; Liang, G.; Dong, B.; Zhi, C. Energy density issues of flexible energy storage devices. Energy Storage Mater 2020, 28, 264–292. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible Energy-Storage Devices: Design Consideration and Recent Progress; Wiley-VCH Verlag: Weinheim, Germany, 2014. [Google Scholar] [CrossRef]
- Corzo, D.; Tostado-Blázquez, G.; Baran, D. Flexible Electronics: Status, Challenges and Opportunities. Front. Electron. 2020, 1, 594003. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Cui, K.; Ge, S.; Cheng, X.; Yan, M.; Yu, J.; Liu, H. Flexible Electronics Based on Micro/Nanostructured Paper; Wiley-VCH Verlag: Weinheim, Germany, 2018. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Brown, R.M. Cellulose Structure and Biosynthesis: What is in Store for the 21st Century? J. Polym. Sci. Part A Polym. Chem. 2004, 42, 487–495. [Google Scholar] [CrossRef]
- Zhu, H.; Fang, Z.; Preston, C.; Li, Y.; Hu, L. Transparent paper: Fabrications, properties, and device applications. Energy Environ. Sci. 2013, 7, 269–287. [Google Scholar] [CrossRef]
- Jabbour, L.; Chaussy, D.; Eyraud, B.; Beneventi, D. Highly conductive graphite/carbon fiber/cellulose composite papers. Compos. Sci. Technol. 2012, 72, 616–623. [Google Scholar] [CrossRef]
- Koehly, R.; Curtil, D.; Van De Ven, T.G.M.; Wanderley, M.M. Carbon Black Loaded Paper: A intelligent substrate for Electronic Sensors Design. In Proceedings of the International Conference on Advances in Printing and Media Technology, Zagreb, Croatia, 5 November 2007; pp. 285–292. [Google Scholar]
- Ding, C.; Qian, X.; Shen, J.; An, X. Preparation and characterization of conductive paper via in-situ polymerization of pyrrole. Bioresources 2010, 5, 303–315. [Google Scholar] [CrossRef]
- Johnston, J.H.; Moraes, J.; Borrmann, T. Conducting polymers on paper fibres. Synth. Met. 2005, 153, 65–68. [Google Scholar] [CrossRef]
- Schreck, M.; Deshmukh, R.; Tervoort, E.; Niederberger, M. Impregnation of Cellulose Fibers with Copper Colloids and Their Processing into Electrically Conductive Paper. Chem. Mater. 2022, 34, 43–52. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, J.; Kou, T.; Song, Y.; Liu, T.; Li, Y. Paper-Based Electrodes for Flexible Energy Storage Devices; Wiley-VCH Verlag: Weinheim, Germany, 2017. [Google Scholar] [CrossRef]
- Kumar, V.; Forsberg, S.; Engström, A.-C.; Nurmi, M.; Andres, B.; Dahlström, C.; Toivakka, M. Conductive nanographite-nanocellulose coatings on paper. Flex. Print. Electron. 2017, 2, 035002. [Google Scholar] [CrossRef]
- Tang, Y.; He, Z.; Mosseler, J.A.; Ni, Y. Production of highly electro-conductive cellulosic paper via surface coating of carbon nanotube/graphene oxide nanocomposites using nanocrystalline cellulose as a binder. Cellulose 2014, 21, 4569–4581. [Google Scholar] [CrossRef]
- Lepoutre, P. The structure of paper coatings: An update. Prog. Org. Coat. 1989, 17, 89–106. [Google Scholar] [CrossRef]
- El Ouatani, L.; Dedryvère, R.; Ledeuil, J.-B.; Siret, C.; Biensan, P.; Desbrières, J.; Gonbeau, D. Surface film formation on a carbonaceous electrode: Influence of the binder chemistry. J. Power Sources 2009, 189, 72–80. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production-A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Engquist, I.; Wang, X.; Fall, A.; Mengistie, D.; Calvie, E.; Granberg, H.; Gustafsson, G.; Berggren, M.; Engquist, I. Highly conducting nanographite-filled paper fabricated via standard papermaking techniques. ACS Appl. Mater. Interfaces 2020, 12, 48828–48835. [Google Scholar] [CrossRef]
- Kossyrev, P. Carbon black supercapacitors employing thin electrodes. J. Power Sources 2012, 201, 347–352. [Google Scholar] [CrossRef]
- Bonnefoi, L.; Simon, P.; Fauvarque, J.F.; Sarrazin, C.; Sarrau, J.F.; Dugast, A. Electrode compositions for carbon power supercapacitors. J. Power Sources 1999, 80, 149–155. [Google Scholar] [CrossRef]
- Buqa, H.; Holzapfel, M.; Krumeich, F.; Veit, C.; Novák, P. Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J. Power Sources 2006, 161, 617–622. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, S.; Yuan, J.; Qin, X.; Deng, Y.; Qu, R.; Haarberg, G.M. Effects of various binders on supercapacitor performances. Int. J. Electrochem. Sci. 2016, 11, 8270–8279. [Google Scholar] [CrossRef]
- Hu, L.; Choi, J.W.; Yang, S.J.Y.; La Mantia, F.; Cui, L.-F.; Cu, Y. Highly conductive paper for energy-storage devices. Proc. Natl. Acad. Sci. USA 2009, 106, 21490–21494. [Google Scholar] [CrossRef] [PubMed]
- Kordás, K.; Mustonen, T.; Tóth, G.; Jantunen, H.; Lajunen, M.; Soldano, C.; Talapatra, S.; Kar, S.; Vajtai, R.; Ajayan, P.M. Inkjet printing of electrically conductive patterns of carbon nanotubes. Small 2006, 2, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Mosseler, J.A.; He, Z.; Ni, Y. Imparting cellulosic paper of high conductivity by surface coating of dispersed graphite. Ind. Eng. Chem. Res. 2014, 53, 10119–10124. [Google Scholar] [CrossRef]
- Vahey, D.W.; Zhu, J.Y.; Houtman, C.J. On Measurements of Effective Residual Ink Concentration (ERIC) of Deinked Papers Using Kubelka-Munk Theory. Prog. Pap. Recycl. 2006, 16, 3–12. [Google Scholar]
- Körkkö, M.; Haapala, A.; Ämmälä, A.; Liimatainen, H.; Niinimäkí, J. Effect of wavelength selection on determination of ink content and ink elimination at 700 or 950 nm. TAPPI J. 2014, 13, 45–52. [Google Scholar] [CrossRef]
- Subramanian, S.; Øye, G. Aqueous carbon black dispersions stabilized by sodium lignosulfonates. Colloid Polym. Sci. 2021, 299, 1223–1236. [Google Scholar] [CrossRef]
- Chauhan, A.P.S.; Chawla, K. Comparative studies on Graphite and Carbon Black powders, and their dispersions. J. Mol. Liq. 2016, 221, 292–297. [Google Scholar] [CrossRef]
- Legrand, G.; Manneville, S.; McKinley, G.H.; Divoux, T. Dual Origin of Viscoelasticity in Polymer-Carbon Black Hydrogels: A Rheometry and Electrical Spectroscopy Study. Macromolecules 2023, 56, 2298–2308. [Google Scholar] [CrossRef]
- Fardim, P. Paper and Surface Chemistry-Part 2-Coating and Printability; Science Direct Working Paper; Universidade Estadual de Campinas (UNICAMP): Campinas, Brazil, 2002. [Google Scholar]
- Dickson, R.J. Adhesion and Cohesion in Coated Paper. Doctoral Dissertation, The University of Maine, Orono, ME, USA, 1997. [Google Scholar]


| 98% Solvent (Composition) | 2% Solids (Composition) | |||||
|---|---|---|---|---|---|---|
| Condition Evaluated | Formulation | Binder | Isopropyl Alcohol ACS Grade (wt%) | DI Water (wt%) | Carbon Black (wt%) | Binder (wt%) |
| Kossyrev [22] formulation | 1 | PTFE and latex (separately) | 99.9 | 0.10 | 90 | 10 |
| CB/binder ratio | 2 | PTFE and | 97.7 | 0.3 | 80 | 20 |
| 3 | latex | 97.6 | 0.4 | 70 | 30 | |
| 4 | (separately) | 97.3 | 0.7 | 50 | 50 | |
| Alcohol concentration | 5 | 70 | 30 | 90 | 10 | |
| 6 | PTFE | 55 | 45 | 90 | 10 | |
| 7 | 40 | 60 | 90 | 10 | ||
| Alcohol concentration | 8 | Latex | 70 | 30 | 90 | 10 |
| 9 | 55 | 45 | 90 | 10 | ||
| 10 | 40 | 60 | 90 | 10 | ||
| Alcohol concentration | 11 | 42 | 58 | 90 | 10 | |
| 12 | CMC | 33 | 67 | 90 | 10 | |
| 13 | 24 | 76 | 90 | 10 | ||
| CB/binder ratio (at different alcohol %) | 14 | 42 | 58 | 82 | 18 | |
| 15 | CMC | 33 | 67 | 82 | 18 | |
| 16 | 24 | 76 | 82 | 18 | ||
| Formulation | DI Water (wt%) | Solids (wt%) | Solids Composition | |
|---|---|---|---|---|
| CB (wt%) | CMC (wt%) | |||
| 17 | 96 | 4 | 88 | 13 |
| 18 | 93 | 7 | 88 | 13 |
| 19 | 86 | 14 | 88 | 13 |
| Property | Basis Weight (g/m2) | Caliper (µm) | Density (g/cm3) | Contact Angle (°) | Air Permeability (s/100mL) | Roughness (µm) |
|---|---|---|---|---|---|---|
| Average | 44.94 | 64 | 705.55 | 91.31 | 68.70 | 5.61 |
| Standard Deviation | 0.45 | 0.87 | 1.01 × 10−5 | 2.98 | 3.02 | 0.44 |
| Condition Evaluated | Formulation | Binder | Resistance (kΩ) | Standard Deviation | ERIC | Standard Deviation | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kossyrev [22] formulation | 1 | PTFE and latex (separately) | - | - | - | - | ||||
| CB/binder ratio | 2 | PTFE and | PTFE | Latex | PTFE | Latex | PTFE | Latex | PTFE | Latex |
| latex | 10.38 | 6.24 | 1.78 | 4.81 | - | - | - | - | ||
| 3 | (separately) | 9.77 | 3.81 | 4.26 | 1.46 | - | - | - | - | |
| 4 | 5.07 | 1.74 | 0.32 | 0.28 | - | - | - | - | ||
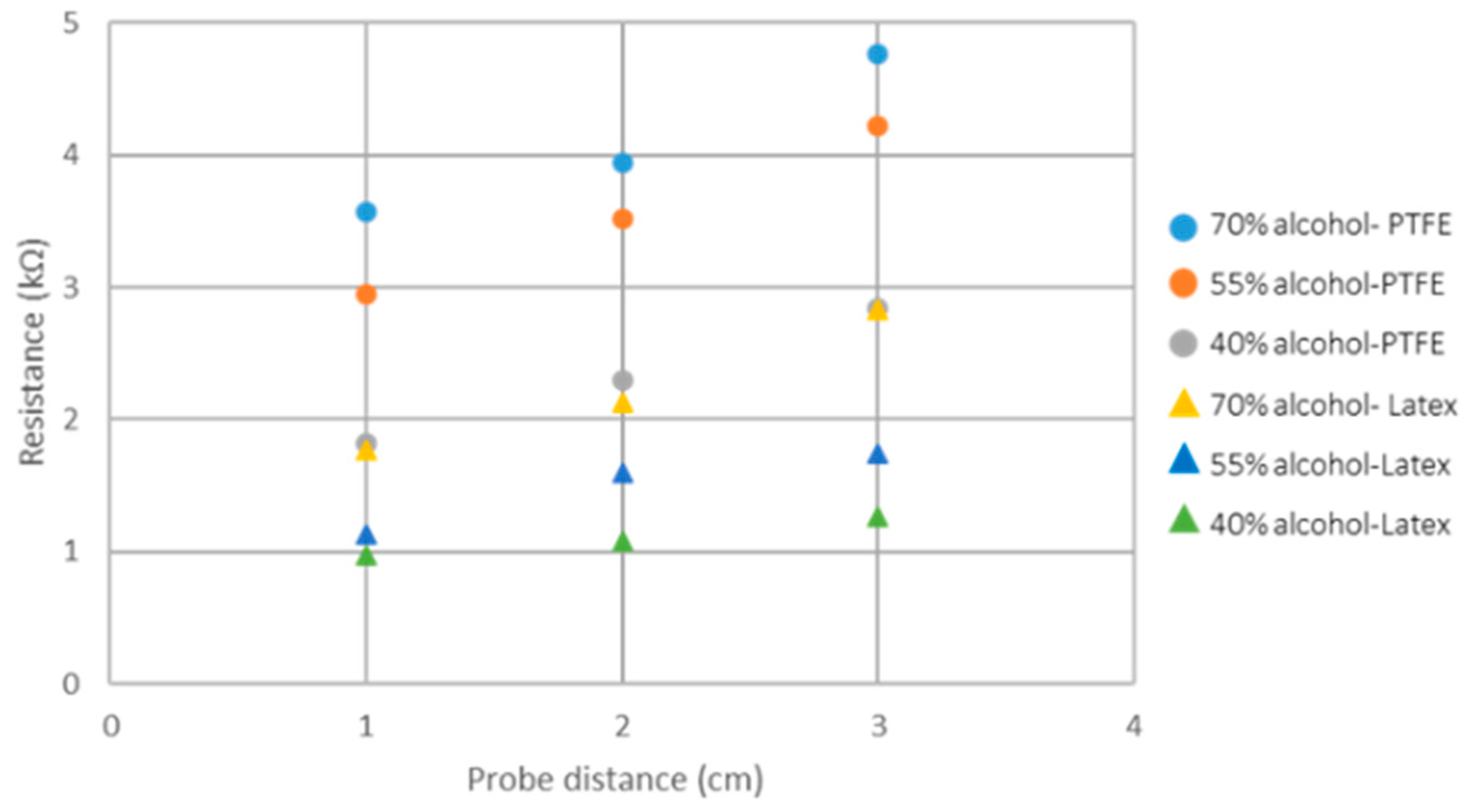
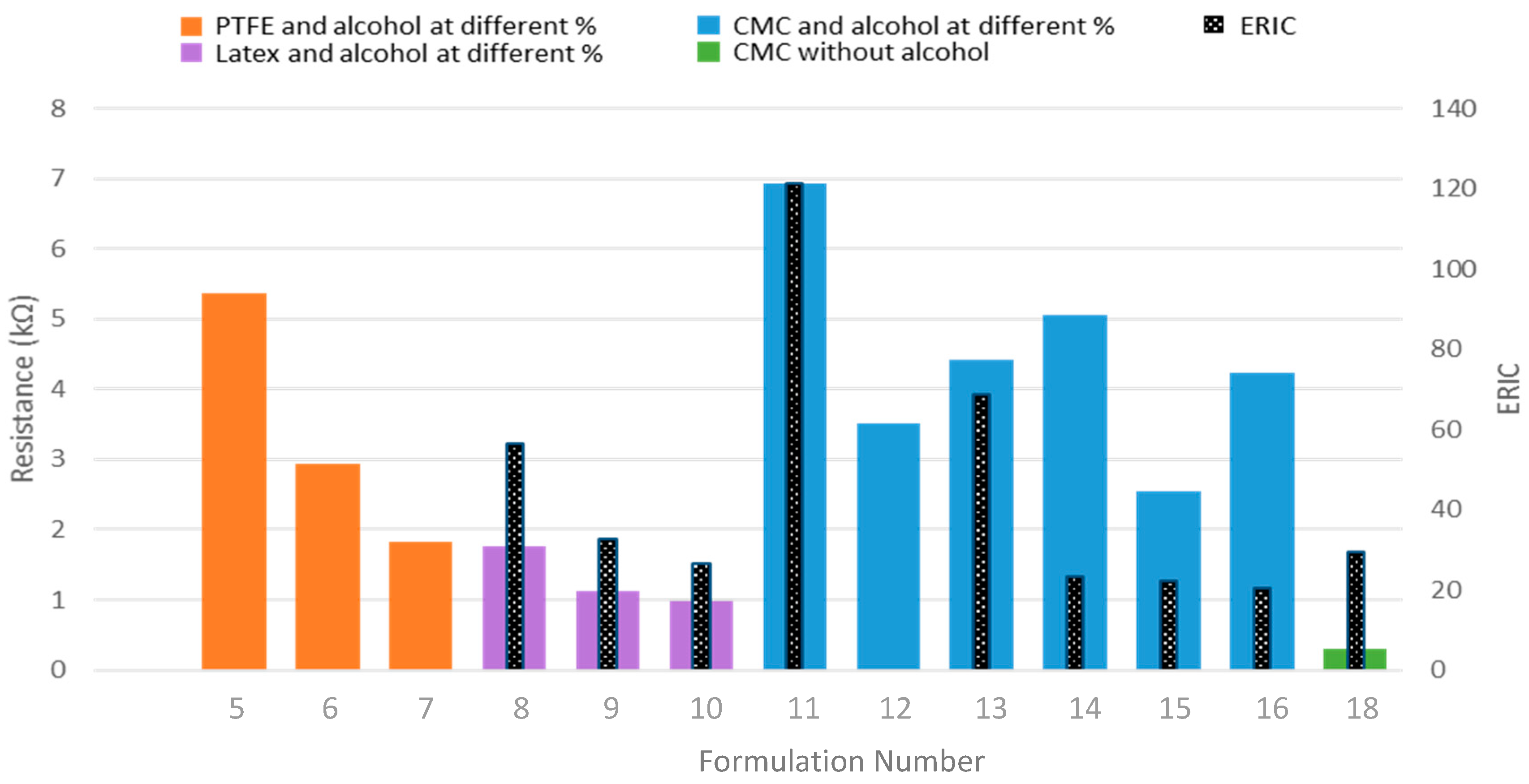
| Alcohol concentration | 5 | 5.37 | 0.2 | - | - | |||||
| 6 | PTFE | 2.94 | 0.6 | - | - | |||||
| 7 | 1.82 | 0.25 | - | - | ||||||
| Alcohol concentration | 8 | Latex | 1.77 | 0.37 | 56.31 | 15.66 | ||||
| 9 | 1.13 | 0.17 | 32.62 | 8.38 | ||||||
| 10 | 0.97 | 0.03 | 26.44 | 3.42 | ||||||
| Alcohol concentration | 11 | 6.94 | 1.41 | 121.19 | 22.94 | |||||
| 12 | CMC | 3.52 | 0.73 | - | - | |||||
| 13 | 4.42 | 0.70 | 68.85 | 21.12 | ||||||
| CB/binder ratio (at different alcohol %) | 14 | 5.06 | 0.60 | 23.12 | 3.24 | |||||
| 15 | CMC | 2.55 | 0.28 | 22.33 | 3.4 | |||||
| 16 | 4.24 | 0.61 | 20.22 | 4.66 | ||||||
| Using CMC as binder and dispersant | 18 | CMC | 0.29 | 0.56 | 29.49 | 2.13 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millan, A.; Morales, A.; Venditti, R.A.; Pawlak, J.J. Coating Formulations Based on Carbon Black: An Alternative to Develop Environmentally Friendly Conductive Cellulose Paper. Materials 2025, 18, 2708. https://doi.org/10.3390/ma18122708
Millan A, Morales A, Venditti RA, Pawlak JJ. Coating Formulations Based on Carbon Black: An Alternative to Develop Environmentally Friendly Conductive Cellulose Paper. Materials. 2025; 18(12):2708. https://doi.org/10.3390/ma18122708
Chicago/Turabian StyleMillan, Adriana, Anny Morales, Richard A. Venditti, and Joel J. Pawlak. 2025. "Coating Formulations Based on Carbon Black: An Alternative to Develop Environmentally Friendly Conductive Cellulose Paper" Materials 18, no. 12: 2708. https://doi.org/10.3390/ma18122708
APA StyleMillan, A., Morales, A., Venditti, R. A., & Pawlak, J. J. (2025). Coating Formulations Based on Carbon Black: An Alternative to Develop Environmentally Friendly Conductive Cellulose Paper. Materials, 18(12), 2708. https://doi.org/10.3390/ma18122708







