The Effect of Zr and Y on the Corrosion Behavior of T6-Treated Al–Si–Mg Alloys
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
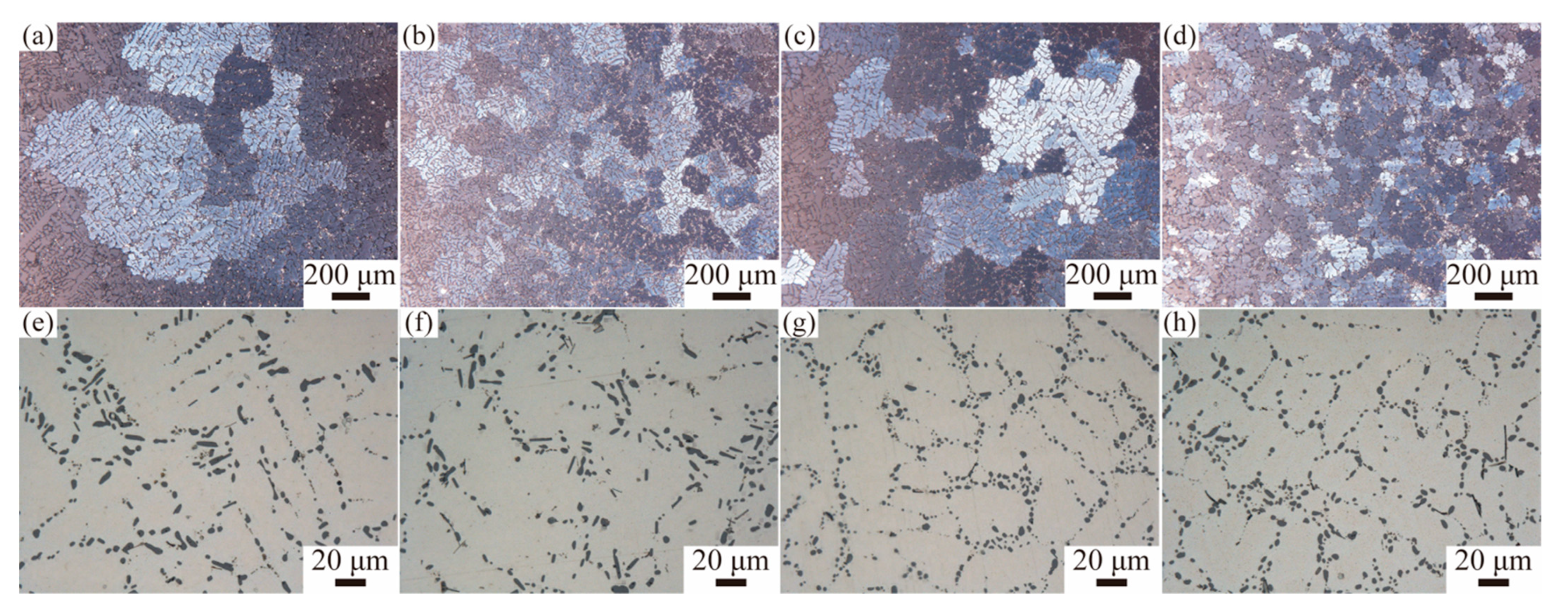
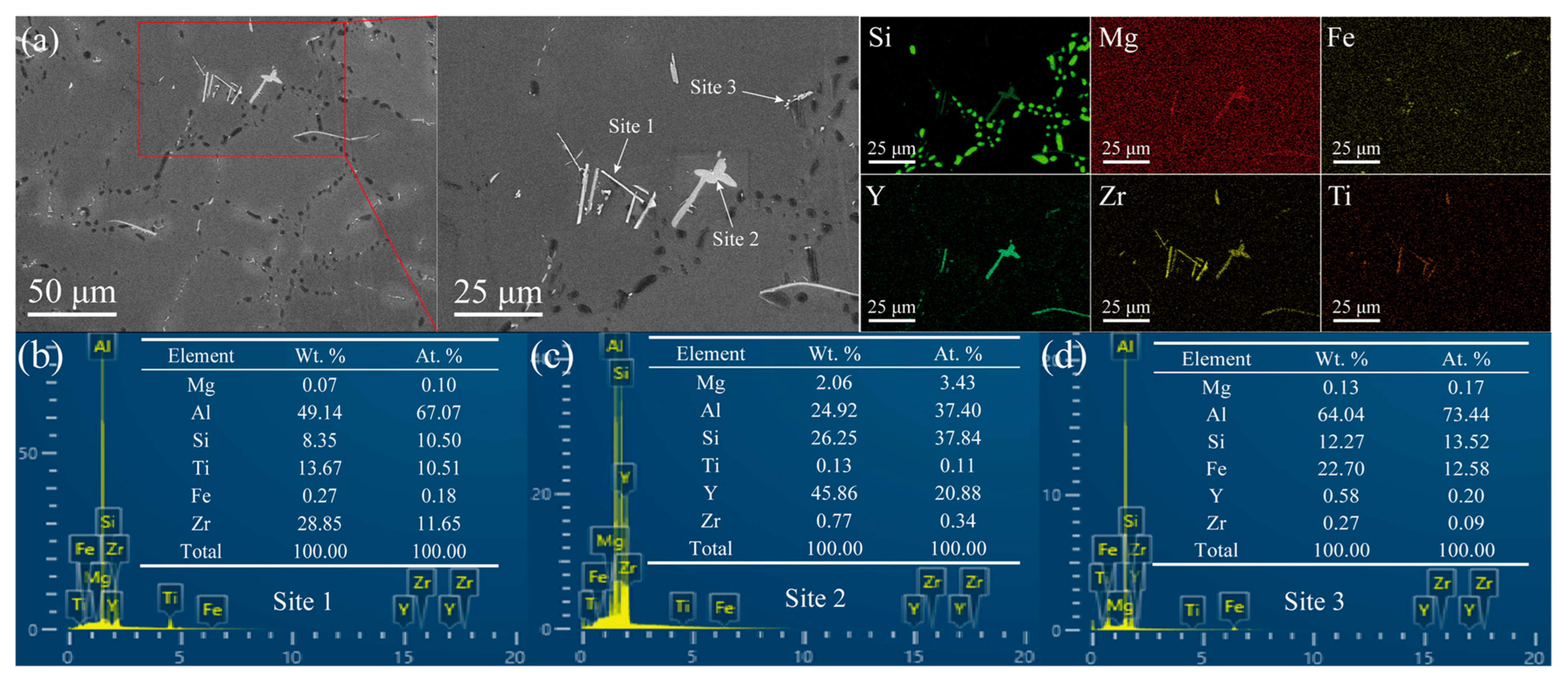
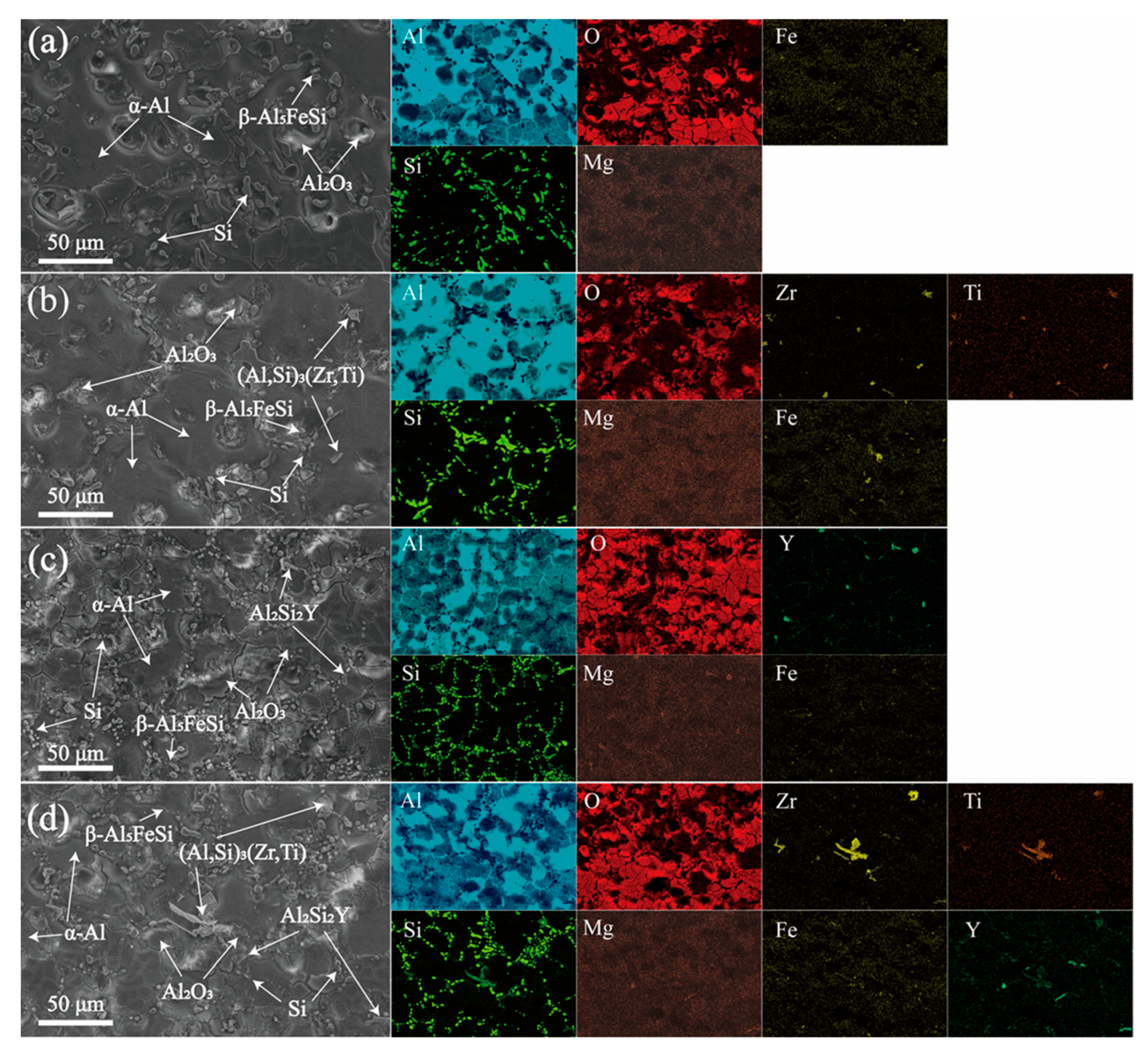
3.1. Microstructure Studies
3.2. Corrosion Behavior
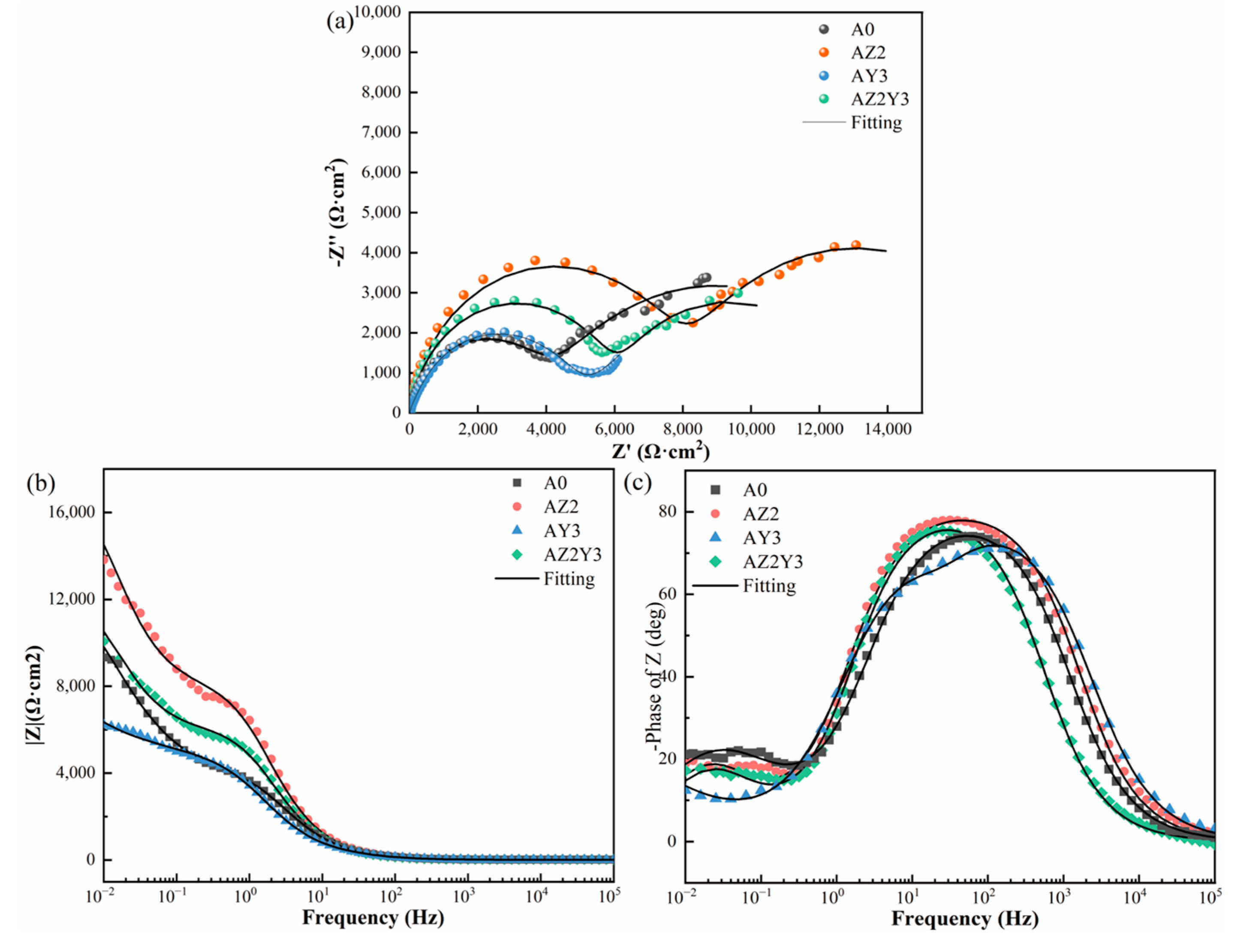
3.2.1. EIS Analysis
3.2.2. Potentiodynamic Polarization Analysis
3.2.3. Immersion Corrosion Test
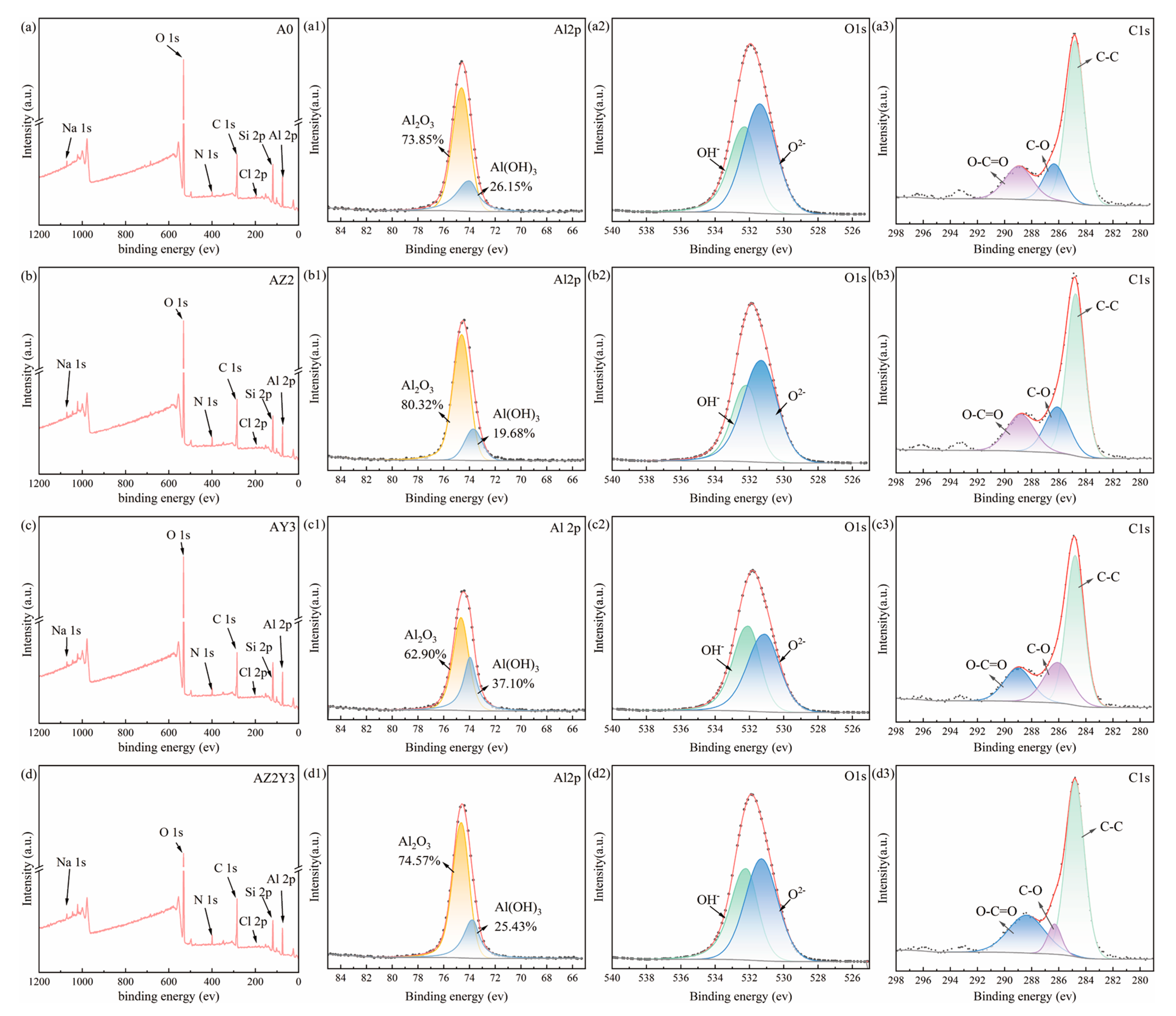
3.3. XPS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, S.; Liu, Y.; Yang, B.; Fu, Y.; Gao, M.; Guan, R. Effect of Stirring Speed and Temperature on the Microstructure and Mechanical Properties of A356 Alloy under Vacuum. Vacuum 2023, 212, 112059. [Google Scholar] [CrossRef]
- Teng, D.; Zhang, G.; Zhang, S.; Li, J.; Jia, H.; He, Q.; Guan, R. Microstructure Evolution and Strengthening Mechanism of A356/Al-X-Ce(Ti, C) System by Inoculation Treatment. J. Mater. Res. Technol. 2024, 28, 1233–1246. [Google Scholar] [CrossRef]
- Kang, H.; Jang, H.; Oh, S.; Yoon, P.; Lee, G.; Park, J.; Kim, E.; Choi, Y. Effects of Solution Treatment Temperature and Time on the Porosities and Mechanical Properties of Vacuum Die-Casted and T6 Heat-Treated Al–Si–Mg Alloy. Vacuum 2021, 193, 110536. [Google Scholar] [CrossRef]
- Xu, S.-L.; Jia, H.-L.; Zha, M.; Zhou, X.-L.; Gao, D.; Ma, P.-K.; Wang, D. The Effect of B and Sb on the Corrosion Behavior of T6-Treated Al–Si–Mg Alloys. J. Mater. Res. Technol. 2024, 30, 3611–3621. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Q.; Jia, Z.; Xing, Y.; Ding, L.; Wang, X. The Intergranular Corrosion Behavior of 6000-Series Alloys with Different Mg/Si and Cu Content. Appl. Surf. Sci. 2017, 405, 489–496. [Google Scholar] [CrossRef]
- Gollapudi, S. Grain Size Distribution Effects on the Corrosion Behaviour of Materials. Corros. Sci. 2012, 62, 90–94. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the Relationship between Grain Size and Corrosion Rate of Metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Chen, Z.; Kang, H.; Fan, G.; Li, J.; Lu, Y.; Jie, J.; Zhang, Y.; Li, T.; Jian, X.; Wang, T. Grain Refinement of Hypoeutectic Al-Si Alloys with B. ACTA Mater. 2016, 120, 168–178. [Google Scholar] [CrossRef]
- Timpel, M.; Wanderka, N.; Schlesiger, R.; Yamamoto, T.; Lazarev, N.; Isheim, D.; Schmitz, G.; Matsumura, S.; Banhart, J. The Role of Strontium in Modifying Aluminium–Silicon Alloys. ACTA Mater. 2012, 60, 3920–3928. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, L.; Jiang, H.; Zhao, J.; He, J. Effect Mechanisms of Micro-Alloying Element La on Microstructure and Mechanical Properties of Hypoeutectic Al-Si Alloys. J. Mater. Sci. Technol. 2020, 47, 142–151. [Google Scholar] [CrossRef]
- Kang, J.; Su, R.; Wu, D.Y.; Liu, C.H.; Li, T.; Wang, L.S.; Narayanaswamy, B. Synergistic Effects of Ce and Mg on the Microstructure and Tensile Properties of Al-7Si-0.3Mg-0.2Fe Alloy. J. Alloys Compd. 2019, 796, 267–278. [Google Scholar] [CrossRef]
- Mao, F.; Yan, G.; Xuan, Z.; Cao, Z.; Wang, T. Effect of Eu Addition on the Microstructures and Mechanical Properties of A356 Aluminum Alloys. J. Alloys Compd. 2015, 650, 896–906. [Google Scholar] [CrossRef]
- Limmaneevichitr, C.; Eidhed, W. Fading Mechanism of Grain Refinement of Aluminum–Silicon Alloy with Al–Ti–B Grain Refiners. Mater. Sci. Eng. A 2003, 349, 197–206. [Google Scholar] [CrossRef]
- Liao, H.; Sun, G. Mutual Poisoning Effect between Sr and B in Al–Si Casting Alloys. Scr. Mater. 2003, 48, 1035–1039. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Jie, J.; Wei, Z. Effects of Yttrium and Heat Treatment on the Microstructure and Tensile Properties of Al–7.5Si–0.5Mg Alloy. Mater. Des. 2011, 32, 1617–1622. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Y. Effect of Yttrium on the Microstructure of a Semi-Solid A356 Al Alloy. Rare Met. 2008, 27, 536–540. [Google Scholar] [CrossRef]
- Colombo, M.; Gariboldi, E.; Morri, A.; Tonelli, D. SKPFM Investigations of Intermetallic Compounds of Innovative Er- and Zr-Containing Al–Si–Mg Alloys and Their Influence on Corrosion Localization in Saline Solution. Mater. Corros. 2019, 70, 1570–1577. [Google Scholar] [CrossRef]
- Zou, Y.; Yan, H.; Yu, B.; Hu, Z. Effect of Rare Earth Yb on Microstructure and Corrosion Resistance of ADC12 Aluminum Alloy. Intermetallics 2019, 110, 106487. [Google Scholar] [CrossRef]
- Farahany, S.; Ourdjini, A.; Bakhsheshi-rad, H.R. Microstructure, Mechanical Properties and Corrosion Behavior of Al–Si–Cu–Zn–X (X = Bi, Sb, Sr) Die Cast Alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 28–38. [Google Scholar] [CrossRef]
- Ru, Z.; Wei, L.; Liu, M.; Pi, Z.; Han, C.; Qiu, M.; Li, E.; Huang, H.; Li, D. Effect of Y and Zr addition on the intergranular corrosion of Al-Mg alloy after sensitization treatment. Micron 2025, 191, 103789. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, M.; Liu, Y.; Cai, B. Microstructures and Corrosion Behaviors of Al–6.5Si–0.45Mg–xSc Casting Alloy. Trans. Nonferrous Met. Soc. China 2022, 32, 424–435. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Wang, M. Microstructures and Corrosion Resistances of Hypoeutectic Al-6.5Si-0.45 Mg Casting Alloy with Addition of Sc and Zr. Mater. Chem. Phys. 2022, 276, 125321. [Google Scholar] [CrossRef]
- Ye, P.; Jiang, F.; Wu, F.; Ye, K.; Fan, Y. Effects of Zr and Y Additions on Microstructure and Mechanical Properties of Cast A356 Alloy. J. Mater. Res. Technol. 2024, 30, 6355–6365. [Google Scholar] [CrossRef]
- Liu, M.; Schmutz, P.; Uggowitzer, P.J.; Song, G.; Atrens, A. The Influence of Yttrium (Y) on the Corrosion of Mg–Y Binary Alloys. Corros. Sci. 2010, 52, 3687–3701. [Google Scholar] [CrossRef]
- Zhao, M.-C.; Schmutz, P.; Brunner, S.; Liu, M.; Song, G.; Atrens, A. An Exploratory Study of the Corrosion of Mg Alloys during Interrupted Salt Spray Testing. Corros. Sci. 2009, 51, 1277–1292. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, T.; Dong, B.; Yuan, Z.; Huang, Z.; Yan, F.; Zu, F. Simultaneous Refinement of α-Al and Modification of Si in Al–Si Alloy Achieved via the Addition of Y and Zr. J. Mater. Res. Technol. 2024, 30, 1822–1833. [Google Scholar] [CrossRef]
- Zheng, Q.; Wu, J.; Jiang, H.; Zhang, L.; Zhao, J.; He, J. Effect of Micro-Alloying Element La on Corrosion Behavior of Al-Mg-Si Alloys. Corros. Sci. 2021, 179, 109113. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wu, B.; Guo, X.W.; Wang, Y.J.; Chen, D.; Zhang, Y.C.; Du, K.; Oguzie, E.E.; Ma, X.L. Unmasking Chloride Attack on the Passive Film of Metals. Nat. Commun. 2018, 9, 2559. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, S.; Chen, Q.; Xin, X.; Song, Z.; Chen, Z.; Wu, C.; Ren, X.; Meng, C. Innovative Heterostructure Enhance Mechanical Strength and Corrosion Resistance in A356/6061 Dissimilar Aluminum Alloy MIG Welded Joints. Vacuum 2025, 235, 114125. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of Effective Capacitance and Film Thickness from Constant-Phase-Element Parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Geng, R.; Jia, S.-Q.; Qiu, F.; Zhao, Q.-L.; Jiang, Q.-C. Effects of Nanosized TiC and TiB2 Particles on the Corrosion Behavior of Al-Mg-Si Alloy. Corros. Sci. 2020, 167, 108479. [Google Scholar] [CrossRef]
- Milazzo, G.; Caroli, S.; Braun, R.D. Tables of Standard Electrode Potentials. J. Electrochem. Soc. 1978, 125, 261C. [Google Scholar] [CrossRef]






| Alloy | Si | Mg | Fe | Ti | Y | Zr | Al |
|---|---|---|---|---|---|---|---|
| A0 | 7.03 | 0.29 | 0.095 | 0.13 | 0 | 0 | Balance |
| AZ2 | 6.82 | 0.28 | 0.14 | 0.10 | 0 | 0.2 | Balance |
| AY3 | 6.81 | 0.31 | 0.094 | 0.13 | 0.29 | 0 | Balance |
| AZ2Y3 | 6.86 | 0.29 | 0.14 | 0.11 | 0.27 | 0.19 | Balance |
| Alloy | Major Axis (μm) | Minor Axis (μm) | Aspect Ratio |
|---|---|---|---|
| A0 | 6.18 ± 4.38 | 2.59 ± 1.02 | 2.45 ± 1.61 |
| AZ2 | 5.35 ± 3.37 | 2.24 ± 0.81 | 2.46 ± 1.48 |
| AY3 | 2.47 ± 1.09 | 1.76 ± 0.69 | 1.41 ± 0.36 |
| AZ2Y3 | 3.17 ± 1.59 | 2.08 ± 0.80 | 1.52 ± 0.49 |
| Alloy | Rs (Ω cm2) | Q (μS sn cm−2) | n | Rf (Ω cm2) | Q1 (μS sn cm−2) | n1 | Rct (Ω cm2) |
|---|---|---|---|---|---|---|---|
| A0 | 9.92 | 22.0 | 0.90 | 4269 | 990 | 0.71 | 8460 |
| AZ2 | 9.77 | 17.5 | 0.91 | 8272 | 645 | 0.82 | 10240 |
| AY3 | 9.83 | 34.2 | 0.90 | 4161 | 1130 | 0.80 | 6820 |
| AZ2Y3 | 10.11 | 20.1 | 0.92 | 6122 | 795 | 0.83 | 9956 |
| Alloy | Ecorr (V) | Jcorr (μA/cm2) | Rp (KΩ cm2) | Pi (mm/year) |
|---|---|---|---|---|
| A0 | −0.830 | 1.07 | 27.5 | 0.0244 |
| AZ2 | −0.779 | 0.51 | 72.5 | 0.0117 |
| AY3 | −0.896 | 2.01 | 22.4 | 0.0459 |
| AZ2Y3 | −0.789 | 0.71 | 40.3 | 0.0162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, P.; Wu, F.; Jiang, F. The Effect of Zr and Y on the Corrosion Behavior of T6-Treated Al–Si–Mg Alloys. Materials 2025, 18, 2705. https://doi.org/10.3390/ma18122705
Ye P, Wu F, Jiang F. The Effect of Zr and Y on the Corrosion Behavior of T6-Treated Al–Si–Mg Alloys. Materials. 2025; 18(12):2705. https://doi.org/10.3390/ma18122705
Chicago/Turabian StyleYe, Pengcheng, Feifei Wu, and Feng Jiang. 2025. "The Effect of Zr and Y on the Corrosion Behavior of T6-Treated Al–Si–Mg Alloys" Materials 18, no. 12: 2705. https://doi.org/10.3390/ma18122705
APA StyleYe, P., Wu, F., & Jiang, F. (2025). The Effect of Zr and Y on the Corrosion Behavior of T6-Treated Al–Si–Mg Alloys. Materials, 18(12), 2705. https://doi.org/10.3390/ma18122705






