Effect of Ageing on the Mechanical Properties of Dental Resin with and Without Bisphenol A
Abstract
1. Introduction
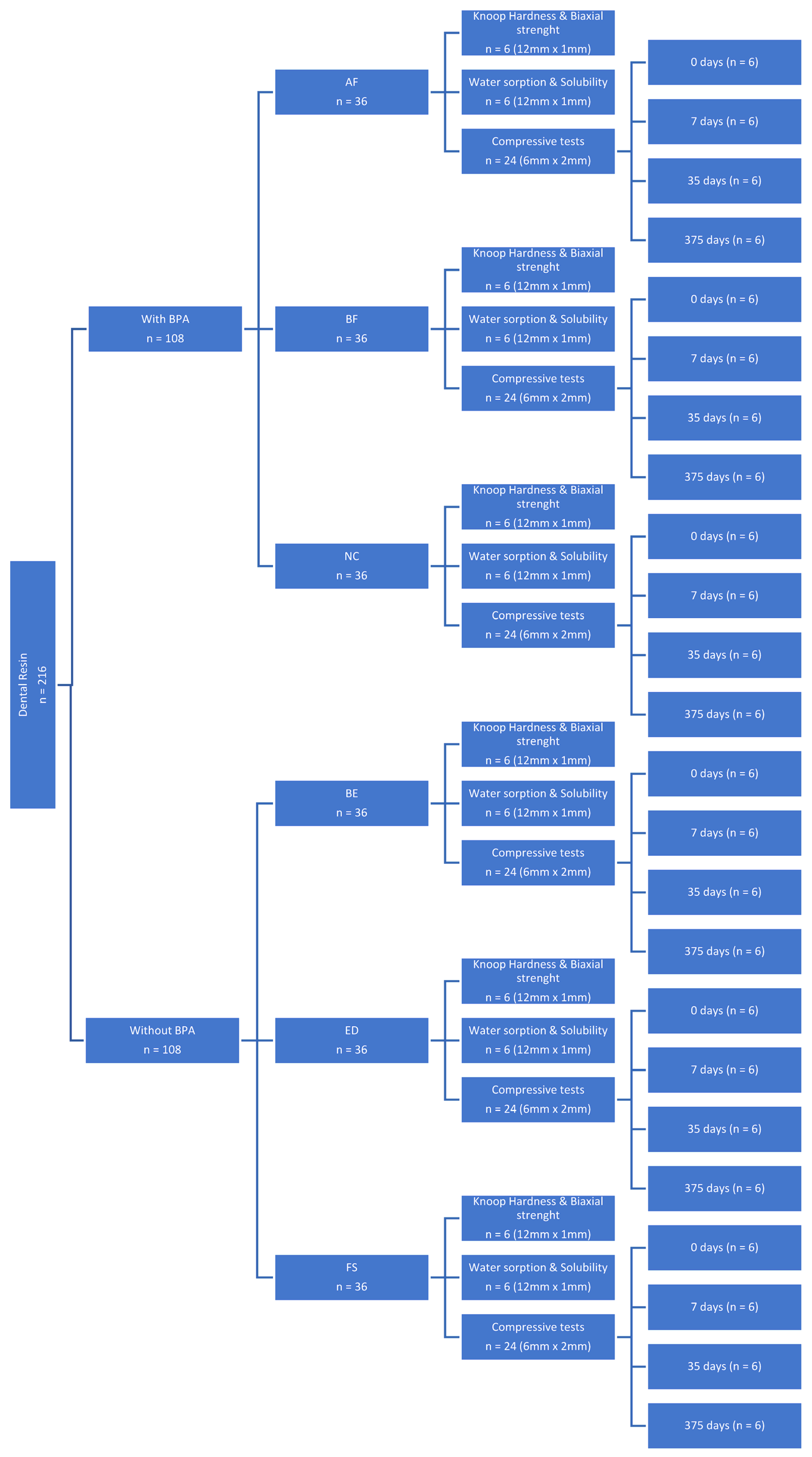
2. Materials and Methods
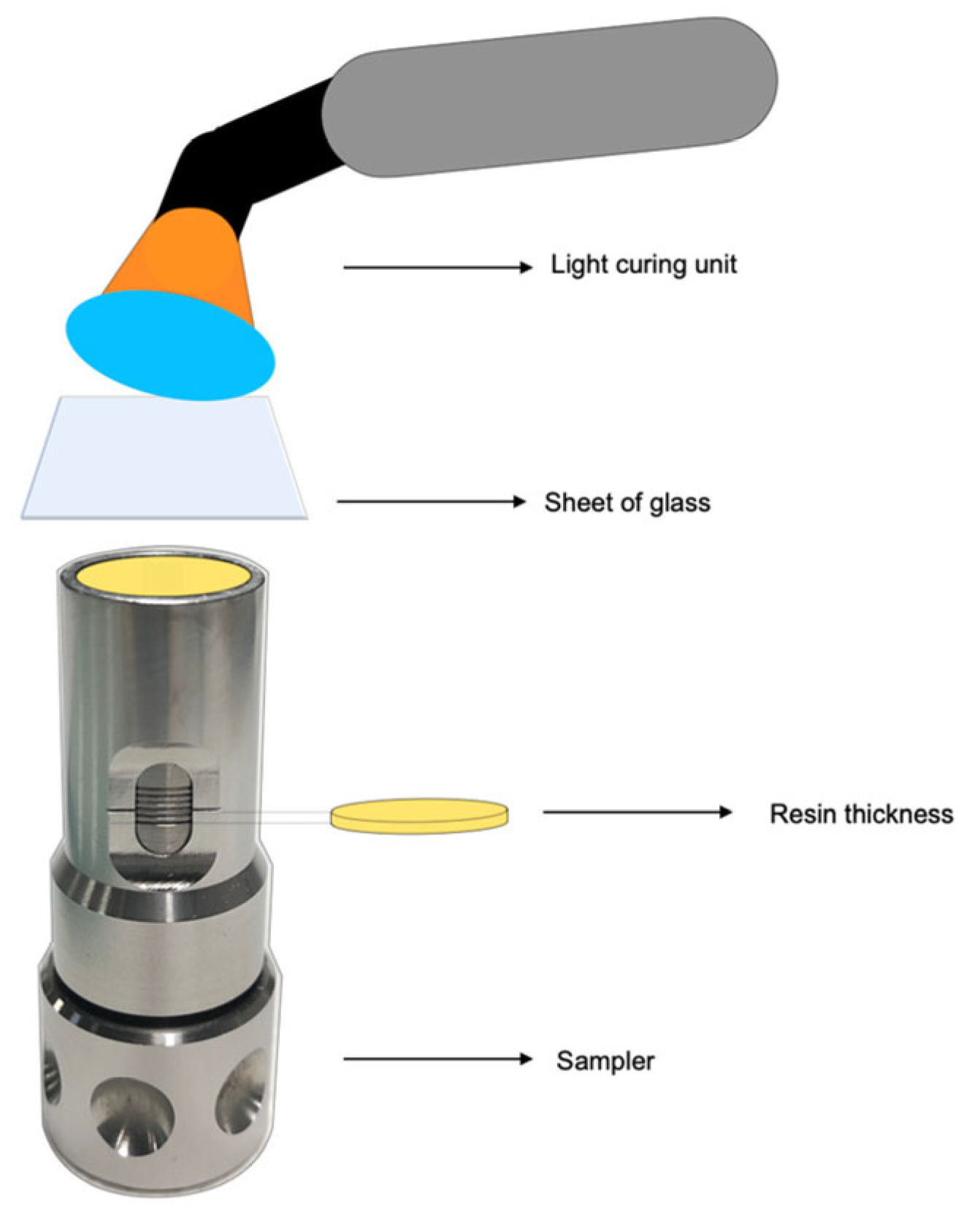
2.1. Preparation of Specimens
2.2. Test of Specimens
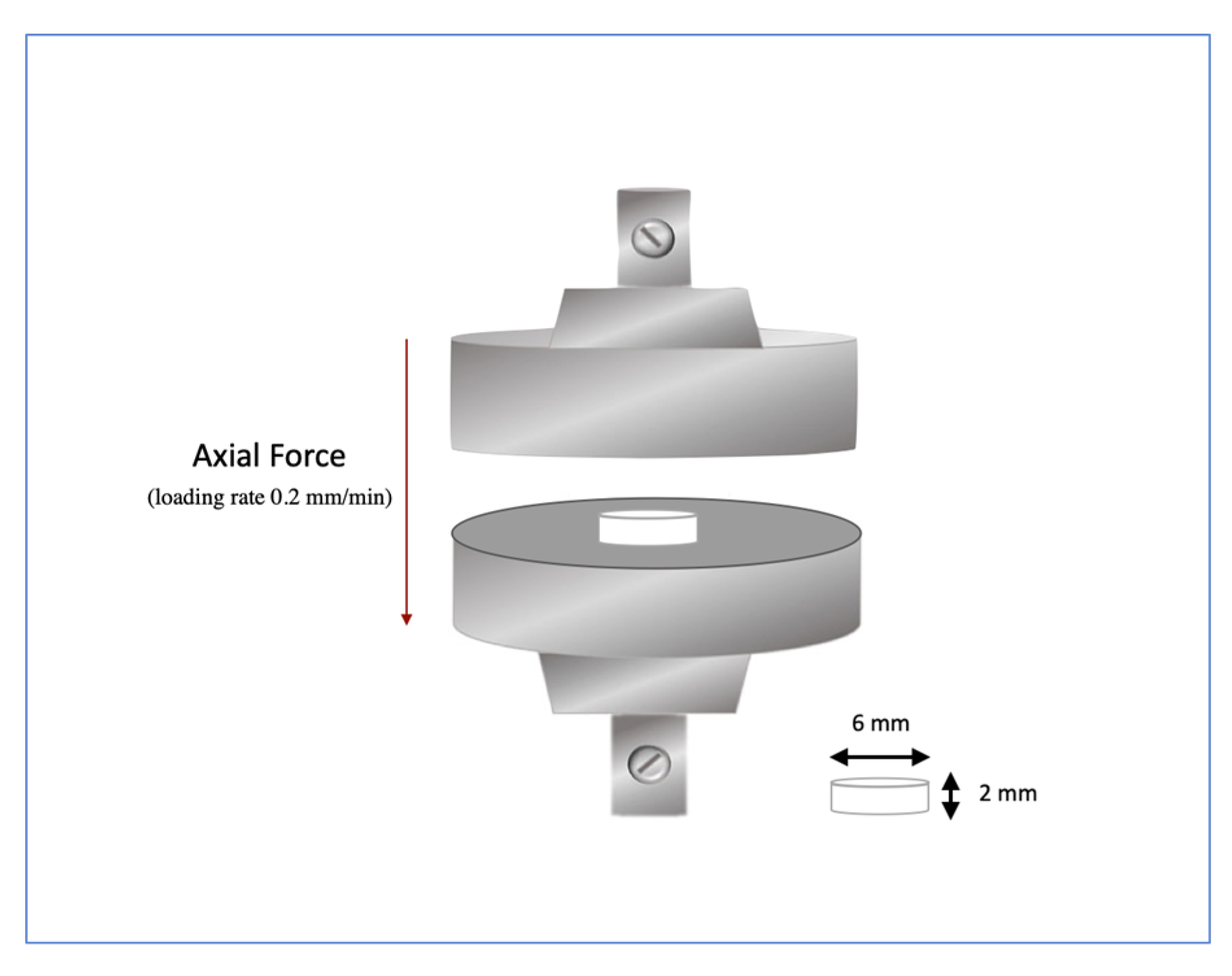
2.2.1. Knoop Hardness and Biaxial Strength
2.2.2. Water Sorption and Solubility
2.2.3. Compressive Tests Procedure
2.3. Statistical Analysis
3. Results
3.1. Knoop Hardness and Biaxial Strength
3.2. Water Sorption and Solubility
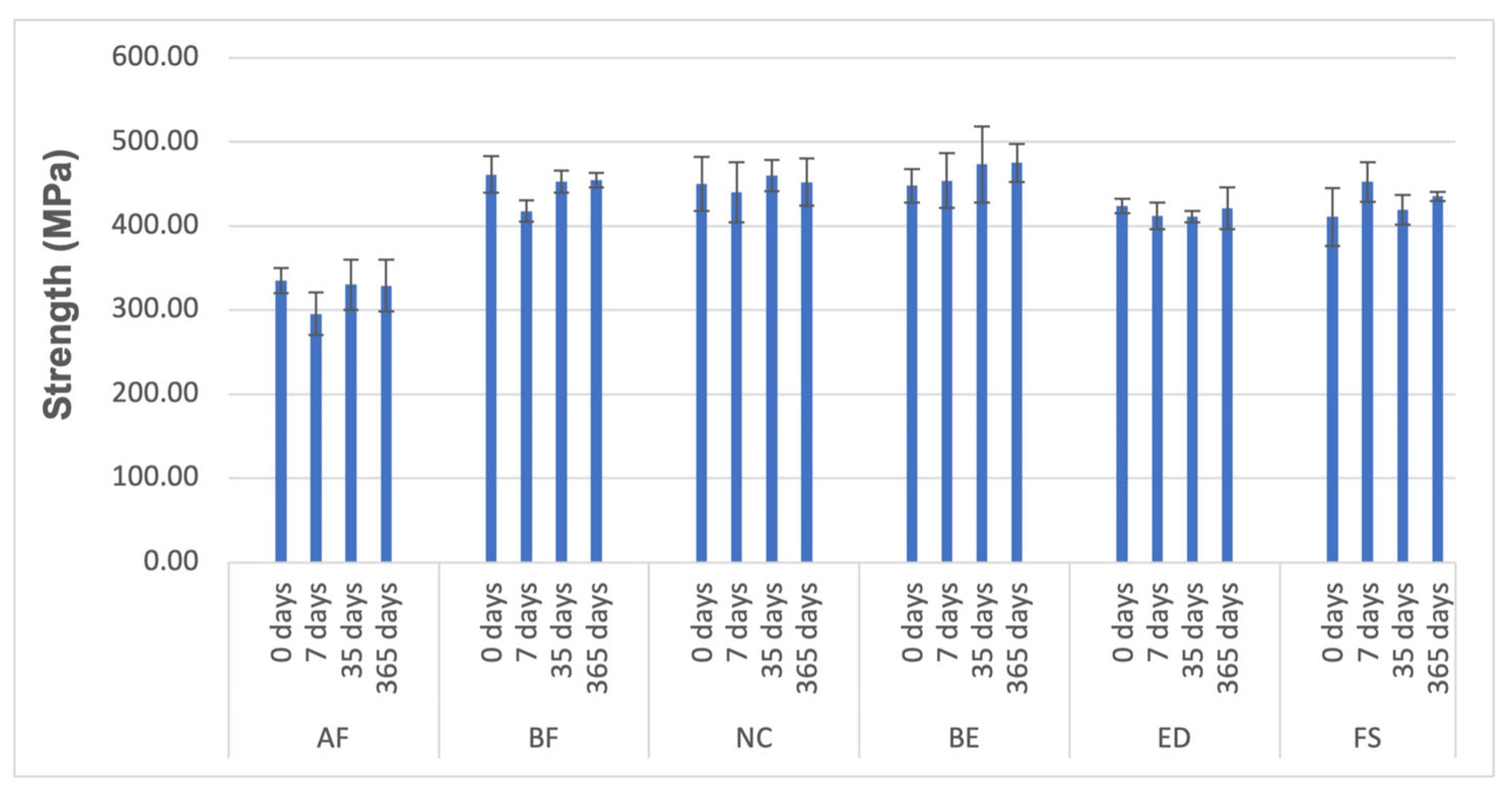
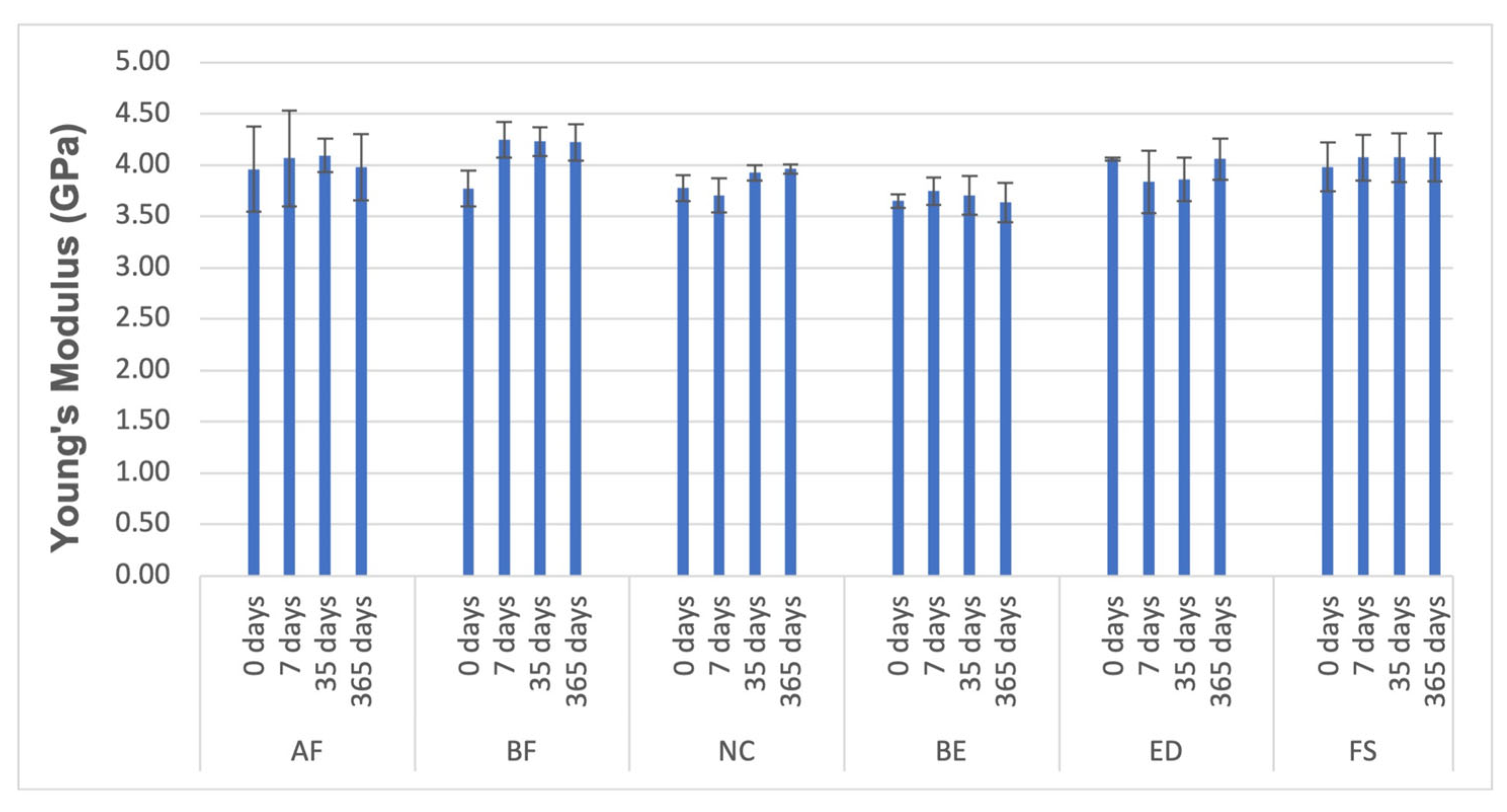
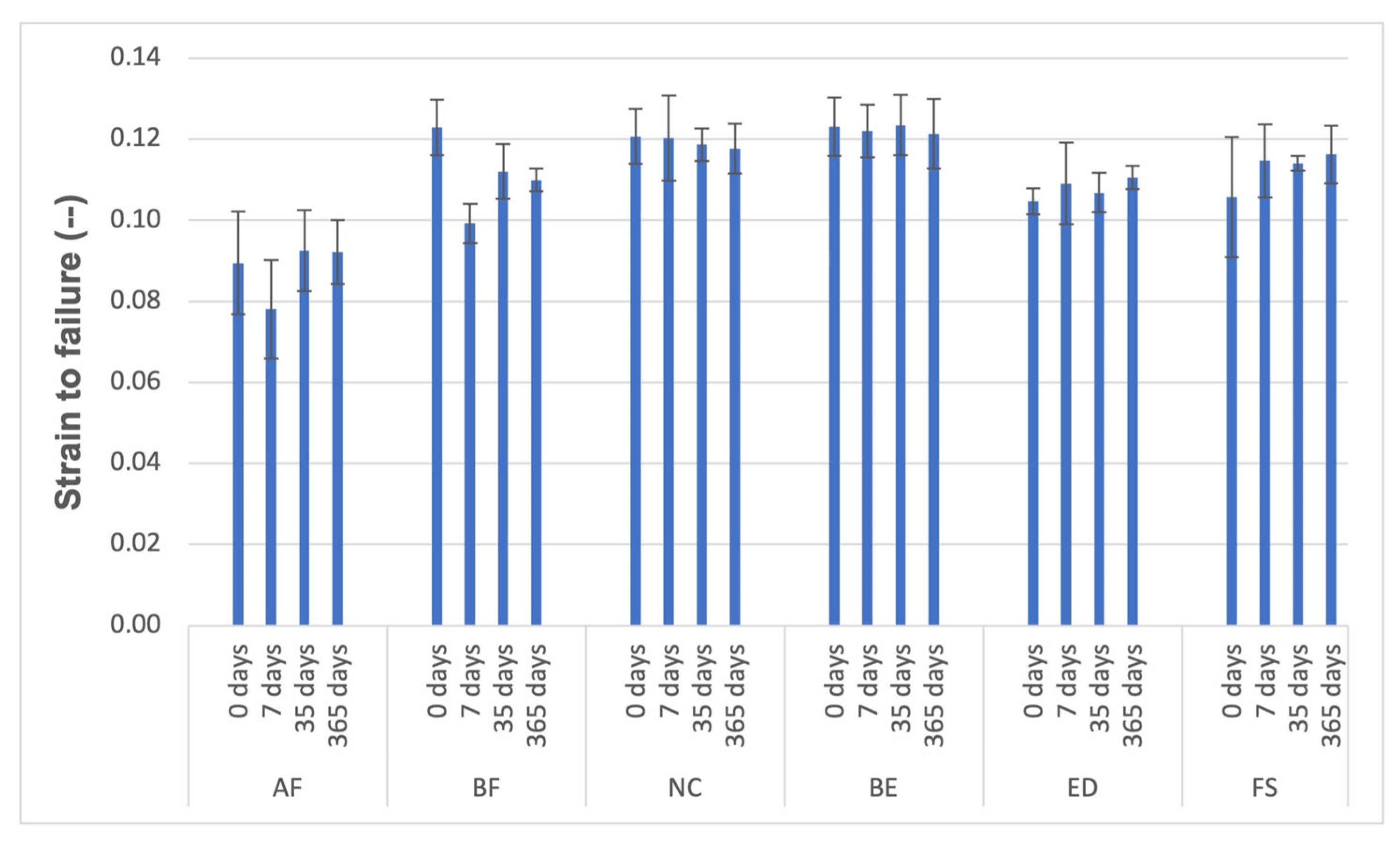
3.3. Compressive Tests
4. Discussion
5. Conclusions
- No significant differences exist between groups of resins containing BPA and those free from this monomer and its derivatives. Therefore, the absence of BPA in resins may not affect the chemical–mechanical performance of composite resins.
- The AF group is a promising Bis-GMA-free and Ormocer®-based material; however, it does not perform mechanically comparably to conventional Bis-GMA-containing resin–matrix composites. Additionally, AF seems more prone to mechanical degradation effects caused by water, particularly in the early stages of diffusion. The inorganic matrix may exert more influence than the organic matrix regarding the studied parameters, where BPA and its derivatives are in their composition.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPA | bisphenol A |
| Bis-GMA | bisphenol A-diglycidyl methacrylate |
| Bis-DMA | bisphenol A-dimethacrylate |
| Bis-EMA | ethoxylated bisphenol A methacrylate |
| Bis-PMA | propoxylated bisphenol A-dimethacrylate |
| BADGE | bisphenol A diglycidyl ether |
| PC Bis-GMA | polycarbonate-modified Bis-GMA |
| Bis-MPEPP | bisphenol A polyethoxy methacrylate |
| TEGDMA | triethylene glycol dimethacrylate |
| UDMA | urethane dimethacrylate |
| ORMOCER | organically modified ceramic |
| FUDMA | fluorinated urethane dimethacrylate |
| AF | Admira® Fusion |
| BF | Enamel Plus HRI BIO Function |
| NC | Experimental resin |
| BE | BRILLIANT EverGlowTM |
| ED | IPS Empress Direct |
| FS | FiltekTM Supreme XTE |
| CS | compressive strength |
| WSL | water solubility |
| WS | water sorption |
| E | elastic modulus |
| E′ | loss modulus |
| DC | degree conversion |
References
- Cho, K.; Rajan, G.; Farrar, P.; Prentice, L.; Prusty, B.G. Dental resin composites: A review on materials to product realizations. Compos. Part B Eng. 2022, 230, 109495. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- WHO; Petersen, P.E.; Baez, R.; Kwan, S.; Ogawa, H. Future Use of Materials for Dental Restoration: Report of the Meeting Convened at WHO HQ, Geneva, Switzerland, 16–17 November 2009; World Health Organization: Geneva, Switzerland, 2009; ISBN 9789241500647. [Google Scholar]
- Rocha, L.; Garcez, J.; Tiritan, M.E.; da Silva, L.F.M.; Pinho, T. Maxillary lateral incisor agenesis and microdontia: Minimally invasive symmetric and asymmetric esthetic rehabilitation. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2022, 62, 41–51. [Google Scholar] [CrossRef]
- Cordeiro, M.; Souza, J.C.M.; Gomes, A.T.P.C.; Correia, P.; Fidalgo-Pereira, R. Toxicity of resin-matrix composites in a dental clinical setting. Odontology 2025. [Google Scholar] [CrossRef]
- Rajkumar, D.S.; Padmanaban, R. Impact of Bisphenol A and Analogues Eluted from Resin-based Dental Materials on Cellular and Molecular Processes: An Insight on Underlying Toxicity Mechanisms. J. Appl. Toxicol. 2025, 45, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Rocha, L.; Ribeiro-Gonçalves, L.; Henriques, B.; Özcan, M.; Tiritan, M.E.; Souza, J.C.M. An integrative review on the toxicity of Bisphenol A (BPA) released from resin composites used in dentistry. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1942–1952. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations. Joint FAO/WHO Expert Meeting to Review Toxicological and Health Aspects of Bisphenol A: Final Report, Including Report of Stakeholder Meeting on Bisphenol A, 1–5 November 2010, Ottawa, Canada; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Koulaouzidou, E.A.; Roussou, K.; Sidiropoulos, K.; Nikolaidis, A.; Kolokuris, I.; Tsakalof, A.; Tsitsimpikou, C.; Kouretas, D. Investigation of the chemical profile and cytotoxicity evaluation of organic components eluted from pit and fissure sealants. Food Chem. Toxicol. 2018, 120, 536–543. [Google Scholar] [CrossRef]
- Maserejian, N.N.; Trachtenberg, F.L.; Wheaton, O.B.; Calafat, A.M.; Ranganathan, G.; Kim, H.-Y.Y.; Hauser, R. Changes in urinary bisphenol A concentrations associated with placement of dental composite restorations in children and adolescents. J. Am. Dent. Assoc. 2016, 147, 620–630. [Google Scholar] [CrossRef]
- Khan, N.G.; Correia, J.; Adiga, D.; Rai, P.S.; Dsouza, H.S.; Chakrabarty, S.; Kabekkodu, S.P. A comprehensive review on the carcinogenic potential of bisphenol A: Clues and evidence. Environ. Sci. Pollut. Res. 2021, 28, 19643–19663. [Google Scholar] [CrossRef] [PubMed]
- Dursun, E.; Fron-Chabouis, H.; Attal, J.-P.; Raskin, A. Bisphenol A Release: Survey of the Composition of Dental Composite Resins. Open Dent. J. 2016, 10, 446–453. [Google Scholar] [CrossRef]
- Habib, E.; Wang, R.; Wang, Y.; Zhu, M.; Zhu, X.X.; Wang, Y.; Zhu, M.; Zhu, X.X.; Wang, Y.; Zhu, M.; et al. Inorganic fillers for dental resin composites: Present and future. ACS Biomater. Sci. Eng. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Meimand, M.N.; Tsoi, J.K.H.; Burrow, M.F.; He, J.; Cho, K. A comparative study on the mechanical and antibacterial properties of BPA-free dental resin composites. Dent. Mater. 2024, 40, e31–e39. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Fidalgo-Pereira, R.; Torres, O.; Carvalho, O.; Henriques, B.; Özcan, M.; Souza, J.C.M. The impact of inorganic fillers, organic content, and polymerization mode on the degree of conversion of monomers in resin-matrix cements for restorative dentistry: A scoping review. Clin. Oral Investig. 2024, 28, 454. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.D.; Karabela, M.M.; Vouvoudi, E.C. Physical properties of current dental nanohybrid and nanofill light-cured resin composites. Dent. Mater. 2011, 27, 598–607. [Google Scholar] [CrossRef]
- Chen, M.-H. Update on dental nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef]
- Boaro, L.C.; Gonçalves, F.; Guimarães, T.C.; Ferracane, J.L.; Pfeifer, C.S.; Braga, R.R. Sorption, solubility, shrinkage and mechanical properties of “low-shrinkage” commercial resin composites. Dent. Mater. 2013, 29, 398–404. [Google Scholar] [CrossRef]
- De Nys, S.; Turkalj, M.; Duca, R.C.; Covaci, A.; Elskens, M.; Godderis, L.; Vanoirbeek, J.; Van Meerbeek, B.; Van Landuyt, K.L. Level of BPA contamination in resin composites determines BPA release. Dent. Mater. 2024, 40, 1025–1030. [Google Scholar] [CrossRef]
- Schmalz, G.; Widbiller, M. Biocompatibility of Amalgam vs Composite—A Review. Oral Health Prev. Dent. 2022, 20, 149–156. [Google Scholar] [CrossRef]
- Pfeifer, C.S.; Shelton, Z.R.; Braga, R.R.; Windmoller, D.; Machado, J.C.; Stansbury, J.W. Characterization of dimethacrylate polymeric networks: A study of the crosslinked structure formed by monomers used in dental composites. Eur. Polym. J. 2011, 47, 162–170. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M.; Chrószcz, M.W.; Chladek, G. Novel Urethane-Dimethacrylate Monomers and Compositions for Use as Matrices in Dental Restorative Materials. Int. J. Mol. Sci. 2020, 21, 2644. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, R.; Olea-Serrano, M.F.; Novillo-Fertrell, A.; Rivas, A.; Pazos, P.; Pedraza, V.; Navajas, J.-M.M.; Olea, N. Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography. Environ. Health Perspect. 2000, 108, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Floyd, C.J.E.; Dickens, S.H. Network structure of Bis-GMA- and UDMA-based resin systems. Dent. Mater. 2006, 22, 1143–1149. [Google Scholar] [CrossRef]
- Korkut, B.; Türkmen, C. Longevity of direct diastema closure and recontouring restorations with resin composites in maxillary anterior teeth: A 4-year clinical evaluation. J. Esthet. Restor. Dent. 2021, 33, 590–604. [Google Scholar] [CrossRef]
- da Costa, J.B.; Frazier, K.; Duong, M.-L.; Khajotia, S.; Kumar, P.; Urquhart, O. Defective restoration repair or replacement: An American Dental Association Clinical Evaluators Panel survey. J. Am. Dent. Assoc. 2021, 152, 329–330.e2. [Google Scholar] [CrossRef]
- Kalra, S.; Singh, A.; Gupta, M.; Chadha, V. Ormocer: An aesthetic direct restorative material; An in vitro study comparing the marginal sealing ability of organically modified ceramics and a hybrid composite using an ormocer-based bonding agent and a conventional fifth-generation bonding agent. Contemp. Clin. Dent. 2012, 3, 48–53. [Google Scholar] [CrossRef]
- Gregor, L.; Krejci, I.; Di Bella, E.; Feilzer, A.J.; Ardu, S. Silorane, ormocer, methacrylate and compomer long-term staining susceptibility using ΔE and ΔE 00 colour-difference formulas. Odontology 2016, 104, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. A Historical Perspective on Dental Composite Restorative Materials. J. Funct. Biomater. 2024, 15, 173. [Google Scholar] [CrossRef]
- Yap, A.U.J.; Wong, N.Y.; Siow, K.S. Composite Cure and Shrinkage Associated With High Intensity Curing Light. Oper. Dent. 2020, 28, 357–364. [Google Scholar]
- Silva, T.M.D.; Sales, A.L.L.S.; Pucci, C.R.; Borges, A.B.; Torres, C.R.G. The combined effect of food-simulating solutions, brushing and staining on color stability of composite resins. Acta Biomater. Odontol. Scand. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Hongsathavij, R.; Yosvimol Kuphasuk, K.R. Effectiveness of platelet-rich fibrin in the management of pain and delayed wound healing. Eur. J. Dent. 2017, 11, 192–195. [Google Scholar] [CrossRef]
- Dey, T.; Naughton, D. Nano-porous sol-gel derived hydrophobic glass coating for increased light transmittance through green-house. Mater. Res. Bull. 2019, 116, 126–130. [Google Scholar] [CrossRef]
- Quinn, J.B.; Quinn, G.D. Indentation brittleness of ceramics: A fresh approach. J. Mater. Sci. 1997, 32, 4331–4346. [Google Scholar] [CrossRef]
- ISO 4049:2009; 2019 Dentistry—Polymer-Based Restorative Materials, 5th ed. ISO: Geneva, Switzerland, 2019.
- Kim, K.-H.; Ong, J.L.; Okuno, O. The effect of filler loading and morphology on the mechanical properties of contemporary composites. J. Prosthet. Dent. 2002, 87, 642–649. [Google Scholar] [CrossRef]
- Fick, A. On liquid diffusion. J. Memb. Sci. 1995, 100, 33–38. [Google Scholar] [CrossRef]
- Wilson, K.S.; Antonucci, J.M. Interphase structure-property relationships in thermoset dimethacrylate nanocomposites. Dent. Mater. 2006, 22, 995–1001. [Google Scholar] [CrossRef]
- Bouschlicher, M.R.; Rueggeberg, F.A.; Wilson, B.M. Correlation of bottom-to-top surface microhardness and conversion ratios for a variety of resin composite compositions. Oper. Dent. 2004, 29, 698–704. [Google Scholar]
- Bilge, K.; İpek, İ. Effects of different LED light curing units on the degree of conversion and microhardness of different composites: FT-IR and SEM-EDX analysis. Polym. Bull. 2024, 81, 13825–13838. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Jeon, G.-H.; Jang, C.-M.; Seol, H.-J.; Kim, H.-I. Evaluation of polymerization of light-curing hybrid composite resins. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 106–113. [Google Scholar] [CrossRef]
- Pereira, S.G.; Osorio, R.; Toledano, M.; Nunes, T.G. Evaluation of two Bis-GMA analogues as potential monomer diluents to improve the mechanical properties of light-cured composite resins. Dent. Mater. 2005, 21, 823–830. [Google Scholar] [CrossRef]
- Mandikos, M.N.; McGivney, G.P.; Davis, E.; Bush, P.J.; Carter, J.M. A comparison of the wear resistance and hardness of indirect composite resins. J. Prosthet. Dent. 2001, 85, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, L.A.; Kerby, R.E.; Clelland, N.; Lee, J. Hardness and degree of conversion of posterior packable composites. Oper. Dent. 2004, 29, 642–649. [Google Scholar] [PubMed]
- Szalewski, L.; Wójcik, D.; Sowa, M.; Vivcharenko, V.; Pałka, K. Influence of Low pH on the Microhardness and Roughness Surface of Dental Composite—A Preliminary Study. Materials 2024, 17, 3443. [Google Scholar] [CrossRef]
- Yu, B.; Liu, F.; He, J. Preparation of low shrinkage methacrylate-based resin system without Bisphenol A structure by using a synthesized dendritic macromer (G-IEMA). J. Mech. Behav. Biomed. Mater. 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Kopacz, K.; Ranoszek-Soliwoda, K.; Sokolowski, J.; Bociong, K. Towards the Standardization of Artificial Aging Protocols for Dental Composites: Evaluation of Proposed Methods. J. Funct. Biomater. 2025, 16, 49. [Google Scholar] [CrossRef]
- Costella, A.M.; Trochmann, J.L.; Oliveira, W.S. Water sorption and diffusion coefficient through an experimental dental resin. J. Mater. Sci. Mater. Med. 2010, 21, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.D.; Karabela, M.M.; Vouvoudi, E.C. Dynamic thermomechanical properties and sorption characteristics of two commercial light cured dental resin composites. Dent. Mater. 2008, 24, 737–743. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Antonucci, J.M. Dimethacrylate monomers with varied fluorine contents and distributions. Dent. Mater. 1999, 15, 166–173. [Google Scholar] [CrossRef]
- Kumar, N.; Sangi, L. Water sorption, solubility, and resultant change in strength among three resin-based dental composites. J. Investig. Clin. Dent. 2014, 5, 144–150. [Google Scholar] [CrossRef]
- Musanje, L.; Shu, M.; Darvell, B.W. Water sorption and mechanical behaviour of cosmetic direct restorative materials in artificial saliva. Dent. Mater. 2001, 17, 394–401. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Marker, V.A. Solvent degradation and reduced fracture toughness in aged composites. J. Dent. Res. 1992, 71, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Condon, J.R. Rate of elution of leachable components from composite. Dent. Mater. 1990, 6, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liao, M.; Liu, F.; Huang, X.; Mai, S.; He, J. Preparation of Bis-GMA free dental resin composites with anti-adhesion effect against Streptococcus mutans using synthesized fluorine-containing methacrylate (DFMA). J. Mech. Behav. Biomed. Mater. 2022, 131, 105263. [Google Scholar] [CrossRef]
- Chun, K.; Choi, H.; Lee, J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 2014, 5, 1758736014520809. [Google Scholar] [CrossRef]
- de Moraes, R.R.; Gonçalves, L.d.S.; Lancellotti, A.C.; Consani, S.; Correr-Sobrinho, L.; Sinhoreti, M.A. Nanohybrid resin composites: Nanofiller loaded materials or traditional microhybrid resins? Oper. Dent. 2009, 34, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhu, W.; Liu, F.; He, J. Preparation of a Bis-GMA-Free dental resin system with synthesized fluorinated dimethacrylate monomers. Int. J. Mol. Sci. 2016, 17, 2014. [Google Scholar] [CrossRef]
- Da Fonte Porto Carreiro, A.; Dos Santos Cruz, C.A.; Vergani, C.E. Hardness and compressive strength of indirect composite resins: Effects of immersion in distilled water. J. Oral Rehabil. 2004, 31, 1085–1089. [Google Scholar] [CrossRef]
- Tarumi, H.; Torii, M.; Tsuchitani, Y. Relationship between particle size of barium glass filler and water sorption of light-cured composite resin. Dent. Mater. J. 1995, 14, 37–44. [Google Scholar] [CrossRef]


| Group | Brand | Code | Lot number | Classification |
|---|---|---|---|---|
| AF | Admira® Fusion | A2 enamel | 19076721 | Nanohybrid-ORMOCER |
| BF | Enamel Plus HRI BIO Function | A2 enamel | 2018003080 | Nanohybrid |
| NC | Experimental resin | ---- | C1971 | Nanohybrid |
| BE | BRILLIANT EverGlowTM | A2/B2 | J18375 | Submicron hybrid |
| ED | IPS Empress Direct | A2 enamel | Y28474 | Nanohybrid |
| FS | FiltekTM Supreme XTE | CT enamel | NA28205 | Nanofilled |
| Group | Organic Matrix | Filler | Filler by Weight (%) | Filler Dimension (μm) | Water Sorption (μg/mm3) | Water Solubility (μg/mm3) | |
|---|---|---|---|---|---|---|---|
| Without BPA | AF | ORMOCER® resin | SiO2 Ba-Al-B-Si-glass fillers | 84 | 2.5 to 3.0 | 13.4 | ≤0.1 |
| BF | UDMA, TCDDMA, no co monomers, and no Bis-GMA | Glass filler, high dispersion silicon dioxide, fluorine | 74 | 0.2 to 3.0 | 15.27 | 0.31 | |
| NC | Not available | Barium glass submicron | Not available | Not available | Not available | Not available | |
| With BPA | BE | Bis-GMA TEGDMA Bis-EMA | ZnO Amorphous silica sillers | 79 | 0.4 to 0.7 | 15.1 | <0.1 |
| ED | Bis-GMA UDMA TCDD | Ba-Al-Si-glass YbF3, SiO2/ZrO2, MO, Nanomodifier | 78 | 0.1 to 0.3 | 19.6 | <0.1 | |
| FS | Bis-GMA UDMA TEGDMA Bis-EMA | ZrO2/SiO2 cluster SiO2 nano-scale fillers | 72.5 | 0.6 to 20 | Not available | Not available |
| Material | Irradiated Face | Opposite Face | ||
|---|---|---|---|---|
| Knoop Hardness (Mean ± SD) | Coefficient (%) | Knoop Hardness (Mean ± SD) | Coefficient (%) | |
| AF | 57.31 ± 3.88 | 7 | 53.92 ± 2.75 | 5 |
| BF | 54.44 ± 5.62 | 10 | 52.60 ± 4.53 | 9 |
| NC | 53.18 ± 6.42 | 12 | 39.13 ± 1.70 | 4 |
| BE | 49.18 ± 5.60 | 11 | 40.08 ± 2.43 | 6 |
| ED | 48.95 ± 5.36 | 11 | 41.36 ± 2.99 | 7 |
| FS | 48.19 ± 4.37 | 9 | 42.31 ± 2.26 | 5 |
| Material | Flexural Strength [MPa] | Coefficient (%) |
|---|---|---|
| AF | 133.7 ± 13.90 | 10 |
| BF | 164.0 ± 26.30 | 16 |
| NC | 165.9 ± 13.10 | 8 |
| BE | 150.0 ± 15.00 | 10 |
| ED | 146.9 ± 12.90 | 9 |
| FS | 178.9 ± 18.50 | 10 |
| Material | Water Sorption (µg/mm3) | ||
|---|---|---|---|
| Brand | 7 Days | 36 Days | |
| AF | 13.4 | 18.19 ± 0.93 | 18.16 ± 1.03 |
| BF | 15.27 | 12.23 ± 0.82 | 14.08 ± 0.75 |
| NC | Not available | 15.89 ± 0.83 | 19.16 ± 1.21 |
| BE | 15.1 | 15.80 ± 1.55 | 18.02 ± 0.76 |
| ED | 19.6 | 19.47 ± 0.82 | 20.05 ± 0.89 |
| FS | Not available | 31.17 ± 3.53 | 33.69 ± 4.23 |
| Material | Water Solubility (µg/mm3) | |
|---|---|---|
| Brand | Study | |
| AF | ≤0.1 | 2.29 ± 0.53 |
| BF | 0.31 | 1.55 ± 1.10 |
| NC | Not available | 1.28 ± 0.53 |
| BE | <0.1 | 1.48 ± 0.75 |
| ED | <0.1 | 3.48 ± 1.88 |
| FS | Not available | 1.11 ± 0.40 |
| Material | D (m2/s) | M∞ (%) |
|---|---|---|
| AF | 9.8 × 10−13 | 0.63 |
| BF | 7.5 × 10−13 | 0.57 |
| NC | 3.8 × 10−13 | 0.84 |
| BE | 8.4 × 10−13 | 0.59 |
| ED | 9.3 × 10−13 | 0.83 |
| FS | 5.2 × 10−13 | 0.12 |
| Material | Strength (MPa) | Young’s Modulus (GPa) | Strain to Failure (-) |
|---|---|---|---|
| AF | 295.55 ± 25.45 | 4.07 ± 0.47 | 0.08 ± 0.01 |
| BF | 417.73 ± 12.81 | 4.25 ± 0.18 | 0.10 ± 0.00 |
| NC | 440.52 ± 35.82 | 3.71 ± 0.16 | 0.12 ± 0.01 |
| BE | 453.89 ± 32.73 | 3.75 ± 0.13 | 0.12 ± 0.01 |
| ED | 411.87 ± 16.04 | 3.84 ± 0.31 | 0.11 ± 0.01 |
| FS | 452.59 ± 23.41 | 4.07 ± 0.22 | 0.11 ± 0.01 |
| Material | Strength (MPa) | Young’s Modulus (GPa) | Strain to Failure (-) |
|---|---|---|---|
| AF | 330.11 ± 29.58 | 4.10 ± 0.17 | 0.09 ± 0.01 |
| BF | 452.98 ± 13.37 | 4.23 ± 0.14 | 0.11 ± 0.01 |
| NC | 460.05 ± 18.91 | 3.93 ± 0.08 | 0.12 ± 0.00 |
| BE | 473.33 ± 45.24 | 3.70 ± 0.19 | 0.12 ± 0.01 |
| ED | 411.10 ± 6.70 | 3.86 ± 0.21 | 0.11 ± 0.00 |
| FS | 419.10 ± 17.84 | 4.07 ± 0.24 | 0.11 ± 0.00 |
| Material | Strength (MPa) | Young’s Modulus (GPa) | Strain to Failure (-) |
|---|---|---|---|
| AF | 328.93 ± 30.65 | 3.98± 0.32 | 0.09 ± 0.01 |
| BF | 454.85 ± 8.73 | 4.22 ± 0.18 | 0.11 ± 0.00 |
| NC | 452.32 ± 28.46 | 3.96 ± 0.04 | 0.12 ± 0.01 |
| BE | 475.23 ± 22.82 | 3.64 ± 0.19 | 0.12 ± 0.01 |
| ED | 421.25 ± 24.70 | 4.06 ± 0.20 | 0.11 ± 0.00 |
| FS | 435.30 ± 5.41 | 4.07 ± 0.23 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes-Rocha, L.; Torres, O.; Garcez, J.; Carbas, R.J.C.; Borges, C.; Gonçalves, V.M.F.; Tiritan, M.E.; Medeiros, I.S.; Pinho, T.; da Silva, L.F.M. Effect of Ageing on the Mechanical Properties of Dental Resin with and Without Bisphenol A. Materials 2025, 18, 2704. https://doi.org/10.3390/ma18122704
Lopes-Rocha L, Torres O, Garcez J, Carbas RJC, Borges C, Gonçalves VMF, Tiritan ME, Medeiros IS, Pinho T, da Silva LFM. Effect of Ageing on the Mechanical Properties of Dental Resin with and Without Bisphenol A. Materials. 2025; 18(12):2704. https://doi.org/10.3390/ma18122704
Chicago/Turabian StyleLopes-Rocha, Lígia, Orlanda Torres, Joana Garcez, Ricardo J. C. Carbas, Catarina Borges, Vírginia M. F. Gonçalves, Maria Elizabeth Tiritan, Igor Studart Medeiros, Teresa Pinho, and Lucas F. M. da Silva. 2025. "Effect of Ageing on the Mechanical Properties of Dental Resin with and Without Bisphenol A" Materials 18, no. 12: 2704. https://doi.org/10.3390/ma18122704
APA StyleLopes-Rocha, L., Torres, O., Garcez, J., Carbas, R. J. C., Borges, C., Gonçalves, V. M. F., Tiritan, M. E., Medeiros, I. S., Pinho, T., & da Silva, L. F. M. (2025). Effect of Ageing on the Mechanical Properties of Dental Resin with and Without Bisphenol A. Materials, 18(12), 2704. https://doi.org/10.3390/ma18122704













