The Engineered Synthesis and Enhancement of Nitrogen and Chlorine Co-Doped Fluorescent Carbon Dots for the Sensitive Detection of Quercetin
Abstract
1. Introduction
2. Materials and Methods
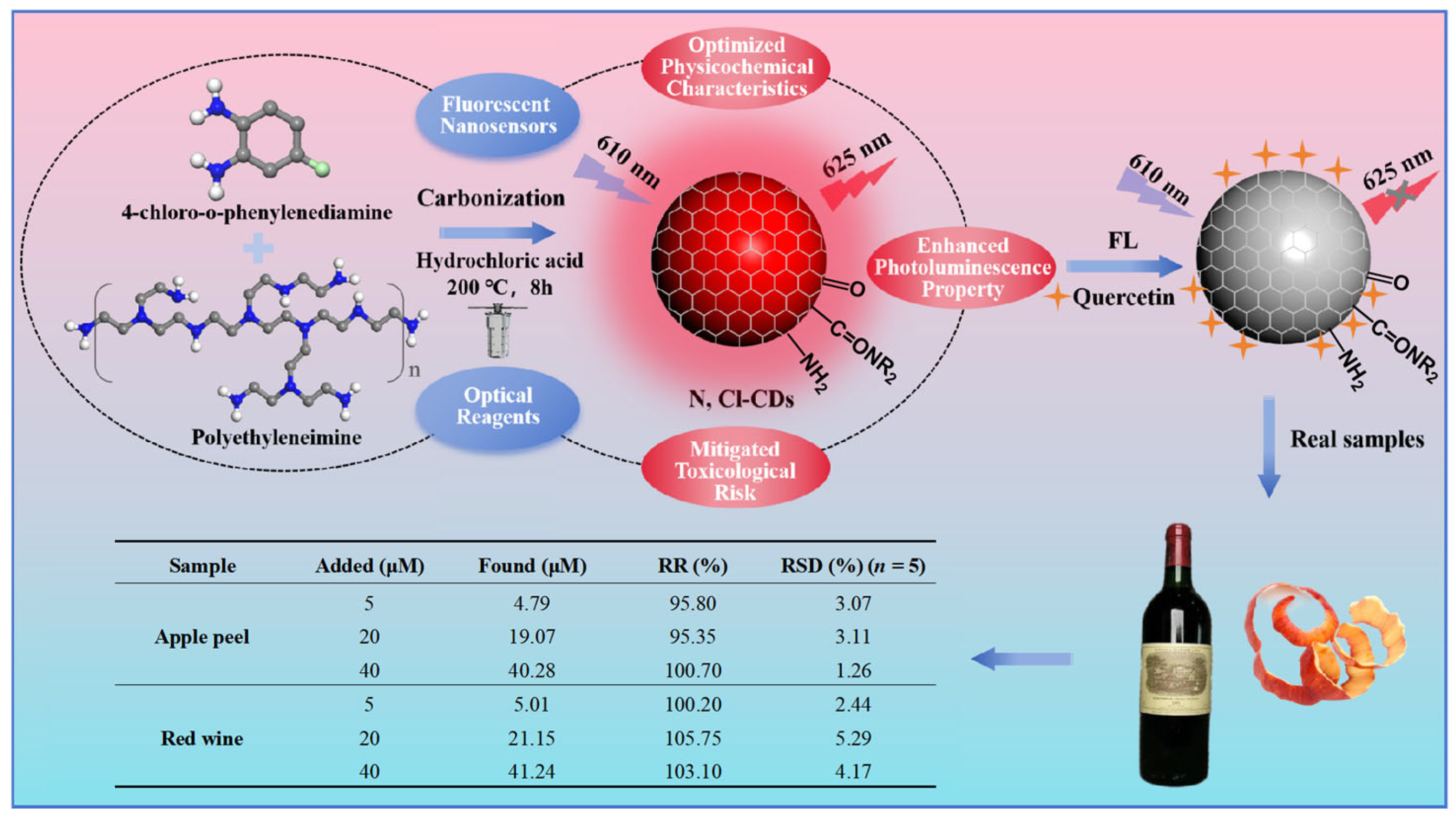
2.1. Preparation of N, Cl-CDs
2.2. Detection of Quercetin Based on N, Cl-CDs
2.3. Actual Sample Preparation
3. Results and Discussion
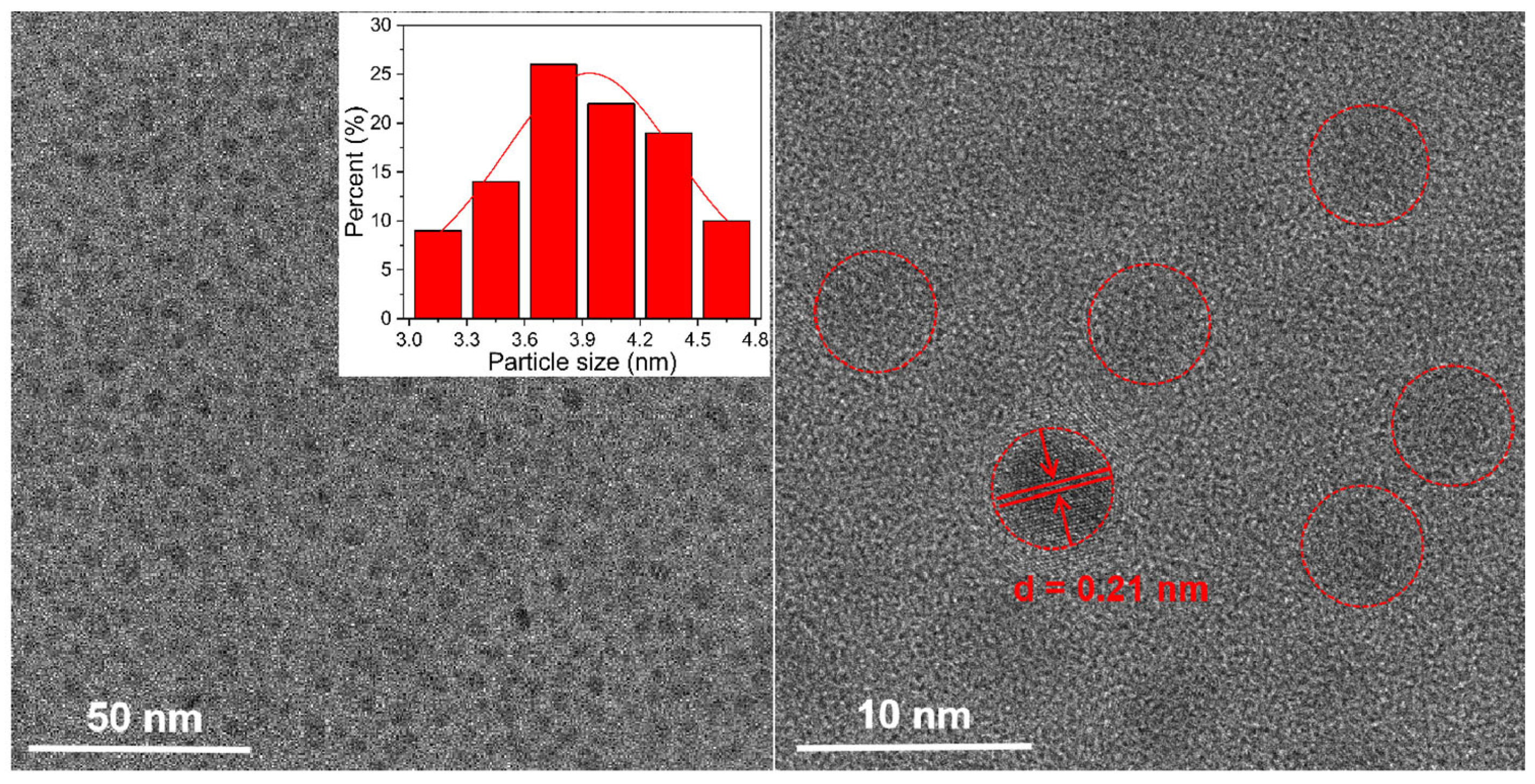
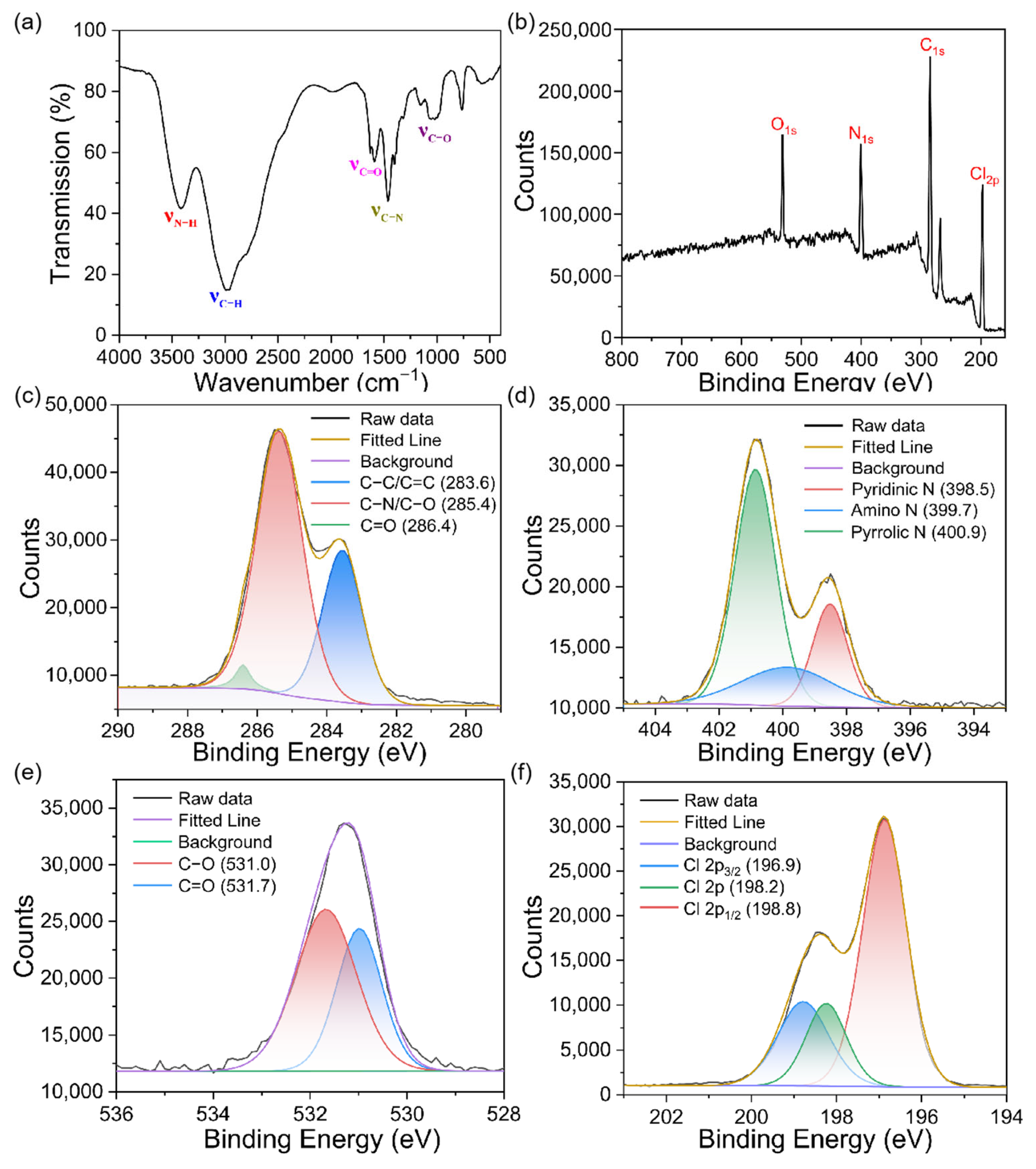
3.1. Characterization of N, Cl-CDs
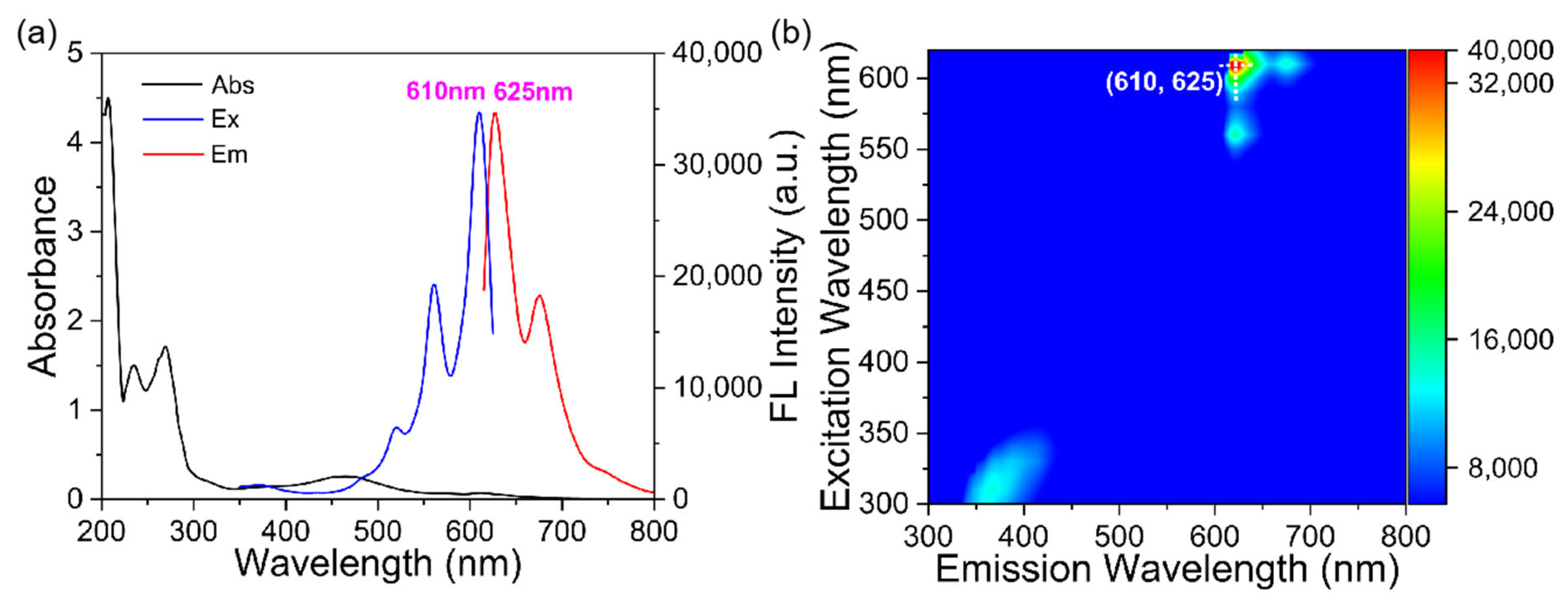
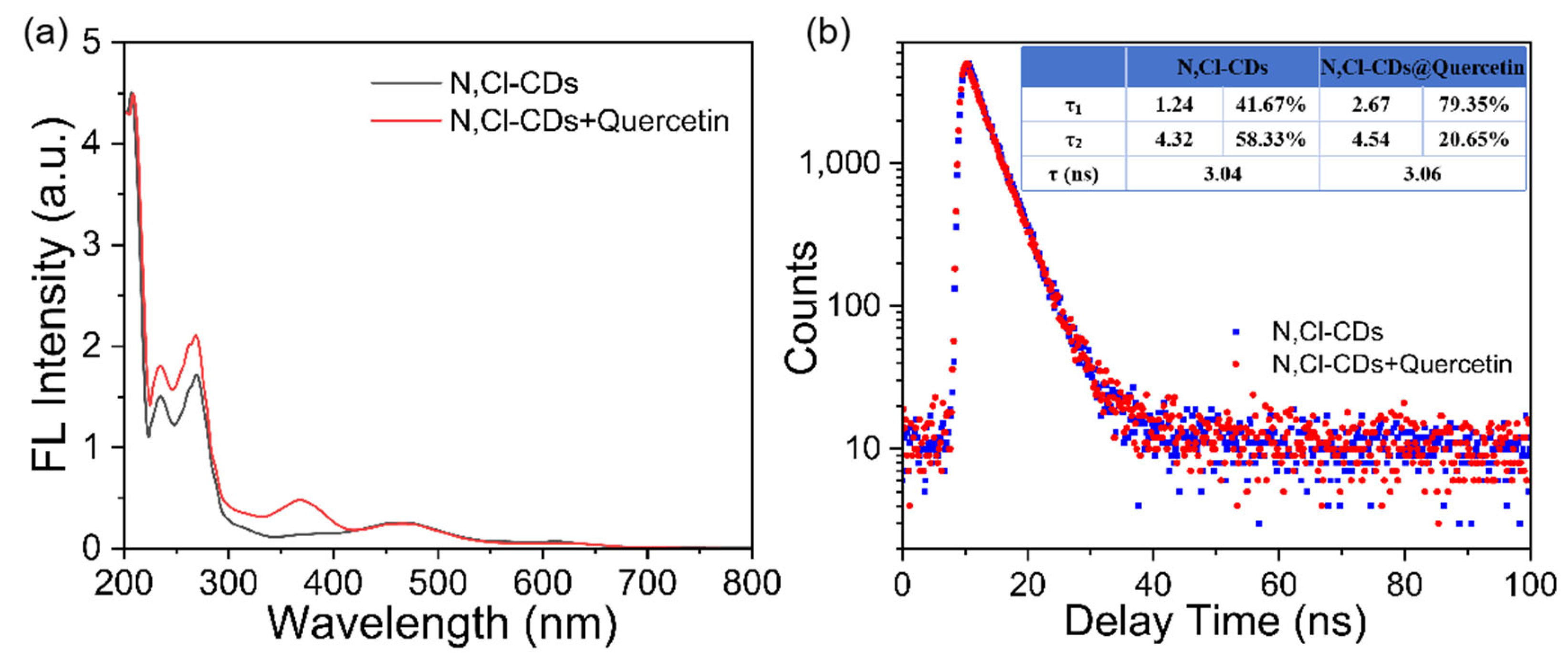
3.2. Optical Characteristics of N, Cl-CDs
3.3. Fluorescent Behavior of N, Cl-CDs in Response to Quercetin
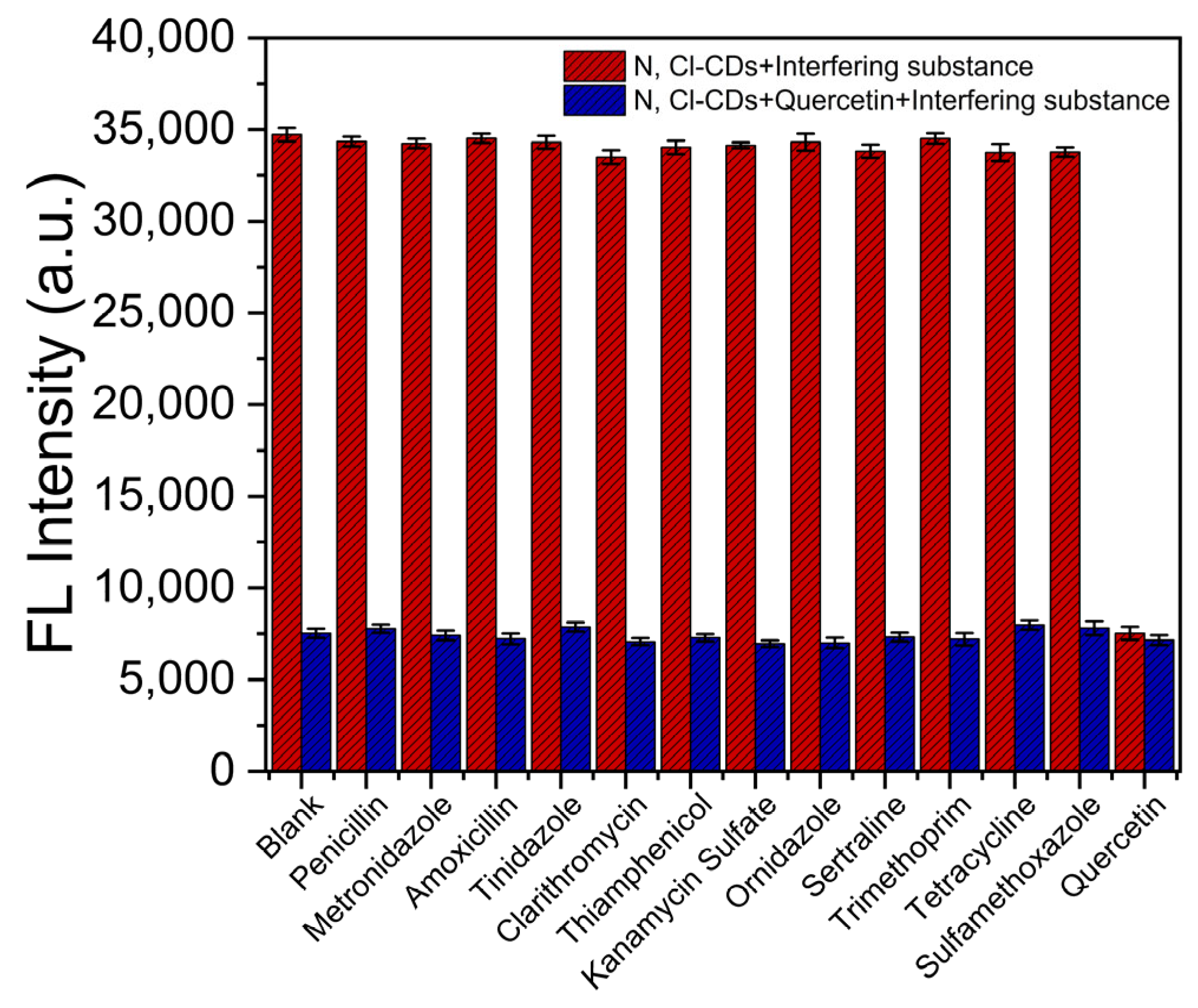
3.4. Recovery of Quercetin from Actual Samples
3.5. Sensing Mechanism of Quercetin by N, Cl-CDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mansour, F.R.; Abdallah, I.A.; Bedair, A.; Hamed, M. Analytical Methods for the Determination of Quercetin and Quercetin Glycosides in Pharmaceuticals and Biological Samples. Crit. Rev. Anal. Chem. 2025, 55, 187–212. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- Heřmánková, E.; Zatloukalová, M.; Biler, M.; Sokolová, R.; Bancířová, M.; Tzakos, A.G.; Křen, V.; Kuzma, M.; Trouillas, P.; Vacek, J. Redox properties of individual quercetin moieties. Free. Radic. Biol. Med. 2019, 143, 240–251. [Google Scholar] [CrossRef]
- Buljeta, I.; Ćorković, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Application of Citrus and Apple Fibers for Formulation of Quercetin/Fiber Aggregates: Impact of Quercetin Concentration. Plants 2022, 11, 3582. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Watanabe, K. Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov. Today 2016, 21, 632–639. [Google Scholar] [CrossRef]
- Algandaby, M.M. Quercetin attenuates cisplatin-induced ovarian toxicity in rats: Emphasis on anti-oxidant, anti-inflammatory and anti-apoptotic activities. Arab. J. Chem. 2021, 14, 103191. [Google Scholar] [CrossRef]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharmacol. 2017, 8, 336. [Google Scholar] [CrossRef]
- Özsoy Gökbilen, S.; Becer, E.; Vatansever, H.S. Senescence-mediated anticancer effects of quercetin. Nutr. Res. 2022, 104, 82–90. [Google Scholar] [CrossRef]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef]
- Da Silva, A.B.; Cerqueira Coelho, P.L.; das Neves Oliveira, M.; Oliveira, J.L.; Oliveira Amparo, J.A.; da Silva, K.C.; Soares, J.R.P.; Pitanga, B.P.S.; dos Santos Souza, C.; de Faria Lopes, G.P.; et al. The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav. Immun. 2020, 85, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, Y.; Wakabayashi, K. Report on the mutagenicity of flavone derivatives and their contribution to advancing scientific knowledge. Proc. Jpn. Acad. Ser. B 2024, 100, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Numata, Y.; Tanaka, H. Quantitative analysis of quercetin using Raman spectroscopy. Food Chem. 2011, 126, 751–755. [Google Scholar] [CrossRef]
- Pilařová, V.; Plachká, K.; Chrenková, L.; Najmanová, I.; Mladěnka, P.; Švec, F.; Novák, O.; Nováková, L. Simultaneous determination of quercetin and its metabolites in rat plasma by using ultra-high performance liquid chromatography tandem mass spectrometry. Talanta 2018, 185, 71–79. [Google Scholar] [CrossRef]
- Gao, F.; Hong, W.; Xu, B.; Wang, Y.; Lu, L.; Zhao, Z.; Zhang, C.; Deng, X.; Tang, J. MXene Nanosheets Decorated with Pt Nanostructures for the Selective Electrochemical Detection of Quercetin. ACS Appl. Nano Mater. 2023, 6, 6869–6878. [Google Scholar] [CrossRef]
- Duhan, J.; Bisht, A.; Kumar, H.; Obrai, S. Graphitic nitride co-doped with sodium and sulphur as fluorescent and UV–Vis probe for quercetin detection. Surf. Interfaces 2024, 52, 104927. [Google Scholar] [CrossRef]
- Feng, S.; Tang, F.; Wu, F.; Zhang, J. One-pot synthesis of nano Zr-based metal-organic frameworks for fluorescence determination of quercetin and Hg2+. Food Chem. 2024, 432, 137173. [Google Scholar] [CrossRef]
- Iranmanesh, H.; Afshar, E.A.; Taher, M.A.; Karaman, C.; Karimi-Maleh, H. Detection and Determination of Quercetin in Sour Cherry and Black Tea Samples by Fluorescent Probe Prepared by Green Hydrothermal Method Using Achillea Millefolium Extract. Top. Catal. 2024, 67, 864–872. [Google Scholar] [CrossRef]
- Alafeef, M.; Srivastava, I.; Aditya, T.; Pan, D. Carbon Dots: From Synthesis to Unraveling the Fluorescence Mechanism. Small 2024, 20, 2303937. [Google Scholar] [CrossRef]
- Zhou, Y.; Benetti, D.; Tong, X.; Jin, L.; Wang, Z.M.; Ma, D.; Zhao, H.; Rosei, F. Colloidal carbon dots based highly stable luminescent solar concentrators. Nano Energy 2018, 44, 378–387. [Google Scholar] [CrossRef]
- Kumar, V.B.; Sahu, A.K.; Mohsin, A.S.M.; Li, X.; Gedanken, A. Refractive-Index Tuning of Highly Fluorescent Carbon Dots. ACS Appl. Mater. Interfaces 2017, 9, 28930–28938. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; An, M.; Zhao, X.; Wang, Y.; Hasan, M. Advances in Fluorescent Sensing Carbon Dots: An Account of Food Analysis. ACS Omega 2023, 8, 9031–9039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, D.; Huang, H.; Liu, L.; Zhou, Y.; Xia, X.; Deng, K.; Liu, X. Preparation of Gold–Carbon Dots and Ratiometric Fluorescence Cellular Imaging. ACS Appl. Mater. Interfaces 2016, 8, 6646–6655. [Google Scholar] [CrossRef]
- Mutuyimana, F.P.; Liu, J.; Nsanzamahoro, S.; Na, M.; Chen, H.; Chen, X. Yellow-emissive carbon dots as a fluorescent probe for chromium(VI). Microchim. Acta 2019, 186, 163. [Google Scholar] [CrossRef]
- Cai, T.-T.; Liu, B.; Pang, E.-N.; Ren, W.-J.; Li, S.-J.; Hu, S.-L. A review on the preparation and applications of coal-based fluorescent carbon dots. New Carbon Mater. 2020, 35, 646–666. [Google Scholar] [CrossRef]
- Koutsogiannis, P.; Thomou, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Advances in fluorescent carbon dots for biomedical applications. Adv. Phys. X 2020, 5, 1758592. [Google Scholar] [CrossRef]
- Saikia, M.; Hazarika, A.; Roy, K.; Khare, P.; Dihingia, A.; Konwar, R.; Saikia, B.K. Waste-derived high-yield biocompatible fluorescent carbon quantum dots for biological and nanofertiliser applications. J. Environ. Chem. Eng. 2023, 11, 111344. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.L.; Li, C.M.; Huang, C.Z. Fluorescent carbon dots functionalization. Adv. Colloid Interface Sci. 2019, 270, 165–190. [Google Scholar] [CrossRef]
- Paydar, S.; Feizi, F.; Shamsipur, M.; Barati, A.; Chehri, N.; Taherpour, A.; Jamshidi, M. An ideal ratiometric fluorescent probe provided by the surface modification of carbon dots for the determination of Pb2+. Sens. Actuators B Chem. 2022, 369, 132243. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Microchim. Acta 2018, 185, 424. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Zhu, F.; Zhao, X.; Yang, H.; Chen, X. A reusable P, N-doped carbon quantum dot fluorescent sensor for cobalt ion. Sens. Actuators B Chem. 2018, 260, 156–164. [Google Scholar] [CrossRef]
- Shi, L.; Li, L.; Li, X.; Zhang, G.; Zhang, Y.; Dong, C.; Shuang, S. Excitation-independent yellow-fluorescent nitrogen-doped carbon nanodots for biological imaging and paper-based sensing. Sens. Actuators B Chem. 2017, 251, 234–241. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, P.; Wu, Y.; Su, X.; Liu, H.; Zhu, G.; Zhou, Q. Fast and efficient “on-off-on” fluorescent sensor from N-doped carbon dots for detection of mercury and iodine ions in environmental water. Sci. Total Environ. 2022, 827, 154357. [Google Scholar] [CrossRef]
- Fu, Q.; Sun, S.; Li, N.; Lu, K.; Dong, Z. Based on halogen-doped carbon dots: A review. Mater. Today Chem. 2023, 34, 101769. [Google Scholar] [CrossRef]
- Wu, M.; Li, J.; Wu, Y.; Gong, X.; Wu, M. Design of a Synthetic Strategy to Achieve Enhanced Fluorescent Carbon Dots with Sulfur and Nitrogen Codoping and Its Multifunctional Applications. Small 2023, 19, 2302764. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, X.; Zhu, Q.; Han, X.; Tang, Y.; Liao, S.; Guo, Z.; Wang, Z.; Bi, W.; Xu, Q.; et al. Exploring multi-element co-doped carbon dots as dual-mode probes for fluorescence/CT imaging. Chem. Eng. J. 2023, 470, 144042. [Google Scholar] [CrossRef]
- Kaleem, W.; Kumar, A.; Panda, D. Luminescent N, S-Doped Carbon Nanodot: An Effective Two-Fluorophore System of Pyridone and Thiazolopyridone. J. Phys. Chem. C 2018, 122, 26722–26732. [Google Scholar] [CrossRef]

| Sample | Added (μM) | Found (μM) | RR (%) | RSD (%) (n = 5) |
|---|---|---|---|---|
| Apple peel | 5 | 4.79 | 95.80 | 3.07 |
| 20 | 19.07 | 95.35 | 3.11 | |
| 40 | 40.28 | 100.70 | 1.26 | |
| Red wine | 5 | 5.01 | 100.20 | 2.44 |
| 20 | 21.15 | 105.75 | 5.29 | |
| 40 | 41.24 | 103.10 | 4.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Miao, X.; Wang, L.; Hong, S.; Gao, Y.; Wang, X. The Engineered Synthesis and Enhancement of Nitrogen and Chlorine Co-Doped Fluorescent Carbon Dots for the Sensitive Detection of Quercetin. Materials 2025, 18, 2669. https://doi.org/10.3390/ma18112669
Jiao Y, Miao X, Wang L, Hong S, Gao Y, Wang X. The Engineered Synthesis and Enhancement of Nitrogen and Chlorine Co-Doped Fluorescent Carbon Dots for the Sensitive Detection of Quercetin. Materials. 2025; 18(11):2669. https://doi.org/10.3390/ma18112669
Chicago/Turabian StyleJiao, Yuan, Xuewen Miao, Lizhang Wang, Shasha Hong, Yifang Gao, and Xin Wang. 2025. "The Engineered Synthesis and Enhancement of Nitrogen and Chlorine Co-Doped Fluorescent Carbon Dots for the Sensitive Detection of Quercetin" Materials 18, no. 11: 2669. https://doi.org/10.3390/ma18112669
APA StyleJiao, Y., Miao, X., Wang, L., Hong, S., Gao, Y., & Wang, X. (2025). The Engineered Synthesis and Enhancement of Nitrogen and Chlorine Co-Doped Fluorescent Carbon Dots for the Sensitive Detection of Quercetin. Materials, 18(11), 2669. https://doi.org/10.3390/ma18112669







