Blackseed Oil Supplemented Caseinate–Carboxymethyl Chitosan Film Membrane for Improving Shelf Life of Grape Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Edible Membrane/Film/Coating
2.1.1. Preparation of Edible Film
2.1.2. Preparation of Sample Coating
3. Characterization of Films
3.1. Physicochemical Properties
3.1.1. Particle Size and Zeta Potential Measurement
3.1.2. Thickness
3.1.3. Moisture Content
3.1.4. Water Solubility
3.1.5. Water Vapor Permeability (WVP)
3.1.6. Color and Opacity Index
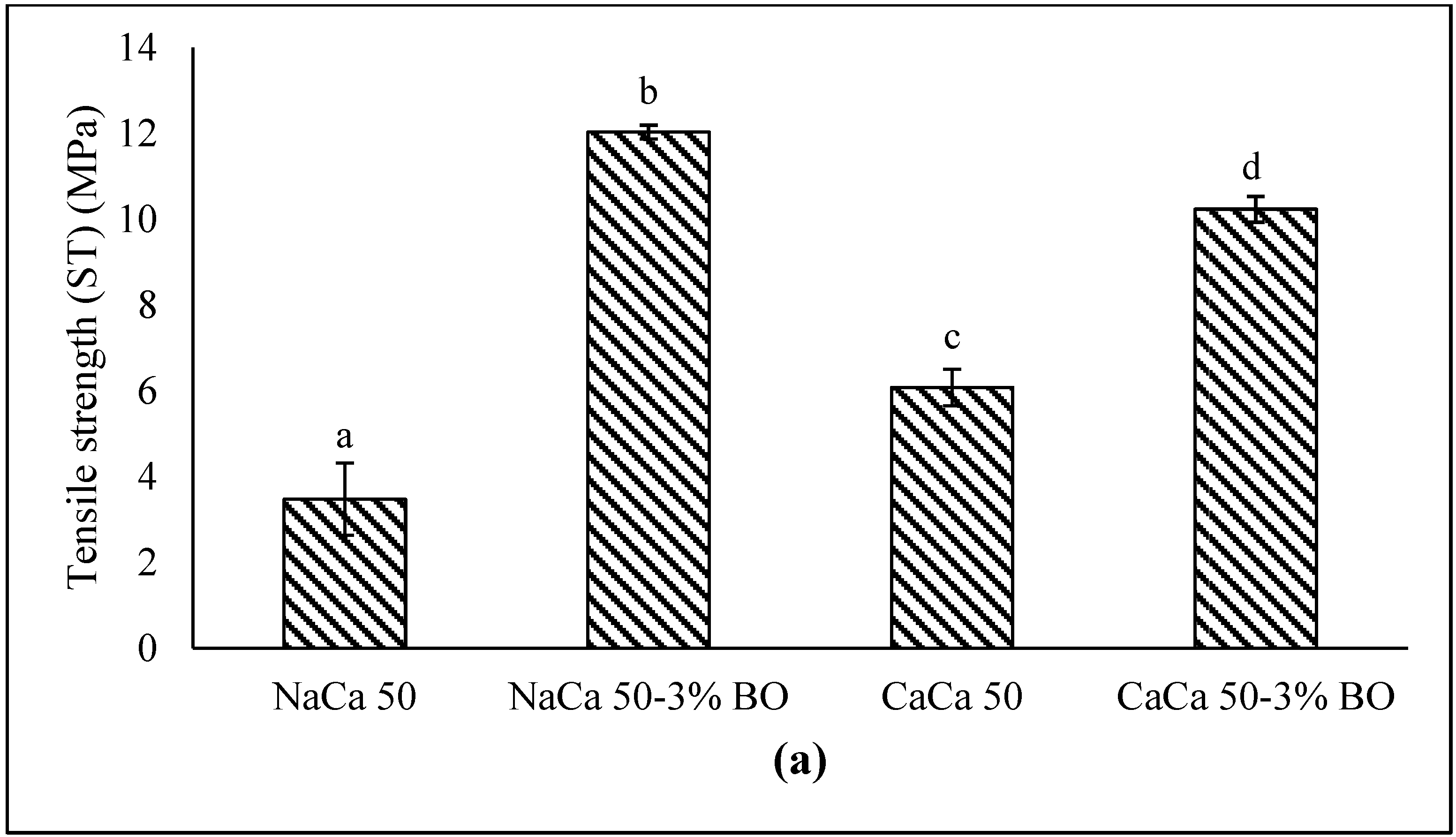
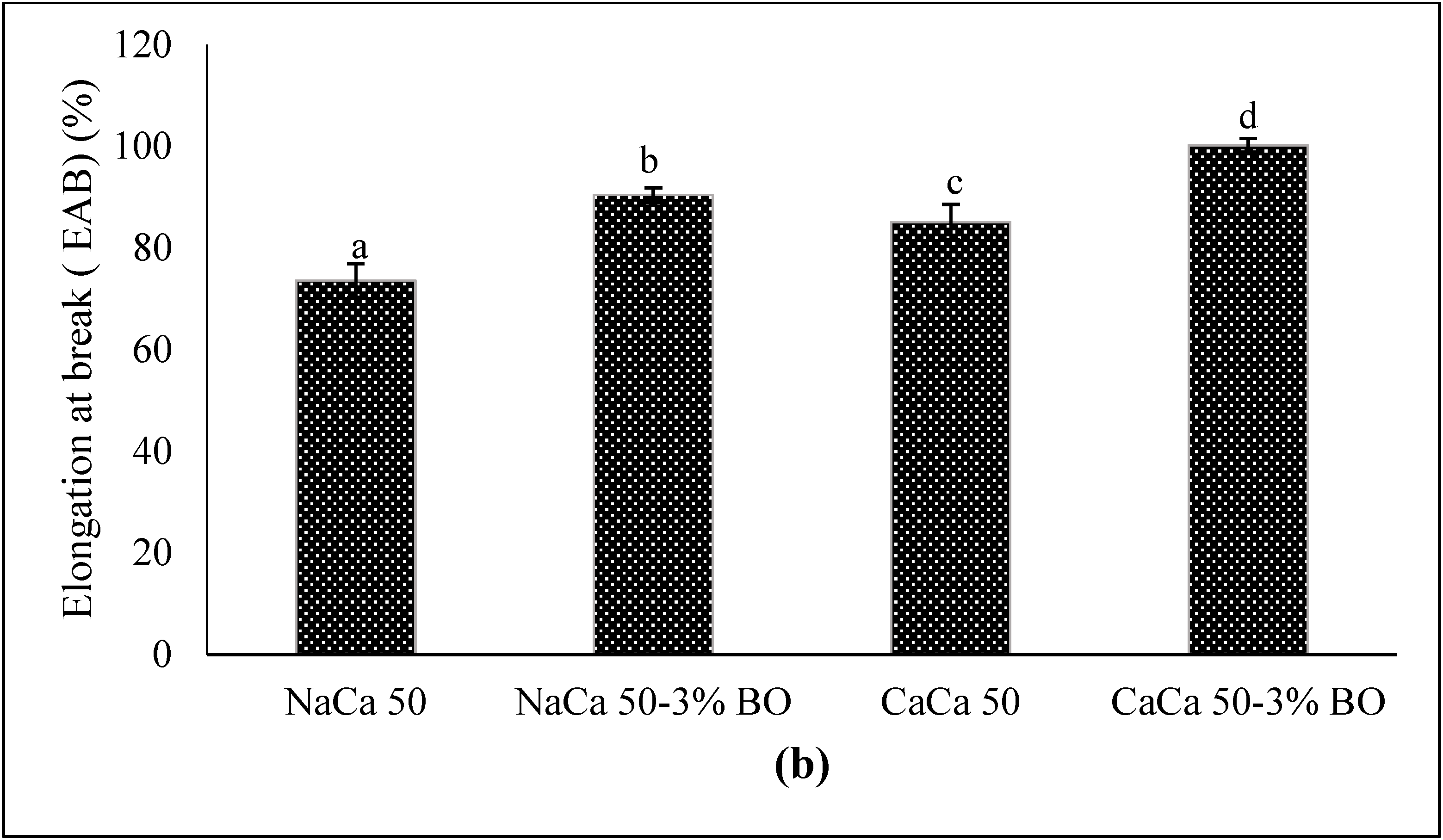
3.1.7. Mechanical Properties
3.1.8. Antioxidant Activities
DPPH Free Radical Scavenging Activity Assay
ABTS Radical Scavenging Activity Assay
3.1.9. Structural Properties
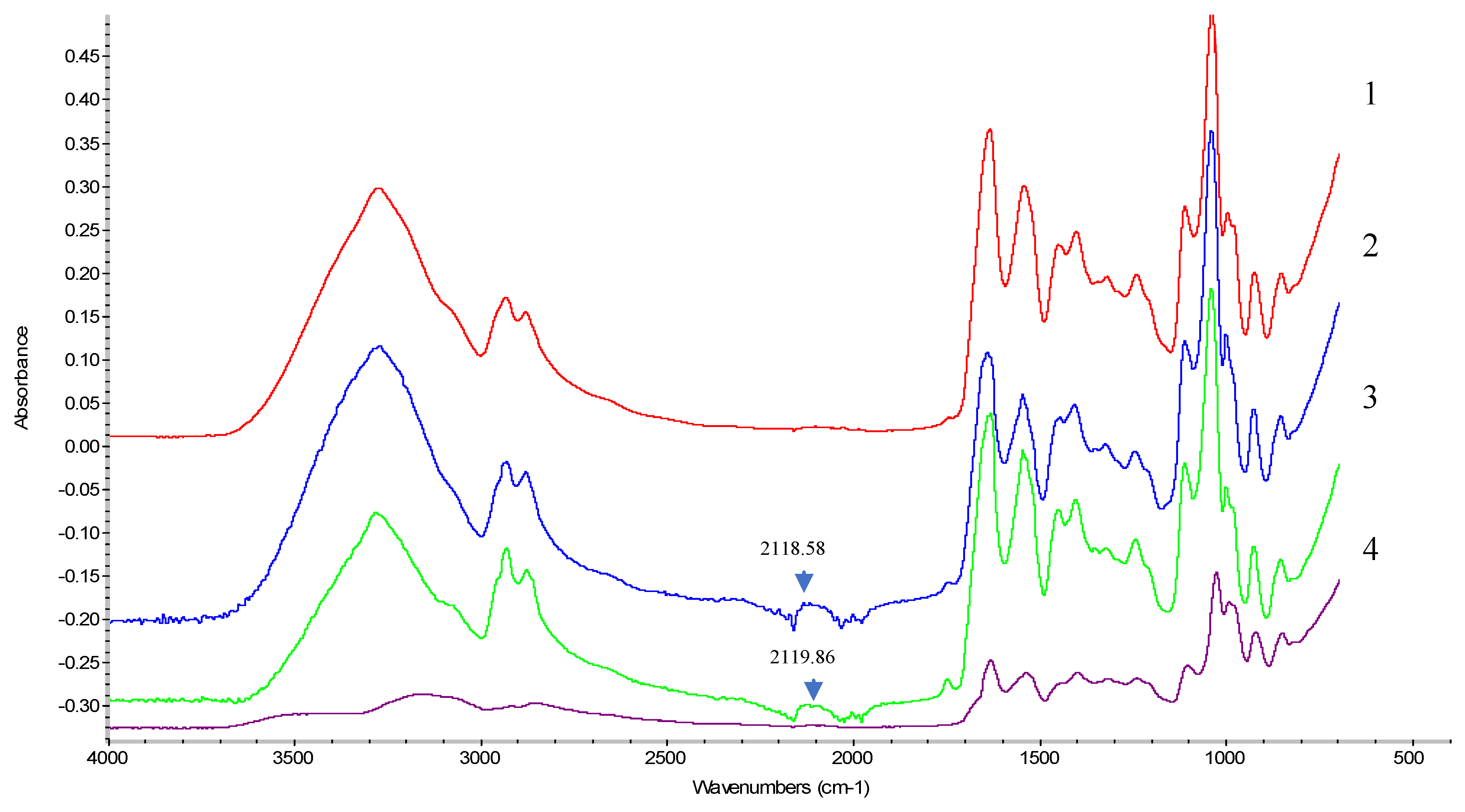
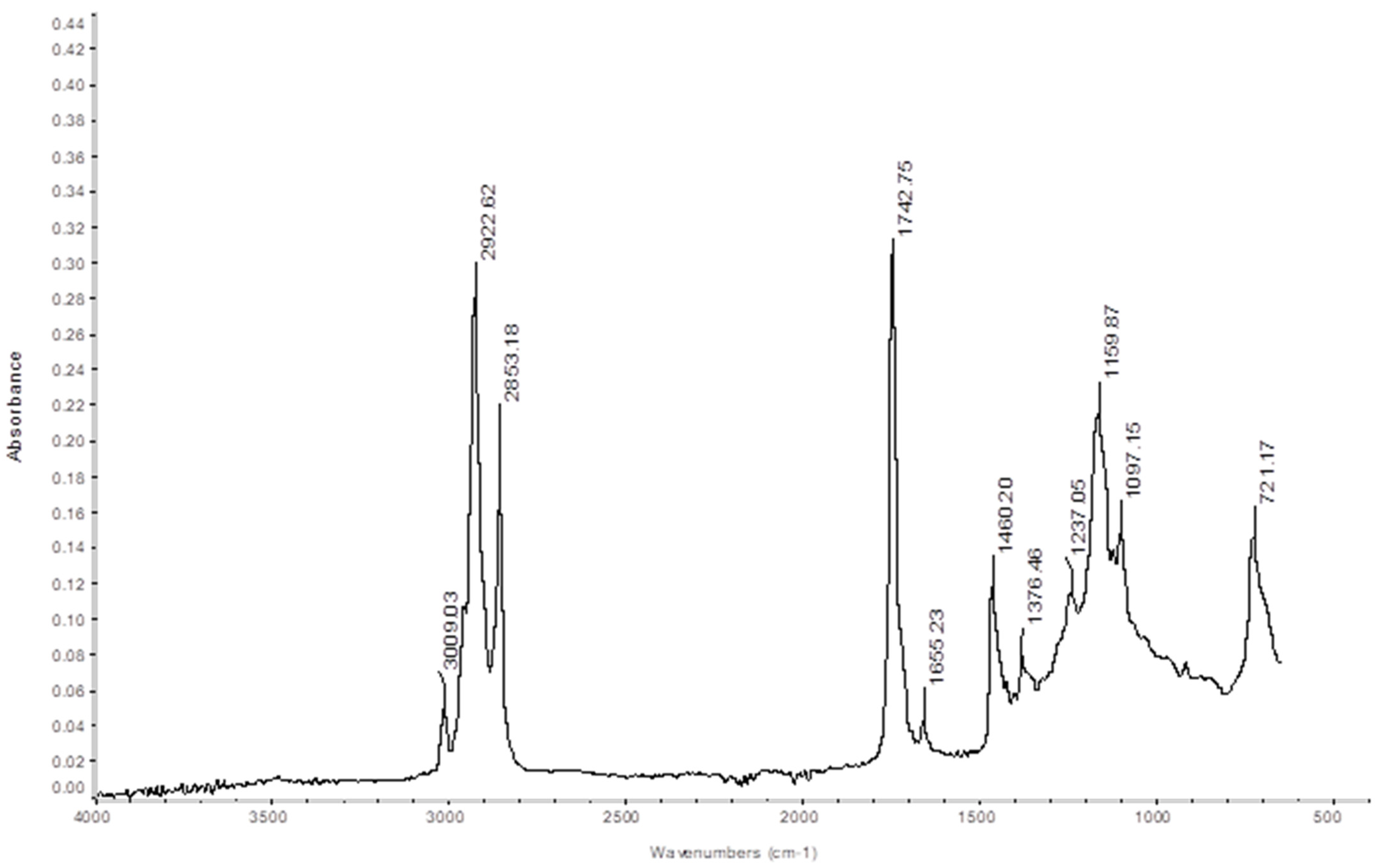
Fourier Transform Infrared Spectroscopy (FT-IR)
Scanning Electron Microscopy (SEM)
4. Physicochemical Properties
4.1. pH, Brix, Titratable Acidity, and Weight Loss
4.2. Color Measurement
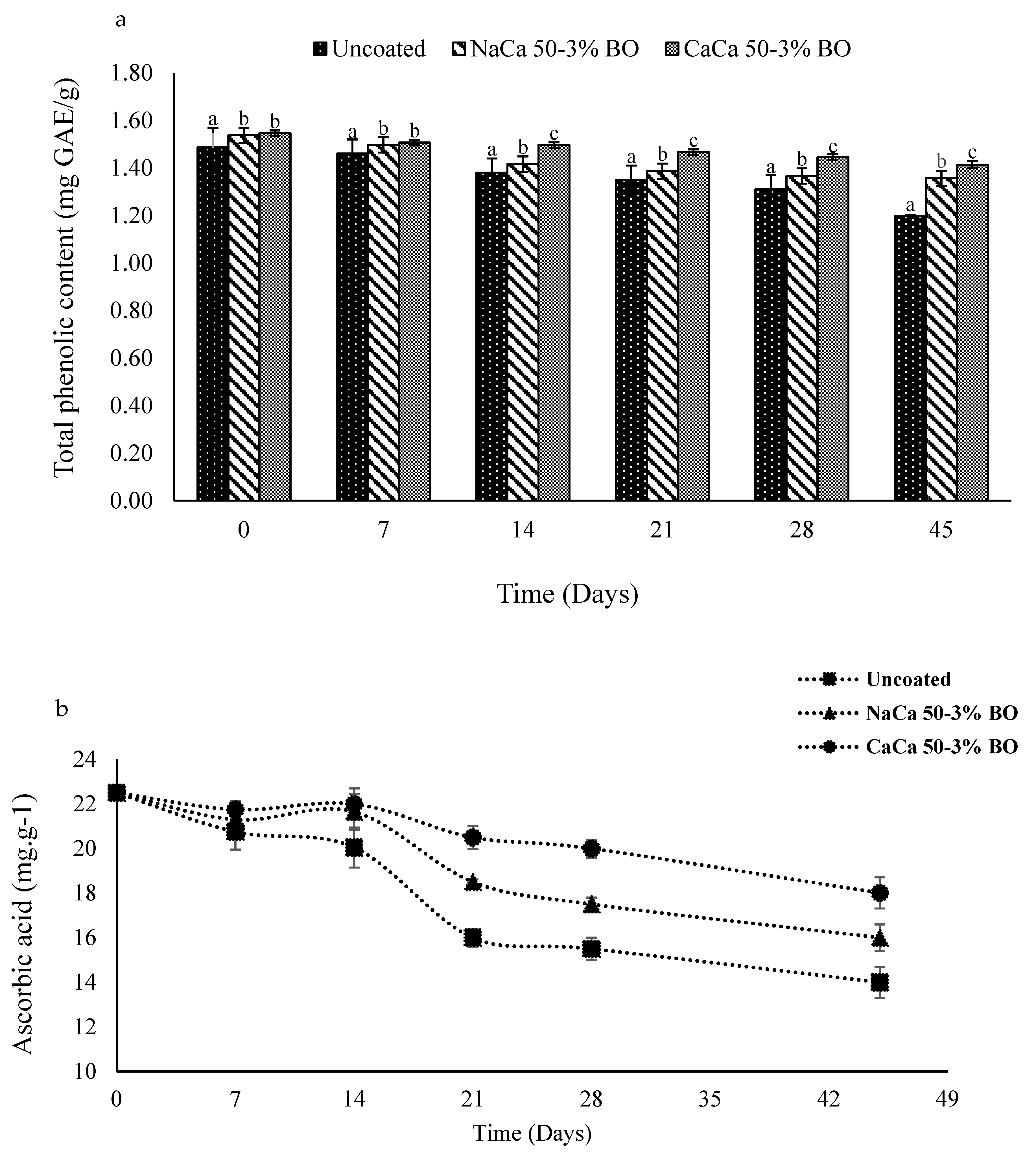
4.3. Determination of Total Phenolic and Ascorbic Acid
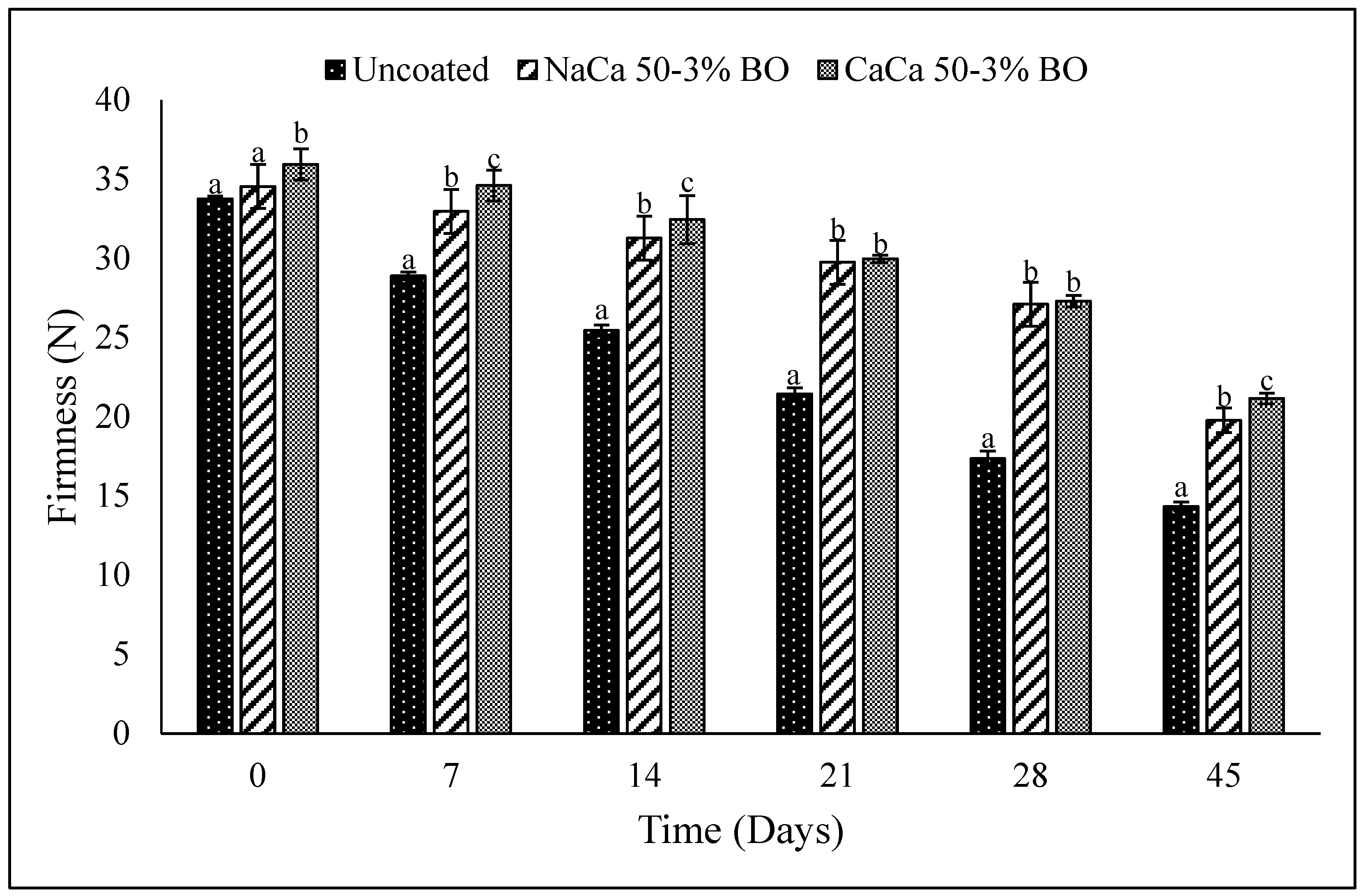
4.4. Texture Evaluation
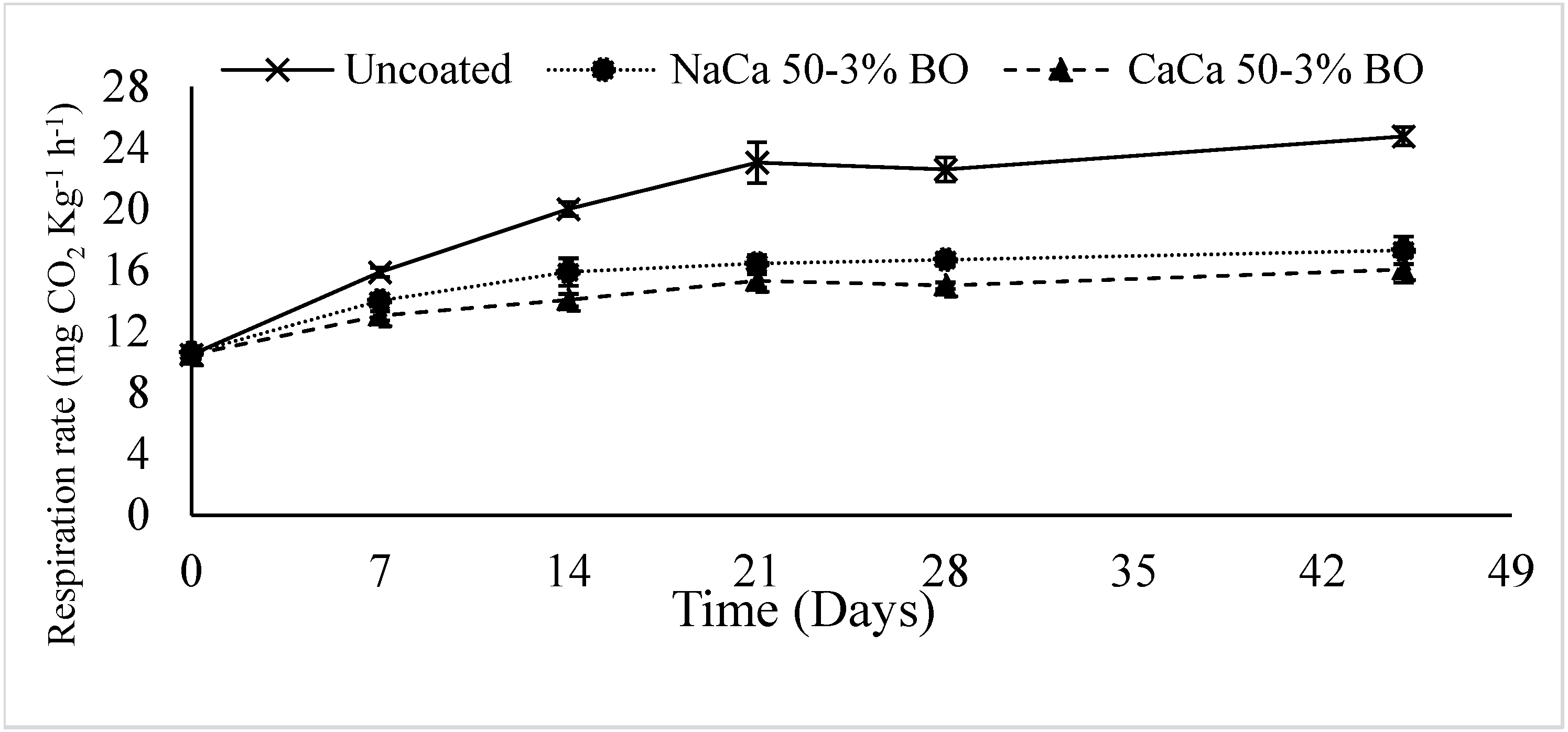
4.5. Respiration Rate
4.6. Microbial Growth
4.7. Statistical Analyses
5. Results and Discussion
5.1. Characterization of Film Properties
5.1.1. Particle Size and Zeta Potential Measurement
5.1.2. Physicochemical Properties
5.1.3. Color and Opacity Index
5.1.4. Mechanical Properties
5.1.5. Antioxidant Activities
5.1.6. FTIR Analysis
5.1.7. Scanning Electron Microscopy (SEM)
6. Quality Parameters of Tomatoes with and Without Coating
6.1. pH, Total Soluble Solids, Titratable Acidity, and Weight Loss
6.2. Color Parameters
6.3. Total Phenolic and Ascorbic Acid
6.4. Texture
6.5. Respiration Rate
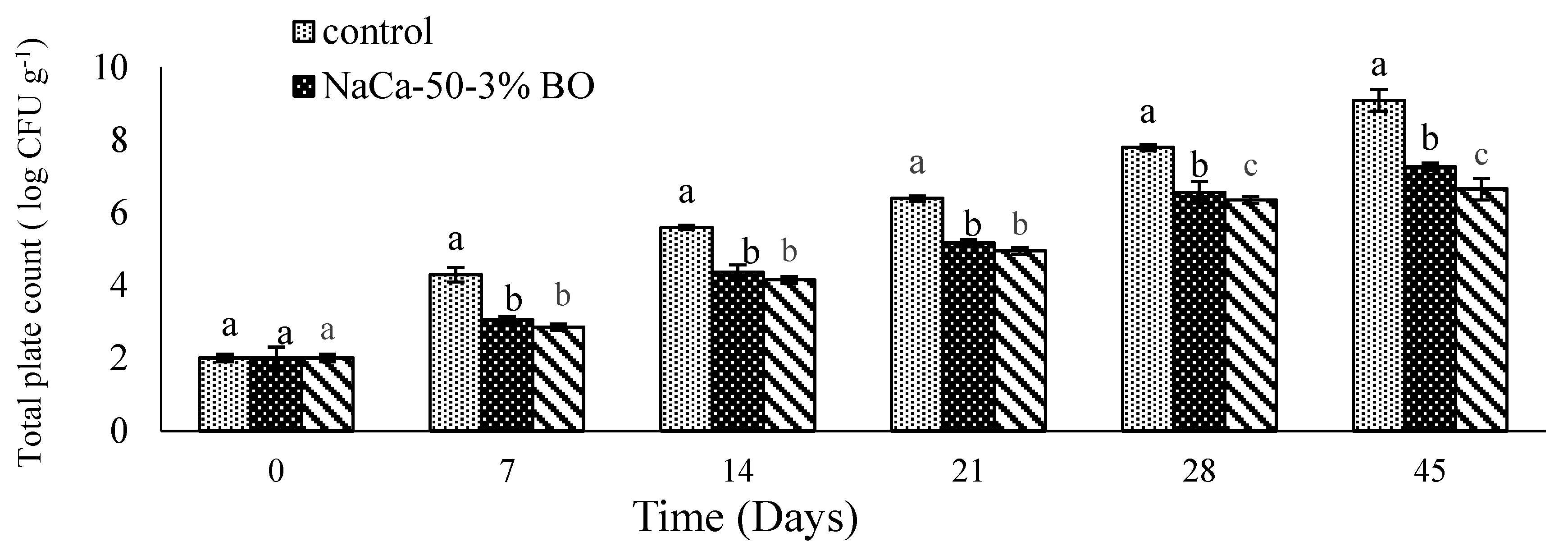
6.6. Microbial Quality
6.7. Visual Appearance
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abedi, E.; Sayadi, M.; Oliyaei, N. Fabrication and characterization of emulsion-based edible film containing cinnamon essential oil using Chia seed mucilage. Int. J. Biol. Macromol. 2024, 266, 131173. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.F.R.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Recent highlights in sustainable bio-based edible films and coatings for fruit and vegetable applications. Foods 2024, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, Y.; Wang, H.; Liu, W.; Cheong, K.; Teng, B. Characterization of seaweed polysaccharide-based bilayer films containing essential oils with antibacterial activity. LWT 2021, 150, 111961. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Rachtanapun, C.; Jantrawut, P.; Thanakkasaranee, S.; Kasi, G.; Tantala, J.; Panraksa, P.; Chaiwarit, T. Carboxymethyl chitosan-based materials in packaging food, pharmaceutical, and cosmetics. In Multifaceted Carboxymethyl Chitosan Derivatives: Properties and Biomedical Applications; Springer Nature: Cham, Switzerland, 2023; p. 139. [Google Scholar]
- Wang, Q.; Li, C.; Qaio, Y.; Hao, Y.; Gong, Z.; Wu, Y.; Liu, X. Improving physical stability of microalga protein-based emulsion under acidic and neutral conditions via carboxymethyl chitosan complexation. Food Chem. 2024, 23, 101690. [Google Scholar]
- Li, L.; Baig, M.I.M.; de Vos, W.; Lindhoud, S. Preparation of sodium carboxymethyl cellulose-chitosan complex membranes through sustainable aqueous phase separation. Appl. Polym. Mater. 2023, 5, 1810–1818. [Google Scholar] [CrossRef]
- Iamareerat, B.; Singh, M.; Sadiq, M.B.; Anal, A.K. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nano clay as food packaging material. J. Food Sci. Technol. 2018, 55, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G.; Omer, A.M.; Tamer, T.M. Evaluation of antimicrobial and antioxidant activities for cellulose acetate films incorporated with rosemary and aloe vera essential oils. J. Food Sci. Technol. 2019, 3, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Casalini, S.; Baschetti, M.G. The use of essential oils in chitosan or cellulose-based materials for the production of active food packaging solutions: A review. J. Sci. Food Agric. 2022, 3, 1021–1041. [Google Scholar] [CrossRef] [PubMed]
- Makouie, S.; Alizadeh, M.; Maleki, O.; Khosrowshahi, A. Investigation of physicochemical properties and oxidative stability of encapsulated Nigella sativa seed oil. Flavour Fragr. J. 2021, 2, 233–242. [Google Scholar] [CrossRef]
- Mohammed, S.J.; Amin, H.H.H.; Aziz, S.B.; Sha, A.M.; Hassan, S.; Abdul Aziz, J.M.; Rahman, H.S. Structural characterization, antimicrobial activity, and in vitro cytotoxicity effect of blackseed oil. Evid.-Based Complement. Altern. Med. 2019, 2019, 6515671. [Google Scholar] [CrossRef] [PubMed]
- Duguma, H.T. Potential applications and limitations of edible coatings for maintaining tomato quality and shelf life. Int. J. Food Sci. Technol. 2022, 57, 1353–1366. [Google Scholar] [CrossRef]
- Jhanani, G.K.; AlSalhi, M.S.; Naveena, T.; Shanmuganathan, R. As assessment of shelf-life increasing competence of pectin (zucchini) based edible coating on tomatoes. Environ. Res. 2024, 258, 119368. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Shah, Y.A.; Al-Harrasi, A.; Jawad, M.; Koca, E.; Aydemir, L.Y. Novel applications of black pepper essential oil as an antioxidant agent in sodium caseinate and chitosan based active edible films. Int. J. Biol. Macromol. 2024, 254, 128045. [Google Scholar] [CrossRef] [PubMed]
- Roshandel-Hesrai, N.; Mokaber-Esfahani, M.; Taleghani Aand Akbari, R. Investigation of physiochemical properties, antimicrobial and antioxidant activity of edible films based on chitosan/casein containing Origanum vulgare L. essential oil and its effect on quality maintenance of cherry tomato. Food Chem. 2022, 396, 133650. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, H.; Wang, Y.; Wang, H.; Wang, L.; Elfallen, W.; Yu, D. Effect of soybean protein-isolate-catechin edible film and coating on the storage quality of bean bun. Food Biosci. 2024, 57, 103613. [Google Scholar] [CrossRef]
- Galus, S.; Mikus, M.; Ciurzynska, A.; Domian, E.; Kowalska, J.; Marzec, A.; Kowalska, H. The effect of whey protein-based edible coatings incorporated with lemon and lemongrass essential oils on the quality attributes of fresh-cut pears during storage. Coatings 2021, 11, 745. [Google Scholar] [CrossRef]
- ASTM D638-10; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2010.
- Guan, T.; Li, N.; Zhang, G.; Xue, P. Characterization and evaluation of sodium alginate-based edible films by incorporation of star anise ethanol extract/hydroxypropyl--cyclodextrin inclusion complex. Food Packag. Shelf Life 2022, 31, 100785. [Google Scholar] [CrossRef]
- Popovic, S.; Pericin, D.; Vastag, Z.; Lazic, V.; Popovic, L. Pumpkin oil cake protein isolate films as potential gas barrier coating. J. Food Eng. 2012, 110, 374–379. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 14th ed.; Association of Official Analytical Chemistry: Washington, DC, USA, 1998. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemistry: Washington, DC, USA, 1990. [Google Scholar]
- Vieira, J.M.; Flores-Lopez, M.L.; Rodrguez, D.J.; SOUSA, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan-aloe vera coating on postharvest quality of blueberry (vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, C.S. Edible composite bi-layer coating based on whey protein isolate, xanthan gum and clove oil for prolonging shelf life of tomatoes. Meas. Food 2021, 2, 100005. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Marquez, G.R.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.L.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/ pectin edible films. Food Sci. Technol. 2017, 75, 124–130. [Google Scholar]
- Maftoonzad, N. Evaluation of Edible Films and Coatings for Extending the Postharvest Shelf Life of Avocado. McGill University Libraries, [Montreal]. 2006. Available online: https://central.bac-lac.gc.ca/.item?id=TC-QMM-102678&op=pdf&app=Library (accessed on 22 May 2025).
- Jaderi, Z.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Effect of glycerol and sorbitol on a novel biodegradable edible film based on Malva sylvestris flower gum. Food Sci. Nutr. 2023, 2, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ni, Z.; Thakur, K.; Zhang, J.; Hu, F.; Wei, Z. Preparation and characterization of clove essential oil loaded nanoemulsion and pickering emulsion active pullulan-gelatin based edible film. Int. J. Biol. Macromol. 2021, 181, 528–539. [Google Scholar] [CrossRef]
- Rocca-Smith, J.R.; Marcuzzo, E.; Karbowiak, T.; Centa, J.; Giacometti, M.; Scapin, F.; Venir, E.; Sensidoni, A.; Debeaufort, F. Effect of lipid incorporation on functional properties of wheat gluten based edible films. J. Cereal Sci. 2016, 69, 275–282. [Google Scholar] [CrossRef]
- Wai, S.N.; How, Y.H.; Saleena, L.A.; Degraeve, P.; Oulahal, N.; Pui, L.P. Chitosan-sodium caseinate composite edible film incorporated with probiotic limos lactobacillus fermentum: Physical properties, viability, and antibacterial properties. Foods 2022, 22, 3583. [Google Scholar] [CrossRef]
- Lee, J.; Lee, E.; Han, J. Enhancement of the water-resistance properties of an edible film prepared from mung bean starch via the incorporation of sunflower seed oil. Sci. Rep. 2020, 10, 13622. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Q.; Critzer, F.; Davidson, P.M.; Zhong, Q. Physical and antibacterial properties of alginate film containing cinnamon bark oil and soybean oil. Food Sci. Technol. 2015, 64, 423–430. [Google Scholar] [CrossRef]
- Chen, K.; Tian, R.; Xu, G.; Wu, G.; Liu, Y.; Jiang, F. Characterization of konjac glucomannan/curdlan edible coatings and the preservation effect on cherry tomatoes. Int. J. Biol. Macromol. 2023, 232, 12359. [Google Scholar] [CrossRef]
- Zimet, P.; Mombru, A.W.; Mombru, D.; Castro, A.; Villanueva, J.P.; Pardo, H.; Rufo, C. Physico-chemical and antilisterial properties of nisin-incorporated chitosan/ carboxymethyl chitosan films. Carbohydr. Polym. 2019, 219, 334–343. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y.; et al. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mano preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef] [PubMed]
- How, Y.H.; Lim, E.M.; Kon, L.; Kee, P.; Pui, L. Development of carboxymethyl cellulose-chitosan based antibacterial films incorporating a Persicaria minor Huds. essential oil nanoemulsion. Sustain. Food Technol. 2024, 2, 400. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Rakkapao, N.; Lekjing, S. Physicochemical and antimicrobial characterization of chitosan and native glutinous rice starch-based composite edible films: Influence of different essential oils incorporation. Membranes 2023, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Shah, Y.A.; Al-Harrasi, A.; Alhadhrami, A.S.; AlHashmi, D.S.H.; Jawad, M.; Diblan, S.; Al Dawery, S.K.H.; Esatbeyoglu, T.; Anwer, M.K.; et al. Characterization of biodegradable films based on guar gum and calcium caseinate incorporated with clary sage oil: Rheological, physicochemical, antioxidant, and antimicrobial properties. J. Agric. Food Res. 2024, 15, 100948. [Google Scholar] [CrossRef]
- University of Illinois, Springfield. UIS: Introduction to Organic Spectroscopy. LibreTexts. Available online: https://chem.libretexts.org/Courses/University_of_Illinois_Springfield/Introduction_to_Organic_Spectroscopy (accessed on 22 May 2025).
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 9, 1073–1101. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 8, 549–559. [Google Scholar] [CrossRef]
- Barreto, T.A.; Andrade, S.C.; Maciel, J.F.; Arcanjo, N.M.; Madruga, M.S.; Meireles, B.; Magnani, M. A chitosan coating containing essential oil from Origanum vulgare L., to control postharvest mold infections and keep the quality of cherry tomatoes fruit. J. Front. Microbiol. 2016, 7, 1724. [Google Scholar] [CrossRef]
- Pobiega, K.; Przyby, J.L.; Zubermilk, J.; Gniewosz, M. Prolonging the shelf life of cherry tomatoes by pullulan coating with ethanol extract of propolis during refrigerated storage. Food Bioprocess Technol. 2020, 13, 1447–1461. [Google Scholar] [CrossRef]
- Guo, X.; Chen, B.; Wu, X.; Li, J.; Sun, Q. Utilization of cinnamaldehde and zinc oxide nanoparticles in a carboxymethylcellulose-based composite coating to improve the postharvest quality of cherry tomatoes. Int. J. Biol. Macromol. 2020, 160, 175–182. [Google Scholar] [CrossRef]
- Palumbo, M.; Attolico, G.; Capozzi, V.; Cozzolino, R.; Corvino, A.; Chiara, M.L.V.; Pace, B.; Pelosi, S.; Ricci, I.; Romaniello, R.; et al. Emerging postharvest technologies to enhance the shelf-life of fruit and vegetable: An overview. Foods 2022, 11, 3925. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Barciela, P.; Carpena, M.; Prieto, M.A. Edible coatings as a natural packaging system to improve fruit and vegetable shelf life and quality. Foods 2023, 12, 3570. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Veloz, L.M.; Calderon-Santoyo, M.; Carvajal-Millan, E.; Martinez-Robinson, K.; Ragazzo-Sanchez, J.A. Artocarpus heterophyllus Lam. Leaf extracts added to pectin-based edible coating for Alternaria sp. control in tomato. Food Sci. Technol. 2022, 156, 113022. [Google Scholar] [CrossRef]
- Jin, Z.T.; Fan, X.; Mukhopadhyay, S. Antimicrobial coating with organic acids and essential oil for the enhancement of safety and shelf life of grape tomatoes. Int. J. Food Microbiol. 2022, 378, 109827. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Salas, L.; Exposito-Almellon, X.; Borras-Linares, I.; Lozano-Sanchez, J.; Segura-Carretero, A. Design of experiments for green and GRAS solvent extraction of phenolic compounds from food industry by-product—A systematic review. Trends Anal. Chem. 2024, 171, 117536. [Google Scholar] [CrossRef]
- Cice, D.; Ferrara, E.; Pecoraro, M.T.; Capriolo, G.; Petriccione, M. An innovative layer-by-layer edible coating to regulate oxidative stree and ascorbate-glutathione cycle in fresh-cut melon. Horticulturae 2024, 10, 465. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Chai, Y.; Guo, C.; Luo, C.; Chen, D.; Cheng, X.; Wang, F.; Huang, C. Chitosan-pullulan films enriched with Artemisia annua essential oil: Characterization and application in grape presentation. Int. J. Biol. Macromol. 2023, 243, 125216. [Google Scholar] [CrossRef]
- Mondal, K.; Goud, V.V.; Katiyar, V. Effect of waste green algal biomass extract incorporated chitosan-based edible coating on the shelf life and quality attributes of tomato. Food Sci. Technol. 2022, 2, 1151–1165. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H.H. Sensory Evaluation of Food: Principles and Practices; Chapman and Hall: New York, NY, USA, 1998. [Google Scholar]
- Ochida, C.O.; Itodo, A.U.; Nwanganga, P.A. A review on postharvest storage, processing and preservation of tomatoes (Lycopersicon esculentum Mill.). Asian Food Sci. J. 2019, 2, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Cui, Q.L.; Wang, Y.; Shi, F.; Liu, Y.; Liu, J.; Nie, G. Effect of carboxymethyl chitosan-gelatin based edible coatings on the quality and antioxidant properties of sweet cherry during postharvest storage. Sci. Hortic. 2021, 289, 110462. [Google Scholar] [CrossRef]
- Careli-Gondim, I.; Mesquita, T.C.; Vilas Boas, E.V.B.; Caliari, M.; Junior, M.S.S. The effect of active coating and refrigerated storage on the quality of avocado cultivar, Quintal. J. Food Sci. Technol. 2020, 1, 143–151. [Google Scholar] [CrossRef]
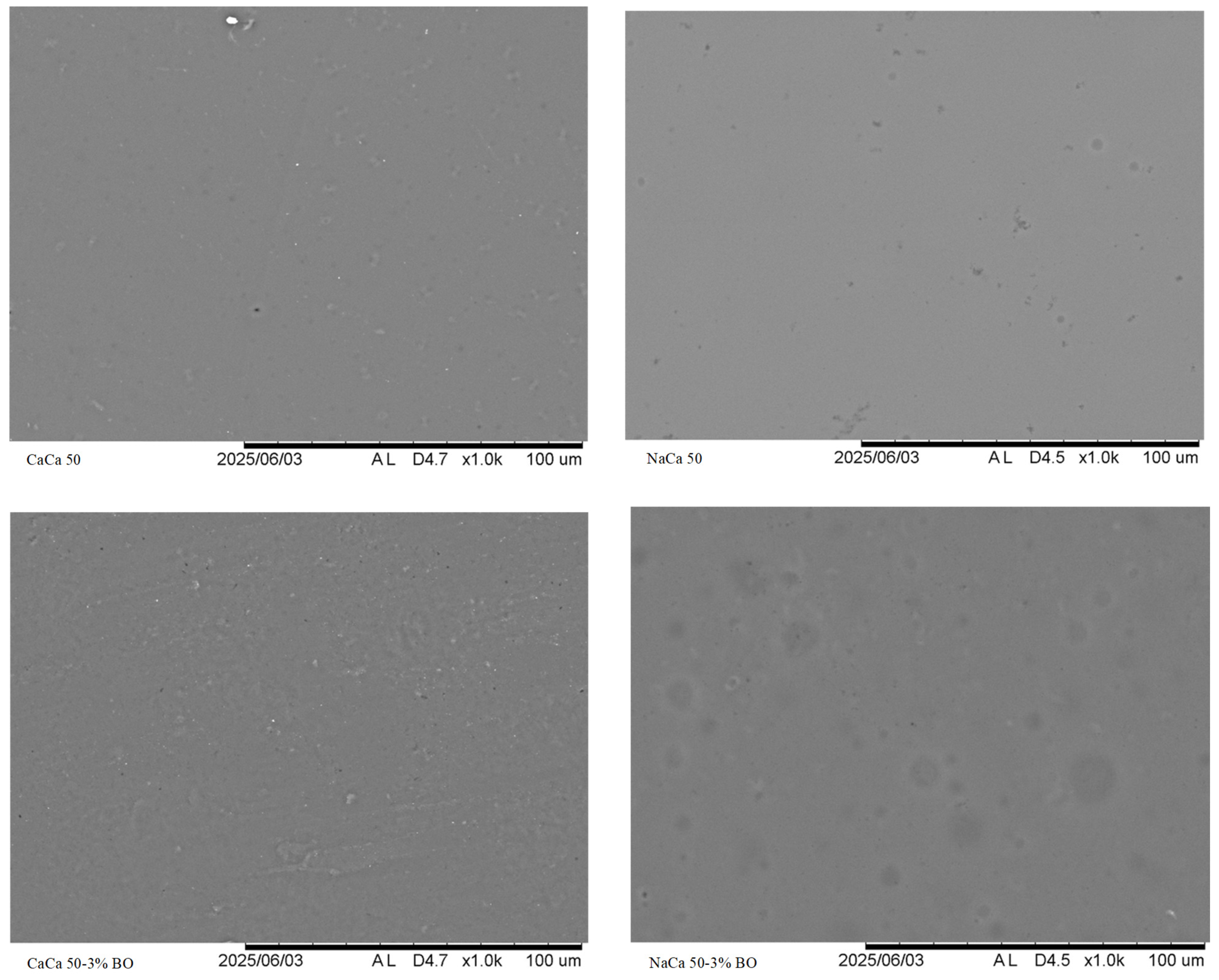


| Sample | Thickness (mm) | Water Solubility (%) | Moisture Content (%) | WVP (g kPa−1 h−1 m−2) |
|---|---|---|---|---|
| NaCa-CMCH | 0.154 0.01 a | 37.01 0.70 a | 21.30 0.11 a | 6.62 0.60 a |
| NaCa-CMCH-BO | 0.130 0.02 b | 22.20 0.11 b | 12.65 0.02 b | 4.33 0.08 b |
| CaCa-CMCH | 0.151 0.10 a | 31.08 0.32 c | 16.44 0.06 c | 6.01 0.40 c |
| CaCa-CMCH-BO | 0.129 0.04 b | 25.09 0.29 d | 10.33 0.08 d | 3.01 0.04 d |
| Sample | L* | a* | b* | E | YI | Opacity Index |
|---|---|---|---|---|---|---|
| NaCa-CMCH | 91.2 0.06 a | −1.14 0.41 a | 4.75 0.58 a | 7.45 0.72 a | 6.87 0.83 a | 0.63 0.02 a |
| NaCa-CMCH-BO | 82.6 0.67 b | 2.08 0.15 b | 31.1 0.06 b | 35.3 0.31 b | 44.9 0.01 b | 1.22 0.13 b |
| CaCa-CMCH | 91.8 ± 0.20 a | −0.95 ± 0.05 c | 3.44 ± 0.09 c | 6.09 ± 0.10 c | 4.98 0.13 c | 0.37 0.03 c |
| CaCa-CMCH-BO | 87.7 1.87 c | 0.49 1.01 d | 23.5 1.55 d | 26.5. 0.43 d | 33.9 1.03 d | 2.36 0.09 d |
| Treatment | Storage Time (days) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 45 | ||
| pH | Control | 4.82 0.01 Aa | 4.90 0.01 Bb | 4.93 0.01 Cb | 5.00 0.02 Dd | 5.07 0.04 Eb | 5.16 0.01 Fc |
| NaCa-CMCH-BO | 4.81 0.01 Aa | 4.82 0.04 Aa | 4.91 0.01 Bb | 4.94 0.02 Cb | 5.00 0.01 Da | 5.07 0.01 Eb | |
| CaCa-CMCH-BO | 4.81 0.01 Ab | 4.83 0.01 Ca | 4.85 0.01 Ca | 4.60 0.01 Aa | 4.99 0.01 Da | 5.01 0.02 Da | |
| TSS | Control | 4.42 0.03 Ac | 4.55 0.02 Bb | 4.78 0.02 Cc | 4.86 0.05 Db | 5.11 0.02 Eb | 5.29 0.03 Fc |
| NaCa-CMCH-BO | 4.29 0.01 Ab | 4.36 0.02 Ba | 4.49 0.01 Cb | 4.52 0.02 Da | 5.67 0.01 Fc | 4.88 0.01 Ea | |
| CaCa-CMCH-BO | 4.22 0.01 Aa | 4.35 0.01 Ba | 4.47 0.04 Ca | 4.52 0.02 Da | 4.65 0.01 Ea | 4.90 0.02 Fb | |
| TA | Control | 0.65 0.05 Da | 0.52 0.01 Cc | 0.51 0.01 Cc | 0.48 0.01 Bb | 0.47 0.01 Bb | 0.44 0.01 Ac |
| NaCa-CMCH-BO | 0.65 0.04 Da | 0.51 0.01 Cb | 0.50 0.01 Cb | 0.47 0.01 Ba | 0.46 0.02 Bb | 0.43 0.01 Ab | |
| CaCa-CMCH-BO | 0.65 0.03 Da | 0.49 0.01 Ca | 0.48 0.01 Ca | 0.46 0.01 Ca | 0.44 0.02 Ba | 0.38 0.01 Aa | |
| Treatments | Days of Storage | ||||||
|---|---|---|---|---|---|---|---|
| L* | 0 | 7 | 14 | 21 | 28 | 45 | |
| Control | 33.41 0.90 a | 32.85 0.02 a | 28.06 0.08 a | 27.96 0.41 a | 25.77 0.33 a | 23.93 2.01 a | |
| NaCa-CMCH-BO | 28.11 0.04 b | 27.09 0.07 b | 26.88 0.8 b | 26.19 0.08 b | 26.01 0.45 a | 25.08 0.21 b | |
| CaCa-CMCH-BO | 32.01 0.02 a | 31.50 0.01 c | 29.69 0.03 c | 31.55 0.52 c | 31.02 0.88 b | 30.21 0.07 c | |
| a* | 0 | 7 | 14 | 21 | 28 | 45 | |
| Control | 22.30 0.03 a | 21.35 0.04 a | 20.16 0.7 a | 20.09 0.01 a | 19.55 0.9 a | 18.34 0.16 a | |
| NaCa-CMCH-BO | 18.32 0.01 b | 16.15 0.11 b | 15.09 0.04 b | 18.01 0.21 b | 18.88 1.03 a | 18.06 0.11 a | |
| CaCa-CMCH-BO | 20.09 0.01 a | 20.35 0.06 a | 19.36 0.03 c | 20.89 0.07 c | 21.01 0.70 b | 20.99 0.53 b | |
| b* | 0 | 7 | 14 | 21 | 28 | 45 | |
| Control | 19.96 0.06 a | 18.16 0.09 a | 16.97 0.27 a | 15.88 0.07 a | 15.33 0.85 a | 13.09 0.33 a | |
| NaCa-CMCH-BO | 15.60 0.02 a | 13.60 0.02 b | 14.22 0.06 b | 15.78 0.30 a | 15.05 0.11 a | 13.83 0.69 a | |
| CaCa-CMCH-BO | 17.18 0.01 c | 17.56 0.03 a | 16.62 0.06 a | 17.59 0.03 b | 16.87 0.07 b | 16.55 0.08 b | |
| a*/b* | 0 | 7 | 14 | 21 | 28 | 45 | |
| Control | 1.17 0.04 a | 1.18 0.06 a | 1.19 0.49 a | 1.26 0.04 a | 1.27 0.87 a | 1.40 0.25 a | |
| NaCa-CMCH-BO | 1.17 0.01 a | 1.19 0.05 a | 1.05 0.05 b | 1.14 0.26 b | 1.25 0.56 b | 1.30 0.40 b | |
| CaCa-CMCH-BO | 1.16 0.01 a | 1.16 0.05 b | 1.16 0.04 c | 1.18 0.04 c | 1.24 0.28 b | 1.27 0.30 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.M.A.; Ramaswamy, H.S. Blackseed Oil Supplemented Caseinate–Carboxymethyl Chitosan Film Membrane for Improving Shelf Life of Grape Tomato. Materials 2025, 18, 2653. https://doi.org/10.3390/ma18112653
Mohamed AMA, Ramaswamy HS. Blackseed Oil Supplemented Caseinate–Carboxymethyl Chitosan Film Membrane for Improving Shelf Life of Grape Tomato. Materials. 2025; 18(11):2653. https://doi.org/10.3390/ma18112653
Chicago/Turabian StyleMohamed, Amal M. A., and Hosahalli S. Ramaswamy. 2025. "Blackseed Oil Supplemented Caseinate–Carboxymethyl Chitosan Film Membrane for Improving Shelf Life of Grape Tomato" Materials 18, no. 11: 2653. https://doi.org/10.3390/ma18112653
APA StyleMohamed, A. M. A., & Ramaswamy, H. S. (2025). Blackseed Oil Supplemented Caseinate–Carboxymethyl Chitosan Film Membrane for Improving Shelf Life of Grape Tomato. Materials, 18(11), 2653. https://doi.org/10.3390/ma18112653









