Multi-Scale Investigation of Fly Ash Aggregates (FAAs) in Concrete: From Macroscopic Physical–Mechanical Properties to Microscopic Structure of Hydration Products
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
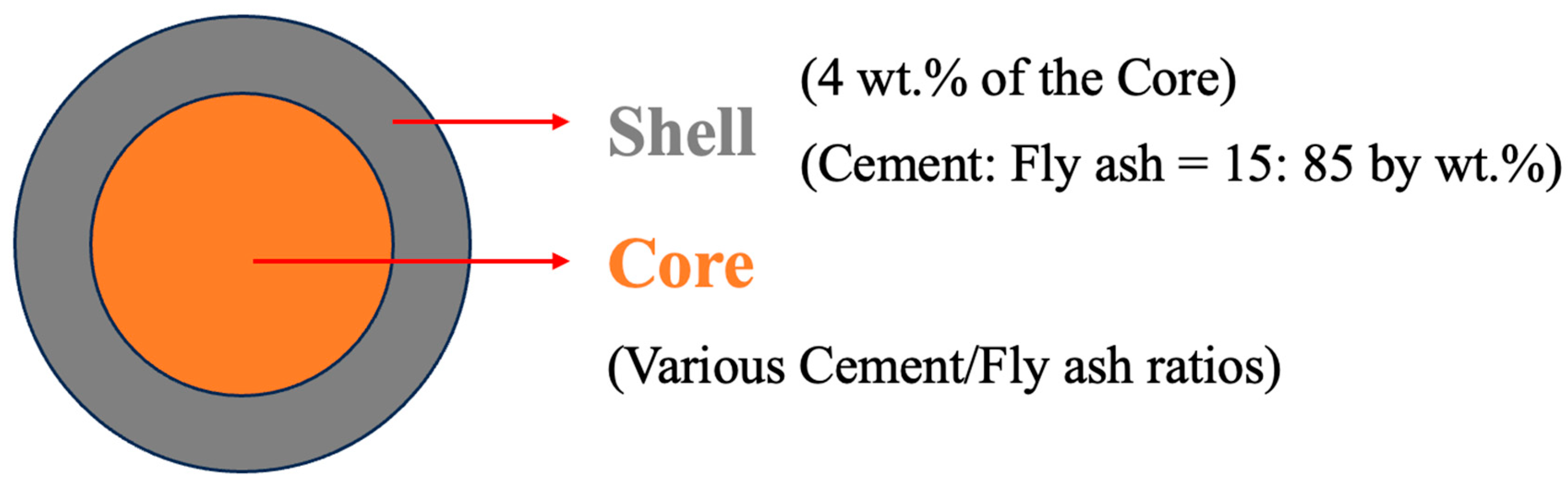
2.2. Sample Preparation
2.3. Testing
3. Results and Discussion
3.1. Macro Physico-Mechanical Properties of Fly Ash Aggregates (FAAs)
3.1.1. Water Absorption
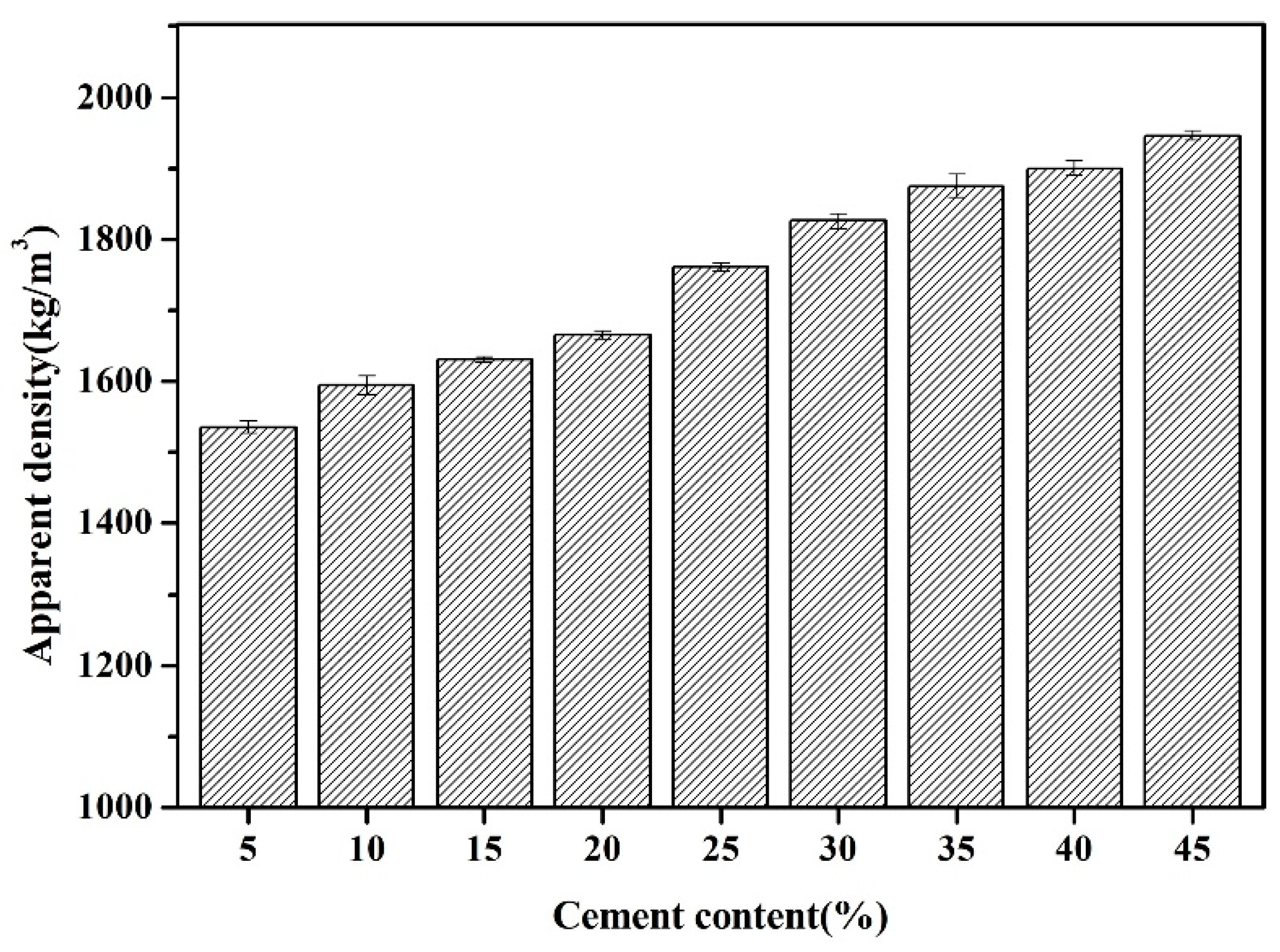
3.1.2. Apparent Density
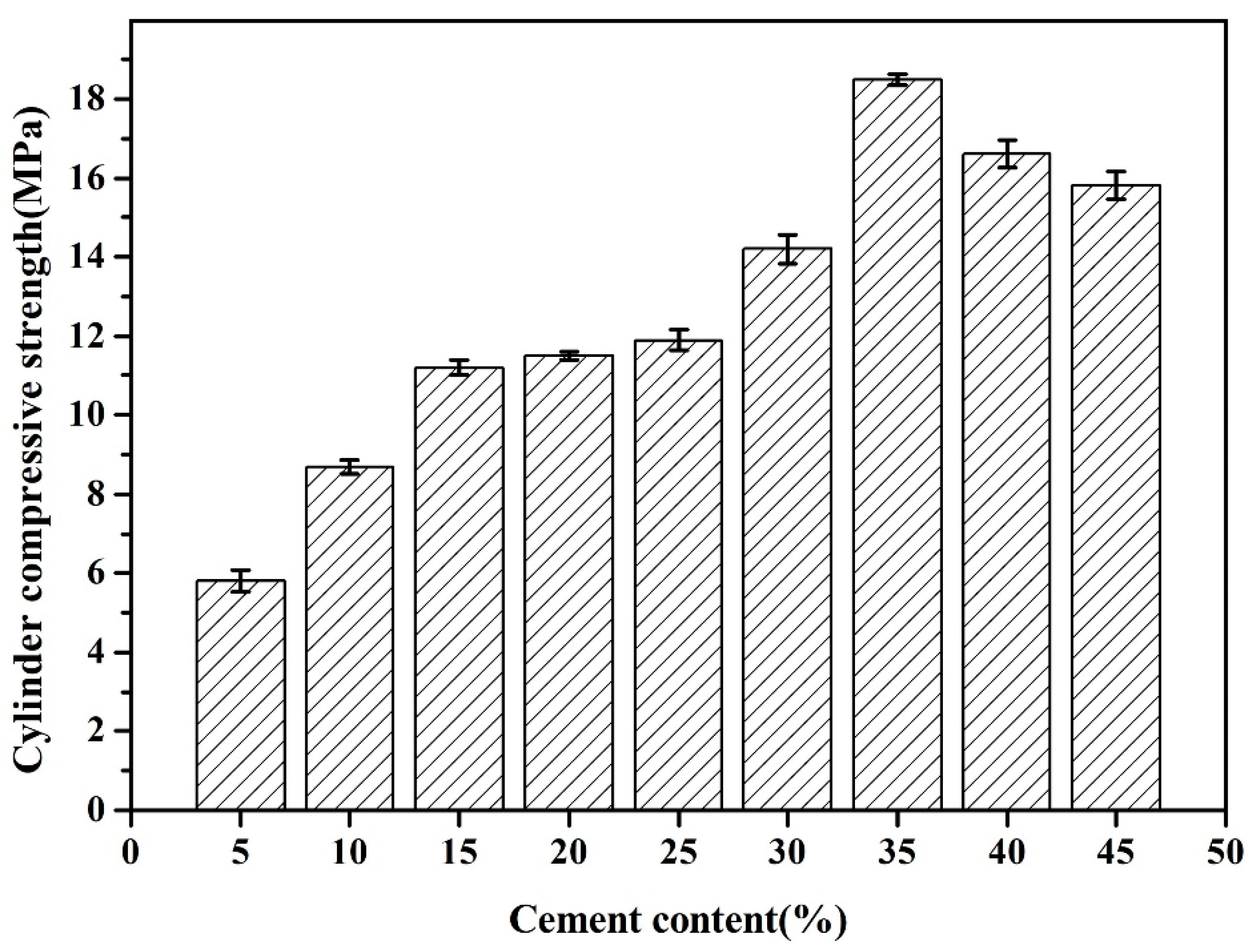
3.1.3. Cylinder Compressive Strength
3.2. Micro-Structure of Fly Ash Aggregates
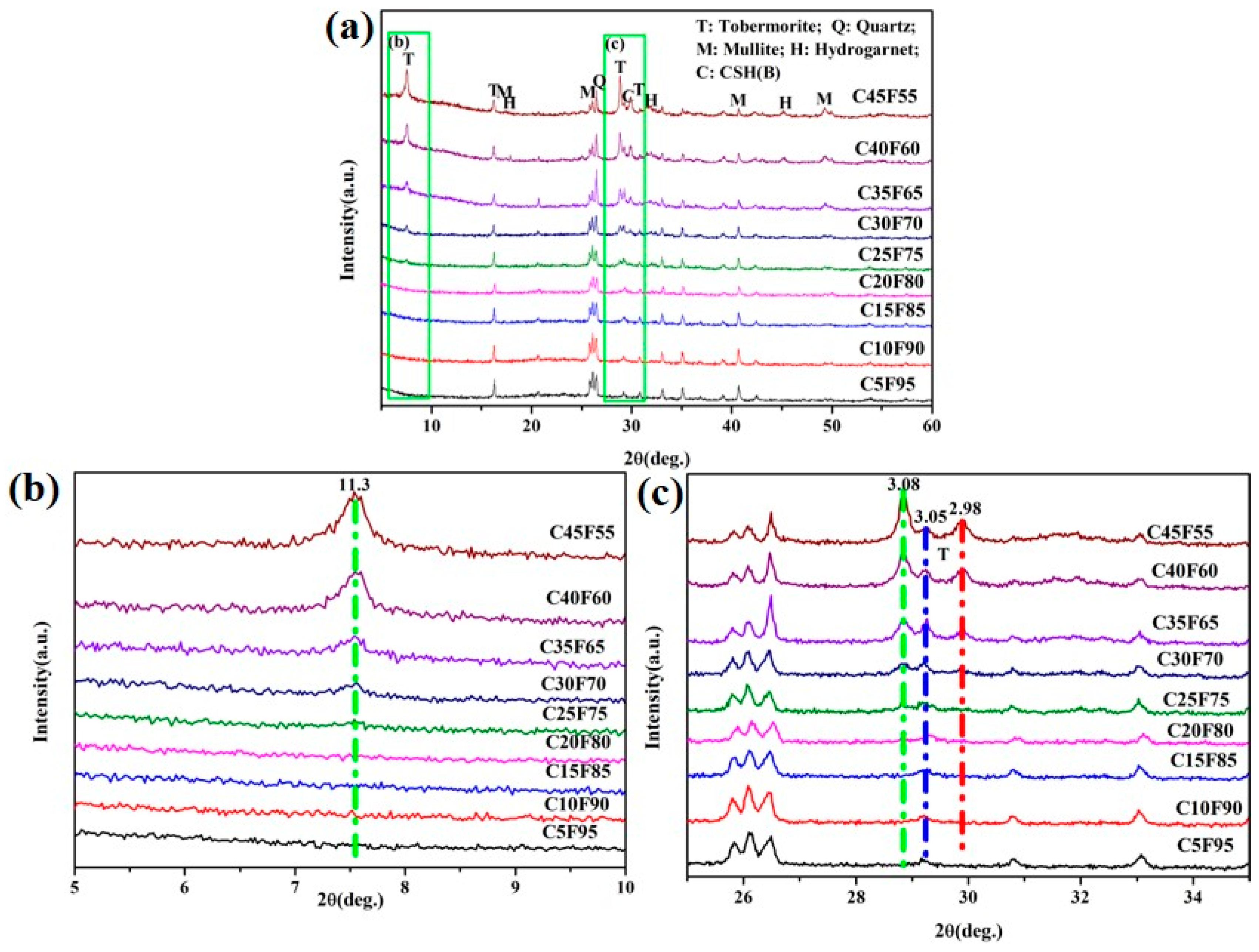
3.2.1. XRD Patterns
3.2.2. Insoluble Matter
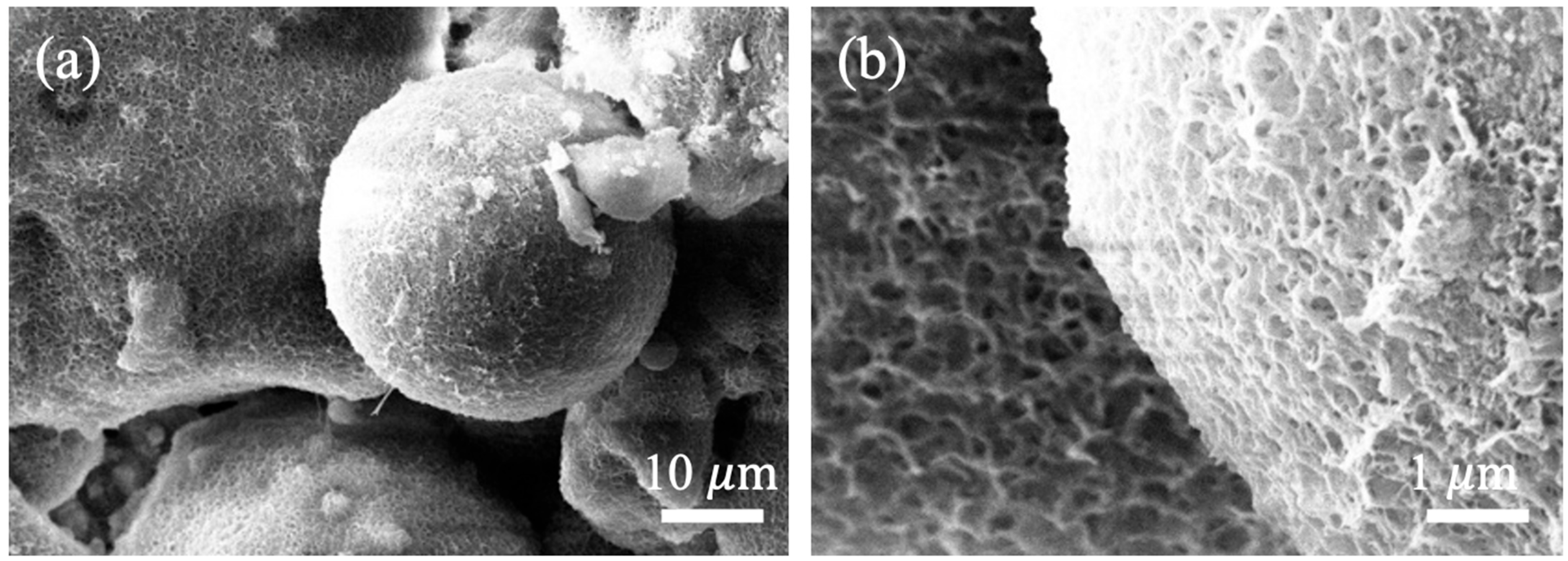
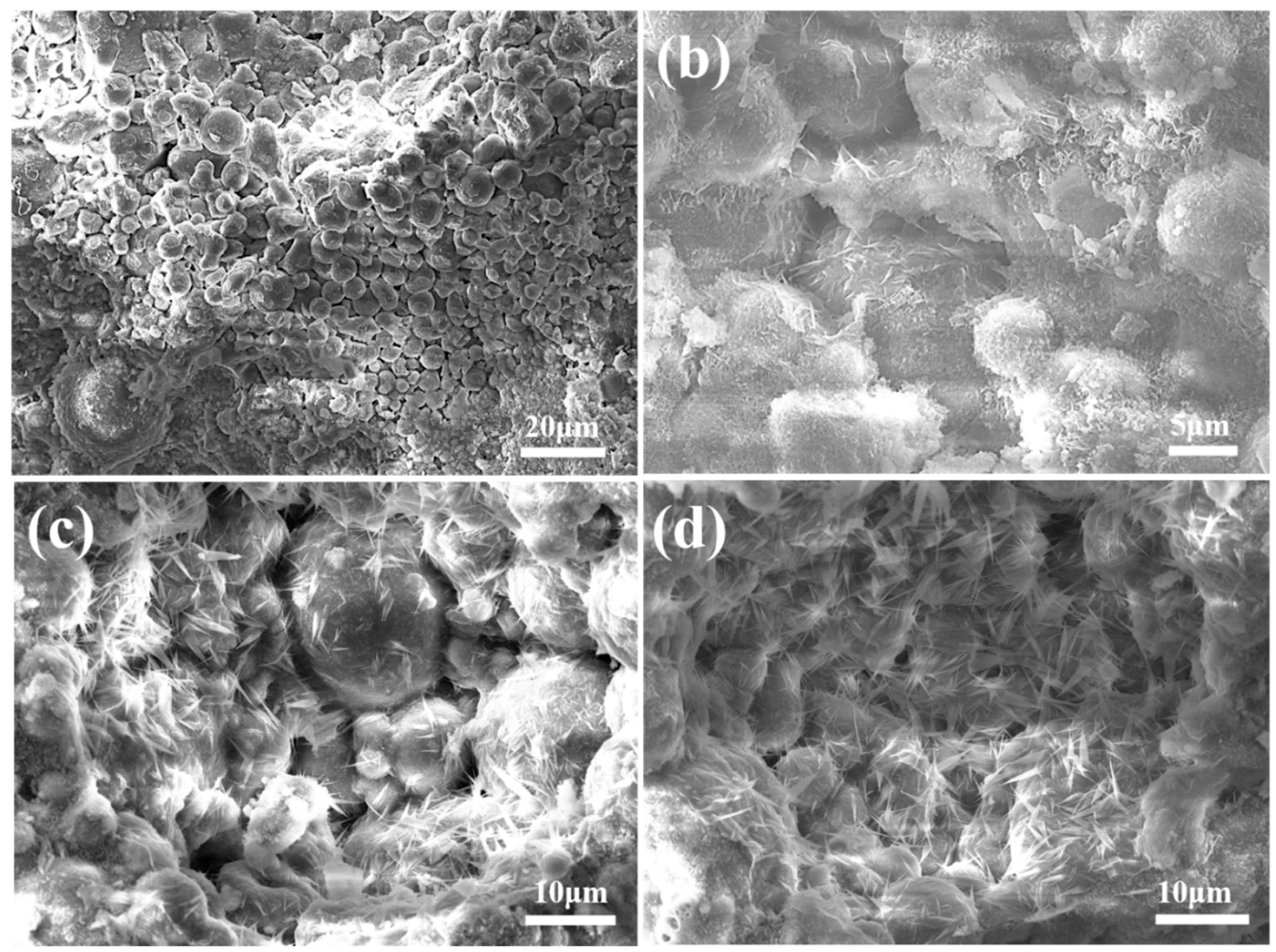
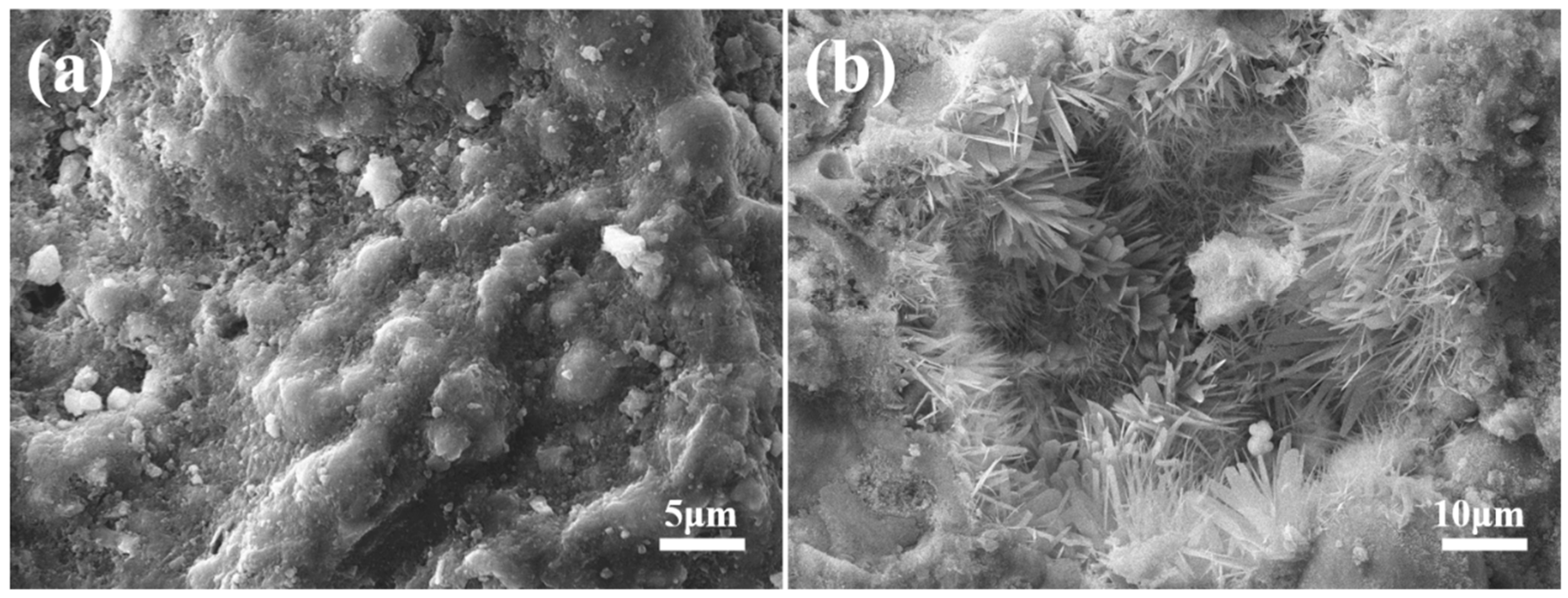
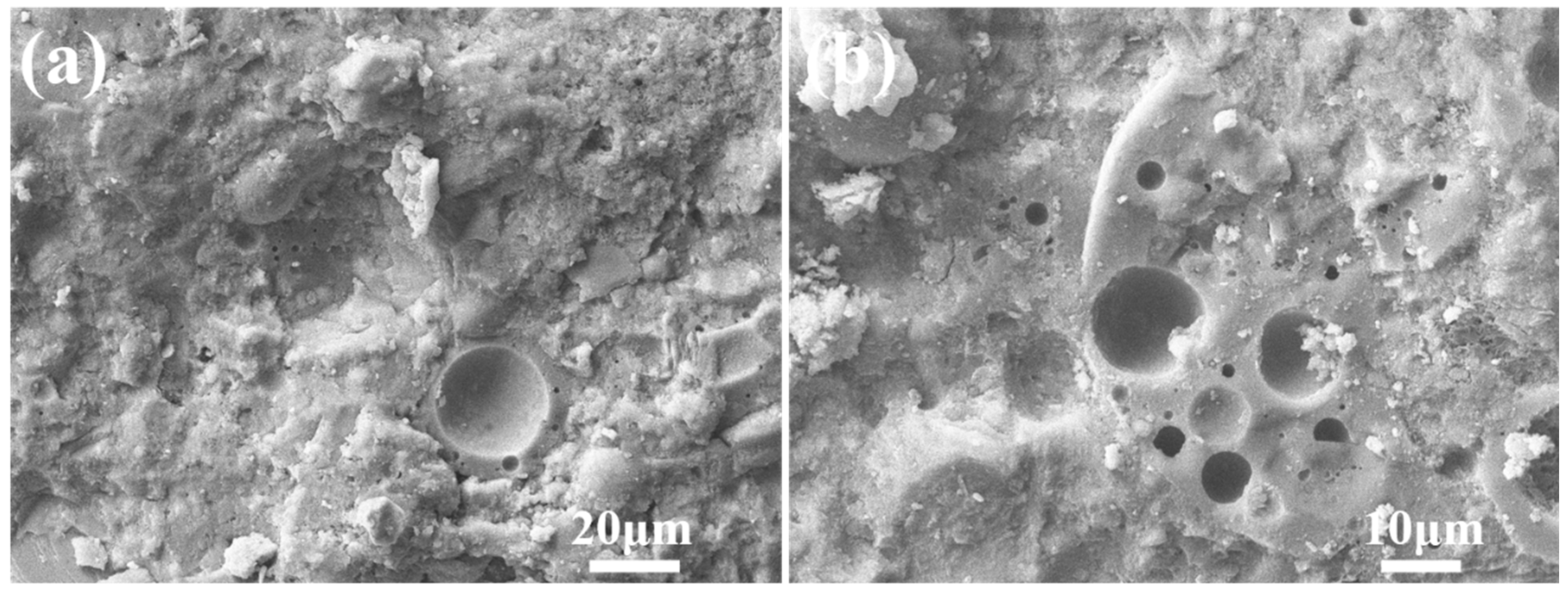
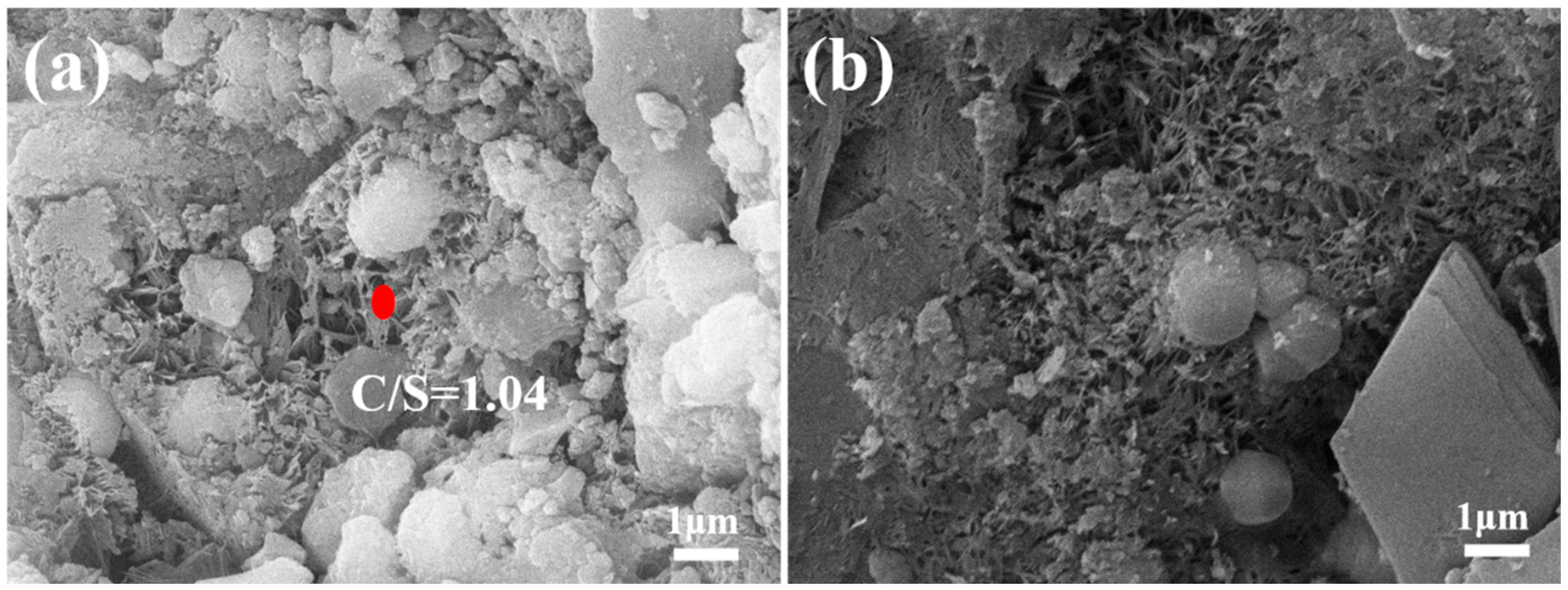
3.2.3. Microstructure of Autoclaved Aggregates
3.3. Mechanism of Strength Development in Fly Ash Aggregates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, C.-G.; Jiao, C.-J.; Zhang, X.-C.; Lin, W.-C.; Chen, X.-F. Fly Ash-Supported Photocatalysts: Synthesis, Applications, and Advances in Modification Technology. Crystals 2025, 15, 223. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Li, W.; Nie, Y.; Wang, F.; Bian, R.; Wang, H.; Wang, Y.; Gong, Z.; Lu, J.; et al. Solidification/Stabilization Pre-Treatment Coupled with Landfill Disposal of Heavy Metals in MSWI Fly Ash in China: A Systematic Review. J. Hazard. Mater. 2024, 478, 135479. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-K.; Lu, W.-C.; Chen, Y.-L.; Shen, Y.-H. Production of Autoclaved Aerated Concrete by Using Municipal Solid Waste Incinerator Fly Ash as an Alternative Raw Material. Case Stud. Constr. Mater. 2024, 21, e03914. [Google Scholar] [CrossRef]
- Chen, X.-F.; Quan, C.-Q.; Jiao, C.-J. Experimental Study of Chloride Resistance of Polypropylene Fiber Reinforced Concrete with Fly Ash and Modeling. Materials 2021, 14, 4417. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, F.; Khushnood, R.A.; Amjad, H.; Ahmad, F. Feasibility Study of Expanded Clay Aggregate Lightweight Concrete for Nonstructural Applications. Adv. Civil Eng. 2024, 2024, 8263261. [Google Scholar] [CrossRef]
- Lu, J.-X. Recent Advances in High Strength Lightweight Concrete: From Development Strategies to Practical Applications. Constr. Build. Mater. 2023, 400, 132905. [Google Scholar] [CrossRef]
- Chen, X.-F.; Jiao, C.-J. Effect of Construction Wastes on the Rheo-Physical Behavior of Photocatalytic Mortar. Case Stud. Constr. Mater. 2022, 16, e01049. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Qiao, Z.; Deng, H.; Xu, L.; Wu, K.; Yang, Z.; Tang, L. The Toxic Leaching Behavior of MSWI Fly Ash Made Green and Non-Sintered Lightweight Aggregates. Constr. Build. Mater. 2023, 373, 130809. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Yang, F.; Zhang, W.; Xu, L.; Wu, K.; Tang, L.; Yang, Z. Resourceful Utilization of Quarry Tailings in the Preparation of Non-Sintered High-Strength Lightweight Aggregates. Constr. Build. Mater. 2022, 334, 127444. [Google Scholar] [CrossRef]
- Kumar, P.; Dinakar, P.; Saravanan, T.J. Development and Mechanical Performance of Self-Compacting Lightweight Aggregate Concrete Using Sintered Fly Ash Aggregates. J. Mater. Civil Eng. 2024, 36, 4024174. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Mohammed, M.S.; Heikal, M. Ultra-Lightweight Porous Materials Fabrication and Hazardous Lead-Stabilization through Alkali-Activation/Sintering of Different Industrial Solid Wastes. J. Clean. Prod. 2020, 244, 118742. [Google Scholar] [CrossRef]
- Khater, G.A.; Nabawy, B.S.; El-Kheshen, A.A.; Abdel-Baki, M.; Farag, M.M. Utilizing of Solid Waste Materials for Producing Porous and Lightweight Ceramics. Mater. Chem. Phys. 2022, 280, 125784. [Google Scholar] [CrossRef]
- Yu, L.; Wang, S.; Qiao, Z.; Xu, L.; Wu, K.; Li, P.; Yang, Z. Effect of Curing Time and Temperature on the Mechanical Properties of Green and Ultra-High-Strength Non-Sintered Aggregate via Autoclave Technology. Constr. Build. Mater. 2023, 374, 130874. [Google Scholar] [CrossRef]
- Deng, X.; Li, J.; Du, D.; Wang, T. Manufacturing Non-Sintered Ceramsite from Dredged Sediment, Steel Slag, and Fly Ash for Lightweight Aggregate: Production and Characterization. Environ. Sci. Pollut. Res. 2024, 31, 15078–15090. [Google Scholar] [CrossRef]
- Ma, H.; Cui, C.; Li, X.; Sun, Z. Study of High Performance Autoclaved Shell-Aggregate from Propylene Oxide Sludge. Constr. Build. Mater. 2011, 25, 3030–3037. [Google Scholar] [CrossRef]
- GB/T 17431.2-2010; Test Methods for Lightweight Aggregates—Part 2: Lightweight Aggregate Tests. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2010.
- JC/T 478.2-2013; Standard Test Methods for Building Lime. Part 2: Methods for Chemical Analysis. National Standards of People’s Republic of China: Beijing, China, 2013.
- GB/T 12960-2019; Quantitative Determination of Constituents of Cement. National Standard of the People’s Republic of China: Beijing, China, 2019.
- Abdelbaset, A.M.; Katunský, D.; Zeleňáková, M.; El-Feky, M.H. Mechanical Properties Stabilization of Low Plasticity Kaolin Soil Using Fly Ash and Hydrated Lime. Case Stud. Constr. Mater. 2024, 21, e03662. [Google Scholar] [CrossRef]
- Cetin, B.; Aydilek, A.H.; Guney, Y. Stabilization of Recycled Base Materials with High Carbon Fly Ash. Resour. Conserv. Recycl. 2010, 54, 878–892. [Google Scholar] [CrossRef]
- Zhu, C.; Niu, J.; Li, J.; Wan, C.; Peng, J. Effect of Aggregate Saturation Degree on the Freeze–Thaw Resistance of High Performance Polypropylene Fiber Lightweight Aggregate Concrete. Constr. Build. Mater. 2017, 145, 367–375. [Google Scholar] [CrossRef]
- Mohammed, T.; Aguayo, F.; Nodehi, M.; Ozbakkaloglu, T. Engineering Properties of Structural Lightweight Concrete Containing Expanded Shale and Clay with High Volume Class F and Class C Fly Ash. Struct. Concr. 2023, 24, 4029–4046. [Google Scholar] [CrossRef]
- Rashad, A.M. A Short Manual on Natural Pumice as a Lightweight Aggregate. J. Build. Eng. 2019, 25, 100802. [Google Scholar] [CrossRef]
- Tanyildizi, H. The Investigation of Microstructure and Strength Properties of Lightweight Mortar Containing Mineral Admixtures Exposed to Sulfate Attack. Measurement 2016, 77, 143–154. [Google Scholar] [CrossRef]
- He, Z.; Zhu, X.; Wang, J.; Mu, M.; Wang, Y. Comparison of CO2 Emissions from OPC and Recycled Cement Production. Constr. Build. Mater. 2019, 211, 965–973. [Google Scholar] [CrossRef]
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Chen, Z.; Xu, M.; Li, H.; Sanjayan, J.G. Analysis of Theoretical Carbon Dioxide Emissions from Cement Production: Methodology and Application. J. Clean. Prod. 2022, 334, 130270. [Google Scholar] [CrossRef]
- GB/T 17431.1-2010; Lightweight Aggregate and Its Methods-Part 1: Lightweight Aggregate. National Standards of People’s Republic of China: Beijing, China, 2010.
- Yuli, W.; Hang, H.; Xiaoxing, L. Influences of Aggregate Micro Fines on the Packing of Fresh Mortar and the Performances of Mortar. Compos. B Eng. 2019, 164, 493–498. [Google Scholar] [CrossRef]


| Component | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O |
|---|---|---|---|---|---|---|
| Fly ash | 53.47 | 30.48 | 4.73 | 2.94 | 0.95 | - |
| Cement | 20.30 | 5.61 | 3.25 | 64.00 | 1.17 | - |
| Notation | Core | Shell | Thickness | |
|---|---|---|---|---|
| Cement | Fly Ash | Fly Ash/Cement | Shell/Core | |
| C5F95 | 5 | 95 | 85:15 | 4 |
| C10F90 | 10 | 90 | 85:15 | 4 |
| C15F85 | 15 | 85 | 85:15 | 4 |
| C20F80 | 20 | 80 | 85:15 | 4 |
| C25F75 | 25 | 75 | 85:15 | 4 |
| C30F70 | 30 | 70 | 85:15 | 4 |
| C35F65 | 35 | 65 | 85:15 | 4 |
| C40F60 | 40 | 60 | 85:15 | 4 |
| C45F55 | 45 | 55 | 85:15 | 4 |
| Notation | Ca/Si a | R (wt.%) | Ca/Si b |
|---|---|---|---|
| C5F95 | 0.12 | 81.88 | 0.69 |
| C10F90 | 0.19 | 72.09 | 0.69 |
| C15F85 | 0.27 | 70.24 | 0.90 |
| C20F80 | 0.35 | 62.86 | 0.93 |
| C25F75 | 0.43 | 56.37 | 0.99 |
| C30F70 | 0.52 | 49.58 | 1.04 |
| C35F65 | 0.62 | 31.56 | 0.91 |
| C40F60 | 0.73 | 25.72 | 0.98 |
| C45F55 | 0.85 | 17.96 | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-F.; Zhang, X.-C.; Peng, Y. Multi-Scale Investigation of Fly Ash Aggregates (FAAs) in Concrete: From Macroscopic Physical–Mechanical Properties to Microscopic Structure of Hydration Products. Materials 2025, 18, 2651. https://doi.org/10.3390/ma18112651
Chen X-F, Zhang X-C, Peng Y. Multi-Scale Investigation of Fly Ash Aggregates (FAAs) in Concrete: From Macroscopic Physical–Mechanical Properties to Microscopic Structure of Hydration Products. Materials. 2025; 18(11):2651. https://doi.org/10.3390/ma18112651
Chicago/Turabian StyleChen, Xue-Fei, Xiu-Cheng Zhang, and Ying Peng. 2025. "Multi-Scale Investigation of Fly Ash Aggregates (FAAs) in Concrete: From Macroscopic Physical–Mechanical Properties to Microscopic Structure of Hydration Products" Materials 18, no. 11: 2651. https://doi.org/10.3390/ma18112651
APA StyleChen, X.-F., Zhang, X.-C., & Peng, Y. (2025). Multi-Scale Investigation of Fly Ash Aggregates (FAAs) in Concrete: From Macroscopic Physical–Mechanical Properties to Microscopic Structure of Hydration Products. Materials, 18(11), 2651. https://doi.org/10.3390/ma18112651






