Geopolymer Concretes with Organic Phase Change Materials—Analysis of Thermal Properties and Microstructure
Abstract
1. Introduction
1.1. Geopolymers—Benefits and Production from Waste Materials
1.2. Organic PCMs—Properties and Use in Modifying Binders and Geopolymers
1.3. Balancing Thermal Functionality and Structural Integrity
2. Materials and Methods
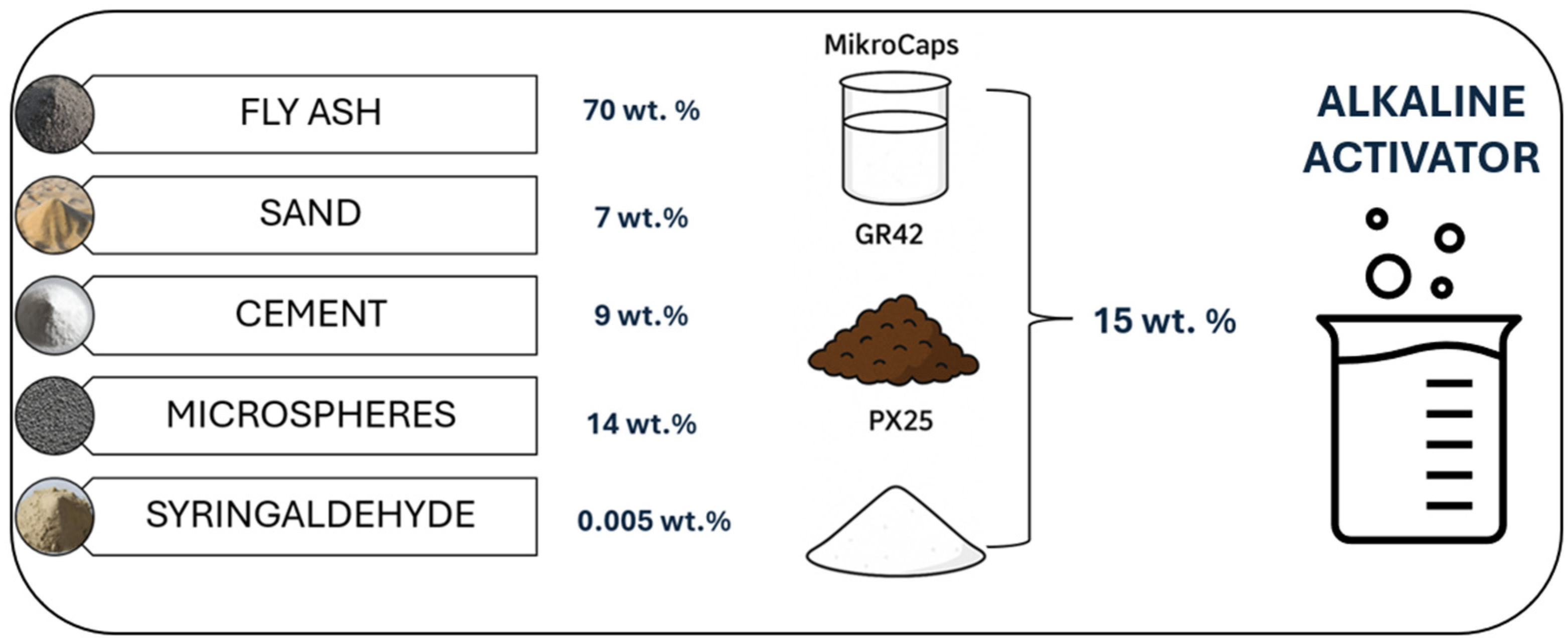
2.1. Raw Materials for the Manufacture of Geopolymer Concretes
2.2. Phase Change Materials
2.3. Preparations of Geopolymer Concretes
2.4. Density Measurements
2.5. Procedure for Water Leachability Testing
2.6. Acquiring λ, CV, Cp, and α of Geopolymer Concretes with PCMs
2.7. Microscopic Observations
3. Results
3.1. Density of Geopolymer Concretes with PCMs
3.2. Water Leachability of Geopolymer Concretes with PCMs
3.3. λ, CV, Cp, and α of Geopolymer Concretes with PCMs
3.4. Microstructure of Geopolymer Concretes with PCMs
4. Discussion
5. Conclusions
- The introduction of various types of PCMs (MikroCaps, GR42, PX25) affected the physicochemical and thermal properties of geopolymers in different ways. This observation confirms that the characteristics of a specific PCM are crucial in shaping the final parameters of the composite.
- The addition of PCM reduced the density of the composites. This phenomenon can be attributed to the modification of the rheological properties of the mixture and changes in the degree of density and distribution of solid components.
- All samples satisfied the criteria for non-hazardous waste disposal, demonstrating levels of elemental leachability significantly below acceptable standards. At the same time, the strongly alkaline reaction of the mixtures (pH > 10) facilitated polycondensation processes; however, it might also influence the mobility of certain ions, including heavy metals.
- PCMs affected thermal conductivity to varying extents. The most significant decrease in thermal conductivity was noted for MikroCaps, with a reduction of 22.2%, whereas PX25 resulted in an increase of 13.2%. This observation suggests that the thermal conductivity of the material is indirectly dependent on its microstructure and porosity, rather than being solely contingent upon the conductivity of the PCM.
- The observed increase in specific heat for certain samples (MC, PX25) suggests their potential for thermal energy storage. At the same time, the changes in specific heat by volume remained non-negligible, indicating the limited effect of PCMs on their long-term energy storage capacity per unit volume.
- The parameter of thermal diffusivity exhibited the most significant changes in the samples with MikroCaps (−20.7%) and PX25 (+12.4%). This observation leads to the conclusion that different PCMs can be utilized for both thermal insulation, characterized by slower heat transport, and dynamic thermal control, which is marked by faster conduction.
- The selection of a suitable PCM should be subject to specific functional requirements, whether the objective is to enhance thermal insulation, augment thermal capacity, or dynamically respond to fluctuations in ambient temperature. The findings of the study reveal significant potential for the incorporation of geopolymers with PCM additives in next-generation construction materials, particularly in relation to sustainability and energy efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurian, S.T.N.; Dominic, J. Experimental study on rubberized geopolymer concrete with sugarcane bagasse ash. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2024; Volume 529, p. 01027. [Google Scholar] [CrossRef]
- Ganesan, N.; Abraham, R.; Raj, S. Durability characteristics of steel fibre reinforced geopolymer concrete. Constr. Build. Mater. 2015, 93, 471–476. [Google Scholar] [CrossRef]
- Zubarev, K.; Shcherban’, E.M.; Stel’makh, S.A.; Beskopylny, A.N.; El’shaeva, D.; Chernil’nik, A.; Zakieva, N.I.; Pimenova, E.V.; Shilov, A.A. Structure and properties improvement by recipe factors of geopolymer basalt fiber reinforced concrete for building enclosing structures. Buildings 2024, 14, 743. [Google Scholar] [CrossRef]
- Aris, R.H.; Bachtiar, E.; Makbul, R. Workability dan sifat mekanik self compacting geopolimer concrete (SCGC). Civilla J. Tek. Sipil Univ. Islam Lamongan 2021, 6, 267. [Google Scholar] [CrossRef]
- Matsimbe, J.; Dinka, M.; Olukanni, D.; Musonda, I. Geopolymer: A Systematic Review of Methodologies. Materials 2022, 15, 6852. [Google Scholar] [CrossRef]
- Wong, L.S. Durability Performance of Geopolymer Concrete: A Review. Polymers 2022, 14, 868. [Google Scholar] [CrossRef] [PubMed]
- Parathi, S.; Nagarajan, P.; Pallikkara, S.A. Ecofriendly geopolymer concrete: A comprehensive review. Clean Technol. Environ. Policy 2021, 23, 1701–1713. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, X.; Wang, F.; Wang, J. Mechanical Properties and Durability of Geopolymer Recycled Aggregate Concrete: A Review. Polymers 2023, 15, 615. [Google Scholar] [CrossRef]
- Siddique, S.; Gupta, V.; Chaudhary, S.; Park, S.; Jang, J.-G. Influence of the Precursor, Molarity and Temperature on the Rheology and Structural Buildup of Alkali-Activated Materials. Materials 2021, 14, 3590. [Google Scholar] [CrossRef]
- Mohamed, O.A. Effects of the Curing Regime, Acid Exposure, Alkaline Activator Dosage, and Precursor Content on the Strength Development of Mortar with Alkali-Activated Slag and Fly Ash Binder: A Critical Review. Polymers 2023, 15, 1248. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Alkali-Activated Materials and Geopolymer: A Review of Common Precursors and Activators Addressing Circular Economy. Circ. Econ. Sust. 2022, 2, 165–196. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Nisar, K.S.; Abdel-Aty, A.-H.; Yahia, I.S. Evaluation of the Effect of Granite Waste Powder by Varying the Molarity of Activator on the Mechanical Properties of Ground Granulated Blast-Furnace Slag-Based Geopolymer Concrete. Polymers 2022, 14, 306. [Google Scholar] [CrossRef]
- Ghafoor, M.T.; Khan, Q.S.; Qazi, A.U.; Sheikh, M.N.; Hadi, M. Influence of alkaline activators on the mechanical properties of fly ash based geopolymer concrete cured at ambient temperature. Constr. Build. Mater. 2021, 273, 121752. [Google Scholar] [CrossRef]
- Farhan, K.Z.; Johari, M.A.M.; Demirboğa, R. Impact of fiber reinforcements on properties of geopolymer composites: A review. J. Build. Eng. 2021, 44, 102628. [Google Scholar] [CrossRef]
- Li, W.; Shumuye, E.D.; Tang, S.; Wang, Z.; Zerfu, K. Eco-friendly fibre reinforced geopolymer concrete: A critical review on the microstructure and long-term durability properties. Case Stud. Constr. Mater. 2022, 16, e00894. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.B. Steel fibre reinforced geopolymer concrete (SFRGC) with improved microstructure and enhanced fibre-matrix interfacial properties. Constr. Build. Mater. 2017, 139, 286–307. [Google Scholar] [CrossRef]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental impact assessment of fly ash and silica fume based geopolymer concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Imtiaz, L.; Kashif-ur-Rehman, S.; Alaloul, W.S.; Nazir, K.; Javed, M.F.; Aslam, F.; Musarat, M.A. Life Cycle Impact Assessment of Recycled Aggregate Concrete, Geopolymer Concrete, and Recycled Aggregate-Based Geopolymer Concrete. Sustainability 2021, 13, 13515. [Google Scholar] [CrossRef]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent progress in environmentally friendly geopolymers: A review. Sci. Total Environ. 2021, 762, 143166. [Google Scholar] [CrossRef]
- Shehata, N.; Mohamed, O.; Sayed, E.T.; Abdelkareem, M.A.; Olabi, A. Geopolymer concrete as green building materials: Recent applications, sustainable development and circular economy potentials. Sci. Total Environ. 2022, 836, 155577. [Google Scholar] [CrossRef]
- De Oliveira, L.B.; De Azevedo, A.R.; Marvila, M.T.; Pereira, E.C.; Fediuk, R.; Vieira, C.M.F. Durability of geopolymers with industrial waste. Case Stud. Constr. Mater. 2022, 16, e00839. [Google Scholar] [CrossRef]
- Das, D.; Gołąbiewska, A.; Rout, P.K. Geopolymer bricks: The next generation of construction materials for sustainable environment. Constr. Build. Mater. 2024, 445, 137876. [Google Scholar] [CrossRef]
- Amran, M.; Huang, S.; Debbarma, S.; Rashid, R.S. Fire resistance of geopolymer concrete: A critical review. Constr. Build. Mater. 2022, 324, 126722. [Google Scholar] [CrossRef]
- Shill, S.K.; Al-Deen, S.; Ashraf, M.; Hutchison, W. Resistance of fly ash based geopolymer mortar to both chemicals and high thermal cycles simultaneously. Constr. Build. Mater. 2020, 239, 117886. [Google Scholar] [CrossRef]
- Astuti, P. The mechanical performance evaluation of fly-ash derived geopolymer mortar for concrete patch repair. Multidiscip. Sci. J. 2024, 7, 2025161. [Google Scholar] [CrossRef]
- Waqas, R.M.; Butt, F.; Zhu, X.; Jiang, T.; Tufail, R.F. A Comprehensive Study on the Factors Affecting the Workability and Mechanical Properties of Ambient Cured Fly Ash and Slag Based Geopolymer Concrete. Appl. Sci. 2021, 11, 8722. [Google Scholar] [CrossRef]
- Kumar, G.; Mishra, S.S. Effect of GGBFS on Workability and Strength of Alkali-activated Geopolymer Concrete. Civ. Eng. J. 2021, 7, 1036–1049. [Google Scholar] [CrossRef]
- Laskar, S.M.; Talukdar, S. Preparation and tests for workability, compressive and bond strength of ultra-fine slag based geopolymer as concrete repairing agent. Constr. Build. Mater. 2017, 154, 176–190. [Google Scholar] [CrossRef]
- Salehi, S.; Khattak, J.; Saleh, F.K.; Igbojekwe, S. Investigation of mix design and properties of geopolymers for application as wellbore cement. J. Pet. Sci. Eng. 2019, 178, 133–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wu, Z.; Lu, Y.; Kang, A.; Xiao, P. Optimal design of geopolymer grouting material for semi-flexible pavement based on response surface methodology. Constr. Build. Mater. 2021, 306, 124779. [Google Scholar] [CrossRef]
- Oderji, S.Y.; Chen, B.; Shakya, C.; Ahmad, M.R.; Shah, S.F.A. Influence of superplasticizers and retarders on the workability and strength of one-part alkali-activated fly ash/slag binders cured at room temperature. Constr. Build. Mater. 2019, 229, 116891. [Google Scholar] [CrossRef]
- Kotsay, G.; Grabowski, P. Properties of geopolymers based on metakaolin and soda-lime waste glass. Materials 2023, 16, 5392. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Tsai, Y.; Lin, Y.; Ho, P.; Kuo, C. Experimental and numerical investigation of the mechanical properties of a fiber-reinforced geopolymer mortar blast resistant panel. Polymers 2023, 15, 3440. [Google Scholar] [CrossRef]
- Azimi, E.A.; Abdullah, M.M.A.B.; Ming, L.Y.; Yong, H.C.; Hussin, K.; Aziz, I.H. Review of dolomite as precursor of geopolymer materials. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016; Volume 78, p. 01090. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Vimonsatit, V. Compressive strength of fly-ash-based geopolymer concrete at elevated temperatures. Fire Mater. 2014, 39, 174–188. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Hanafi, M.; Merkl, J.; Lacaillerie, J.d.d. Evolution of the microstructure of unconsolidated geopolymers by thermoporometry. J. Am. Ceram. Soc. 2020, 104, 1581–1591. [Google Scholar] [CrossRef]
- Sarmin, S.N. The influence of different wood aggregates on the properties of geopolymer composites. Key Eng. Mater. 2016, 723, 74–79. [Google Scholar] [CrossRef]
- Ou, Z.W.; Zhou, S.Y.; Xu, C.X.; Zhang, Y.; Yang, J.C.; Li, Y.X. Research and application of geopolymer cementitious material progress and review. Appl. Mech. Mater. 2013, 405–408, 2903–2911. [Google Scholar] [CrossRef]
- Luna-Galiano, Y.; Fernández-Pereira, C.; Vale, J. Stabilization/solidification of a municipal solid waste incineration residue using fly ash-based geopolymers. J. Hazard. Mater. 2011, 185, 373–381. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Pandiyan, T.; Elavenil, S. Development of sustainable geopolymer concrete by synergistic utilization of jarosite and GGBS. Mater. Res. Express 2024, 11, 115508. [Google Scholar] [CrossRef]
- Mehta, G.; Garg, A.; Sharma, S.K. Self-compacting geopolymer concrete using class f fly ash. IOP Conf. Ser. Earth Environ. Sci. 2024, 1327, 012005. [Google Scholar] [CrossRef]
- Liyana, J.; Abdullah, M.M.A.B.; Kamarudin, H.; Ruzaidi, C.; Rashid, A.A. Effect of fly ash/alkaline activator ratio and sodium silicate/naoh ratio on fly ash geopolymer coating strength. Key Eng. Mater. 2013, 594–595, 146–150. [Google Scholar] [CrossRef]
- Baltazar, L.G.; Henriques, F.; Temporão, D.; Cidade, M.T. Experimental assessment of geopolymer grouts for stone masonry strengthening. Key Eng. Mater. 2019, 817, 507–513. [Google Scholar] [CrossRef]
- Saukani, M.; Lisdawati, A.N.; Irawan, H.; Iqbal, R.M.; Nurjaya, D.M.; Astutiningsih, S. Effect of Nano-Zirconia Addition on Mechanical Properties of Metakaolin-Based Geopolymer. J. Compos. Sci. 2022, 6, 293. [Google Scholar] [CrossRef]
- Bērziņš, A.; Morozovs, A.; Groß, U.; Iejavs, J. Mechanical properties of wood-geopolymer composite. Eng. Rural Dev. 2017, 16, 1167–1173. [Google Scholar] [CrossRef]
- Peng, K.; Wang, H.; Wan, P.; Wang, J.; Luo, H.; Zhou, S.; Li, X.; Yang, J. Graphene modified montmorillonite based phase change material for thermal energy storage with enhanced interfacial thermal transfer. Chem. Sel. 2020, 5, 6040–6047. [Google Scholar] [CrossRef]
- Han, G.G.D.; Li, H.; Grossman, J.C. Optically-controlled long-term storage and release of thermal energy in phase-change materials. Nat. Commun. 2017, 8, 1446. [Google Scholar] [CrossRef]
- Singh, S.P.; Bhat, V. Applications of organic phase change materials for thermal comfort in buildings. Rev. Chem. Eng. 2014, 30, 521–538. [Google Scholar] [CrossRef]
- Peng, K.; Zhang, J.; Yang, H.; Ouyang, J. Acid-hybridized expanded perlite as a composite phase-change material in wallboards. RSC Adv. 2015, 5, 66134–66140. [Google Scholar] [CrossRef]
- Cui, Y.; Xie, J.; Liu, J.; Wang, J.; Chen, S. A review on phase change material application in building. Adv. Mech. Eng. 2017, 9, 168781401770082. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Qiao, Y.; Liu, J.; Wang, M. Building envelope with phase change materials. In Zero and Net Zero Energy; IntechOpen Limited: London, UK, 2019. [Google Scholar] [CrossRef]
- Li, Y.; Sang, G.; Shi, H. Potential applications of phase-change materials (PCM) in building energy efficiency. J. New Mater. Electrochem. Syst. 2014, 17, 123–127. [Google Scholar] [CrossRef]
- Felix, P.G.; Velavan, R.; Kannan, K. Investigations on phase change materials for thermal energy storage (TES) system for low-temperature steam applications. Int. Res. J. Multidiscip. Technovation 2020, 2, 9–20. [Google Scholar] [CrossRef]
- Liu, Z.; Zou, R.; Lin, Z.; Gui, X.; Chen, R.; Lin, J.; Shang, Y.; Cao, A. Tailoring carbon nanotube density for modulating electro-to-heat conversion in phase change composites. Nano Lett. 2013, 13, 4028–4035. [Google Scholar] [CrossRef]
- Guo, C.X. Application study of nanoparticle-enhanced phase change material in ceiling board. Adv. Mater. Res. 2010, 150–151, 723–726. [Google Scholar] [CrossRef]
- Fořt, J.; Trník, A.; Pavlík, Z. Influence of the heating and cooling rate on thermal performance of cement-lime plaster with PCM admixture. Key Eng. Mater. 2016, 677, 150–154. [Google Scholar] [CrossRef]
- Asadi, I.; Baghban, M.H.; Hashemi, M.; Izadyar, N.; Sajadi, B. Phase change materials incorporated into geopolymer concrete for enhancing energy efficiency and sustainability of buildings: A review. Case Stud. Constr. Mater. 2022, 17, e01162. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Pasupathy, K.; Sanjayan, J. Synthesis and properties of thermally enhanced aerated geopolymer concrete using form-stable phase change composite. J. Build. Eng. 2021, 40, 102756. [Google Scholar] [CrossRef]
- Hassan, A.; Rashid, Y.; Mourad, A.-H.I.; Ismail, N.; Laghari, M.S. Thermal and Structural Characterization of Geopolymer-Coated Polyurethane Foam—Phase Change Material Capsules/Geopolymer Concrete Composites. Materials 2019, 12, 796. [Google Scholar] [CrossRef]
- Hassan, A.; Mourad, A.I.; Rashid, Y.; Ismail, N.; Laghari, M.S. Thermal and structural performance of geopolymer concrete containing phase change material encapsulated in expanded clay. Energy Build. 2019, 191, 72–81. [Google Scholar] [CrossRef]
- Kathirvel, P.; Thangavelu, M.; Gopalan, R.; Mohan, K.S.R. Bond characteristics of reinforcing steel embedded in geopolymer concrete. IOP Conf. Ser. Earth Environ. Sci. 2017, 80, 012001. [Google Scholar] [CrossRef]
- Rathinavel, N.; Aleem, A.; Murugesan, A. Unveiling the promise of phase change materials in geopolymer composites. In Next Generation Materials for Sustainable Engineering; IGI Global Scientific Publishing: New York, NY, USA, 2024; pp. 11–39. [Google Scholar] [CrossRef]
- Łach, M.; Pławecka, K.; Bąk, A.; Adamczyk, M.; Bazan, P.; Kozub, B.; Korniejenko, K.; Lin, W. Review of solutions for the use of phase change materials in geopolymers. Materials 2021, 14, 6044. [Google Scholar] [CrossRef] [PubMed]
- Cao, V.D.; Bui, T.Q.; Kjøniksen, A. Thermal analysis of multi-layer walls containing geopolymer concrete and phase change materials for building applications. Energy 2019, 186, 115792. [Google Scholar] [CrossRef]
- Pilehvar, S.; Cao, V.D.; Szczotok, A.M.; Valentini, L.; Salvioni, D.; Magistri, M.; Pamies, R.; Kjøniksen, A. Mechanical properties and microscale changes of geopolymer concrete and Portland cement concrete containing micro-encapsulated phase change materials. Cem. Concr. Res. 2017, 100, 341–349. [Google Scholar] [CrossRef]
- Pilehvar, S.; Szczotok, A.M.; Rodríguez, J.F.; Valentini, L.; Lanzón, M.; Pamies, R.; Kjøniksen, A. Effect of freeze-thaw cycles on the mechanical behavior of geopolymer concrete and Portland cement concrete containing micro-encapsulated phase change materials. Constr. Build. Mater. 2019, 200, 94–103. [Google Scholar] [CrossRef]
- Pilehvar, S.; Cao, V.D.; Szczotok, A.M.; Carmona, M.; Valentini, L.; Lanzón, M.; Pamies, R.; Kjøniksen, A. Physical and mechanical properties of fly ash and slag geopolymer concrete containing different types of micro-encapsulated phase change materials. Constr. Build. Mater. 2018, 173, 28–39. [Google Scholar] [CrossRef]
- Marczyk, J.; Przybek, A.; Setlak, K.; Bazan, P.; Łach, M. Energy-efficient insulating geopolymer foams with the addition of phase change materials. ACS Omega 2025, 10, 2488–2500. [Google Scholar] [CrossRef]
- Przybek, A.; Łach, M.; Bogucki, R.; Ciemnicka, J.; Prałat, K.; Koper, A.; Korniejenko, K.; Masłoń, A. Energy-Efficient Geo-polymer Composites Containing Phase-Change Materials—Comparison of Different Contents and Types. Materials 2024, 17, 4712. [Google Scholar] [CrossRef]
- Rangan, P.R. Effect of curing time and liquid alkaline activators on compressive strength development of fly ash and bamboo leaf ash-based geopolymer concrete. Int. J. GEOMATE 2024, 27, 112–119. [Google Scholar] [CrossRef]
- Darmawan, M.S. Comparative study of flexural performance of geopolymer and portland cement concrete beam using finite element analysis. Int. J. GEOMATE 2022, 23, 1–9. [Google Scholar] [CrossRef]
- Kastiukas, G.; Zhou, X.; Castro-Gomes, J. Development and optimisation of phase change material-impregnated lightweight aggregates for geopolymer composites made from aluminosilicate rich mud and milled glass powder. Constr. Build. Mater. 2016, 110, 201–210. [Google Scholar] [CrossRef]
- Hassan, A.; Ismail, N.; Mourad, A.I.; Rashid, Y.; Laghari, M.S. Preparation and characterization of expanded clay-paraffin wax-geo-polymer composite material. Materials 2018, 11, 2191. [Google Scholar] [CrossRef] [PubMed]
- Gençel, O.; Harja, M.; Sarı, A.; Hekimoğlu, G.; Ustaoğlu, A.; Sütçü, M.; Erdogmus, E.; Kaplan, G.; Bayraktar, O.Y. Development, characterization, and performance analysis of shape-stabilized phase change material included-geopolymer for passive thermal management of buildings. Int. J. Energy Res. 2022, 46, 21841–21855. [Google Scholar] [CrossRef]
- Moustapha, B.E.; Bonnet, S.; Khelidj, A.; Maranzana, N.; Frœlich, D.; Khalifa, A.; Babah, I.A. Effects of microencapsulated phase change materials on chloride ion transport properties of geopolymers incorporating slag and, metakaolin, and cement-based mortars. J. Build. Eng. 2023, 74, 106887. [Google Scholar] [CrossRef]
- Okeyinka, O.M.; Oloke, D.; Adebisi, W.A.; Ayininuola, G.M. Investigation into the applicability of brewery sludge residue-ash as a base material for geopolymer concrete. Constr. Build. Mater. 2019, 223, 28–32. [Google Scholar] [CrossRef]
- Yoo, Y.; Martinez, C.J.; Youngblood, J.P. Synthesis and characterization of microencapsulated phase change materials with poly(urea−urethane) shells containing cellulose nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 31763–31776. [Google Scholar] [CrossRef]
- Kong, S.Y.; Yang, X.; Paul, S.C.; Wong, L.S.; Šavija, B. Thermal response of mortar panels with different forms of macro-encapsulated phase change materials: A finite element study. Energies 2019, 12, 2636. [Google Scholar] [CrossRef]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Mahlia, T.; Metselaar, H.S.C. Fabrication and performances of microencapsulated palmitic acid with enhanced thermal properties. Energy Fuels 2015, 29, 1010–1018. [Google Scholar] [CrossRef]
- Yadav, A.; Pandey, A.; Samykano, M.; Kalidasan, B.; Said, Z. A review of organic phase change materials and their adaptation for thermal energy storage. Int. Mater. Rev. 2024, 69, 380–446. [Google Scholar] [CrossRef]
- Skurkytė-Papievienė, V.; Abraitienė, A.; Sankauskaitė, A.; Rubežienė, V.; Baltušnikaitė-Guzaitienė, J. Enhancement of the thermal performance of the paraffin-based microcapsules intended for textile applications. Polymers 2021, 13, 1120. [Google Scholar] [CrossRef]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Duyen, N.B.; Le, H.T.; Carmona, M.; Rodriguez, J.F.; Kjøniksen, A.-L. Influence of microcapsule size and shell polarity on the time-dependent viscosity of geopolymer paste. Ind. Eng. Chem. Res. 2018, 57, 9457–9464. [Google Scholar] [CrossRef]
- Gunasekara, C.; Law, D.W.; Setunge, S. Long term engineering properties of fly ash geopolymer concrete. Sustain. Constr. Mater. Technol. (SCMT) 2016, 1, 85–94. [Google Scholar] [CrossRef]
- El-Dieb, A.S. Cementless concrete for sustainable construction. MOJ Civ. Eng. 2016, 1, 32. [Google Scholar] [CrossRef]
- Przybek, A.; Łach, M. Insulating Innovative Geopolymer Foams with Natural Fibers and Phase-Change Materials—A Review of Solutions and Research Results. Materials 2024, 17, 4503. [Google Scholar] [CrossRef]
- Bąk, A.; Pławecka, K.; Bazan, P.; Łach, M. Influence of the addition of phase change materials on thermal insulation properties of foamed geopolymer structures based on fly ash. Energy 2023, 278, 127624. [Google Scholar] [CrossRef]
- MikroCaps. Available online: https://www.mikrocaps.com/ (accessed on 15 April 2025).
- Rubitherm. Available online: https://www.rubitherm.eu/en/index.php/productcategory/materialkombinationen-gebundene-pcm-gr-px (accessed on 15 April 2025).
- Huang, X.; Zhu, C.; Lin, Y.; Fang, G. Thermal properties and applications of microencapsulated PCM for thermal energy storage: A review. Appl. Therm. Eng. 2019, 147, 841–855. [Google Scholar] [CrossRef]
- Al-Absi, Z.A.; Hafizal, M.I.M.; Ismail, M.; Awang, H.; Al-Shwaiter, A. Properties of PCM-based composites developed for the exterior finishes of building walls. Case Stud. Constr. Mater. 2022, 16, e00960. [Google Scholar] [CrossRef]
- Cárdenas-Ramírez, C.; Jaramillo, F.; Gómez, M. Systematic review of encapsulation and shape-stabilization of phase change material. J. Energy Storage 2020, 30, 101495. [Google Scholar] [CrossRef]
- Sukontasukkul, P.; Nontiyutsirikul, N.; Songpiriyakij, S.; Sakai, K.; Chindaprasirt, P. Use of phase change material to improve thermal properties of lightweight geopolymer panel. Mater. Struct. 2016, 49, 4637–4645. [Google Scholar] [CrossRef]
- Wang, Z.; Su, H.; Zhao, S.; Zhao, N. Influence of phase change material on mechanical and thermal properties of clay geopolymer mortar. Constr. Build. Mater. 2016, 120, 329–334. [Google Scholar] [CrossRef]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Rodriguez, J.F.; Carmona, M.; Al-Manasir, N.; Kjøniksen, A. Microencapsulated phase change materials for enhancing the thermal performance of Portland cement concrete and geopolymer concrete for passive building applications. Energy Convers. Manag. 2017, 133, 56–66. [Google Scholar] [CrossRef]
- Kheradmand, M.; Pacheco-Torgal, F.; Azenha, M. Thermal performance of resource-efficient geopolymeric mortars containing phase change materials. Open Constr. Build. Technol. J. 2018, 12, 217–233. [Google Scholar] [CrossRef]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Valentini, L.; Carmona, M.; Rodriguez, J.F.; Kjøniksen, A. Influence of microcapsule size and shell polarity on thermal and mechanical properties of thermoregulating geopolymer concrete for passive building applications. Energy Convers. Manag. 2018, 164, 198–209. [Google Scholar] [CrossRef]
- Kheradmand, M.; Abdollahnejad, Z.; Pacheco-Torgal, F. Alkali-activated cement-based binder mortars containing phase change materials (PCMs): Mechanical properties and cost analysis. Eur. J. Environ. Civ. Eng. 2018, 24, 1068–1090. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bùi, T.H.; Pham, V.T.H.Q.; Quang, M.; Hoang, M.D.; Le, V.Q. Leaching behavior and immobilization of heavy metals in geopolymer synthesized from red mud and fly ash. Key Eng. Mater. 2018, 777, 518–522. [Google Scholar] [CrossRef]
- Mashifana, T.; Sillanpää, M. The durability and leaching behavior of granulated blast furnace slag, fly ash, and waste foundry sand geopolymers. Key Eng. Mater. 2022, 922, 153–161. [Google Scholar] [CrossRef]
- Kirilovs, E.; Zotova, I.; Kukle, S.; Pugovics, K. Low density hemp shive particleboards for latent thermal energy storage performance. J. Energy Syst. 2021, 5, 1–9. [Google Scholar] [CrossRef]
- Kirilovs, E.; Zotova, I.; Gendelis, E.; Jörg-Gusovius, H.; Kukle, S.; Stramkale, V. Experimental Study of Using Micro-Encapsulated Phase-Change Material Integrated into Hemp Shive Wallboard. Buildings 2020, 10, 228. [Google Scholar] [CrossRef]
- Zhang, Y. Modified computational methods using effective heat capacity model for the thermal evaluation of PCM outfitted walls. Inter. Comm. Heat Mass Trans. 2019, 108, 104278. [Google Scholar] [CrossRef]
- Khattari, Y.; El Rhafiki, T.; Choab, N.; Kousksou, T.; Alaphilippe, M.; Zeraouli, Y. Apparent heat capacity method to investigate heat transfer in a composite phase change material. J. Energy Storage 2020, 28, 101239. [Google Scholar] [CrossRef]
- Nguyen, H.T. Synthesis and characteristics of inorganic polymer materials geopolymerized from ash of brickyard. Mater. Sci. Forum 2019, 961, 45–50. [Google Scholar] [CrossRef]
- Muraleedharan, M.; Nadir, Y. Geopolymer mortar integrated with phase change materials for improvement of thermal efficiency in buildings: A review. Mater. Today Proc. 2021, 44, 878–885. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, K.; Wang, S.; Wang, Z.; Yang, Z.; Shumuye, E.D.; Gong, X. Effect of elevated temperature on mechanical properties of high-volume fly ash-based geopolymer concrete, mortar and paste cured at room temperature. Polymers 2021, 13, 1473. [Google Scholar] [CrossRef] [PubMed]
- Sarker, P.K.; McBeath, S. Fire endurance of steel reinforced fly ash geopolymer concrete elements. Constr. Build. Mater. 2015, 90, 91–98. [Google Scholar] [CrossRef]


| Precursor | Oxide Composition (wt.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | K2O | TiO2 | SO3 | Br | CuO | OsO4 | |
| Fly ash | 59.21 | 31.05 | 3.88 | 2.29 | 2.07 | 0.77 | 0.50 | - | - | - |
| Sand | 98.57 | - | 0.30 | 0.36 | 0.42 | - | 0.24 | - | - | - |
| Cement | - | 82.85 | - | 17.09 | - | - | - | - | - | - |
| Microspheres | 55.38 | 38.22 | 2.44 | 0.67 | 2.15 | 0.85 | 0.12 | - | - | - |
| Material | D10 [mm] | D50 [mm] | D90 [mm] | Average Value [mm] | Standard Deviation [mm] |
|---|---|---|---|---|---|
| Fly ash | 2.45 | 12.87 | 32.12 | 16.21 | 0.03 |
| Sand | 253.72 | 341.97 | 472.38 | 390.65 | 0.02 |
| Cement | 1.62 | 11.39 | 29.90 | 14.70 | 0.01 |
| Microspheres | 18.93 | 53.39 | 88.89 | 56.39 | 0.07 |
| Syringaldehyde | 3.04 | 3.78 | 7.24 | 5.07 | 0.21 |
| Material | D10 [mm] | D50 [mm] | D90 [mm] | Average Value [mm] | Standard Deviation [mm] |
|---|---|---|---|---|---|
| MikroCaps | 2.23 | 5.85 | 9.48 | 6.25 | 0.03 |
| GR42 | 34.21 | 104.73 | 138.49 | 103.10 | 0.05 |
| PX25 | 3.06 | 10.19 | 21.64 | 11.99 | 0.04 |
| Designation of Samples | F.A.—ref. | 15 wt.% MC | 15 wt.% GR42 | 15 wt.% PX25 |
|---|---|---|---|---|
| ρb1 [kg/m3] | 1832.28 | 1601.60 | 1780.30 | 1671.55 |
| ρb2 [kg/m3] | 1834.88 | 1602.65 | 1785.30 | 1675.90 |
| Permissible Leaching Limits * | F.A. —ref. | 15 wt.% MC | |||
|---|---|---|---|---|---|
| Liquid/Solid Phase = 10 L/kg [mg/kg Dry Weight] Baseline Test | mg/kg | mg/kg | |||
| Component | Criteria for Allowing Inert Waste to Be Deposited in an Inert Waste Landfill | Criteria for Allowing Hazardous Waste to Be Disposed of in a Hazardous Waste Landfill | Criteria for Allowing Non-Hazardous and Inert Waste to Be Deposited in a Landfill for Non-Hazardous and Inert Waste | ||
| Arsen (As) | 0.5 | 25 | 2 | 5.5 | 3.3 |
| Bar (Ba) | 20 | 300 | 100 | 0.25 | 0.53 |
| Cadmium (Cd) | 0.04 | 5 | 1 | <0.0020 | <0.0020 |
| Total chromium (Cr) | 0.5 | 70 | 10 | 0.087 | 0.083 |
| Copper (Cu) | 2 | 100 | 50 | 0.22 | 0.37 |
| Mercury (Hg) | 0.01 | 2 | 0.2 | <0.010 | <0.010 |
| Molybdenum (Mo) | 0.5 | 30 | 10 | 3.0 | 1.5 |
| Nickel (Ni) | 0.4 | 40 | 10 | 0.028 | 0.028 |
| Lead (Pb) | 0.5 | 50 | 10 | <0.020 | 0.020 |
| Antimony (Sb) | 0.06 | 5 | 0.7 | <0.020 | <0.020 |
| Selen (Se) | 0.1 | 7 | 0.5 | 0.55 | 0.91 |
| Zinc (Zn) | 4 | 200 | 50 | 0.079 | 0.17 |
| Chlorides (Cl−) | 800 | 25,000 | 15,000 | 130 | 130 |
| Fluorides (F−) | 10 | 500 | 150 | 21 | 22 |
| Sulfates (SO42−) | 1000 | 50,000 | 20,000 | 2800 | 2900 |
| Dissolved organic carbon (DOC) | 500 | 1000 | 800 | 80 | 1000 |
| Dissolved solids (TDS **) | 4000 | 100,000 | 60,000 | 44,000 | 32,000 |
| Chromium (VI) (Cr6+) | 22 | 35 | |||
| pH | 10.6 | 10.6 | |||
| Statistical Parameters | Thermal Properties of Sample F.A.—ref. | |||
|---|---|---|---|---|
| λ [W/(m × K)] | Cv [MJ/(m3 × K)] | Cp [J/(kg × K)] | α [mm2/s] | |
| Quartile, Q1 | 1.1582 | 1.8120 | 987.9 | 0.6379 |
| Median, M | 1.1616 | 1.8145 | 989.3 | 0.6403 |
| Quartile, Q3 | 1.1646 | 1.8170 | 990.7 | 0.6422 |
| Interquartile range, IQR = (Q3 − Q1) | 0.0065 | 0.0050 | 2.7 | 0.0043 |
| Higher outlier, HO = Q3 + 1.5·IQR | 1.1743 | 1.8245 | 994.7 | 0.6486 |
| Lower outlier, LO = Q1 − 1.5·IQR | 1.1485 | 1.8046 | 983.9 | 0.6314 |
| Average value, | 1.1619 | 1.8141 | 989.1 | 0.6403 |
| Standard deviation, s | 0.0047 | 0.0036 | 2.0 | 0.0027 |
| Coefficient of variation, CV [%] | 0.40 | 0.16 | 0.20 | 0.42 |
| Upper critical value, UCV | 1.1671 | 1.8179 | 991.2 | 0.6431 |
| Lower critical value, LCV | 1.1566 | 1.8103 | 987.1 | 0.6375 |
| Statistical parameters | Thermal properties of sample 15 wt.% MC | |||
| λ [W/(m × K)] | Cv [MJ/(m3 × K)] | Cp [J/(kg × K)] | α [mm2/s] | |
| Quartile, Q1 | 0.8642 | 1.7696 | 1104.9 | 0.4838 |
| Median, M | 0.8848 | 1.7784 | 1110.4 | 0.4971 |
| Quartile, Q3 | 0.9455 | 1.7882 | 1116.6 | 0.5304 |
| Interquartile range, IQR = (Q3 − Q1) | 0.0813 | 0.0186 | 11.7 | 0.0466 |
| Higher outlier, HO = Q3 + 1.5·IQR | 1.0674 | 1.8161 | 1134.1 | 0.6004 |
| Lower outlier, LO = Q1 − 1.5·IQR | 0.7424 | 1.7417 | 1087.4 | 0.4139 |
| Average value, | 0.9037 | 1.7786 | 1110.6 | 0.5080 |
| Standard deviation, s | 0.0760 | 0.0131 | 8.2 | 0.0416 |
| Coefficient of variation, CV [%] | 8.18 | 0.73 | 0.73 | 8.00 |
| Upper critical value, UCV | 0.9897 | 1.7924 | 1119.2 | 0.5517 |
| Lower critical value, LCV | 0.8175 | 1.7648 | 1102.0 | 0.4643 |
| Statistical parameters | Thermal properties of sample 15 wt.% GR42 | |||
| λ [W/(m × K)] | Cv [MJ/(m3 × K)] | Cp [J/(kg × K)] | α [mm2/s] | |
| Quartile, Q1 | 1.1042 | 1.7270 | 967.7 | 0.6392 |
| Median, M | 1.1100 | 1.7288 | 968.6 | 0.6424 |
| Quartile, Q3 | 1.1139 | 1.7300 | 969.4 | 0.6450 |
| Interquartile range, IQR = (Q3 − Q1) | 0.0097 | 0.0031 | 1.7 | 0.0058 |
| Higher outlier, HO = Q3 + 1.5·IQR | 1.1284 | 1.7347 | 971.9 | 0.6537 |
| Lower outlier, LO = Q1 − 1.5·IQR | 1.0897 | 1.7224 | 965.1 | 0.6306 |
| Average value, | 1.1090 | 1.7284 | 968.4 | 0.6418 |
| Standard deviation, s | 0.0070 | 0.0023 | 1.3 | 0.0043 |
| Coefficient of variation, CV [%] | 0.65 | 0.13 | 0.13 | 0.67 |
| Upper critical value, UCV | 1.1169 | 1.7308 | 969.8 | 0.6463 |
| Lower critical value, LCV | 1.1011 | 1.7260 | 967.1 | 0.6373 |
| Statistical parameters | Thermal properties of sample 15 wt.% PX25 | |||
| λ [W/(m × K)] | Cv [MJ/(m3 × K)] | Cp [J/(kg × K)] | α [mm2/s] | |
| Quartile, Q1 | 1.2130 | 1.8156 | 1084.2 | 0.6658 |
| Median, M | 1.3100 | 1.8219 | 1088.0 | 0.7178 |
| Quartile, Q3 | 1.3963 | 1.8325 | 1094.4 | 0.7613 |
| Interquartile range, IQR = (Q3 − Q1) | 0.1834 | 0.0170 | 10.2 | 0.0955 |
| Higher outlier, HO = Q3 + 1.5·IQR | 1.6714 | 1.8580 | 1109.7 | 0.9046 |
| Lower outlier, LO = Q1 − 1.5·IQR | 0.9380 | 1.7902 | 1068.9 | 0.5225 |
| Average value, | 1.3148 | 1.8238 | 1089.1 | 0.7195 |
| Standard deviation, s | 0.1057 | 0.0090 | 5.4 | 0.0553 |
| Coefficient of variation, CV [%] | 7.85 | 0.49 | 0.49 | 7.55 |
| Upper critical value, UCV | 1.4346 | 1.8332 | 1094.8 | 0.7775 |
| Lower critical value, LCV | 1.1949 | 1.8144 | 1083.5 | 0.6615 |
| ID | Thermal Properties of Geopolymer Concretes with PCMs | |||
|---|---|---|---|---|
| λ [W/(m × K)] | Cv [MJ/(m3 × K)] | Cp [J/(kg × K)] | α [mm2/s] | |
| F.A.—ref. | 1.1619 ± 0.0047 | 1.8141 ± 0.0036 | 989.1 ± 2.0 | 0.6403 ± 0.0027 |
| 15 wt.% MC | 0.9037 ± 0.0760 | 1.7786 ± 0.0131 | 1110.6 ± 8.2 | 0.5080 ± 0.0416 |
| 15 wt.% GR42 | 1.1090 ± 0.0070 | 1.7284 ± 0.0023 | 968.4 ± 1.3 | 0.6418 ± 0.0043 |
| 15 wt.% PX25 | 1.3148 ± 0.1057 | 1.8238 ± 0.0090 | 1089.1 ± 5.4 | 0.7195 ± 0.0553 |
| Test Sample | Determined Confidence Intervals |
|---|---|
| F.A.—ref. | p (1.1566≤ λ ≤ 1.1671) = 0.95 |
| p (987.1 ≤ Cp ≤ 991.2) = 0.95 | |
| p (0.6375≤ α ≤ 0.6431) = 0.95 | |
| 15 wt.% MC | p (0.8175 ≤ λ ≤ 0.9897) = 0.95 |
| p (1102.0 ≤ Cp ≤ 1119.2) = 0.95 | |
| p (0.4643 ≤ α ≤ 0.5517) = 0.95 | |
| 15 wt.% GR42 | p (1.1011 ≤ λ ≤ 1.1169) = 0.95 |
| p (967.1 ≤ Cp ≤ 969.8) = 0.95 | |
| p (0.6373 ≤ α ≤ 0.6463) = 0.95 | |
| 15 wt.% PX25 | p (1.1949≤ λ ≤ 1.4346) = 0.95 |
| p (1083.5 ≤ Cp ≤ 1094.8) = 0.95 | |
| p (0.6615 ≤ α ≤ 0.7775) = 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybek, A.; Łach, M.; Romańska, P.; Ciemnicka, J.; Prałat, K.; Koper, A. Geopolymer Concretes with Organic Phase Change Materials—Analysis of Thermal Properties and Microstructure. Materials 2025, 18, 2557. https://doi.org/10.3390/ma18112557
Przybek A, Łach M, Romańska P, Ciemnicka J, Prałat K, Koper A. Geopolymer Concretes with Organic Phase Change Materials—Analysis of Thermal Properties and Microstructure. Materials. 2025; 18(11):2557. https://doi.org/10.3390/ma18112557
Chicago/Turabian StylePrzybek, Agnieszka, Michał Łach, Paulina Romańska, Justyna Ciemnicka, Karol Prałat, and Artur Koper. 2025. "Geopolymer Concretes with Organic Phase Change Materials—Analysis of Thermal Properties and Microstructure" Materials 18, no. 11: 2557. https://doi.org/10.3390/ma18112557
APA StylePrzybek, A., Łach, M., Romańska, P., Ciemnicka, J., Prałat, K., & Koper, A. (2025). Geopolymer Concretes with Organic Phase Change Materials—Analysis of Thermal Properties and Microstructure. Materials, 18(11), 2557. https://doi.org/10.3390/ma18112557









