The Effect of Adding Different Elements (Mg, Fe, Cu, and Ce) on the Properties of NiCo2OX for CO-Catalyzed Oxidation
Abstract
1. Introduction
2. Experimental Method
2.1. Preparation of CO Oxidation Catalyst
2.2. Catalyst Activity Test
2.3. Characterization of Catalysts
3. Results and Discussion
3.1. XRD Characterization Results for Catalysts
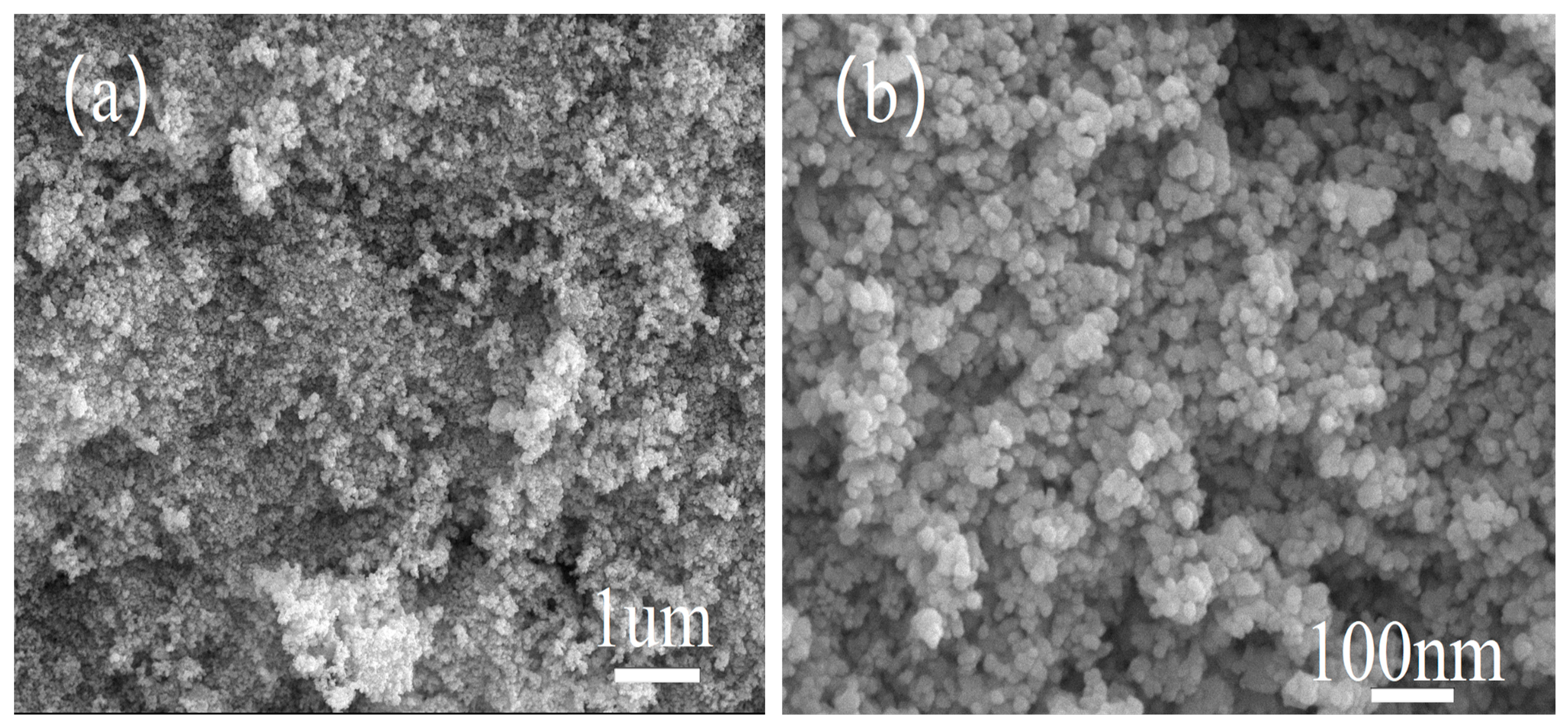
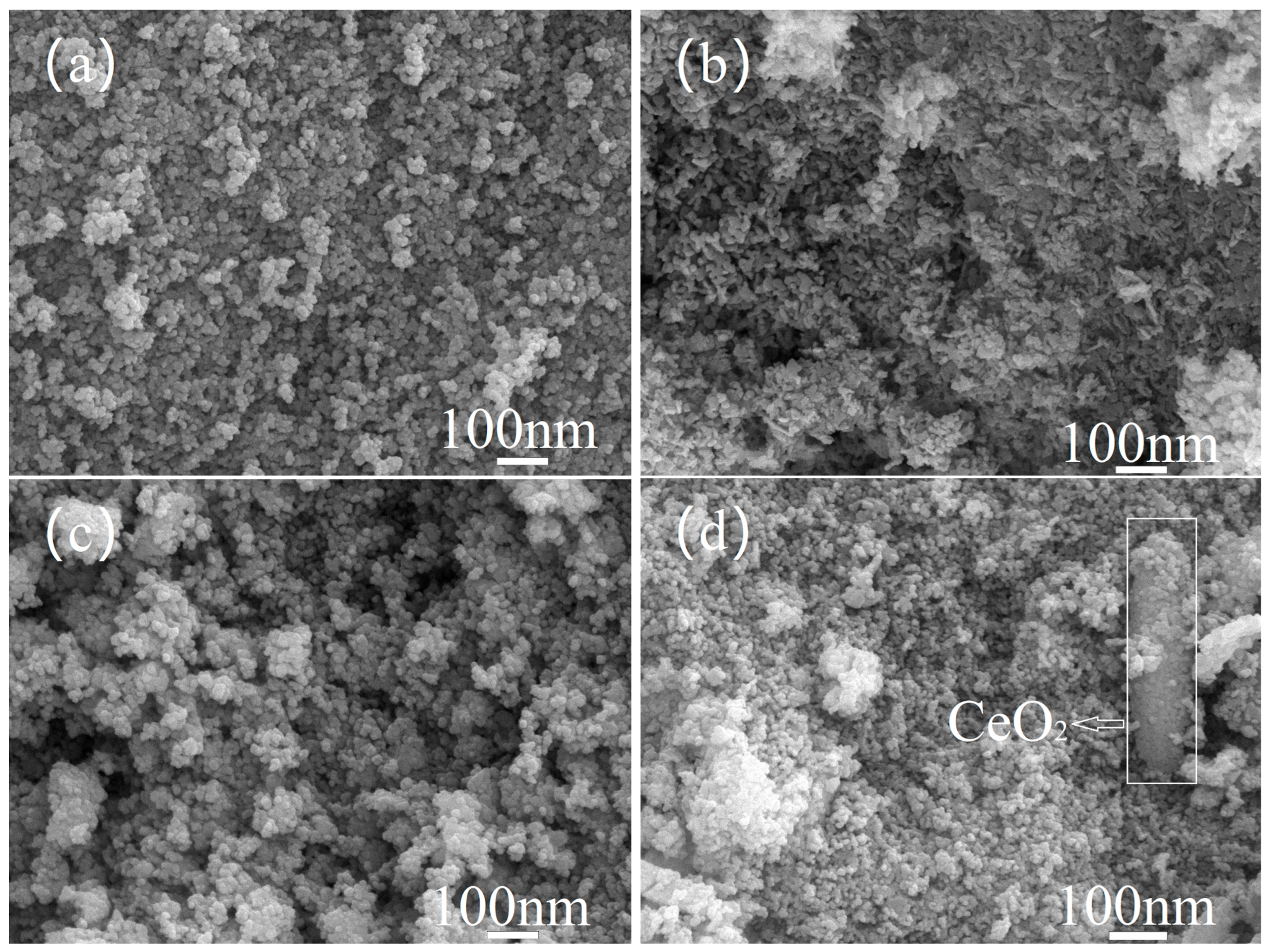
3.2. SEM Characterization of Catalysts
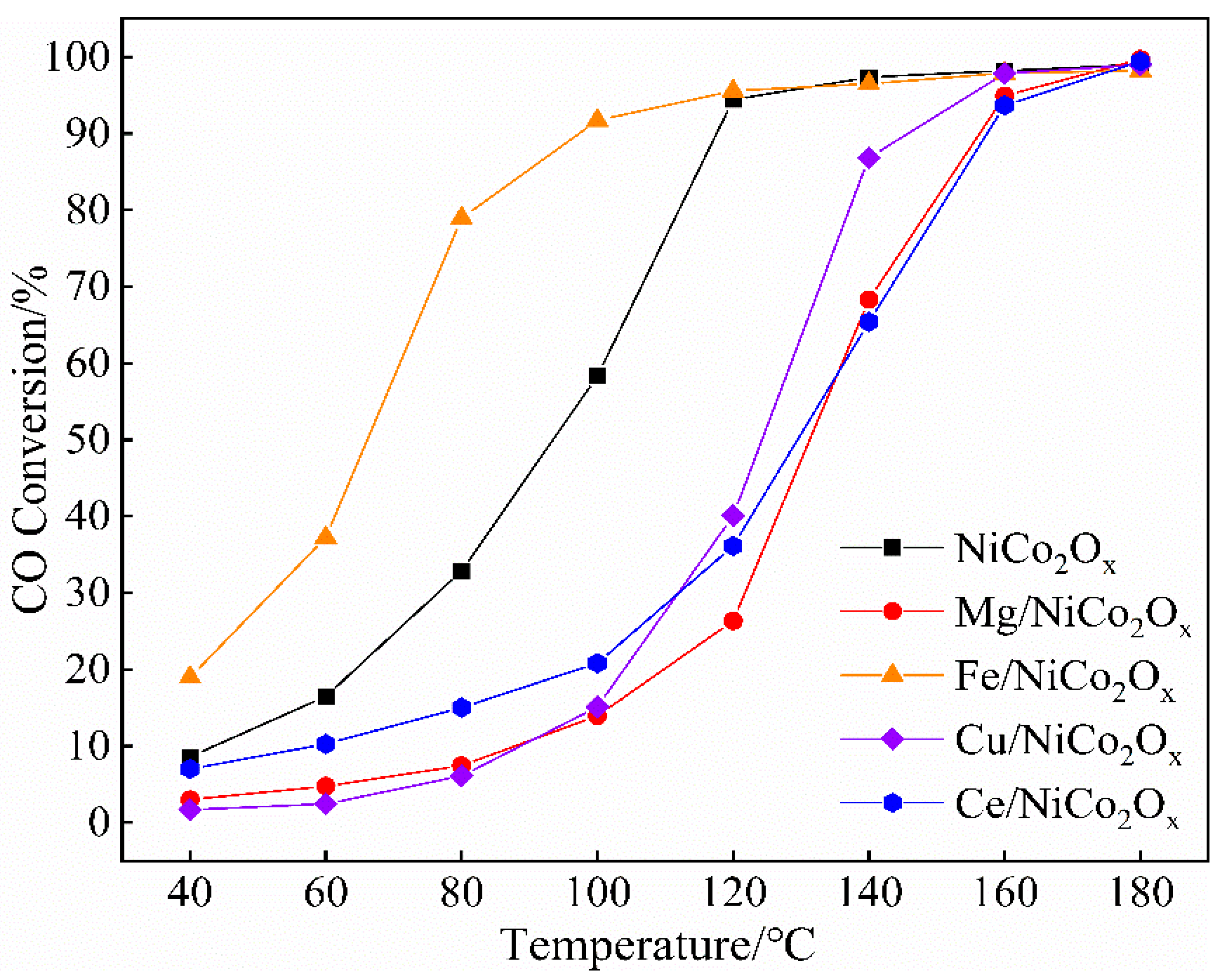
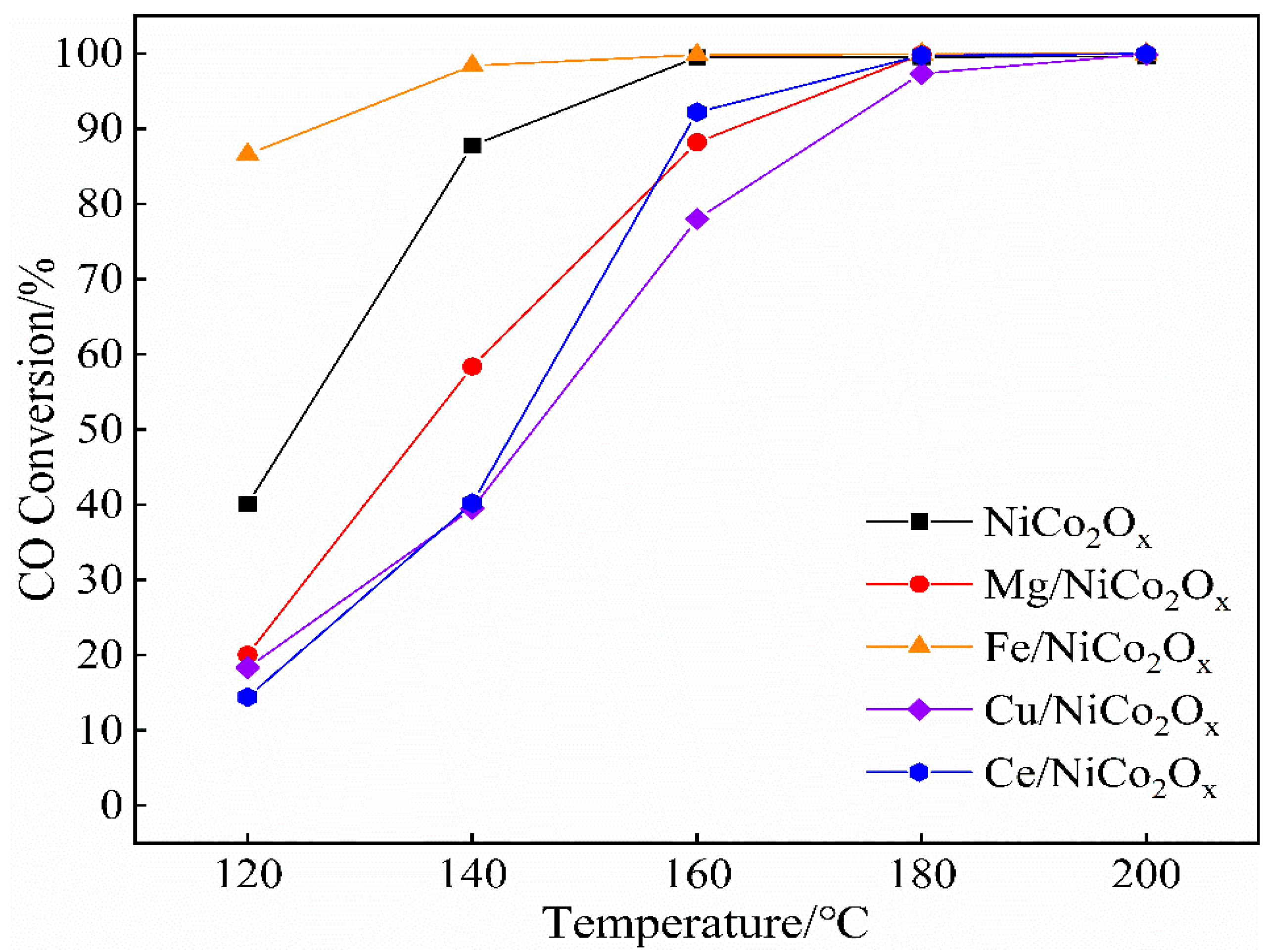
3.3. Catalyst Performance Evaluation
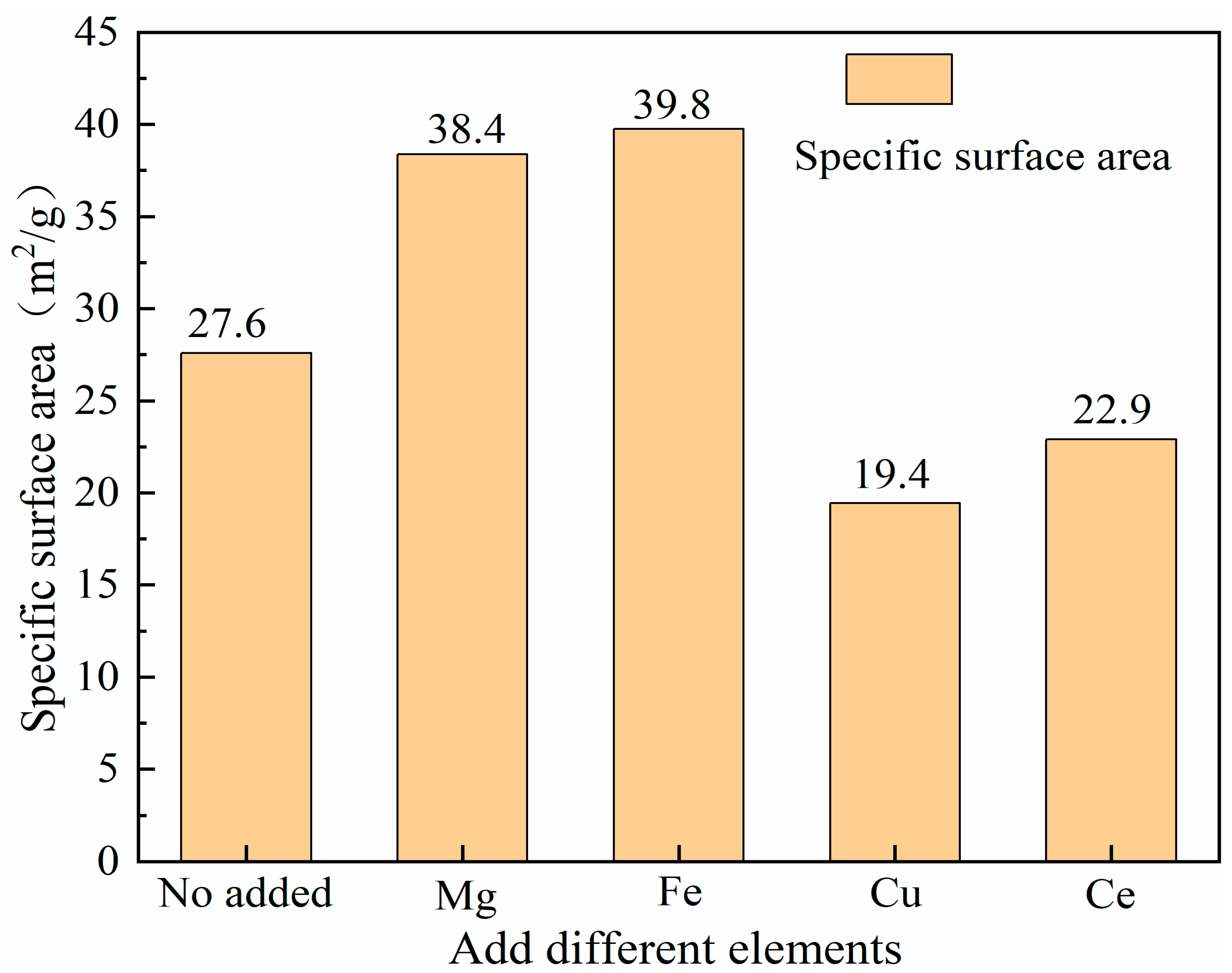
3.4. BET Characterization of Catalysts
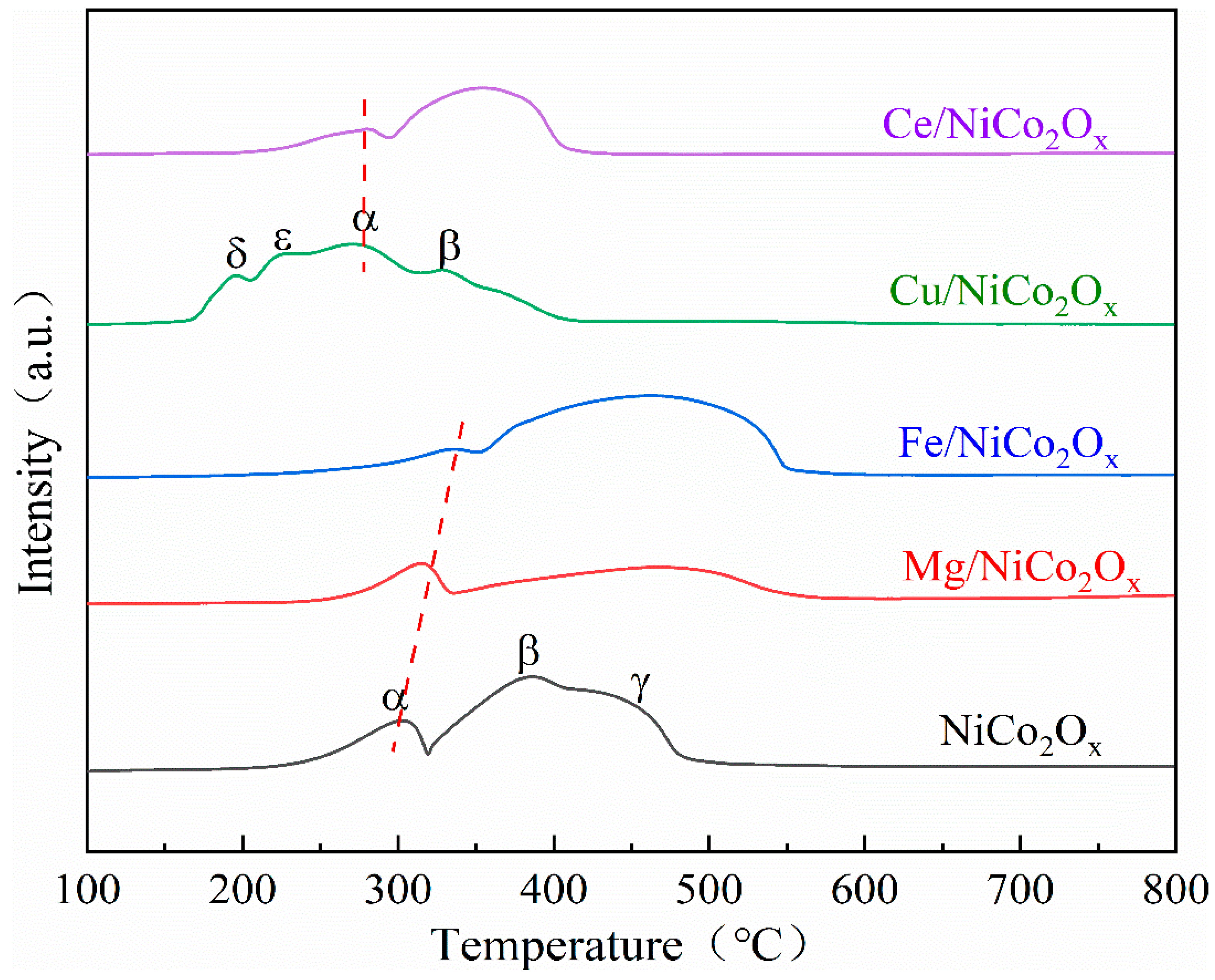
3.5. Characterization of Catalyst H2 Temperature Programmed Reduction (H2-TPR)
3.6. Characterization of Catalyst with CO Temperature-Programmed Desorption (CO-TPD)
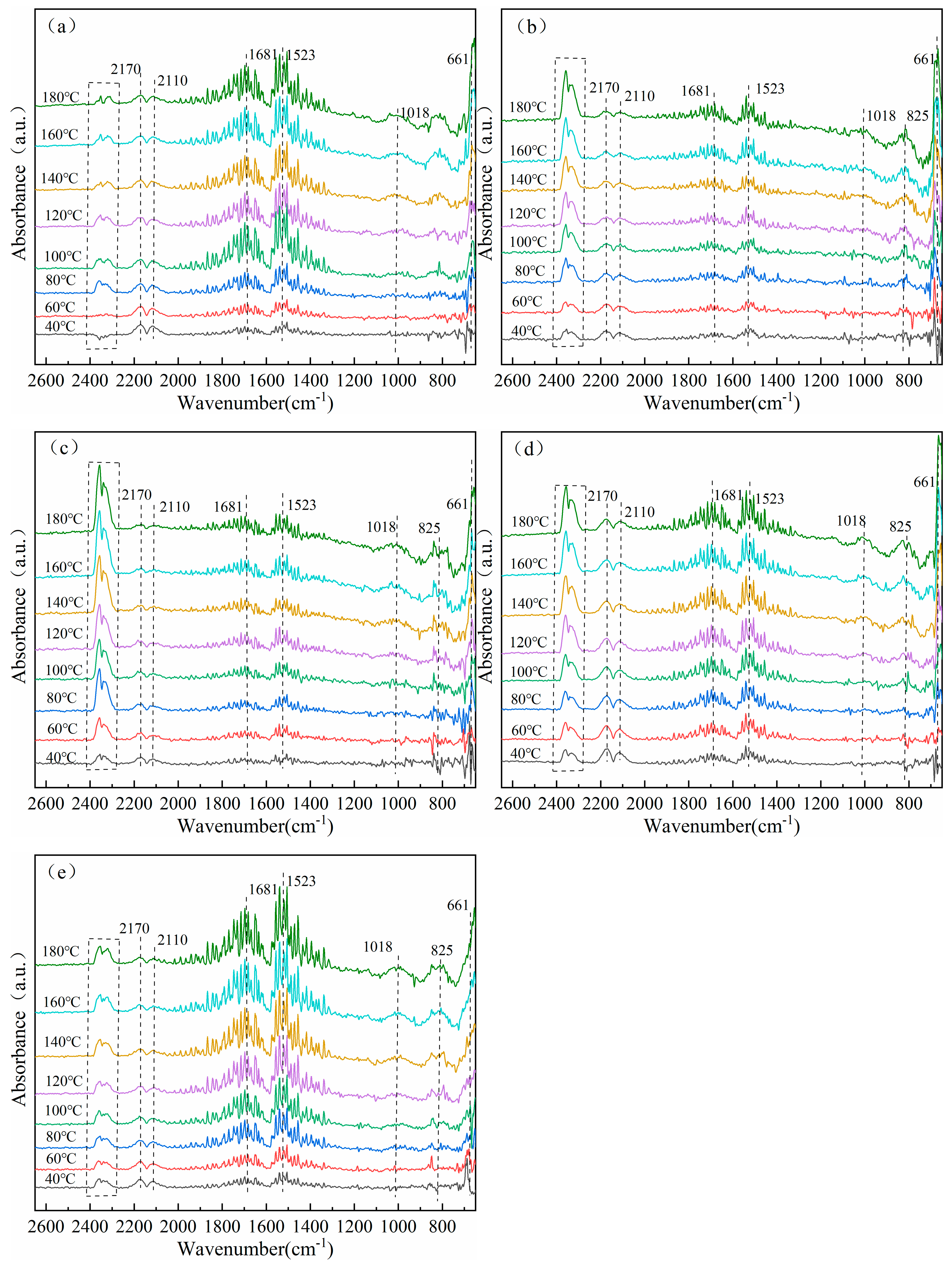
3.7. In Situ Infrared Diffuse Reflection Characterization of Catalysts
4. Study on Water and Sulfur Resistance of NiCo2Ox Catalysts
4.1. Performance of H2O Resistance
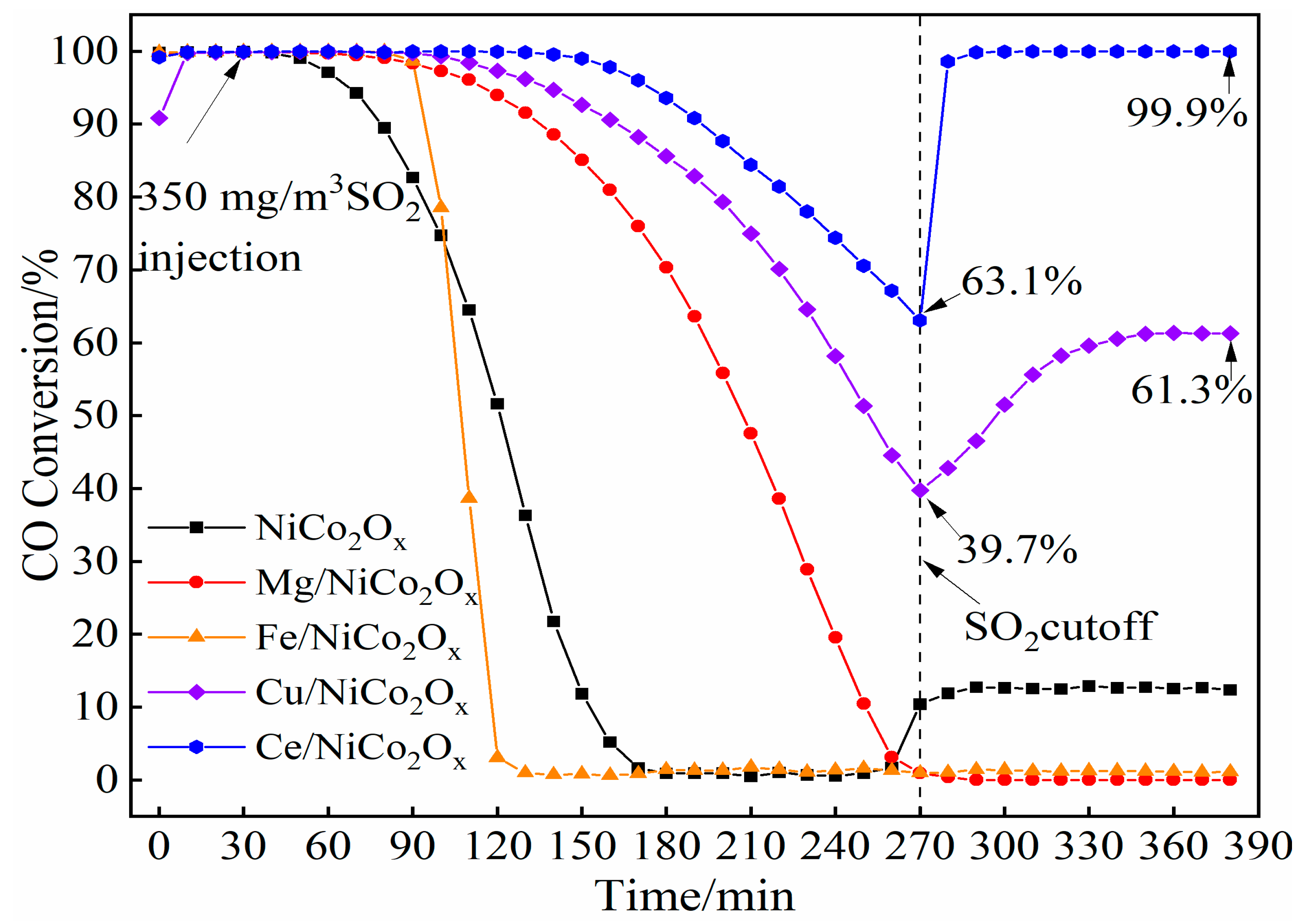
4.2. Performance of SO2 Resistance
4.3. Performance of Hybrid SO2 and H2O
5. Conclusions
- (1)
- NiCo2Ox catalysts were modified by elemental addition. Compared with the undoped catalysts, the Mg-, Cu-, and Ce-doped catalysts all exhibited a significant decrease in CO catalytic performance. Notably, only the Fe-added low-temperature catalytic CO activity gained an enhancement, with a CO conversion of up to 91.7% at 100 °C. Under the oxygen-enriched conditions, the catalyst surface of Fe has good CO catalytic oxidation performance.
- (2)
- Fe addition enhanced water resistance, achieving an efficiency of 98.4%. Under the same temperature and 10% water vapor condition, the catalytic oxidation of CO efficiency of the rest of the catalysts decreased to different degrees. The Ce-added NiCo2Ox catalyst exhibited anti-SO2 performance. The conversion of Ce-added catalyst reached 63.1% after four hours of SO2 exposure, and it was able to recover to 99.9% efficiency after cutting off the SO2 supply.
- (3)
- Under the condition of adding sulfur and water, the anti-sulfur performance of all catalysts was increased, except for the Cu-added catalyst. The performance of Ce-added NiCo2Ox catalysts and was significantly improved, with 94% CO conversion after exposure to SO2 for four hours and H2O for six hours, and the catalytic rate could be restored to 95.9% after cutting off SO2 and water, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.; Zhu, T.; Liu, X. Progress of ultra-low emission technology for key processes of iron and steel industry in China. Iron Steel 2019, 54, 1–11. [Google Scholar]
- Yadava, R.N.; Bhatt, V. Carbon monoxide: Risk assessment, environmental, and health hazard. In Hazardous Gases; Elsevier: Amsterdam, The Netherlands, 2021; pp. 83–96. [Google Scholar]
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Liu, Y.; Liang, Y.; Peng, Y.; Bartlett, S.; Dai, H.; Rostamnia, S.; Li, J. Template-free Scalable Synthesis of Flower-like Co3-MnO4 Spinel Catalysts for Toluene Oxidation. ChemCatChem 2018, 10, 3429–3434. [Google Scholar] [CrossRef]
- Dey, S.; Mehta, N.S. Oxidation of carbon monoxide over various nickel oxide catalysts in different conditions: A review. Chem. Eng. J. Adv. 2020, 1, 100008. [Google Scholar] [CrossRef]
- Waikar, J.; More, P. Co supported on Cex[Al2O3]0.5-x as an effective catalyst for low-temperature CO oxidation: Effect of calcination temperature. Mol. Catal. 2022, 528, 112466. [Google Scholar] [CrossRef]
- Su, Y.; Tang, Z.; Han, W.; Song, Y.; Lu, G. Enhanced Catalytic Performance of Three-Dimensional Ordered Mesoporous Transition Metal (Co, Cu, Fe)-Doped CeO2 Catalysts for CO Catalytic Oxidation. Catal. Surv. Asia 2015, 19, 68–77. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, L.; Zhao, H.; Kang, J.; Chun, T.; Qian, L. Research progress of CO removal in flue gas of typical steel production process. Iron Steel 2023, 58, 24. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, P.A.; Tran, T.T.H.; Chu, T.H.N.; Wang, Y.; Arandiyan, H. Catalytic performance of spinel-type Ni-Co Oxides for Oxidation of Carbon Monoxide and Toluene. Top. Catal. 2023, 66, 117–125. [Google Scholar] [CrossRef]
- Gou, Y.; Liang, X.; Chen, B. Porous Ni–Co bimetal oxides nanosheets and catalytic properties for CO oxidation. J. Alloys Compd. 2013, 574, 181–187. [Google Scholar] [CrossRef]
- Yan, A.; Liu, Y.; Liu, Y.; Li, X.; Lei, Z.; Liu, P. A NaAc-assisted large-scale coprecipitation synthesis and microwave absorption efficiency of Fe3O4 nanowires. Mater. Lett. 2012, 68, 402–405. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Song, L.; Hou, F.; Yang, Y.; Wang, Y.; Liu, N. Enhanced catalytic performance for CO oxidation and preferential CO oxidation over CuO/CeO2 catalysts synthesized from metal organic framework: Effects of preparation methods. Int. J. Hydrogen Energy 2018, 43, 18279–18288. [Google Scholar] [CrossRef]
- Sun, S.; Yang, L.; Pang, G.; Feng, S. Surface properties of Mg doped LaCoO3 particles with large surface areas and their enhanced catalytic activity for CO oxidation. Appl. Catal. A Gen. 2011, 401, 199–203. [Google Scholar] [CrossRef]
- Sabet Sarvestani, N.; Tabasizadeh, M.; Hossein Abbaspour-Fard, M.; Nayebzadeh, H.; Karimi-Maleh, H.; Chu Van, T.; Jafari, M.; Ristovski, Z.; Brown, R.J. Influence of doping Mg cation in Fe3O4 lattice on its oxygen storage capacity to use as a catalyst for reducing emissions of a compression ignition engine. Fuel 2020, 272, 117728. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, Y.; Shi, L.; Mai, H.; Gao, R.; Zhang, J.; Yu, W.; Cao, W. Cu-doped CeO2 spheres: Synthesis, characterization, and catalytic activity. Catal. Commun. 2012, 26, 164–168. [Google Scholar] [CrossRef]
- Zheng, L.; Li, H.-j.; Xu, X.-f. Catalytic decomposition of N2O over Mg-Co composite oxides hydrothermally prepared by using carbon sphere as template. J. Fuel Chem. Technol. 2018, 46, 569–577. [Google Scholar] [CrossRef]
- Shi, X.; Chu, B.; Wang, F.; Wei, X.; Teng, L.; Fan, M.; Li, B.; Dong, L.; Dong, L. Mn-Modified CuO, CuFe2O4, and γ-Fe2O3 Three-Phase Strong Synergistic Coexistence Catalyst System for NO Reduction by CO with a Wider Active Window. ACS Appl. Mater. Interfaces 2018, 10, 40509–40522. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, X.; Wang, X.; Wang, S.; Wu, S. Preparation and characterization of CuO/CeO2 catalysts and their applications in low-temperature CO oxidation. Appl. Catal. A Gen. 2005, 295, 142–149. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Hu, L.; Guo, Y.; Lu, Y.; Feng, J. La doped-Fe2(MoO4)3 with the synergistic effect between Fe2+/Fe3+ cycling and oxygen vacancies enhances the electrocatalytic synthesizing NH3. J. Colloid Interface Sci. 2025, 677, 264–272. [Google Scholar] [CrossRef]
- Lewandowski, M.; Groot, I.M.N.; Shaikhutdinov, S.; Freund, H.J. Scanning tunneling microscopy evidence for the Mars-van Krevelen type mechanism of low temperature CO oxidation on an FeO(111) film on Pt(111). Catal. Today 2012, 181, 52–55. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Kang, J.; Liu, W.; Kuang, Y.; Tan, H.; Yu, Z.; Yang, L.; Yang, X.; Yu, K.; et al. Preparation of Ce-MnOx Composite Oxides via Coprecipitation and Their Catalytic Performance for CO Oxidation. Nanomaterials 2023, 13, 2158. [Google Scholar] [CrossRef]
- Sun, R.-l.; Zhang, S.-r.; An, K.; Song, P.-f.; Liu, Y. Cu1.5Mn1.5O4 spinel type composite oxide modified with CuO for synergistic catalysis of CO oxidation. J. Fuel Chem. Technol. 2021, 49, 799–808. [Google Scholar] [CrossRef]
- Saw, E.T.; Oemar, U.; Tan, X.R.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Bimetallic Ni–Cu catalyst supported on CeO2 for high-temperature water–gas shift reaction: Methane suppression via enhanced CO adsorption. J. Catal. 2014, 314, 32–46. [Google Scholar] [CrossRef]
- Sun, S.; Mao, D.; Yu, J.; Yang, Z.; Lu, G.; Ma, Z. Low-temperature CO oxidation on CuO/CeO2 catalysts: The significant effect of copper precursor and calcination temperature. Catal. Sci. Technol. 2015, 5, 3166–3181. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Wang, R. CeO2 nanorods supported M–Co bimetallic oxides (M = Fe, Ni, Cu) for catalytic CO and C3H8 oxidation. J. Colloid Interface science 2020, 560, 91–102. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Liu, A.; Yu, Y.; Ji, J.; Guo, K.; Wan, H.; Tang, C.; Dong, L. Unravelling the structure sensitivity of CuO/SiO2 catalysts in the NO+ CO reaction. Catal. Sci. Technol. 2020, 10, 3848–3856. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Zhang, G.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, Y.; Lv, D. Interfacial effects promote the catalytic performance of CuCoO2-CeO2 metal oxides for the selective reduction of NO by CO. Chem. Eng. J. 2023, 465, 142856. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, W.; Li, Y. Nanoscale Co-based catalysts for low-temperature CO oxidation. Catal. Sci. Technol. 2015, 5, 1014–1020. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Wei, L.; Chu, B.; Qin, Z.; Jin, G.; Tong, Z.; Dong, L.; Li, B. Preparation of three-dimensionally ordered macroporous MFe2O4 (M = Co, Ni, Cu) spinel catalyst and its simultaneous catalytic application in CO oxidation and NO+ CO reaction. Fuel 2020, 272, 117738. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, B.; Wang, J.; Qu, C.; Sun, H.; Zhang, K.; Liu, M. High-performance hybrid supercapacitors based on self-supported 3D ultrathin porous quaternary Zn-Ni-Al-Co oxide nanosheets. Nano Energy 2016, 28, 475–485. [Google Scholar] [CrossRef]
- AlMohamadi, H.; Smith, K.J. The Impact of CeO2 Loading on the Activity and Stability of PdO/γ-AlOOH/γ-Al2O3 Monolith Catalysts for CH4 Oxidation. Catalysts 2019, 9, 557. [Google Scholar] [CrossRef]
- Dang, H.; Zhao, W.; Wu, R.; Yue, L.; Wang, Y.; Ren, Z.; Zhao, Y. Effect of acid treatment on the catalytic performance of CuO/Cryptomelane catalyst for CO preferential oxidation in H2-rich streams. Int. J. Hydrogen Energy 2023, 48, 27619–27630. [Google Scholar] [CrossRef]
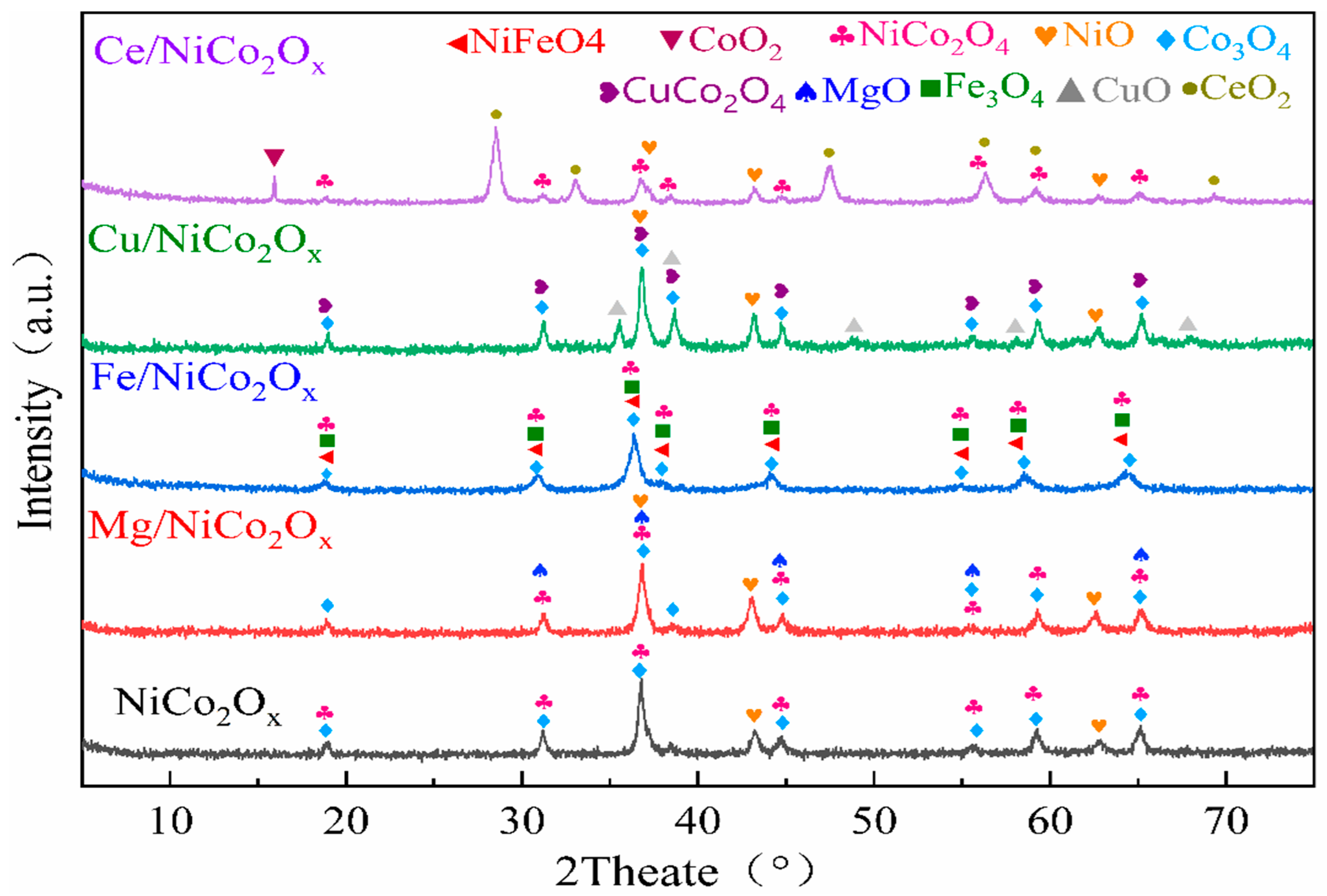


| Catalyzer | Phase Formation |
|---|---|
| Ce/NiCo2O4 | NiCo2O4, NiO, CeO2, CoO2 |
| Cu/NiCo2O4 | NiO, Co3O4, CuO, CuCo2O4 |
| Fe/NiCo2O4 | NiCo2O4, Co3O4, Fe3O4, NiFeO4 |
| Mg/NiCo2O4 | NiCo2O4, NiO, Co3O4, MgO |
| NiCo2O4 | NiCo2O4, NiO, Co3O4 |
| Catalysts | Attribution and Temperature of Reduction Peak | H2 Consumption (mmol/g) | ||||
|---|---|---|---|---|---|---|
| α | β | γ | δ | ε | ||
| Co3O4→CoO | CoO→Co/ NiO→Ni/ Fe2O3→Fe3O4→FeO | CoO→Co | CuO→Cu | Bulk CuO→Cu Bulk CuO→Cu2O | ||
| NiCo2Ox | 303 | 386 | 450 | / | / | 11.5 |
| Mg/NiCo2Ox | 315 | 478 | / | / | / | 6.3 |
| Fe/NiCo2Ox | 333 | 467 | / | / | / | 11.8 |
| Cu/NiCo2Ox | 227 | 332 | / | 195 | 225 | 10.9 |
| Ce/NiCo2Ox | 281 | 352 | / | / | / | 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, Z.; Cao, T.; Yang, J.; Kuang, Y.; Kang, J. The Effect of Adding Different Elements (Mg, Fe, Cu, and Ce) on the Properties of NiCo2OX for CO-Catalyzed Oxidation. Materials 2025, 18, 2554. https://doi.org/10.3390/ma18112554
Wang J, Chen Z, Cao T, Yang J, Kuang Y, Kang J. The Effect of Adding Different Elements (Mg, Fe, Cu, and Ce) on the Properties of NiCo2OX for CO-Catalyzed Oxidation. Materials. 2025; 18(11):2554. https://doi.org/10.3390/ma18112554
Chicago/Turabian StyleWang, Jiefeng, Zhili Chen, Tianqi Cao, Junsheng Yang, Yijian Kuang, and Jiangang Kang. 2025. "The Effect of Adding Different Elements (Mg, Fe, Cu, and Ce) on the Properties of NiCo2OX for CO-Catalyzed Oxidation" Materials 18, no. 11: 2554. https://doi.org/10.3390/ma18112554
APA StyleWang, J., Chen, Z., Cao, T., Yang, J., Kuang, Y., & Kang, J. (2025). The Effect of Adding Different Elements (Mg, Fe, Cu, and Ce) on the Properties of NiCo2OX for CO-Catalyzed Oxidation. Materials, 18(11), 2554. https://doi.org/10.3390/ma18112554






