Abstract
This study focuses on addressing the reflectivity reduction issue in La1–xSrxTiO3+δ during high-temperature preparation, which is caused by oxygen vacancy generation. Bulk samples of CeO2-doped La1–xSrxTiO3+δ with varying doping contents as a second phase and sintering temperatures were prepared. The phase composition, reflectivity, and valence states were thoroughly investigated. Introducing 10 wt.%CeO2 significantly suppressed the formation of oxygen vacancies. Thus, the occurrence of impurity levels caused by oxygen vacancies was reduced. This can further mitigate the reflection decrease caused by impurity levels as photon absorption traps. Additionally, the reduced pore structure achieved at 1450 °C contributed to improved reflectivity compared to pure La1–xSrxTiO3+δ. The findings suggest that this approach has great potential for reducing oxygen vacancies sensitivity in high-reflection ceramics under high-temperature conditions and preserving their optical properties.
1. Introduction
La1–xSrxTiO3+δ (LST) is a perovskite-type oxidation that has garnered significant attention due to its promising applications in various fields, particularly in optics and electronics. This material is particularly noted for its unique electrical and optical properties, which make it suitable for a range of advanced technological applications [1,2,3,4]. The material’s ability to maintain good optical transparency over a wide range of wavelengths is a desirable feature for such applications [2]. In addition, the reflectivity of LST is also of interest. The research of Zhu indicates that LST exhibits high reflectivity. Given its high thermal stability, LST can be potentially applied as a high-reflection material [3]. However, some studies have reported that oxygen vacancies are easily formed and result in an LST material system when the LST material is prepared at a low oxygen partial pressure or in a high-temperature environment [4,5,6]. Meanwhile, the generation of oxygen vacancy leads to a reflectivity reduction in LST. This phenomenon limits the further application of LST in some particular conditions, such as optical material [7,8].
The performance of ceramic materials is often significantly influenced by doping, which serves as a critical approach to enhancing their thermal, optical, mechanical and other properties [9,10,11,12]. Doping involves the intentional introduction of foreign ions into the crystal lattice of a base material, leading to modifications in its electronic structure, defect chemistry, and surface characteristics. These changes can result in improved stability under extreme conditions, enhanced catalytic activity, or tailored optical responses, depending on the specific application requirements.
Among various dopants, CeO2 stands out as a particularly promising candidate due to its unique properties. CeO2 is a well-known oxygen storage material with a cubic fluorite structure that allows for a high concentration of surface oxygen vacancies [13,14]. These oxygen vacancies are not only capable of storing and releasing oxygen but also provide active sites for catalytic reactions [15,16,17]. The ability of CeO2 to reversibly store and release oxygen makes it an attractive candidate for applications in solid oxide fuel cells, catalysis, and gas sensing [18,19]. Given the aforementioned characteristics of CeO2, doping LST with CeO2 presents an innovative solution. Specifically, the addition of CeO2 could enhance the oxygen storage capacity of LST, thereby compensating for its inherent oxygen deficiency. Additionally, the presence of CeO2 may influence the optical properties of LST.
The optimization of doping levels and conditions is crucial to achieving the desired enhancements in performance. For example, excessive doping could lead to the formation of secondary phases or disrupt the lattice structure of LST, thereby compromising its mechanical integrity. On the other hand, insufficient doping may fail to deliver the expected improvements in oxygen storage capacity or optical properties. Therefore, a systematic investigation is required to determine the optimal concentration and distribution of CeO2 within the LST matrix.
In summary, the strategic doping of LST with CeO2 as a second phase offers a promising avenue for overcoming its inherent limitations. In this study, in order to suppress oxygen vacancy generation and maintain the high reflection of LST, the phase, reflectivity, and valence states of CeO2-doped samples with different content and different sintering temperatures were investigated in depth.
2. Materials and Methods
2.1. Sample Preparation
A high-temperature solid-state reaction method was used to prepare the samples with high-purity raw materials: La2O3 (99.99%, Forsman Scientific Co., Ltd., Beijing, China), SrCO3 (AR, Forsman Scientific Co., Ltd., Beijing, China), TiO2 (AR, Forsman Scientific Co., Ltd., Beijing, China), and CeO2 (AR, Forsman Scientific Co., Ltd., Beijing, China). These raw materials of the LST matrix were weighed stoichiometrically according to the previous work [1]. The proportions of LST and CeO2 were weighed and sintered at the corresponding temperature according to the formulation listed in Table 1.

Table 1.
The formulation of LST-CeO2 (LST-CO) samples.
2.2. Characterization
X-ray diffraction (PANalytical Inc., X’Pert PRO MPD, Almelo, The Netherlands) was used to characterize the phase transition of samples with different proportions and the results were analyzed by JADE (version 5.0, Materials Data Inc., Livermore, CA, USA). The surface microstructure of sintered samples was observed by a scanning electron microscope (HITACHI S4800, HITACHI, Tokyo, Japan). The reflectivity of every sample was measured by an ultraviolet–visible–near-infrared (UV–VIS–NIR) spectrophotometer (Cray5000, Palo Alto, CA, USA). Meanwhile, the valence states of Ti and Ce were tested by X-ray photoelectron spectroscopy (PHI 5300, PHI, Lafeyette, LA, USA) in order to explore the optic property changes.
3. Results and Discussion
3.1. Surface Morphology and Optical Analysis of Different CeO2 Content-Doped LST-CO
The surface macro-morphologies of different CeO2-doped LST bulks (LC0, LC1, and LC2) are shown in Figure 1. A visible color difference can be observed with different CeO2-doped LST bulks sintered at 1450 °C. With the 20 wt.% CeO2 doping, the sintered bulk exhibits a significant color change. According to the experience obtained in a previous study, light-colored samples may exhibit a higher reflectivity [3].
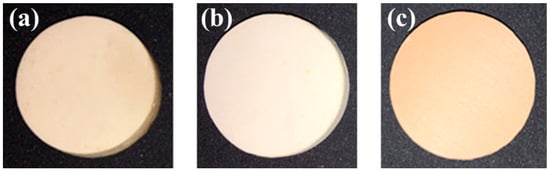
Figure 1.
Surface macro-morphologies of (a) LC0, (b) LC1, and (c) LC2 samples.
Additionally, when observing the surface microstructures of LC0, LC1, and LC2 bulks shown in Figure 2, LC1 and LC2 exhibit less visible pores compared to LC0, which means less absorption will be induced by the porous structure.
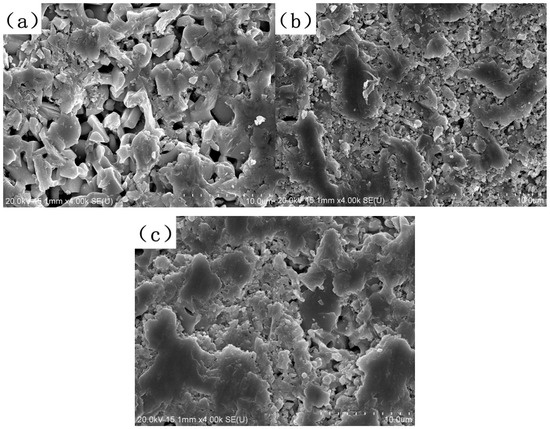
Figure 2.
Surface microstructure of (a) LC0, (b) LC1, and (c) LC2 samples.
From the energy band theory, the optical properties of materials are influenced by the transition and return of electrons between the conduction band and valence band. The appearance of oxygen vacancies leads to the generation of impurity levels below the conduction band. The distance of the generated new impurity levels is less than the bandgap of crystal without oxygen vacancies. Therefore, the subbands behave as trap centers inside the bandgap of the ideal case [20,21]. Some incident photons are absorbed rather than reflected. This is the mechanism of the influence of oxygen vacancy generation on reflectivity.
On the basis of the reflectivity spectra shown in Figure 3, the above conjecture that LC1 bulks have the highest reflectivity has been confirmed. THe LC0 and LC1 bulks show the same variation trend with an obvious reflection from 800 nm to 2500 nm, and the maximum values can reach 92% and 96% at 1064 nm, respectively. The doping of CeO2 does play a positive role in enhancing the reflectivity of LST-CO. The appropriate CeO2 doping should have reduced the generation of oxygen vacancies in LST. This phenomenon also reduced the generation of impurity levels and increased the reflectivity of the sample. However, the highest reflectivity of LC2 is just 80% and a dramatic decrease appears around 2000 nm. This phenomenon may be because at a 20 wt.% doping ratio, the released oxygen of CeO2 escaped as O2 rather than being captured by the LST oxygen vacancies. These impurity levels of oxygen vacancies play a role in absorbing incident photons and preventing reflection [7].
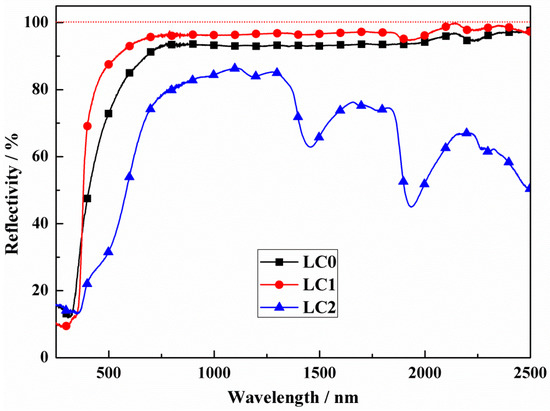
Figure 3.
Reflectivity of LC0, LC1, and LC2 samples.
3.2. Surface Morphology and Optical Property Analysis of Different-Temperature-Sintered LST-CO
Furthermore, a series LST-CO sample with 10 wt.% CeO2 was prepared at different temperatures to further investigate the inhibitory effect of temperature on the generation of oxygen vacancy in LST. Their surface macro-morphologies are listed in Figure 4. With the increase in sintering temperature, the color of the sample surface gradually darkens. Combined with the reflectance spectra shown in Figure 5, it can be seen that there is no obvious difference in reflectivity at 1064 nm of the LC5, LC4, and LC1 samples when the processing temperature is below 1450 °C. Nevertheless, at an ultra-high sintering temperature (1550 °C), a significant decrease in reflectivity appears in the LC3 sample. The appearance of this phenomenon is because at ultra-high temperatures, a large amount of oxygen vacancies are generated and the induced impurity levels dominate the return of photons to the bandgap.
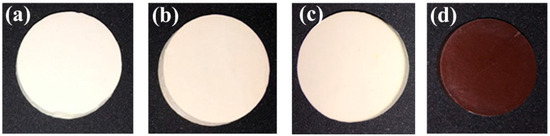
Figure 4.
Surface macro-morphologies of (a) LC5, (b) LC4, (c) LC1, and (d) LC3 samples.
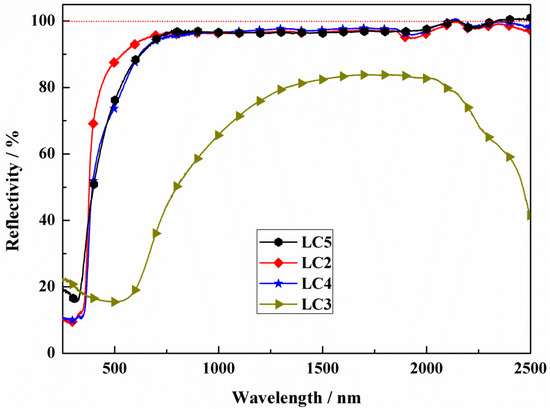
Figure 5.
Reflectivity of LC2, LC3, LC4, and LC5 samples.
According to the XRD pattern shown in Figure 6, the phase structures of LST and CeO2 can be easily detected below 1450 °C, indicating that the LST and CeO2 have good thermal stability and no other new phase transform. However, some diffraction peaks at 1550 °C become weaker than those at any other low temperature, which can be explained by two aspects. On one hand, at that high temperature, a solid solution reaction of CeO2 and LST occurs, meaning that Ce4+ could replace the Ti3+ in LST. On the other hand, a long high-temperature sintering time causes grain growth and grain boundary reduction [22].
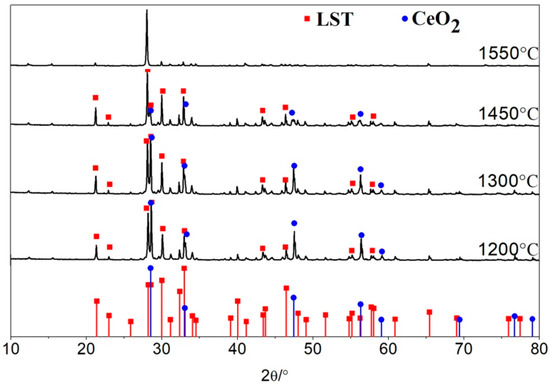
Figure 6.
XRD patterns of LC2, LC3, LC4, and LC5 samples.
In addition, from the microstructure of different-temperature-sintered LST-CO samples shown in Figure 7, the density of the sample shows an obvious enhancement with the increase in temperature. Considering that the mechanical properties of ceramics are positively correlated with their density [23], the material ratio and preparation parameters of the LC2 sample have good potential to improve the application of LST in some special fields with high reflection.
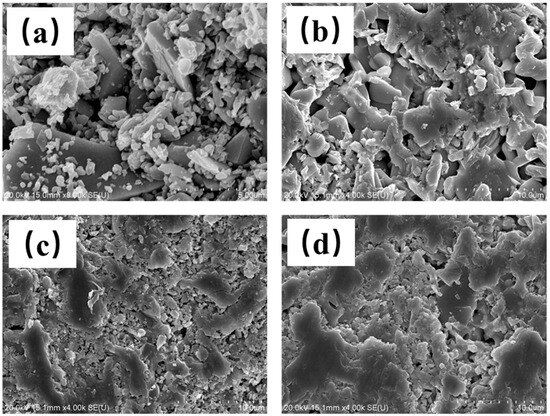
Figure 7.
Surface microstructure of (a) LC5, (b) LC4, (c) LC1, and (d) LC3 samples.
3.3. Phase and XPS Analysis of Different-Temperature-Sintered LST-CO
In order to further investigate the mechanism of the optical properties exhibited above, in-depth research into chemical valence was preceded by X-ray photoelectron spectroscopy (XPS) of 10 wt.% CeO2-doped LST. By analyzing the Ti2p and Ce3d energy bands of samples shown in Figure 8 and Figure 9, the valence states of Ti and Ce can be detected. Since the oxygen vacancy drives the Ti valence state from Ti4+ to Ti3+ and also drives the Ce valence state from Ce4+ to Ce3+ by giving additional electrons, the variation in oxygen vacancy can be calculated by the content of Ti4+ and Ce4+.
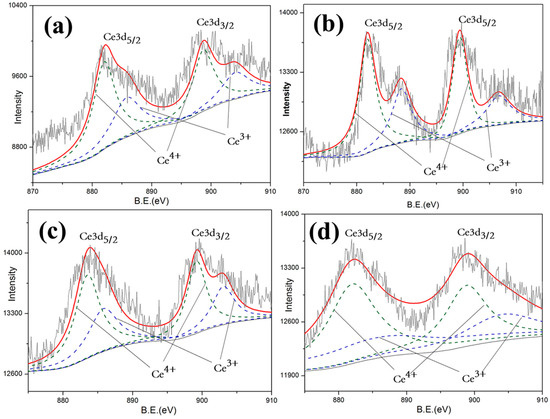
Figure 8.
Total Ce3d spectroscopy of LST-CO samples. (a) LC5, (b) LC4, (c) LC1, and (d) LC3.
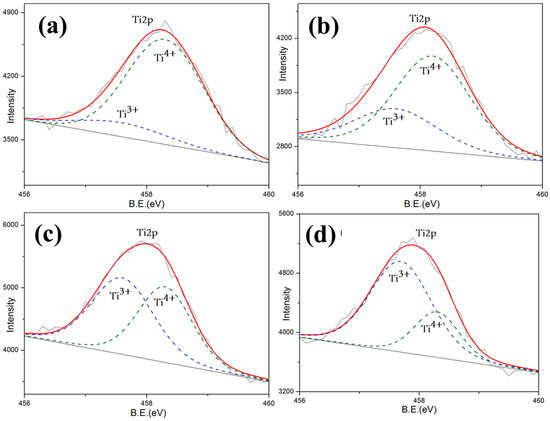
Figure 9.
Total Ti2p spectroscopy of LST-CO samples. (a) LC5, (b) LC4, (c) LC1, and (d) LC3.
The valence state statistics of 10%wt. CeO2-doped LST sintered under different temperatures are shown in Table 1. The valence state variation in Ti and Ce form 4+ to −3+, shown in Reactions (1) and (2), which provides indirect evidence of the formation of oxygen vacancies shown in Reaction (3). According to the law of conservation, the more that reaction (2) occurs, the less reaction (1) occurs. Since the △H of reaction (2) is much lower than that of reaction (1), reaction (2) is more likely to happen at high temperatures. Thus, the number of oxygen vacancies generated in LST is reduced. LST can also obtain the oxygen from CeO2 to slow down the generation of its own oxygen vacancies.
Ti4+ + e → Ti3+ ∆H = −3.86kJ/mol
Ce4+ + e → Ce3+ ∆H = −155.4kJ/mol
O2−→0.5O2 + 2e + Vo (oxygen vacancy)
More Ti4+ and Ce4+ maintaining shown in Table 2 indicates that fewer oxygen vacancies are generated. Oxygen vacancies generated at high temperatures act as absorption centers during the propagation of the light within the crystal, and light phonon scattering easily occurs in crystal structural defects [24,25,26,27]. This result proves that the reason for the dramatic decrease at 1550 °C is the generation of a large amount of oxygen vacancies. On the contrary, in addition to the contribution of the reduced pores of the LC1 structure, it shows a good reflection and high preparation temperature. This is consistent with the reflectivity test results shown in Figure 5.

Table 2.
Valence states of Ti and Ce at different sintering temperatures.
According to the research results, it can be concluded that the improvement of the reflectivity of LST by CeO2 doping as a second phase depends on the doping content and preparation temperature. These two factors directly affect the style of oxygen released by CeO2, either as O2 or captured by LST (oxygen release and capture strategy). Based on all measurements, the sample with 10 wt.% CeO2 doping and sintering at 1450 °C achieved the best optical properties. The trend is shown in Table 3. Compared to other related work, although we did not find the same strategy, the work by Zhao et al. elaborated on another feasible approach to reducing the generation of oxygen vacancies by utilizing multi-element doping [28]. This method increases the generation energy of oxygen vacancies, which can mitigate the impact of oxygen vacancies on optical properties. This strategy enables the sustainment of material reflectance (92% at elevated sintering temperatures (1500 °C). These two methods have successfully suppressed the influence of oxygen vacancies on reflective performance. In the future, we will continue to investigate the feasibility of applying our “oxygen release and capture strategy” to high-temperature and high-reflection coatings/films. Meanwhile, we will also use DFT modeling and first-principles calculation methods to perform a deep investigation.

Table 3.
Reflectivity of LST-CO samples under 1064 nm.
4. Conclusions
The bulk samples of CeO2-doped LST with different doping contents and sintering temperatures were prepared to investigate the effects of CeO2 doping as a second phase on the oxygen-deficient properties and reflection behavior of LST under high-temperature conditions.
Through systematic experiments, it was found that increasing the doping content of CeO2 led to a decrease in reflectivity due to the preferential release of oxygen as O2 rather than capturing by LST oxygen vacancies when the CeO2 doping content was over 10 wt.%. This indicates that the efficiency of oxygen release is higher than oxygen capture in this system. Under 10 wt.% CeO2 doping, the reflectivity exhibits a mountain peak-like trend with increasing preparation temperature. The sample at 1450 °C exhibits the best reflection property, up to 96%. At ultra-high temperatures (1550 °C), the generation of a large number of oxygen vacancies reduces reflection. However, small amounts of CeO2 doping can effectively improve oxygen deficiency and enhance reflection under high-temperature preparation conditions.
The findings demonstrate that CeO2 doping significantly influences the oxygen-related properties and optical behavior of LST at elevated temperatures. This provides valuable insights into designing advanced materials for applications requiring stable oxygen-deficient states and controlled reflectivity. The results suggest potential pathways for optimizing LST-based coatings or devices operating in high-temperature environments. This material can be applied in optical protective fields with high temperature requirements.
Author Contributions
Conceptualization, W.L.; methodology, W.L.; investigation, W.L. and Y.Z.; data curation, W.L. and Z.H.; writing—original draft preparation, W.L.; writing—review and editing, Y.Z. and Z.H.; visualization, W.L.; supervision, Y.Z and Z.H.; project administration, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, W.; Ma, Z.; Gao, L.; Wang, F. Preparation and electrical properties of La0.9Sr0.1TiO3+δ. Materials 2015, 8, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Vilquin, B.; Kanki, T.; Yanagida, T.; Tanaka, H.; Kawai, T. Effect of Sr doping on LaTiO3 thin films. Appl. Surf. Sci. 2005, 244, 494–497. [Google Scholar] [CrossRef]
- Higuchi, T.; Tsukamoto, T.; Taguchi, Y.; Tokura, Y.; Shin, S. Electronic structure for the metal-insulator transition in La1-xSrxTiO3 probed by resonant soft-X-ray emission spectroscopy. Phys. B Condens. Matter 2004, 351, 310–312. [Google Scholar] [CrossRef]
- Yun, J.N.; Zhang, Z.Y.; Yan, J.F.; Wu, Z. Electronic structure and optical properties of La-doped SrTiO3 and Sr2TiO4 by density function theory. Chin. Phys. Lett. 2009, 26, 017107. [Google Scholar]
- Turky, A.O.; Rashad, M.M.; Hassan, A.M.; Elnaggar, E.M.; Bechelany, M. Optical, electrical and magnetic properties of lanthanum strontium manganite La1-xSrxMnO3 synthesized through the citrate combustion method. Phys. Chem. Chem. Phys. 2017, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Gao, L.; Ma, Z.; Liu, Y.; Wang, F. Optical property of La1-xSrxTiO3+δ coatings deposited by plasma spraying technique. Appl. Surf. Sci. 2015, 356, 935–940. [Google Scholar] [CrossRef]
- Tului, M.; Arezzo, F.; Pawlowski, L. Optical Properties of Plasma Sprayed ZnO + Al2O3 Coatings. Surf. Coat. Technol. 2004, 179, 47–55. [Google Scholar] [CrossRef]
- Gao, L.; Ma, Z.; Wang, S.; Wang, F.; Yang, C. Structural and optical properties of La1-xSrxTiO3+δ. Materials 2014, 7, 4982–4993. [Google Scholar] [CrossRef]
- Seok, H.K.; Choi, E.Y.; Cha, P.R.; Son, M.C.; Choi, B.L. Characterization of Plasma-Sprayed Y2O3 Coating and Investigation of Its Visual Aspect Change. Surf. Coat. Technol. 2011, 205, 3341–3346. [Google Scholar] [CrossRef]
- Ma, R.; Li, Z.; Liu, F. The effect of Gd3+ and Ti4+ co-doping on the thermal radiation performance of (Sm1-xGdx)2(Hf1-xTix)2O7 (0 ≤ x ≤ 0.2) ceramic coatings. Cerammic Int. 2019, 45, 16130–16137. [Google Scholar] [CrossRef]
- Sutton, R.J.; Eperon, G.E.; Miranda, L.; Parrott, E.S.; Kamino, B.A.; Patel, J.B.; Hörantner, M.T.; Johnston, M.B.; Haghighirad, A.A.; Moore, D.T.; et al. Bandgaptunable cesium lead halide perovskites with high thermal stability for efficient solar cells. Adv. Energy Mater. 2016, 6, 1502458. [Google Scholar] [CrossRef]
- Xiao, Y.; Jia, W.; Du, M.; Li, Z.; Ma, Y.; Zou, Y.; Ma, C.; Yi, X.; Wang, J.; Li, Y.; et al. High-entropy (YxEr1-x)2(Ti0.2Zr0.2Hf0.2Ge0.2Sn0.2)2O7 oxide: A promising thermal barrier coating material with potential fluorescent Nondestructive Function. Mater. Sci. Eng. B 2025, 316, 118145. [Google Scholar] [CrossRef]
- Pawlowski, L. The Science and Engineering of Thermal Spray Coatings; Wiley: Chichester, UK, 1995; Chapter 8; pp. 484–501. [Google Scholar]
- Zhu, J.; Ma, Z.; Gao, Y.; Gao, L.; Pervak, V.; Wang, L.; Wei, C.; Wang, F. Ablation behavior of plasma-sprayed La1-xSrxTiO3+δ coating irradiated by high-intensity continuous laser. ACS Appl. Mater. Interfaces 2017, 9, 35444–35452. [Google Scholar] [CrossRef]
- Kumar, P.; Jena, P.; Patro, P.K.; Lenka, R.K.; Sinha, A.S.K.; Singh, P.; Singh, R.K. Influence of Lanthanum Doping on Structural and Electrical/Electrochemical Properties of Double Perovskite Sr2CoMoO6 as Anode Materials for Intermediate-Temperature Solid Oxide Fuel Cells. ACS Appl. Mater. Interface 2022, 8, 24659–24667. [Google Scholar] [CrossRef] [PubMed]
- Jaffari, G.H.; Imrana, A.; Bah, M.; Ali, A.; Bhatti, A.S.; Qurashi, U.S.; Shah, S.I. Identification and quantification of oxygen vacancies in CeO2 nanocrystals and their role in formation of F-centers. Appl. Surf. Sci. 2017, 396, 547–553. [Google Scholar] [CrossRef]
- Atran, A.A.; Algethami, J.S.; Hegazy, H.; Hamdy, M.S. Improving the photoluminescence of CeO2 nanoparticles via creating oxygen vacancies in the crystal lattice. Optik 2024, 308, 171818. [Google Scholar] [CrossRef]
- Su, X.H.; Li, W.J.; Chen, D.; Zhang, S.; Lou, C.G.; Tian, Q.; Zhao, J.G.; Zhao, P. Rapid fabrication of oxygen-deficient zirconia by flash sintering treatment. J. Adv. Ceramics. 2024, 13, 1881–1890. [Google Scholar] [CrossRef]
- Yang, J.; Tan, L.; Ji, P.; Sun, F.; Tian, Q.; Su, X. Rapid preparation of Gd2Zr2-xCexO7 waste forms by flash sintering and their chemical durability. J. Eur. Ceram. Soc. 2023, 43, 4950–4957. [Google Scholar] [CrossRef]
- Bandna, B.; Santosh, K.; Lee, H.N.; Rajesh, K. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar]
- Biswas, A.; Li, N.; Jung, M.H.; Lee, Y.W.; Kim, J.S.; Jeong, Y.H. La Doped SrTiO3 Thin Films on SrLaAIO4(001) as Transparent Conductor. J. Appl. Phys. 2013, 113, 183711. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.H.; Huang, C.P.; Zhang, Q.; Chang, C.; Chang, A.; Yao, J. High performance of Ni0.9Mn1.8Mg0.3O4 spinel nanoceramic microbeads via inkjet printing and two step sintering. RSC Adv. 2016, 6, 35118–35123. [Google Scholar] [CrossRef]
- Stokes, R.J. Correlation of mechanical properties with microstructure. Natl. Bur. Stand. Misc. Publ. 1964, 257, 41–72. [Google Scholar]
- Meng, F.; Fan, Z.; Zhang, C.; Hu, Y.; Guan, T.; Li, A. Morphology-controlled synthesis of CeO2 microstructures and their room temperature ferromagnetism. J. Mater. Sci. Technol. 2017, 33, 444–451. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Liu, J.; Tian, Z.; Jing, Y. Regulation of oxygen vacancies in cobalt-cerium oxide catalyst for boosting decontamination of VOCs by catalytic oxidation. Sep. Purif. Technol. 2021, 277, 119505. [Google Scholar] [CrossRef]
- Shi, J.; Li, H.; Genest, A.; Zhao, W.; Qi, P.; Wang, T.; Rupprechter, G. High-performance water gas shift induced by asymmetric oxygen vacancies: Gold clusters supported by ceria-praseodymia mixed oxides. Appl. Catal. B Environ. 2022, 301, 120789. [Google Scholar] [CrossRef]
- Smirnova, I.; Bazhenov, A.; Fursova, T.; Dubovitskii, A.; Uspenskaya, L.; Maksimuk, M. IR-Active Optical Phonons in Pnma-1, Pnma-2 and R3c Phases of LaMnO3+δ. Phys. B 2008, 403, 3896–3902. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, J.; Wang, H.; Ma, Z.; Gao, L.H.; Liu, Y.B.; Liu, Y.; Shu, Y.C.; He, J. Enhanced optical reflectivity and electrical properties in perovskite functional ceramics by inhibiting oxygen vacancy formation. Ceram. Int. 2021, 47, 5549–5558. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).