Carbonation and Chloride Resistance Characteristics of Self-Developed Limestone Calcined Clay Cement (LC3) Derived from Excavated Spoil
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chloride-Binding Capacity
2.3. Rapid Chloride Migration (RCM)
2.4. Accelerated Carbonation
2.5. Microstructural Analyses
3. Results
3.1. Chloride Penetration Resistance
3.2. Carbonation Resistance
4. Discussion of the Durability Performance of Self-Developed LC3
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Ijaz, N.; Ye, W.; Rehman, Z.U.; Ijaz, Z.; Junaid, M.F. Global insights into micro-macro mechanisms and environmental implications of limestone calcined clay cement (LC3) for sustainable construction applications. Sci. Total Environ. 2024, 907, 167794. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yi, P.; Du, H.; Liu, W.; Mi, T.; Huang, L.; Gao, X.; Sun, X.; Xing, F. Activation of locally excavated spoil for utilization in limestone calcined clay cement (LC3). Constr. Build. Mater. 2024, 420, 135518. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. The reaction between metakaolin and limestone and its effect in porosity refinement and mechanical properties. Cem. Concr. Res. 2021, 140, 106307. [Google Scholar] [CrossRef]
- Yu, Y.; Gunasekara, C.; Elakneswaran, Y.; Robert, D.; Law, D.W.; Setunge, S. On the hydration of limestone calcined kaolinitic clay cement and energy-efficient production. Cem. Concr. Compos. 2024, 153, 105698. [Google Scholar] [CrossRef]
- Zunino, F.; Martirena, F.; Scrivener, K. Limestone Calcined Clay Cements (LC3). ACI Mater. J. 2021, 118, 49–56. [Google Scholar]
- Dhandapani, Y.; Santhanam, M. Investigation on the microstructure-related characteristics to elucidate performance of composite cement with limestone-calcined clay combination. Cem. Concr. Res. 2020, 129, 105959. [Google Scholar] [CrossRef]
- Meng, L.; Yi, H.; Park, K.-B.; Lin, R.; Wang, X. Partial replacement of ordinary Portland cement with belite-rich cement to produce limestone calcined clay cement to regulate the hydration process, improve strength, and reduce carbon emissions. Constr. Build. Mater. 2025, 460, 139865. [Google Scholar] [CrossRef]
- Lu, M.; Xia, Y.; Yan, J.; Wang, L. Wet carbonation of MSWI fly ash for sustainable limestone calcined clay cement-type composites. Cem. Concr. Compos. 2025, 156, 105866. [Google Scholar] [CrossRef]
- Wu, W.; He, X.; Yang, W.; Wei, B.; Alam, M.S. Durability and microstructure degradation mechanism of FRP-seawater seasand concrete structures: A review. Constr. Build. Mater. 2023, 391, 131825. [Google Scholar] [CrossRef]
- Dong, S.; Gu, J.; Ouyang, X.; Jang, S.-H.; Han, B. Enhancing mechanical properties, durability and multifunctionality of concrete structures via using ultra-high performance concrete layer: A review. Compos. Part B Eng. 2025, 297, 112329. [Google Scholar] [CrossRef]
- Ler, K.H.; Ma, C.K.; Chin, C.L.; Ibrahim, I.S.; Padil, K.H.; Ghafar, M.A.I.A.; Lenya, A.A. Porosity and durability tests on 3D printing concrete: A review. Constr. Build. Mater. 2024, 446, 137973. [Google Scholar] [CrossRef]
- Rajadesingu, S.; Mendonce, K.C.; Palani, N.; Monisha, P.; Vijayakumar, P.; Ayyadurai, S. Exploring the potential of bacterial concrete: A sustainable solution for remediation of crack and durability enhancement—A critical review. Constr. Build. Mater. 2024, 439, 137238. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, G.; Ye, H.; Zeng, Q.; Zhang, Z.; Tian, Z.; Jin, X.; Jin, N.; Chen, Z.; Wang, J. Corrosion of steel rebar in concrete induced by chloride ions under natural environments. Constr. Build. Mater. 2023, 369, 130504. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Nepal, J.; Das, B.B. Properties; compatibility, environmental benefits and future directions of limestone calcined clay cement (LC3) concrete: A review. J. Build. Eng. 2023, 79, 107794. [Google Scholar] [CrossRef]
- Yang, P.; Dhandapani, Y.; Santhanam, M.; Neithalath, N. Simulation of chloride diffusion in fly ash and limestone-calcined clay cement (LC3) concretes and the influence of damage on service-life. Cem. Concr. Res. 2020, 130, 106010. [Google Scholar] [CrossRef]
- Zolfagharnasab, A.; Ramezanianpour, A.A.; Bahman-Zadeh, F. Investigating the potential of low-grade calcined clays to produce durable LC3 binders against chloride ions attack. Constr. Build. Mater. 2021, 303, 124541. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Santhanam, M. Assessment of pore structure evolution in the limestone calcined clay cementitious system and its implications for performance. Cem. Concr. Compos. 2017, 84, 36–47. [Google Scholar] [CrossRef]
- Maraghechi, H.; Avet, F.; Wong, H.; Kamyab, H.; Scrivener, K. Performance of Limestone Calcined Clay Cement (LC 3) with various kaolinite contents with respect to chloride transport. Mater. Struct. 2018, 51, 125. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, C.; Zhou, Q.; Guo, M.; Hu, R. Unveiling the role of LC3 in chloride penetration resistance of chloride contaminated mortar. Constr. Build. Mater. 2025, 458, 139658. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Gao, X.; Yu, R.; Huang, Y.; Cheng, S. Understanding the chloride binding and diffusion behaviors of marine concrete based on Portland limestone cement-alumina enriched pozzolans. Constr. Build. Mater. 2019, 198, 207–217. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Influence of pH on the chloride binding capacity of Limestone Calcined Clay Cements (LC3). Cem. Concr. Res. 2020, 131, 106031. [Google Scholar] [CrossRef]
- Pillai, R.G.; Gettu, R.; Santhanam, M.; Rengaraju, S.; Dhandapani, Y.; Rathnarajan, S.; Basavaraj, A.S. Service life and life cycle assessment of reinforced concrete systems with limestone calcined clay cement (LC3). Cem. Concr. Res. 2019, 118, 111–119. [Google Scholar] [CrossRef]
- Rengaraju, S.; Pillai, R.G.; Neelakantan, L.; Gettu, R.; Santhanam, M. Chloride-Induced Corrosion Resistance of Steel Embedded in Limestone Calcined Clay Cement Systems. In Calcined Clays for Sustainable Concrete, Proceedings of the 3rd International Conference on Calcined Clays for Sustainable Concrete, New Delhi, India, 15–17 October 2019; Springer: Berlin/Heidelberg, Germany, 2020; pp. 613–619. [Google Scholar]
- Mi, T.; Wang, D.; Li, Y.; Wang, Y. Passivation behaviour of aluminium alloys in limestone calcined clay cement (LC3). Constr. Build. Mater. 2024, 421, 135633. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Y.; Benn, T.; Ataabadi, H.S.; Zhuge, Y. The self-healing performance and mechanism of limestone calcined clay composites coupling with crystalline admixture in chloride-rich environments. Constr. Build. Mater. 2025, 463, 140097. [Google Scholar] [CrossRef]
- Rathnarajan, S.; Dhanya, B.S.; Pillai, R.G.; Gettu, R.; Santhanam, M. Carbonation model for concretes with fly ash, slag, and limestone calcined clay—Using accelerated and five—Year natural exposure data. Cem. Concr. Compos. 2022, 126, 104329. [Google Scholar] [CrossRef]
- Li, L.; Wu, M. An overview of utilizing CO2 for accelerated carbonation treatment in the concrete industry. J. CO2 Util. 2022, 60, 102000. [Google Scholar] [CrossRef]
- Li, L.; Liu, Q.; Dao, V.; Wu, M. Dimensional change of cement paste subjected to carbonation in CO2 sequestration and utilization context: A critical review on the mechanisms. J. CO2 Util. 2023, 70, 102444. [Google Scholar] [CrossRef]
- Leo, E.S.; Alexander, M.G.; Beushausen, H. Compressive strength and durability performance of limestone calcined clay cement concrete made from selected African raw materials. Constr. Build. Mater. 2024, 438, 137012. [Google Scholar] [CrossRef]
- Hay, R.; Celik, K. Comparative performance of OPC and LC3-based composites under combined action of carbonation and chloride exposure. Constr. Build. Mater. 2024, 429, 136428. [Google Scholar] [CrossRef]
- Khan, M.S.; Nguyen, Q.D.; Castel, A. Performance of limestone calcined clay blended cement-based concrete against carbonation. Adv. Cem. Res. 2020, 32, 481–491. [Google Scholar] [CrossRef]
- Bahman-Zadeh, F.; Ramezanianpour, A.A.; Zolfagharnasab, A. Effect of carbonation on chloride binding capacity of limestone calcined clay cement (LC3) and binary pastes. J. Build. Eng. 2022, 52, 104447. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Liu, R.; Zhu, P.; Liu, H.; Wang, X.; Yu, J.; Chen, Y. Chloride penetration of concrete exposed to dry-wet cycle with various dry-wet ratios and temperature. Constr. Build. Mater. 2023, 400, 132883. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Mi, T.; Yu, J.; Xing, F.; Li, W. Corrosion mechanism of reinforcement in LC3 cement pastes under coupled carbonation and chloride attack. Cem. Concr. Compos. 2023, 140, 105080. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Yu, Z.; Chen, P.; Zhang, Y. A review on ultra-high performance seawater sea sand concrete: Hydration, microstructure and properties. Constr. Build. Mater. 2024, 438, 136945. [Google Scholar] [CrossRef]
- ASTM C1152/C1152M-20; Standard Test Method for Acid-Soluble Chloride in Mortar and Concrete. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM C1218/C1218M-20; Standard Test Method for Water-Soluble Chloride in Mortar and Concrete. ASTM International: West Conshohocken, PA, USA, 2020.
- GB/T50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2009.
- Weise, K.; Ukrainczyk, N.; Koenders, E. Pozzolanic reactions of metakaolin with calcium hydroxide: Review on hydrate phase formations and effect of alkali hydroxides, carbonates and sulfates. Mater. Des. 2023, 231, 112062. [Google Scholar] [CrossRef]
- Song, H.; Jeong, Y.; Jun, Y.; Yoon, S.; Oh, J.E. A study of thermal decomposition of phases in cementitious systems using HT-XRD and TG. Constr. Build. Mater. 2018, 169, 648–661. [Google Scholar] [CrossRef]
- Wang, Y.; Ueda, T.; Gong, F.; Zhang, D. Meso-scale mechanical deterioration of mortar due to sodium chloride attack. Cem. Concr. Compos. 2019, 96, 163–173. [Google Scholar] [CrossRef]
- Shah, V.; Scrivener, K.; Bhattacharjee, B.; Bishnoi, S. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M.; Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 2007, 37, 1182–1192. [Google Scholar] [CrossRef]
- Li, Y.Q.; Mi, T.; Liu, W.; Dong, Z.; Dong, B.; Tang, L.; Xing, F. Chemical and mineralogical characteristics of carbonated and uncarbonated cement pastes subjected to high temperatures. Compos. Part B Eng. 2021, 216, 108861. [Google Scholar] [CrossRef]
- Richardson, I.G. The nature of C-S-H in hardened cements. Cem. Concr. Res. 1999, 29, 1131–1147. [Google Scholar] [CrossRef]
- Azar, P.; Patapy, C.; Samson, G.; Cussigh, F.; Frouin, L.; Cyr, M. Effect of natural and accelerated carbonation on microstructure and pH of sodium carbonate alkali-activated slag. Cem. Concr. Res. 2024, 181, 107525. [Google Scholar] [CrossRef]
- Yin, W.; Wei, W.; Wang, X.; Zhu, N. The analysis of concrete carbonated shrinkage and mechanism. Constr. Qual. 2014, 32, 31–35. [Google Scholar]
- Ijaz, N.; Ye, W.; Wang, Q.; Chen, Y.; Rehman, Z.U.; Ijaz, Z.; Khalid, U. A hydraulic binder for reconstituted compacted clay under wet-dry cycles: Low carbon limestone calcined clay cement. J. Rock Mech. Geotech. Eng. 2024, in press.
- Jan, A.; Ferrari, L.; Mikanovic, N.; Ben-Haha, M.; Franzoni, E. Chloride ingress and carbonation assessment of mortars prepared with recycled sand and calcined clay-based cement. Constr. Build. Mater. 2024, 456, 139337. [Google Scholar] [CrossRef]
- Mañosa, J.; Calderón, A.; Salgado-Pizarro, R.; Maldonado-Alameda, A.; Chimenos, J.M. Research evolution of limestone calcined clay cement (LC3), a promising low-carbon binder—A comprehensive overview. Heliyon 2024, 10, e25117. [Google Scholar] [CrossRef]
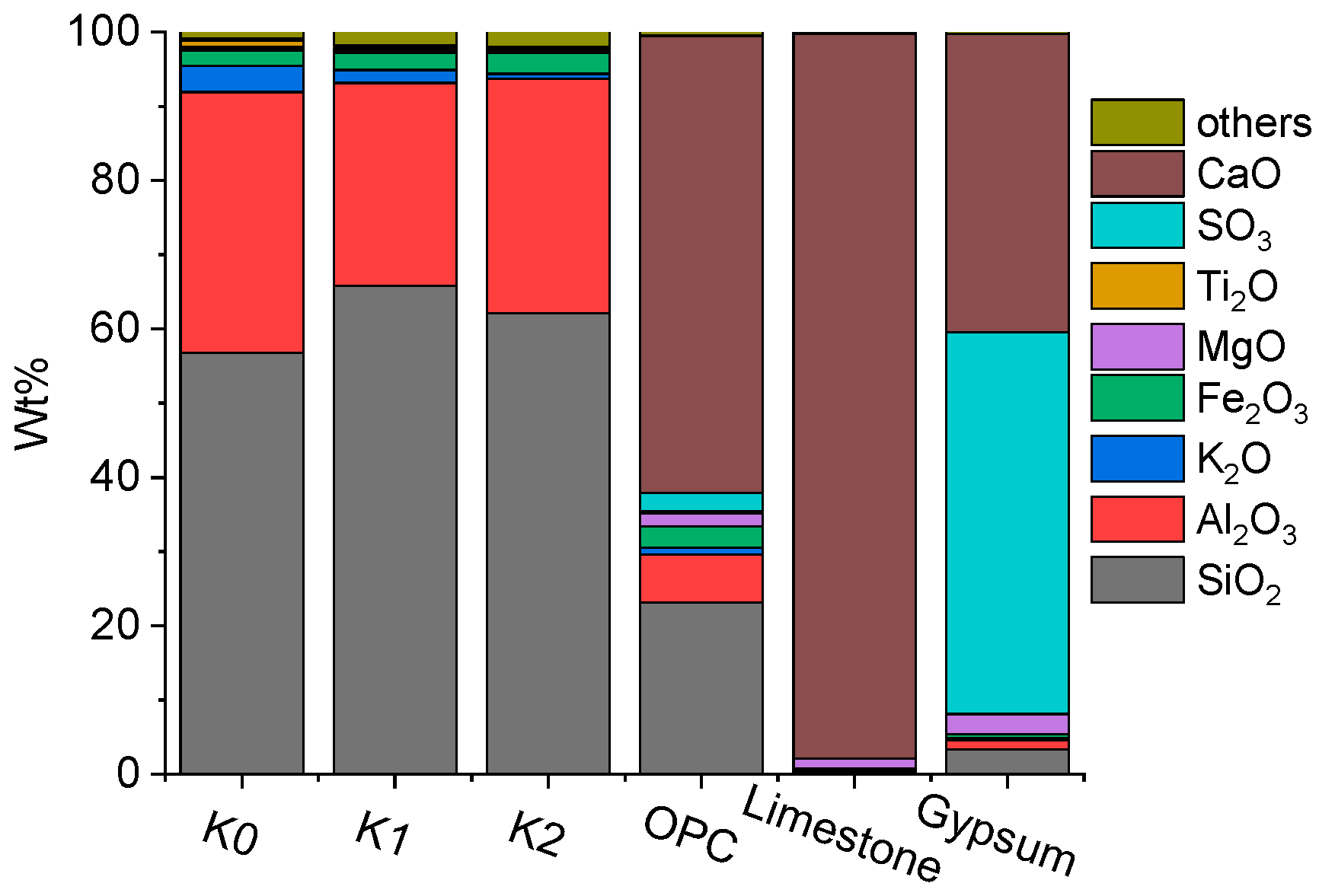
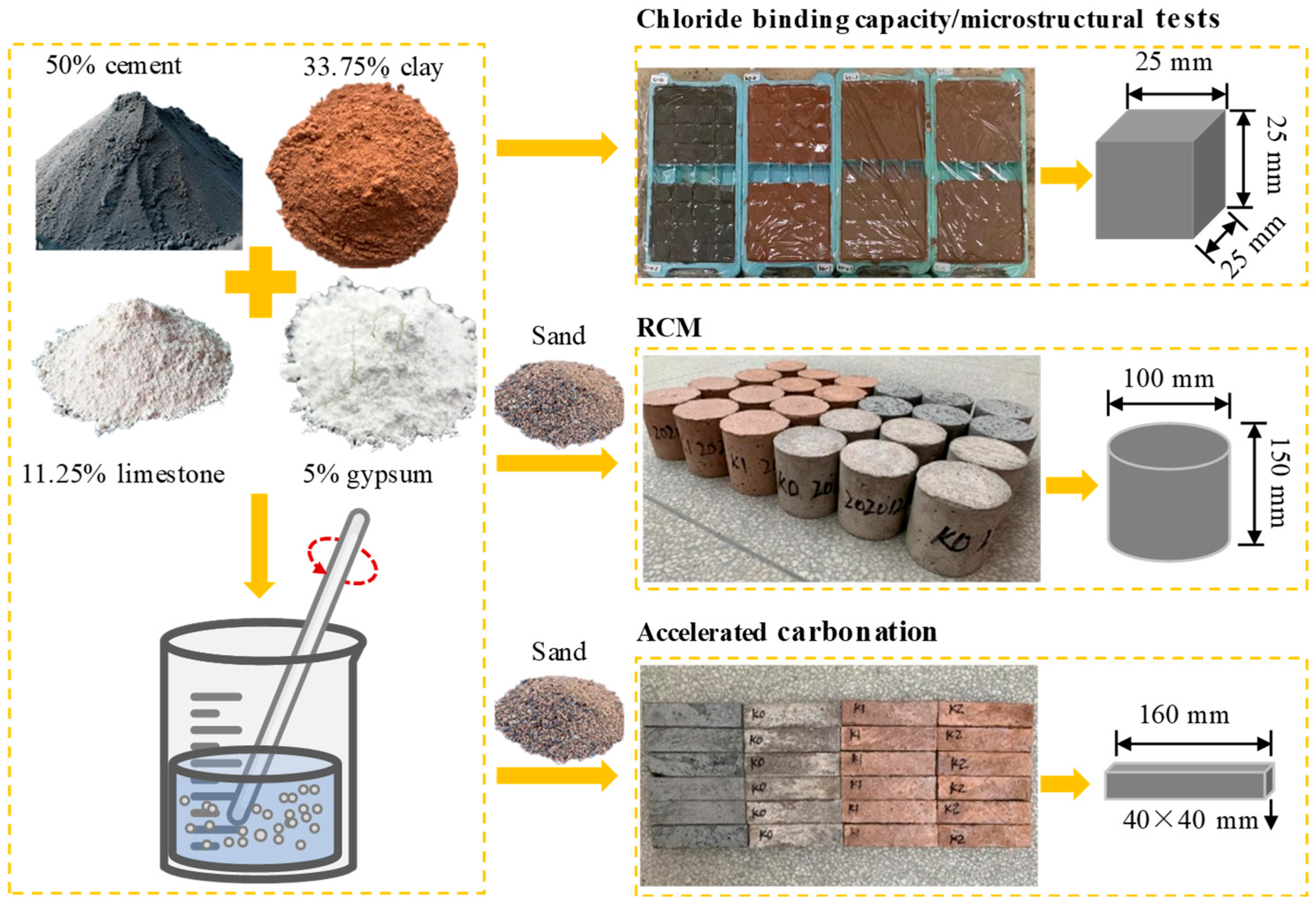
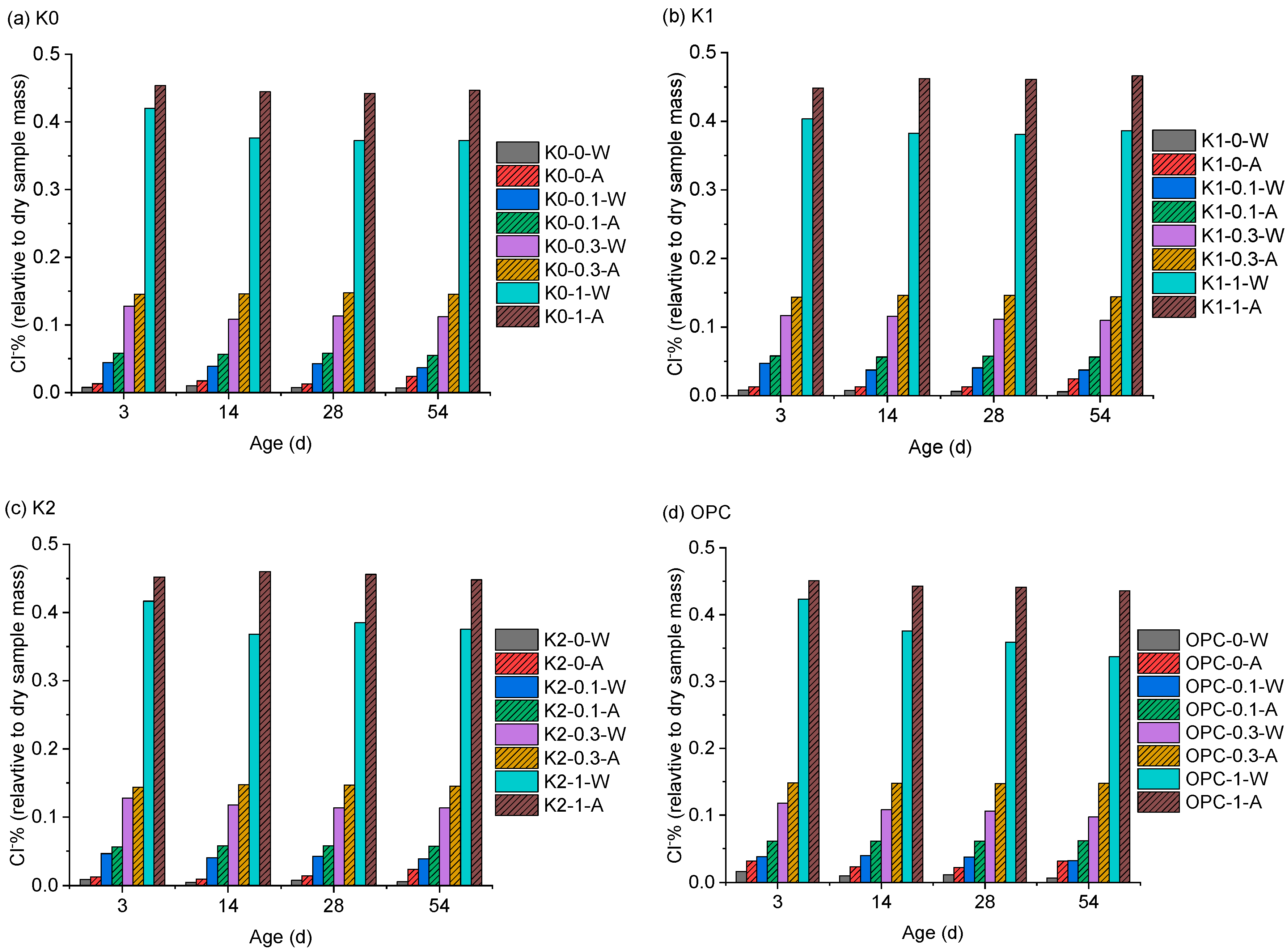


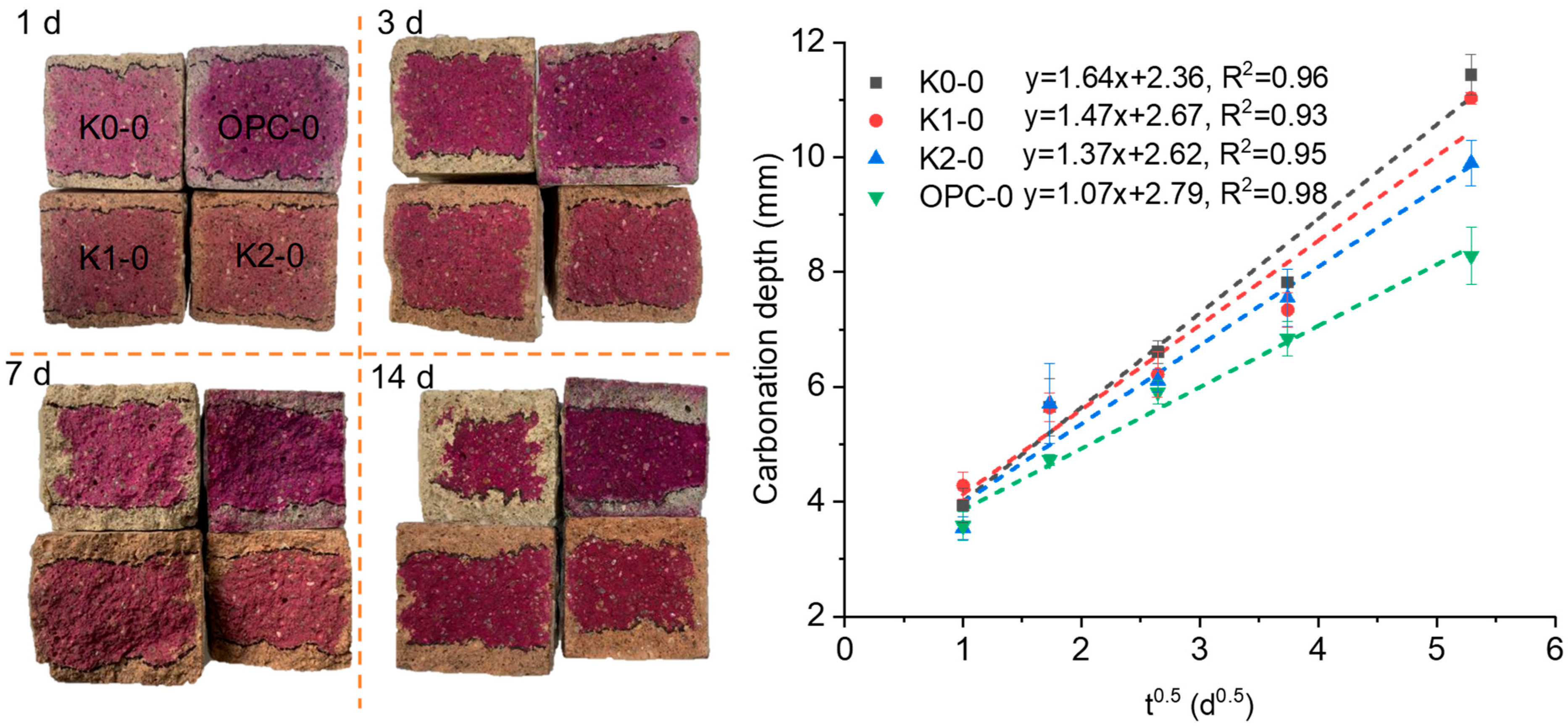
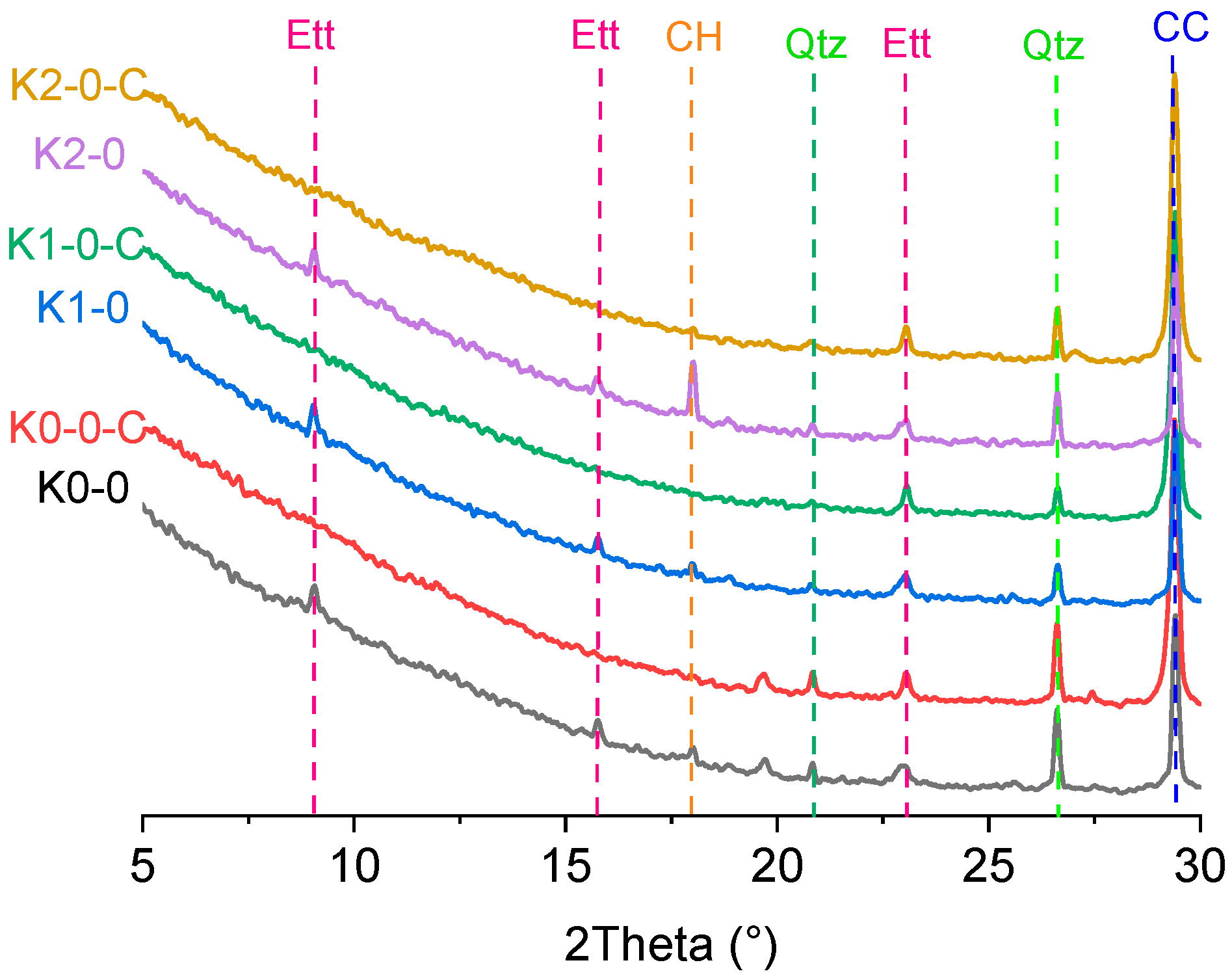
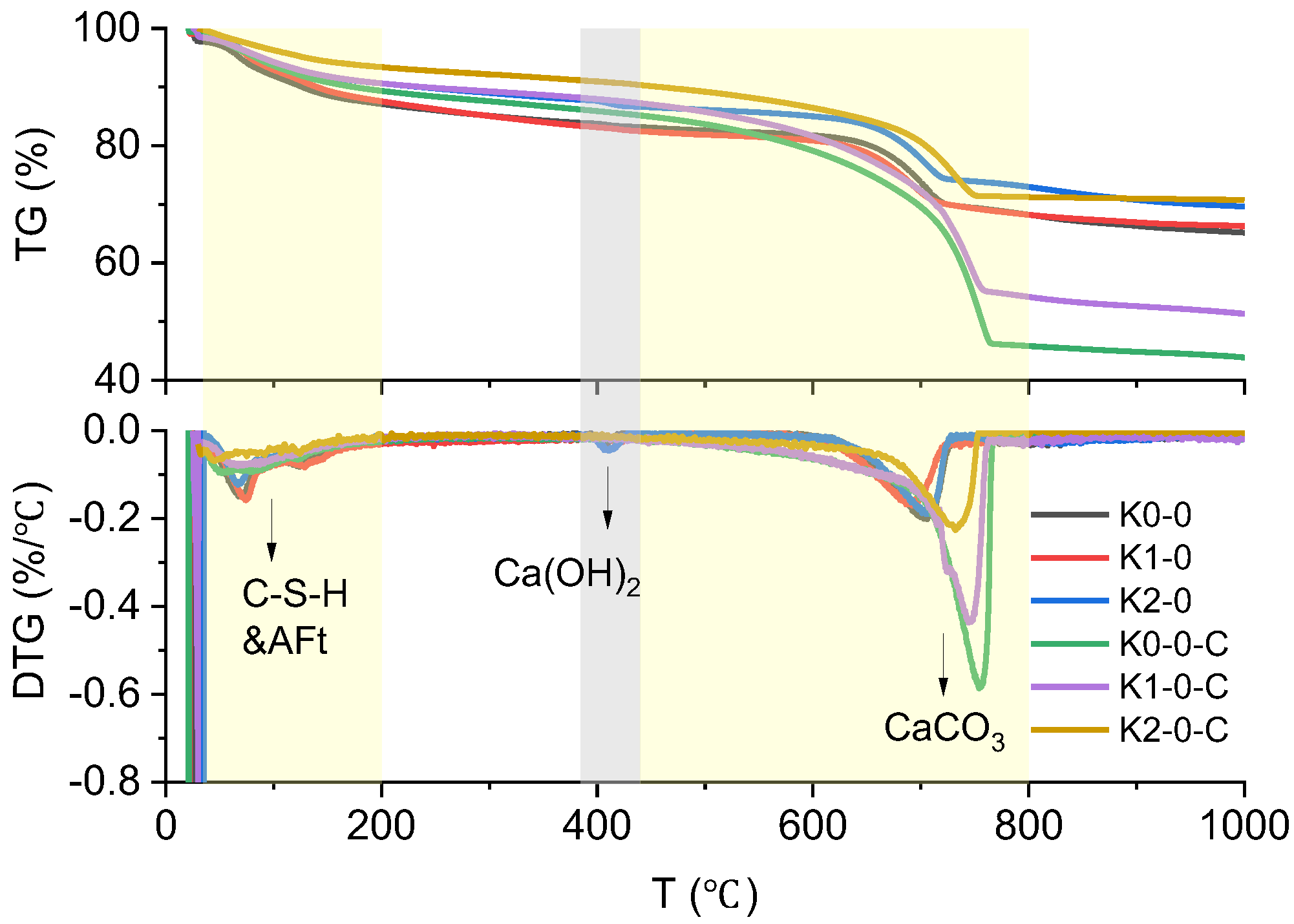
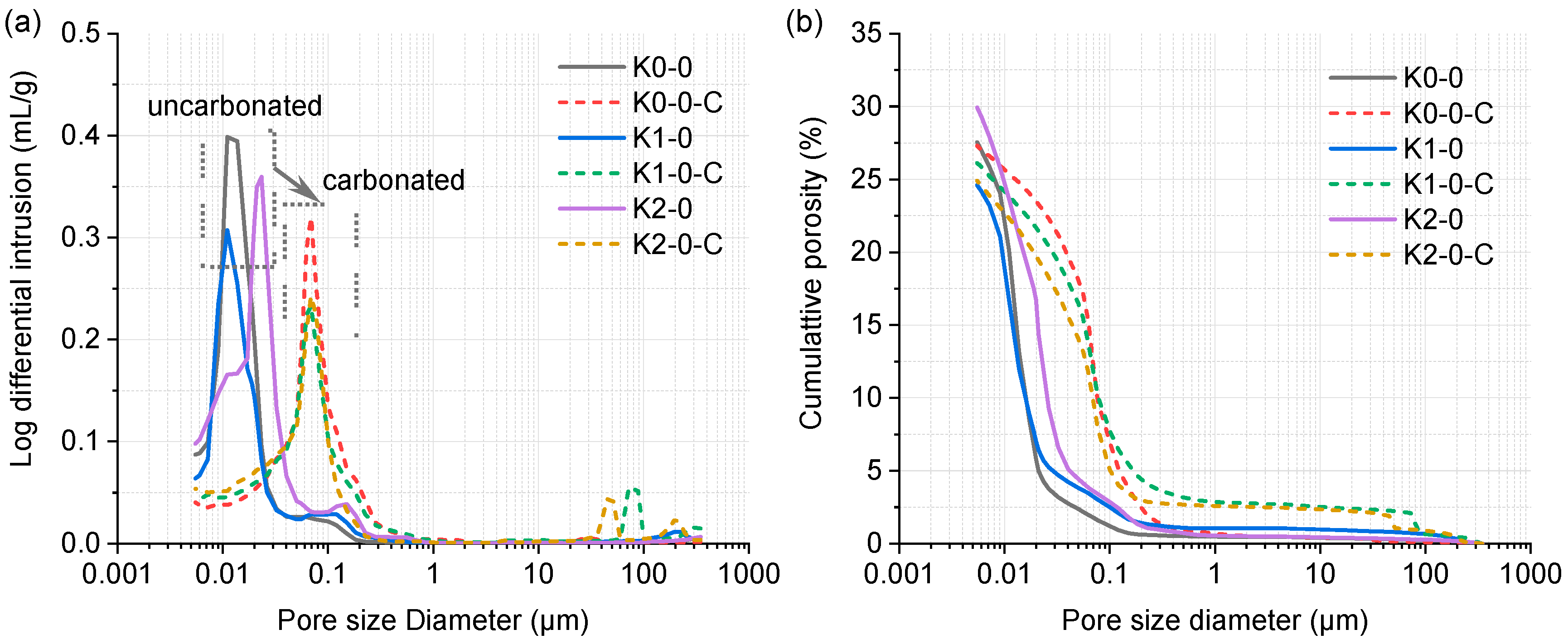
| No. | Clay | OPC (%) | Clay (%) | Limestone (%) | Gypsum (%) | SP 1 (%) | Cl− 2 (%) |
|---|---|---|---|---|---|---|---|
| K0-0 | K0 | 50 | 30 | 15 | 5 | 0.3 | 0 |
| K0-0.1 | K0 | 50 | 30 | 15 | 5 | 0.3 | 0.1 |
| K0-0.3 | K0 | 50 | 30 | 15 | 5 | 0.3 | 0.3 |
| K0-1 | K0 | 50 | 30 | 15 | 5 | 0.3 | 1 |
| K1-0 | K1 | 50 | 30 | 15 | 5 | 0.3 | 0 |
| K1-0.1 | K1 | 50 | 30 | 15 | 5 | 0.3 | 0.1 |
| K1-0.3 | K1 | 50 | 30 | 15 | 5 | 0.3 | 0.3 |
| K1-1 | K1 | 50 | 30 | 15 | 5 | 0.3 | 1 |
| K2-0 | K2 | 50 | 30 | 15 | 5 | 0.18 | 0 |
| K2-0.1 | K2 | 50 | 30 | 15 | 5 | 0.18 | 0.1 |
| K2-0.3 | K2 | 50 | 30 | 15 | 5 | 0.18 | 0.3 |
| K2-1 | K2 | 50 | 30 | 15 | 5 | 0.18 | 1 |
| OPC-0 | - | 100 | / | / | / | / | 0 |
| OPC-0.1 | - | 100 | / | / | / | / | 0.1 |
| OPC-0.3 | - | 100 | / | / | / | / | 0.3 |
| OPC-1 | - | 100 | / | / | / | / | 1 |
| No. | C-S-H & AFt Related Loss (%) | Ca(OH)2 (%) | CaCO3 (%) |
|---|---|---|---|
| K0-0 | 4.4 | 3.0 | 34.0 |
| K0-0-C | 3.9 | 0 | 89.4 |
| K1-0 | 5.0 | 3.7 | 32.4 |
| K1-0-C | 3.3 | 0 | 75.1 |
| K2-0 | 3.3 | 5.2 | 31.1 |
| K2-0-C | 2.7 | 0 | 43.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Miao, L.; Dong, Z.; Jin, Y.; Liu, W.; Gao, F.; Li, Y. Carbonation and Chloride Resistance Characteristics of Self-Developed Limestone Calcined Clay Cement (LC3) Derived from Excavated Spoil. Materials 2025, 18, 2546. https://doi.org/10.3390/ma18112546
Li Y, Miao L, Dong Z, Jin Y, Liu W, Gao F, Li Y. Carbonation and Chloride Resistance Characteristics of Self-Developed Limestone Calcined Clay Cement (LC3) Derived from Excavated Spoil. Materials. 2025; 18(11):2546. https://doi.org/10.3390/ma18112546
Chicago/Turabian StyleLi, Yunyuan, Lixin Miao, Zhijun Dong, Yu Jin, Wei Liu, Fangsheng Gao, and Yongqiang Li. 2025. "Carbonation and Chloride Resistance Characteristics of Self-Developed Limestone Calcined Clay Cement (LC3) Derived from Excavated Spoil" Materials 18, no. 11: 2546. https://doi.org/10.3390/ma18112546
APA StyleLi, Y., Miao, L., Dong, Z., Jin, Y., Liu, W., Gao, F., & Li, Y. (2025). Carbonation and Chloride Resistance Characteristics of Self-Developed Limestone Calcined Clay Cement (LC3) Derived from Excavated Spoil. Materials, 18(11), 2546. https://doi.org/10.3390/ma18112546







