Synthesis and Performance Evaluation of Anti-Washout Admixtures for Underwater Non-Dispersive Concrete Based on Nanosilica
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
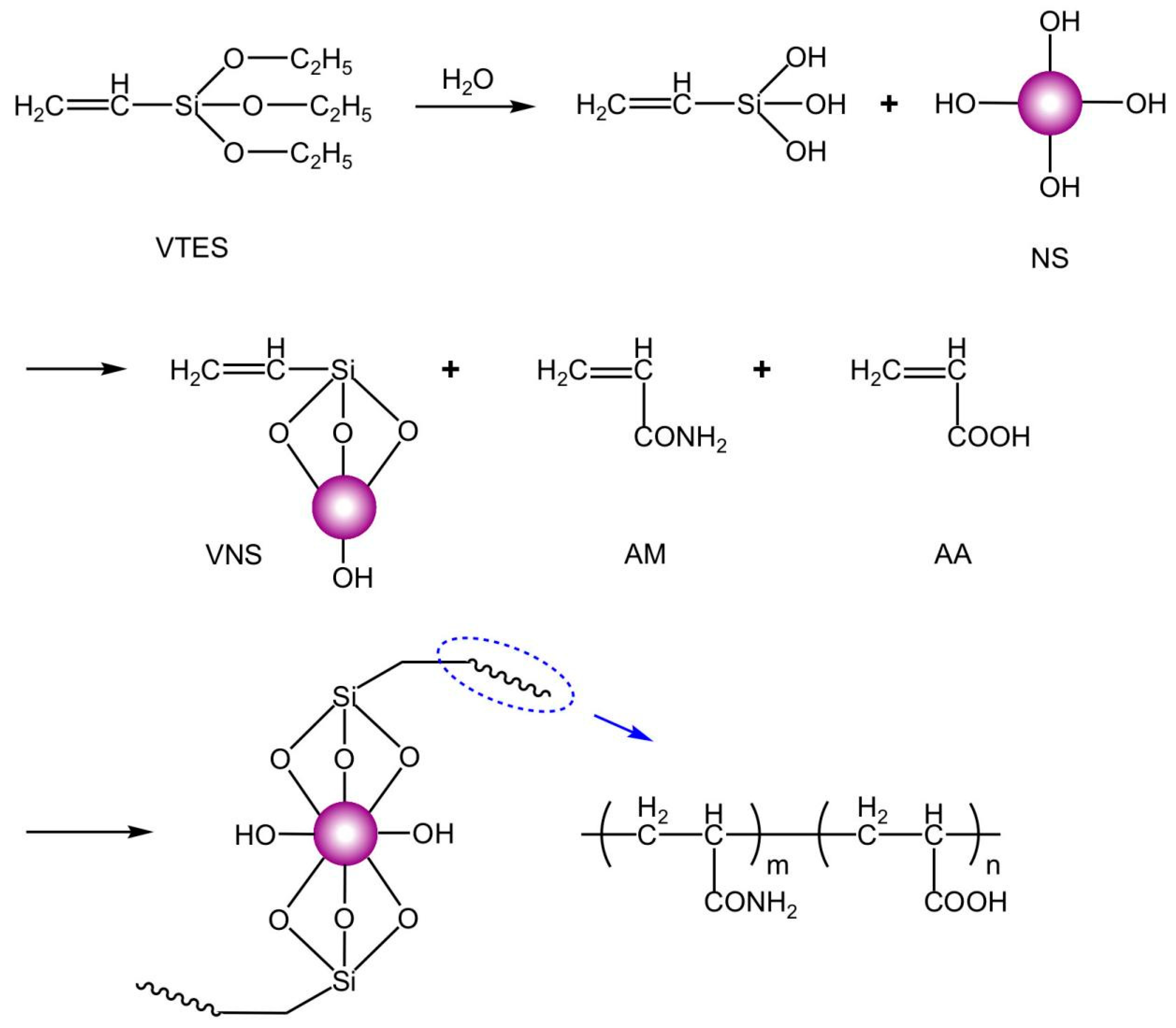
2.2. Polymer Synthesis
2.3. Polymer Characterization
2.4. Preparation of Cement Samples
2.5. Cement Materials Performance Testing
2.5.1. Basic Properties of Cement Paste
2.5.2. Scour Resistance of Cement Mortar
3. Results and Discussion
3.1. Optimization of Process Conditions for VNS-AM-AA
3.2. Characterization of VNS-AM-AA
3.3. Compatibility of Polymers with Water-Reducing Agents
3.4. Anti-Dispersity and Flowability
3.5. Setting Time
3.6. Scour Resistance
3.7. Microscopic Morphology
4. Conclusions
- (a)
- The synthesized VNS-AM-AA achieved the best flocculation effect when NS was selected to be 20 nm, the mass ratio of silane coupling agent VTES to NS was 0.3, the addition of NS was 0.6% of the total mass of the monomer, and the molar ratio of the organic components AM and AA was 3.5:1. The relative molecular weight of the synthesized VNS-AM-AA was about 6 million at this time. FTIR, TEM, and SEM analyses showed that the synthesized polymer was the target product, and it did not easily agglomerate in water with a three-dimensional network effect.
- (b)
- Compatibility experiments showed that VNS-AM-AA was more compatible with the polycarboxylic acid water-reducing agent (SP), and at an SP dosing of 0.6% of cement mass, the loss fluidity in 60 min warp time was only 4 mm, with the best stability of cement paste.
- (c)
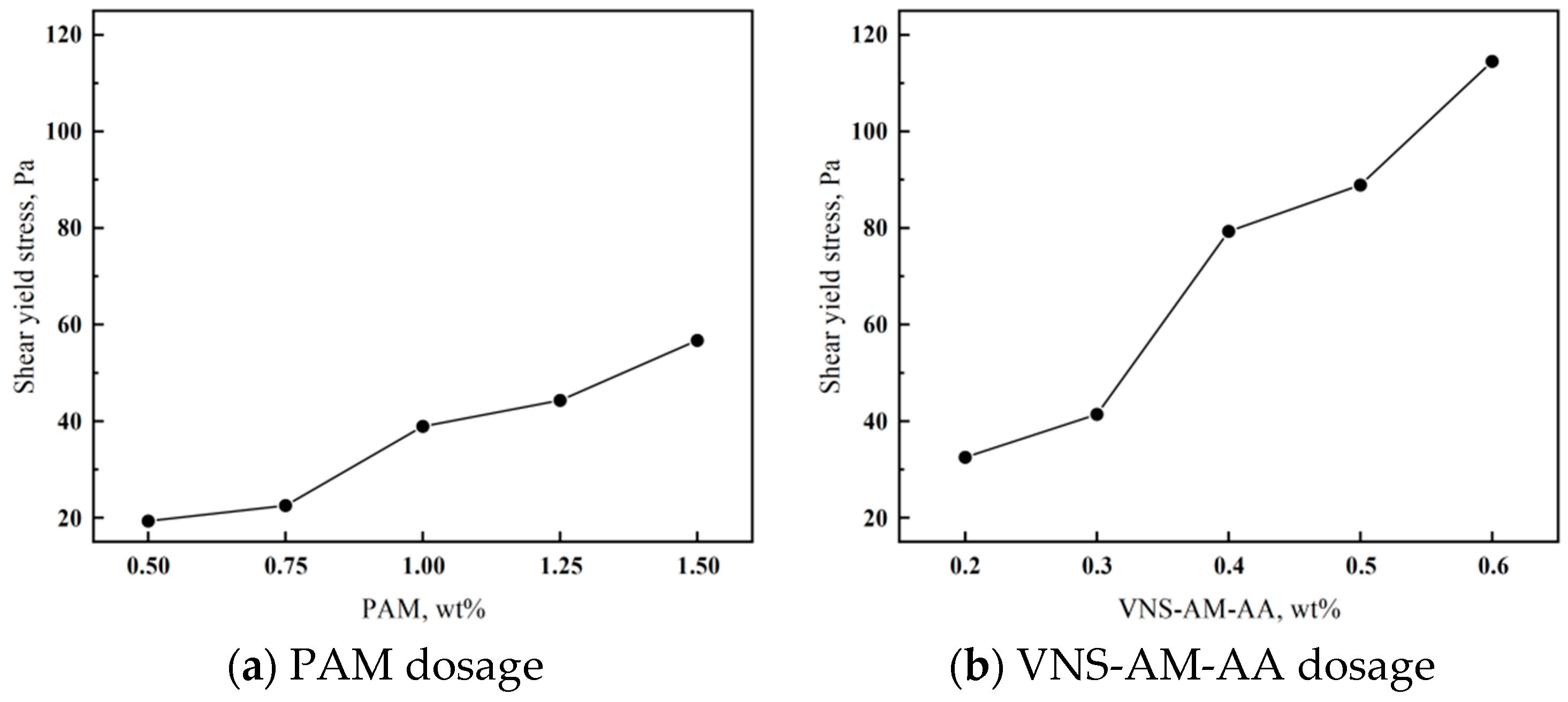
- The anti-dispersity experiment and the flowability experiment showed that the anti-dispersity of VNS-AM-AA was better than that of PAM at a smaller dosage, but its fluidity was more sensitive to the influence of the dosage, and it could be seen that the optimal dosage of VNS-AM-AA for combined fluidity and anti-dispersity was 0.4%. Due to the deeper entanglement of cement particles, the initial setting time of cement paste was delayed by 3–6 h and the final setting time was delayed by 3–9 h after mixing in VNS-AM-AA, which were higher than those of PAM. In actual construction, VNS-AM-AA should be used with a quick-setting agent to reduce the setting time.
- (d)
- The yield shear stress of cement mortar was tested to characterize the scouring resistance of mortar in water, and it was found that VNS-AM-AA could give cement mortar a higher yield shear stress at dosage higher than 0.4%. And the higher the yield shear stress, the less likely that the mortar would be moved by water scouring. So, VNS-AM-AA has the effect of improving the scouring resistance.
- (e)
- Observing the microscopic morphology of the cement mortar with two AWAs after 7d, it can be found that the incorporation of PAM and VNS-AM-AA does not change the type of hydration products, but it will generate complexes to encapsulate the hydration products. Compared with more pores caused by the incorporation of PAM, the incorporation of VNS-AM-AA has relatively fewer pores and a more compact and regular structure, which is beneficial to the improvement of engineering quality.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, H.; Tian, Z.; Zhang, M.; Sun, X.; Ma, Y. Study on testing methods for water resistance of underwater cement paste. Mater. Struct. 2021, 54, 180. [Google Scholar] [CrossRef]
- Palacios, M.; Flatt, R.J. Working mechanism of viscosity-modifying admixtures. In Science and Technology of Concrete Admixtures; Aïtcin, P.-C., Flatt, R.J., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 415–432. [Google Scholar]
- Lu, H.; Sun, X.; Ma, H. Anti-washout concrete: An overview. Constr. Build. Mater. 2022, 344, 128151. [Google Scholar] [CrossRef]
- Yousri, K.M. Self-flowing underwater concrete mixtures. Mag. Concr. Res. 2008, 60, 1–10. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Ming, X.; Chen, B.; Li, Z. Enhancing anti-washout behavior of cement paste by polyacrylamide gelation: From floc properties to mechanism. Cem. Concr. Compos. 2023, 136, 104887. [Google Scholar] [CrossRef]
- Grzeszczyk, S.; Jurowski, K.; Bosowska, K.; Grzymek, M. The role of nanoparticles in decreased washout of underwater concrete. Constr. Build. Mater. 2019, 203, 670–678. [Google Scholar] [CrossRef]
- Huijun, W.; Diao, Z.; Fan, K. Study on durability of non-dispersible concrete in seawater environment. Int. J. Struct. Integr. 2019, 11, 443–452. [Google Scholar] [CrossRef]
- Sha, F.; Lin, C.; Li, Z.; Liu, R. Reinforcement simulation of water-rich and broken rock with Portland cement-based grout. Constr. Build. Mater. 2019, 221, 292–300. [Google Scholar] [CrossRef]
- Horszczaruk, E.; Brzozowski, P. Properties of underwater concretes containing large amount of fly ashes. Procedia Eng. 2017, 196, 97–104. [Google Scholar] [CrossRef]
- Liu, F.; Wang, B.; Wang, M.; Yuan, X. Analysis on pore structure of non-dispersible underwater concrete in saline soil area. J. Renew. Mater. 2021, 9, 723–742. [Google Scholar] [CrossRef]
- Li, X.; Hou, Y.; Gu, X. Study on the effect factors of mechanical properties of porous cement concrete. J. Nanjing For. Univ. 2008, 32, 113–115. [Google Scholar] [CrossRef]
- Jiao, L.; Su, M.; Chen, L.; Wang, Y.; Zhu, H.; Dai, H. Natural cellulose nanofibers as sustainable enhancers in construction cement. PLoS One 2016, 11, e0168422. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Lu, W.; Zhang, Y. Research progress and application prospects of plant fibers in geopolymer concrete: A review. Materials 2025, 18, 2342. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.A.; Chen, S.; Jin, F. Washout resistance of self-protected underwater concrete in freshwater and seawater. Constr. Build. Mater. 2021, 289, 123186. [Google Scholar] [CrossRef]
- Nasr, A.A.; Chen, S.; Wang, Y.; Jin, F.; Qiu, L. Influence of water currents velocity on the strength of a new underwater concrete approach. Constr. Build. Mater. 2022, 356, 129236. [Google Scholar] [CrossRef]
- Hao, D. Survey on the performance of underwater non-dispersive concrete. J. Eng. Res. Reports. 2023, 24, 37–43. [Google Scholar] [CrossRef]
- Su, L.; Ma, B.; Jian, S.; Zhao, Z.; Liu, M. Hydration heat effect of cement pastes modified with hydroxypropyl methyl cellulose ether and expanded perlite. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2013, 28, 122–126. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Wang, P.; Xu, L. Effect of HEMC on the early hydration of Portland cement highlighted by isothermal calorimetry. J. Therm. Anal. Calorim. 2015, 119, 1833–1843. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Q.; Liu, C.; Zhou, M. Properties comparison of mortars with welan gum or cellulose ether. Constr. Build. Mater. 2016, 102, 648–653. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. npj Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Y.; Peng, G.; Chen, Y. A branched hydrophobicity associated with polyacrylamide based on silica: Synthesis and solution properties. J. Polym. Res. 2019, 26, 250. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Yin, H.; Feng, Y.; Quan, H. Comparative studies on enhanced oil recovery: Thermoviscosifying polymer versus polyacrylamide. Energy Fuels 2017, 31, 2479–2487. [Google Scholar] [CrossRef]
- Sjöblom, J.; Dagsgård, C.; Simon, S.; Sørland, G.; Hana, M. Influence of HPAM on W/O emulsion separation properties. J. Dispers. Sci. Technol. 2017, 38, 206–215. [Google Scholar] [CrossRef]
- Li, H.; Yan, N.; Sun, G.; Zheng, H.; Yang, X. Synthesis and flocculation of polyacrylamide with low water absorption for non-dispersible underwater concrete. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2023, 38, 1404–1413. [Google Scholar] [CrossRef]
- Zhong, W.; Zheng, L.; Shen, Y.; Li, W.; Zeng, L. Effect of SiO2 methyl modification on the performance of nondispersible underwater concrete and reinforcement mechanism. Adv. Civ. Eng. 2023, 2023, 7689445. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Y.; Yu, Q.; Zhan, B.; Li, W.; Xiong, C.; Chen, S.; Cheng, L.; Zheng, Y. Microscopic thickening mechanisms of hydroxypropyl methyl cellulose ether anti-washout admixture and its impact on cementitious material rheology and anti-dispersal performance. J. Build. Eng. 2024, 89, 109346. [Google Scholar] [CrossRef]
- GB/T 12005.1-1989; Determination for limiting viscosity number of polyacrylamide. China Standards Press: Beijing, China, 1989.
- GB/T 8077—2012; Methods for testing uniformity of concrete admixture. China Standards Press: Beijing, China, 2012.
- Kawai, T.; Okada, T. Effect of superplasticizer and viscosity-increasing admixture on properties of lightweight aggregate concrete. Spec. Publ. 1989, 119, 583–604. [Google Scholar] [CrossRef]
- Hu, H.M.; Yao, Z.X.; Zeng, S.Y.; Yu, B.B. Experimental research on compatibility between underwater anti-washout admixture and superplasticizer. Key Eng. Mater. 2011, 477, 190–199. [Google Scholar] [CrossRef]
- JTG 3420-2020; Testing methods of cement and concrete for highway engineering. People’s Communications Press: Beijing, China, 2020.
- Lu, H.; Li, C.; Jiang, G.; Fan, H.; Zuo, X.; Liu, H. Rheology properties of underwater cement paste with nonionic polyacrylamide. Iran. J. Sci. Technol. Trans. Civ. Eng. 2023, 47, 1995–2005. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, Y.; Ma, Z.; Gao, F.; Ma, M.; Xu, Z. Effect of polyacrylamide on rheological properties of underwater non-dispersible paste of alkali-activated slag. KSCE J. Civ. Eng. 2022, 26, 236–247. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, G.; Fang, Y. Study on the influence of HPMC on rheological parameters of cement paste and properties of concrete. IOP Conf. Ser. Earth Environ. Sci. 2020, 571, 012148. [Google Scholar] [CrossRef]
- Lachemi, M.; Hossain, K.M.A.; Lambros, V.; Nkinamubanzi, P.C.; Bouzoubaâ, N. Performance of new viscosity modifying admixtures in enhancing the rheological properties of cement paste. Cem. Concr. Res. 2004, 34, 185–193. [Google Scholar] [CrossRef]
- Jarvis, P.; Jefferson, B.; Gregory, J.; Parsons, S.A. A review of floc strength and breakage. Water Res. 2005, 39, 3121–3137. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J. Monitoring particle aggregation processes. Adv. Colloid Interface Sci. 2009, 147-148, 109–123. [Google Scholar] [CrossRef]
- Mirbagheri, M.; Hill, R.J. Sorption and diffusion of moisture in silica-polyacrylamide nanocomposite films. Polymer 2017, 122, 359–371. [Google Scholar] [CrossRef]
- Liu, R.; Pu, W.-F.; Du, D.-J. Synthesis and characterization of core–shell associative polymer that prepared by oilfield formation water for chemical flooding. J. Ind. Eng. Chem. 2017, 46, 80–90. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, H.; Huang, Y.; Zheng, X.; Liu, Z.; An, Y.; Zhao, C.; Liu, Y. Hydrophobic modification of cationic microblocked polyacrylamide and its enhanced flocculation performance for oily wastewater treatment. J. Mater. Sci. 2019, 54, 10024–10040. [Google Scholar] [CrossRef]
- Bessaies-Bey, H.; Khayat, K.H.; Palacios, M.; Schmidt, W.; Roussel, N. Viscosity modifying agents: Key components of advanced cement-based materials with adapted rheology. Cem. Concr. Res. 2022, 152, 106646. [Google Scholar] [CrossRef]
- Yao, H.; Fan, M.; Huang, T.; Yuan, Q.; Xie, Z.; Chen, Z.; Li, Y.; Wang, J. Retardation and bridging effect of anionic polyacrylamide in cement paste and its relationship with early properties. Constr. Build. Mater. 2021, 306, 124822. [Google Scholar] [CrossRef]
- Marchon, D.; Flatt, R.J. Impact of chemical admixtures on cement hydration. In Science and Technology of Concrete Admixtures; Aïtcin, P.-C., Flatt, R.J., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 279–304. [Google Scholar]










| Composition | LOI | CaO | SO2 | SO3 | Fe2O3 | MgO | Al2O3 |
|---|---|---|---|---|---|---|---|
| Content/% | 3.01 | 72.02 | 18.99 | 2.79 | 3.03 | 1.31 | 5.79 |
| Types | Cement (g) | Water (g) | AWAs (wt%) | SP (wt%) |
|---|---|---|---|---|
| PAM | 300 | 120 | 0.5~1.5 | 0.6 |
| VNS-AM-AA | 300 | 120 | 0.2~0.6 | 0.6 |
| Types | Cement (g) | Sand (g) | Water (g) | AWAs (wt%) | SP (wt%) |
|---|---|---|---|---|---|
| PAM | 300 | 343 | 120 | 0.5~1.5 | 0.6 |
| VNS-AM-AA | 300 | 343 | 120 | 0.2~0.6 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Huang, K.; Chu, H.; Li, J. Synthesis and Performance Evaluation of Anti-Washout Admixtures for Underwater Non-Dispersive Concrete Based on Nanosilica. Materials 2025, 18, 2541. https://doi.org/10.3390/ma18112541
Wang J, Huang K, Chu H, Li J. Synthesis and Performance Evaluation of Anti-Washout Admixtures for Underwater Non-Dispersive Concrete Based on Nanosilica. Materials. 2025; 18(11):2541. https://doi.org/10.3390/ma18112541
Chicago/Turabian StyleWang, Jian, Kaijian Huang, Hongyan Chu, and Jianhui Li. 2025. "Synthesis and Performance Evaluation of Anti-Washout Admixtures for Underwater Non-Dispersive Concrete Based on Nanosilica" Materials 18, no. 11: 2541. https://doi.org/10.3390/ma18112541
APA StyleWang, J., Huang, K., Chu, H., & Li, J. (2025). Synthesis and Performance Evaluation of Anti-Washout Admixtures for Underwater Non-Dispersive Concrete Based on Nanosilica. Materials, 18(11), 2541. https://doi.org/10.3390/ma18112541






