Design of a Superhydrophobic Photothermal Shape-Memory Material Based on Carbon-Nanotubes-Doped Resin for Anti-Icing/De-Icing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Preparation of Photothermal Epoxy Substrates
2.2.2. Surface Engineering of CNT/Shape-Memory Epoxy Composites via Laser
2.3. Testing and Characterization
2.3.1. Characterization
2.3.2. Shape-Memory Behavioral Representations
2.3.3. Characterization of Icephobic Efficacy
2.3.4. Photothermal Performance Investigation
3. Results and Discussion
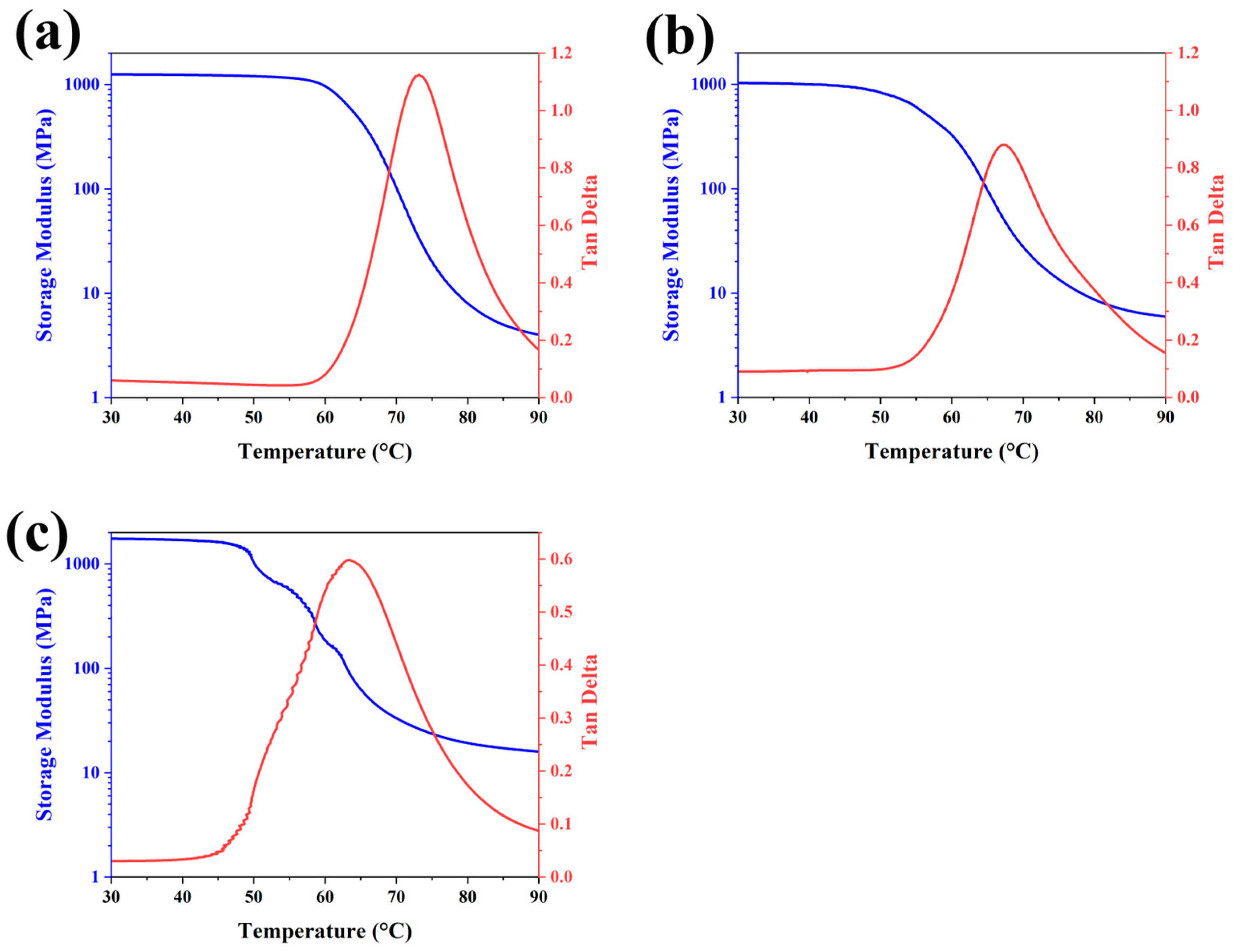
3.1. Shape-Memory Performance
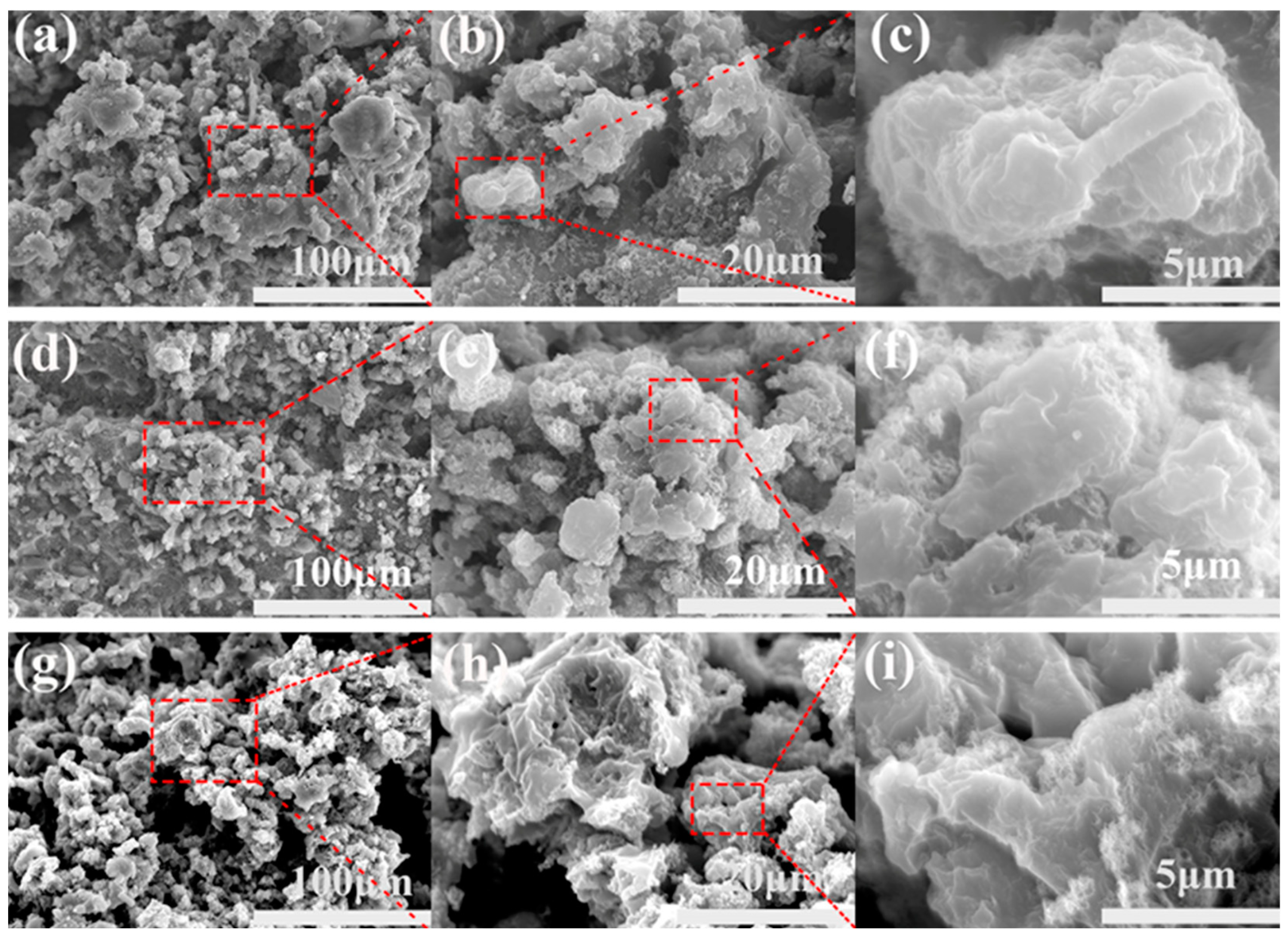
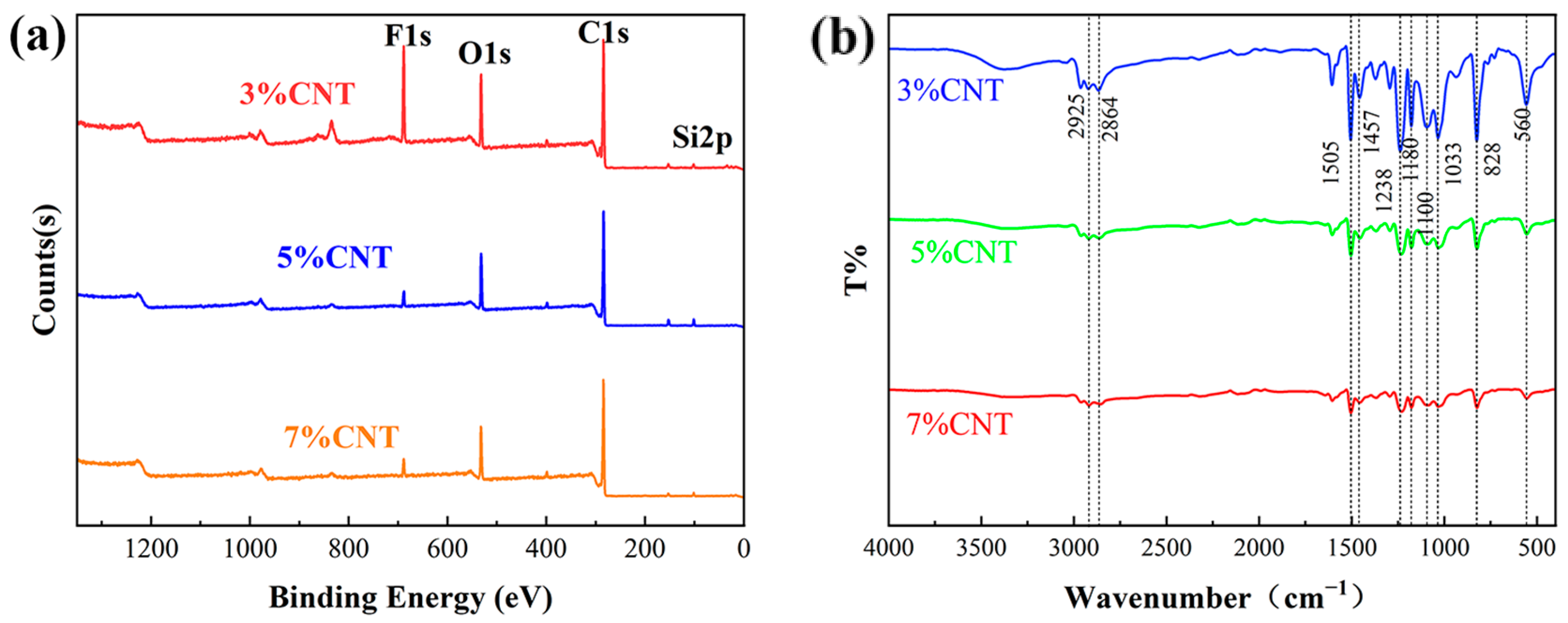
3.2. Physicochemical Analysis of CNT/Shape-Memory Epoxy Composites
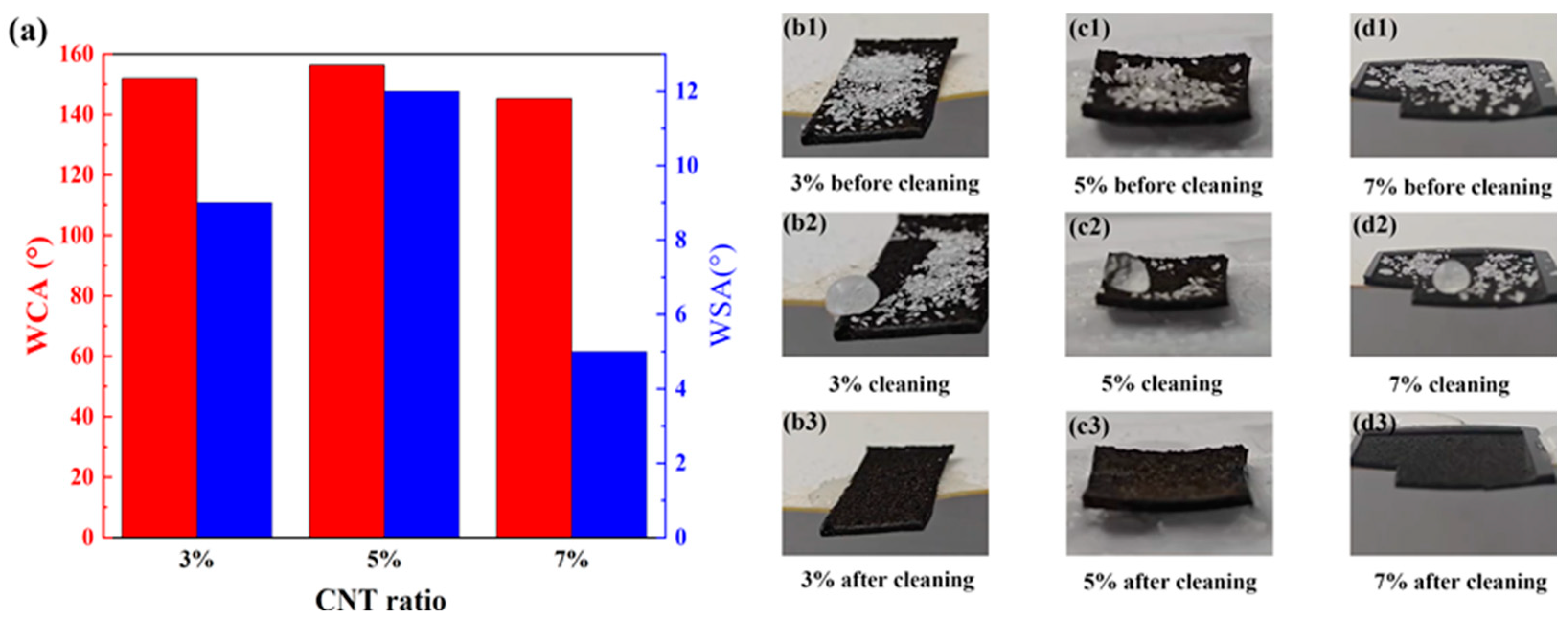
3.3. Self-Cleaning Properties
3.4. Anti-/De-Icing Properties
3.5. Mechanical Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrey, J. Long-Term Trends in Weather-Related Crash Risks. J. Transp. Geogr. 2010, 18, 247–258. [Google Scholar] [CrossRef]
- Bartels-Rausch, T. Ten Things we Need to Know About Ice and Snow. Nature 2013, 494, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.; Liu, Y.; Xu, R.; Liu, S.; Zhou, F. Robust Photothermal Coating Strategy for Efficient Ice Removal. ACS Appl. Mater. Interfaces 2020, 12, 46981–46990. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Eslami-Farsani, R. A Review of Photothermal and Superhydrophobic Polymer Nanocomposites as Anti-Icing Nanocoatings. Polym. Compos. 2025, 46, 5018–5040. [Google Scholar] [CrossRef]
- Parent, O.; Ilinca, A. Anti-Icing and De-Icing Techniques for Wind Turbines: Critical Review. Cold Reg. Sci. Tech. 2011, 65, 88–96. [Google Scholar] [CrossRef]
- Thomas, S.K.; Cassoni, R.P.; MacArthur, C.D. Aircraft Anti-Icing and De-Icing Techniques and Modeling. J. Aircr. 1996, 33, 841–854. [Google Scholar] [CrossRef]
- Bird, J.C.; Dhiman, R.; Kwon, H.; Varanasi, K.K. Reducing the Contact Time of a Bouncing Drop. Nature 2013, 503, 385–388. [Google Scholar] [CrossRef]
- Maitra, T.; Antonini, C.; Tiwari, M.K.; Mularczyk, A.; Imeri, Z.; Schoch, P.; Poulikakos, D. Supercooled Water Drops Impacting Superhydrophobic Textures. Langmuir 2014, 30, 10855–10861. [Google Scholar] [CrossRef]
- Zeng, D.; Li, Y.; Liu, H.; Yang, Y.; Peng, L.; Zhu, C.; Zhao, N. Superhydrophobic Coating Induced Anti-Icing and Deicing Characteristics of an Airfoil. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130824. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, H.; Chen, M. Multi-Scale Superhydrophobic Surface with Excellent Stability and Solar-Thermal Performance for Highly Efficient Anti-Icing and Deicing. Small 2024, 20, 2312226. [Google Scholar] [CrossRef]
- Xuan, S.; Zhuo, L.; Li, G.; Zeng, Q.; Liu, J.; Yu, J.; Chen, L.; Yang, Y.; Liu, S.; Wang, Y.; et al. Micro/Nano Hierarchical Crater-Like Structure Surface with Mechanical Durability and Low-Adhesion for Anti-Icing/Deicing. Small 2024, 20, 2404979. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, Y.; Li, S.; Xiao, A. A Multifunctional Superhydrophobic Phase-Change Photothermal Coating for Concrete Anti-Icing. Polym. Int. 2024. early view. [Google Scholar] [CrossRef]
- Wei, J.; Liang, W.; Mao, M.; Li, B.; Zhang, J. Facile Preparation of Impalement Resistant, Mechanically Robust and Weather Resistant Photothermal Superhydrophobic Coatings for Anti-/De-Icing. Chem.—Asian J. 2024, 19, e202400110. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, J.; He, W.; Xiong, M.; Niu, X.; Li, X.; Yu, D. A Review On Fabrication and Application of Tunable Hybrid Micro–Nano Array Surfaces. Adv. Mater. Interfaces 2023, 10, 2202160. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, D.; Luo, X.; Lai, H.; Liu, Y.; Jiang, L. Superwetting Shape Memory Microstructure: Smart Wetting Control and Practical Application. Adv. Mater. 2021, 33, 2001718. [Google Scholar] [CrossRef]
- Curtis, S.M.; Sielenkämper, M.; Arivanandhan, G.; Dengiz, D.; Li, Z.; Jetter, J.; Hanke, L.; Bumke, L.; Quandt, E.; Wulfinghoff, S.; et al. Tinihf/Sio2/Si Shape Memory Film Composites for Bi-Directional Micro Actuation. Int. J. Smart Nano Mater. 2022, 13, 293–314. [Google Scholar] [CrossRef]
- Yang, S.; He, Y.; Leng, J. Shape Memory Poly (Ether Ether Ketone)S with Tunable Chain Stiffness, Mechanical Strength and High Transition Temperatures. Int. J. Smart Nano Mater. 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Zhang, F.; Wen, N.; Wang, L.; Bai, Y.; Leng, J. Design of 4D Printed Shape-Changing Tracheal Stent and Remote Controlling Actuation. Int. J. Smart Nano Mater. 2021, 12, 375–389. [Google Scholar] [CrossRef]
- Lendlein, A.; Gould, O.E.C. Reprogrammable Recovery and Actuation Behaviour of Shape-Memory Polymers. Nat. Rev. Mater. 2019, 4, 116–133. [Google Scholar] [CrossRef]
- Yang, Q.S.; Liu, X.; Leng, F.F. Effective Thermo-Mechanical Properties and Shape Memory Effect of CNT/SMP Composites. Proc. SPIE 2009, 7493. [Google Scholar]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A Review of Shape Memory Polymers and Composites: Mechanisms, Materials, and Applications. Adv. Mater. 2021, 33, 2000713. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhan, B.; Wang, X.; Liu, Y.; Liu, Y.; Leng, J. Preparation of Superhydrophobic Shape Memory Composites with Uniform Wettability and Morphing Performance. Compos. Sci. Technol. 2024, 247, 110398. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, F.; Wang, L.; Liu, Y.; Leng, J. Recent Advances in Shape Memory Polymers: Multifunctional Materials, Multiscale Structures, and Applications. Adv. Funct. Mater. 2024, 34, 2312036. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Jiao, X.; Song, Y.; Meng, J.; Cheng, Z. Controllable Droplet Sliding On a Smart Shape-Memory Slippery Surface. Chem.—Asian J. 2022, 17, e202200481. [Google Scholar] [CrossRef]
- Lai, H.; Shang, Y.; Cheng, Z.; Lv, T.; Zhang, E.; Zhang, D.; Wang, J.; Liu, Y. Control of Tip Nanostructure On Superhydrophobic Shape Memory Arrays Toward Reversibly Adjusting Water Adhesion. Adv. Compos. Hybrid Mater. 2019, 2, 753–762. [Google Scholar] [CrossRef]
- Li, X.; Zhan, Y.; Li, W.; Huang, Z.; Amirfazli, A. Light- and Heat-Responsive Superhydrophobic Surfaces with Shape Memory Capacity Prepared by 4D Printing. Adv. Eng. Mater. 2024, 26, 2401415. [Google Scholar] [CrossRef]
- Uemura, S.; Matsuo, Y.; Okamatsu, T.; Arita, T.; Shimomura, M.; Hirai, Y. Low-Friction, Superhydrophobic, and Shape-Memory Vulcanized Rubber Microspiked Structures. Adv. Eng. Mater. 2020, 22, 1901226. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, D.; Lv, T.; Lai, H.; Zhang, E.; Kang, H.; Wang, Y.; Liu, P.; Liu, Y.; Du, Y.; et al. Superhydrophobic Shape Memory Polymer Arrays with Switchable Isotropic/Anisotropic Wetting. Adv. Funct. Mater. 2018, 28, 1705002. [Google Scholar] [CrossRef]
- Hao, S.; Zhan, Y.; Li, W.; Zhao, W.; Amirfazli, A. Preparation of Epoxy Resin/Carbon Black Superhydrophobic Photothermal Materials with Shape Memory Capacity for Anti/De-Icing. Adv. Eng. Mater. 2024, 26, 2301948. [Google Scholar] [CrossRef]
- Bai, X.; Yang, Q.; Fang, Y.; Yong, J.; Bai, Y.; Zhang, J.; Hou, X.; Chen, F. Anisotropic, Adhesion-Switchable, and Thermal-Responsive Superhydrophobicity On the Femtosecond Laser-Structured Shape-Memory Polymer for Droplet Manipulation. Chem. Eng. J. 2020, 400, 125930. [Google Scholar] [CrossRef]
- Minakshi, M.; Samayamanthry, A.; Whale, J.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Kumar Shrestha, L. Phosphorous—Containing Activated Carbon Derived From Natural Honeydew Peel Powers Aqueous Supercapacitors. Chem.—Asian J. 2024, 19, e202400622. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Sharma, N.; Ralph, D.; Appadoo, D.; Nallathamby, K. Synthesis and Characterization of Li(Co0.5Ni0.5)Po4 Cathode for Li-Ion Aqueous Battery Applications. Electrochem. Solid-State Lett. 2011, 14, A86. [Google Scholar] [CrossRef]
- Rocha Canella Carneiro, A.; de Souza Ferreira, F.A.; Houmard, M. Easy Functionalization Process Applied to Develop Super-Hydrophobic and Oleophobic Properties on Astm 1200 Aluminum Surface. Surf. Interface Anal. 2018, 50, 1370–1383. [Google Scholar] [CrossRef]
- Yao, Y.; Li, C.; Tao, Z.; Yang, R.; Zhang, H. Experimental and Numerical Study On the Impact and Freezing Process of a Water Droplet On a Cold Surface. Appl. Therm. Eng. 2018, 137, 83–92. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Tian, P.; Li, X.; Gai, D.; Tong, W. Design of a Superhydrophobic Photothermal Shape-Memory Material Based on Carbon-Nanotubes-Doped Resin for Anti-Icing/De-Icing Applications. Materials 2025, 18, 2540. https://doi.org/10.3390/ma18112540
Zhao Y, Tian P, Li X, Gai D, Tong W. Design of a Superhydrophobic Photothermal Shape-Memory Material Based on Carbon-Nanotubes-Doped Resin for Anti-Icing/De-Icing Applications. Materials. 2025; 18(11):2540. https://doi.org/10.3390/ma18112540
Chicago/Turabian StyleZhao, Yingcheng, Pei Tian, Xinlin Li, Di Gai, and Wei Tong. 2025. "Design of a Superhydrophobic Photothermal Shape-Memory Material Based on Carbon-Nanotubes-Doped Resin for Anti-Icing/De-Icing Applications" Materials 18, no. 11: 2540. https://doi.org/10.3390/ma18112540
APA StyleZhao, Y., Tian, P., Li, X., Gai, D., & Tong, W. (2025). Design of a Superhydrophobic Photothermal Shape-Memory Material Based on Carbon-Nanotubes-Doped Resin for Anti-Icing/De-Icing Applications. Materials, 18(11), 2540. https://doi.org/10.3390/ma18112540






