3.1. Evaluation of Cytotoxicity Induced by DEX and CLOB Without a Carrier
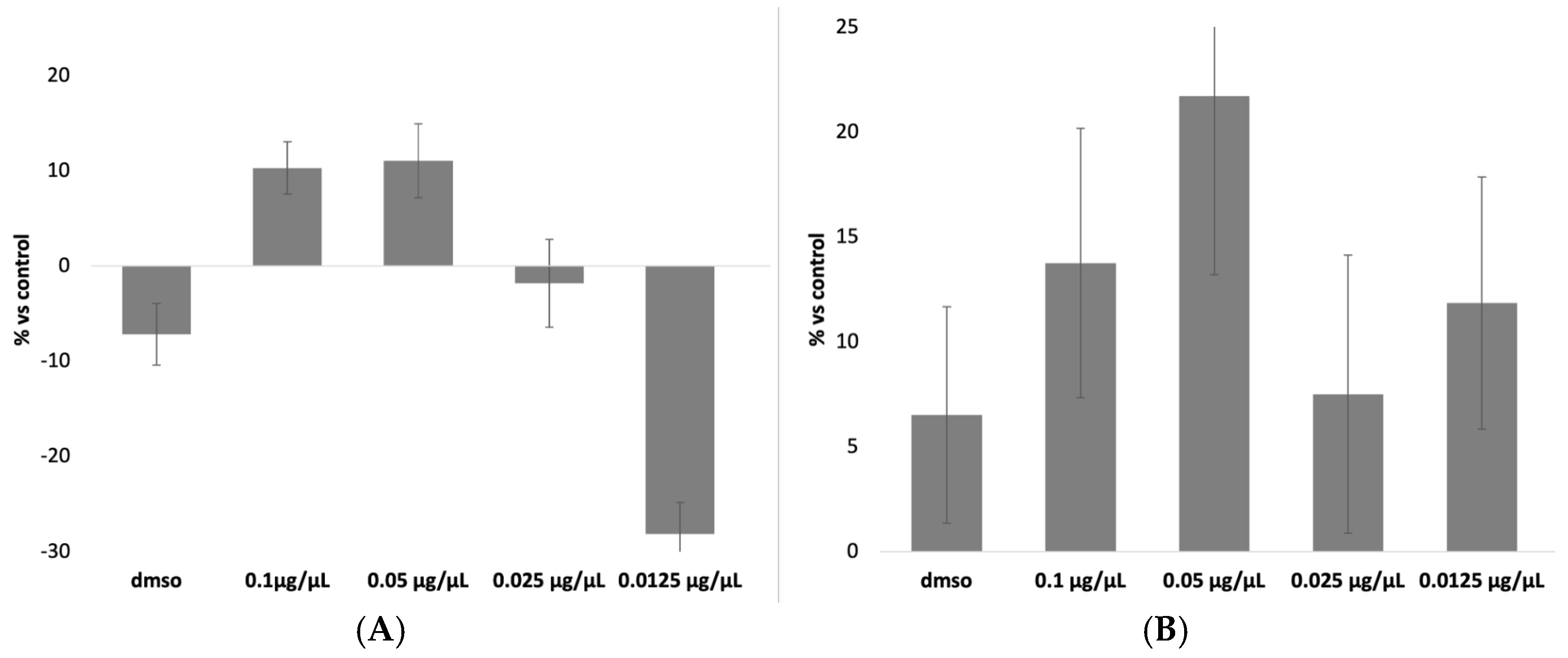
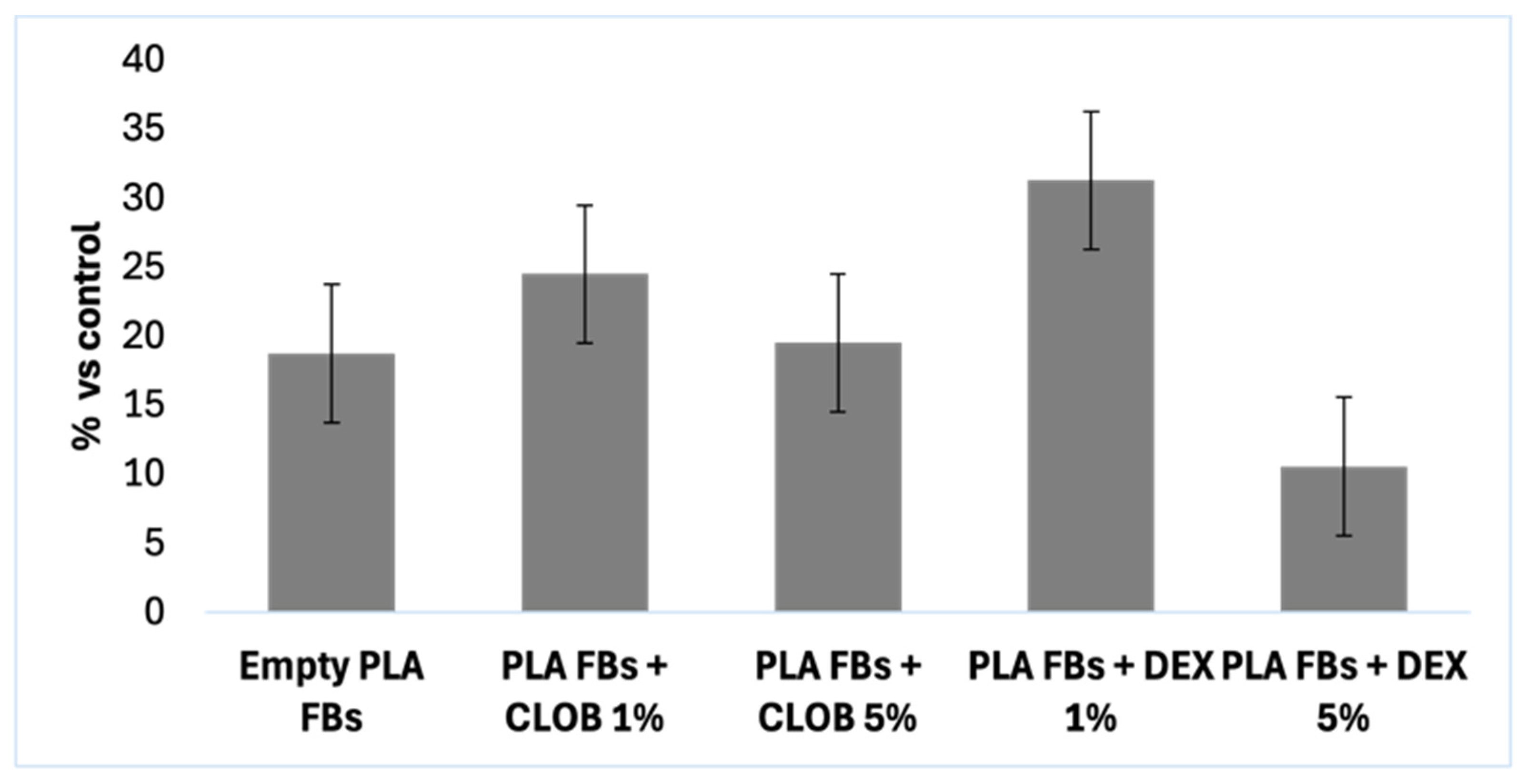
The results of the MTT assay for DEX and CLOB were, as expected, negative for cytotoxic effects (
Figure 1). This outcome aligns with the pharmacological characteristics of these corticosteroids, which primarily exert their effects by modulating inflammatory pathways and immune responses rather than compromising cell viability [
5,
6]. At concentrations approximating therapeutic levels, these compounds are specifically designed to act within a safe therapeutic window, minimizing the risk of cellular damage.
Furthermore, the concentrations employed in the assay were selected to reflect those used in clinical settings and to remain well below the thresholds typically associated with direct cytotoxicity. The negative value observed for the 0.0125 µg/µL concentration indicates that, compared to the control, this concentration did not induce cell mortality but rather showed a slight proliferative effect. Since the results are expressed as a percentage of mortality relative to the control group, a negative value signifies that this concentration was the least toxic, demonstrating no harmful effects on the cells.
Among the tested concentrations, 0.07 µg/µL was identified as the most appropriate for both DEX and CLOB, as it is the closest to the therapeutic dose currently administered in clinical practice. Therefore, this concentration was chosen for subsequent experiments to ensure pharmacological relevance while minimizing the risk of cytotoxic effects unrelated to the carrier system.
3.2. Chromatographic Conditions
To be sure of detecting the exact drug concentration in intracellular and in DMEM conditions, both chromatographic methods were first tested for linearity, using calibrating standard solution mixtures of the DEX and CLOB. Each determination was repeated three times and each calibration curve was performed five times (n = 5). In the selected chromatographic condition, the retention time (RT) for DEX was 15.91 min and for CLOB was 19.64 min.
The obtained regression equations are reported in
Table 1, and the results obtained for the coefficient of determination (R
2) in the linearity tests for DEX (0.9915) and CLOB (0.926) demonstrated suitable levels of linearity for the purposes of this study, with differences reflecting the characteristics of the respective analytical methods.
The R2 for DEX indicates excellent linearity. This result confirms that the analytical method is highly reliable in describing the relationship between concentration and analytical response, with over 99% of the variability explained by the linear regression. Such a high level of linearity meets the most stringent criteria, ensuring that the method is fully suitable for applications requiring high precision and accuracy, such as pharmacokinetic studies or quality control analyses.
The R2 for CLOB, although lower than that for DEX, is still acceptable for the purposes of this study. This level of linearity demonstrates that the method can adequately represent the relationship between concentration and analytical response within the range considered. While it is acknowledged that approximately 7.4% of the variability is not explained by the linear regression, the results are sufficiently robust for the analytical needs of our experimental framework.
In terms of sensitivity, the LOD and LOQ values provide valuable insight into the method’s ability to detect low drug concentrations. The LOD and LOQ values for DEX, which are both lower than those for CLOB, indicate a higher sensitivity for DEX detection. This is particularly important when assessing the pharmacokinetics of DEX in cellular systems, where even very low concentrations may have significant therapeutic or toxicological effects. The high sensitivity ensures that the method can reliably detect DEX at concentrations that are often encountered in biological systems.
For CLOB, the higher LOD and LOQ values (1.8 µg/mL and 5.2 µg/mL, respectively) indicate that the method is less sensitive in comparison to DEX, which is consistent with the lower R2 observed. While these values are still suitable for most experimental applications, it may be necessary to optimize the method for greater sensitivity, particularly in cases where precise quantification of CLOB at low concentrations is critical. Further optimization could involve adjustments to the experimental conditions, such as increasing the sensitivity of the detection system or refining the calibration procedure.
Overall, the results obtained for both DEX and CLOB demonstrate that the analytical methods used in this study are effective and reliable for quantifying these corticosteroids within their expected concentration ranges. However, there are notable differences in sensitivity and linearity between the two compounds, which should be taken into account when interpreting the results. The differences observed in the analytical performance of DEX and CLOB underscore the importance of tailoring analytical methods to the specific properties of the compounds under investigation. This study provides a robust framework for future analyses of these drugs, but as with any analytical method, it is essential to consider the limitations and to continuously refine the approach to enhance precision and accuracy.
3.3. Efficiency of the Drug Recovery in Cell Lysate and DMEM
The amount of active ingredients recovered from intracellular lysates, using HPLC analysis, was 97% for both the drugs (
Table 2).
These results clearly indicate that the intracellular amount of the two drugs is almost completely recovered, suggesting that there are no interactions between the molecules and the cellular contents.
The recovery of DEX and CLOB in DMEM was assessed at two concentrations, both exceeding the LOQ (but always under the limit of the solubility concentration), as summarized in
Table 3. Using the analytical method developed in this study, approximately 80% of DEX and 66% of CLOB were successfully recovered and quantified.
These results highlight differences in the chemical nature of the two drugs, which likely influence their solubility and interaction with the DMEM medium. DEX, a relatively hydrophilic corticosteroid, demonstrates higher recovery rates, consistent with its greater solubility in aqueous media such as DMEM. In contrast, CLOB, characterized by its more lipophilic structure, exhibits lower recovery rates, which may be attributed to its reduced solubility and potential adsorption to container surfaces or other hydrophobic interactions in the medium.
Given the inability to achieve complete recovery for both drugs, it will be necessary to evaluate not only the amount released into the medium but also the residual drug content retained within the fibrous carriers during the release experiments. This comprehensive approach will provide a more accurate assessment of the drug release profile and the efficiency of the delivery system.
3.4. PLA-FBs Physical Characterization
The morphology of PLA-FBs, both empty and loaded with DEX or CLOB at two different concentrations (1% and 5%), was observed using SEM. The micrographs shown in
Figure 2 depict the different PLA-FBs at the same magnification (Mag = 10.00 kx). It can be observed that there are no surface defects and randomly oriented fibers are obtained, as expected. Precipitates on the surface of PLA-FBs loaded with DEX are evident, attributed to the active ingredient itself.
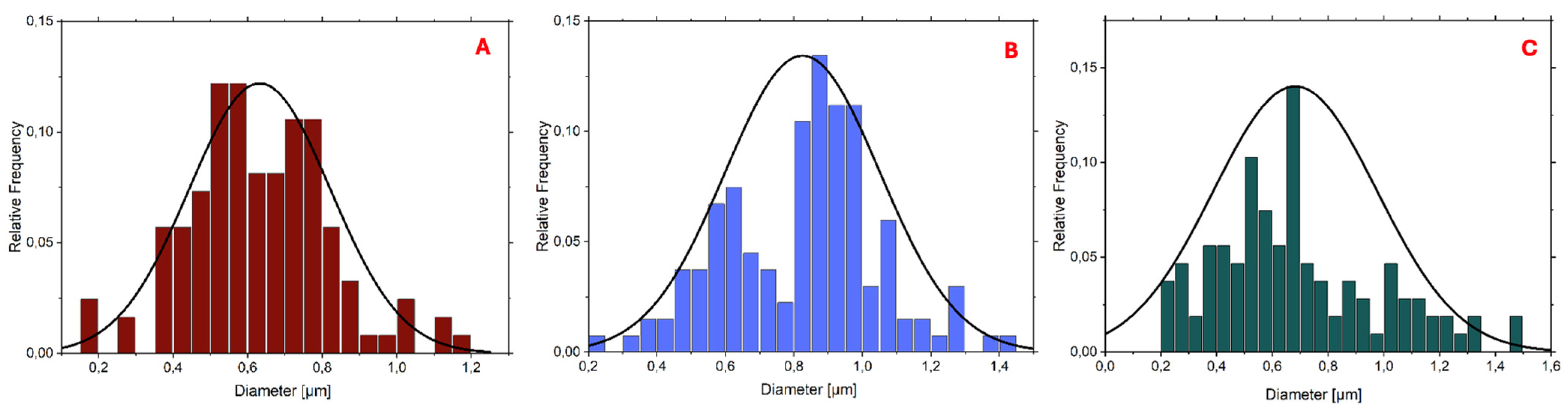
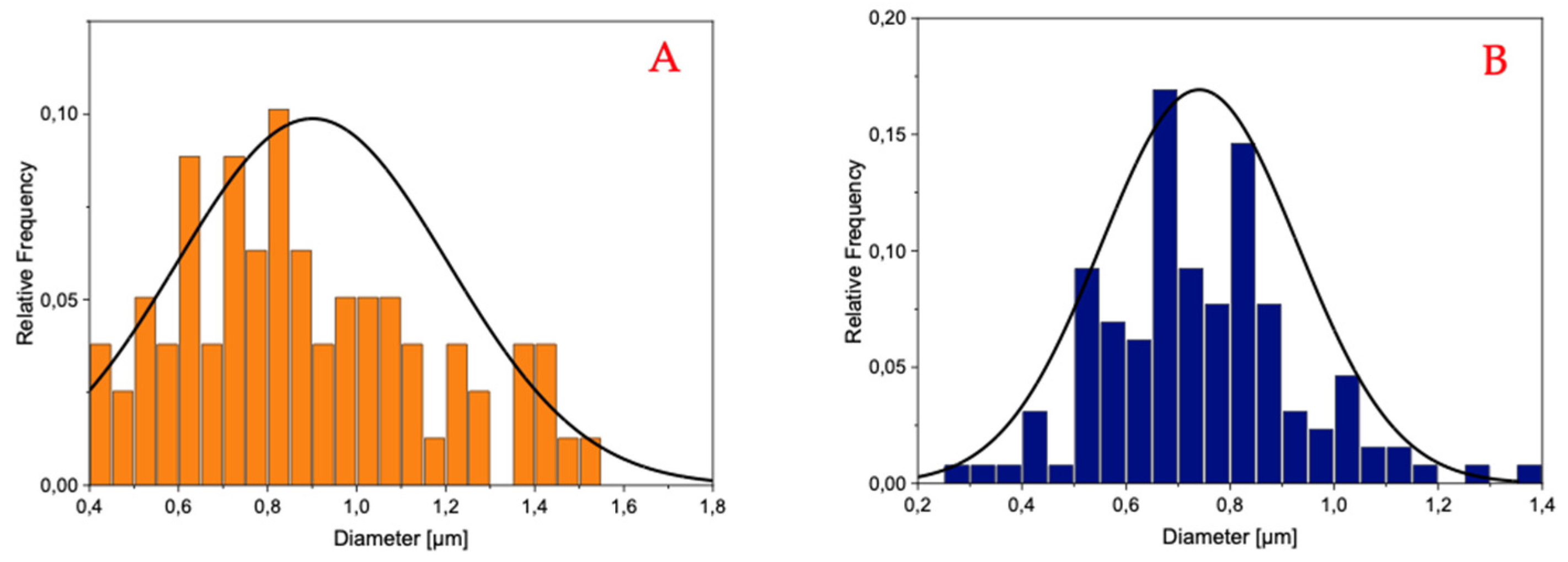
The histograms reported in
Figure 3 and
Figure 4 demonstrate that the PLA-FBs typically have diameters ranging between 0.6 and 0.9 μm, on average.
The SEM analysis revealed the presence of two distinct fiber populations, characterized by significantly different diameters within the same electrospun mat. This bimodal distribution suggests the occurrence of independent fiber formation events during the electrospinning process. Several factors may account for this phenomenon, including possible micro-heterogeneities in the polymer solution, local fluctuations in solvent evaporation rates, or the coexistence of different jet instabilities (stable vs. whipping segments) under the same operating conditions. Additionally, in formulations containing dispersed drugs or additives, localized variations in viscosity or conductivity may contribute to the generation of two separate fiber populations. This observation highlights the complexity and sensitivity of the electrospinning process to subtle physicochemical and environmental factors, and suggests that even within optimized systems, multiple fiber formation pathways can coexist [
28].
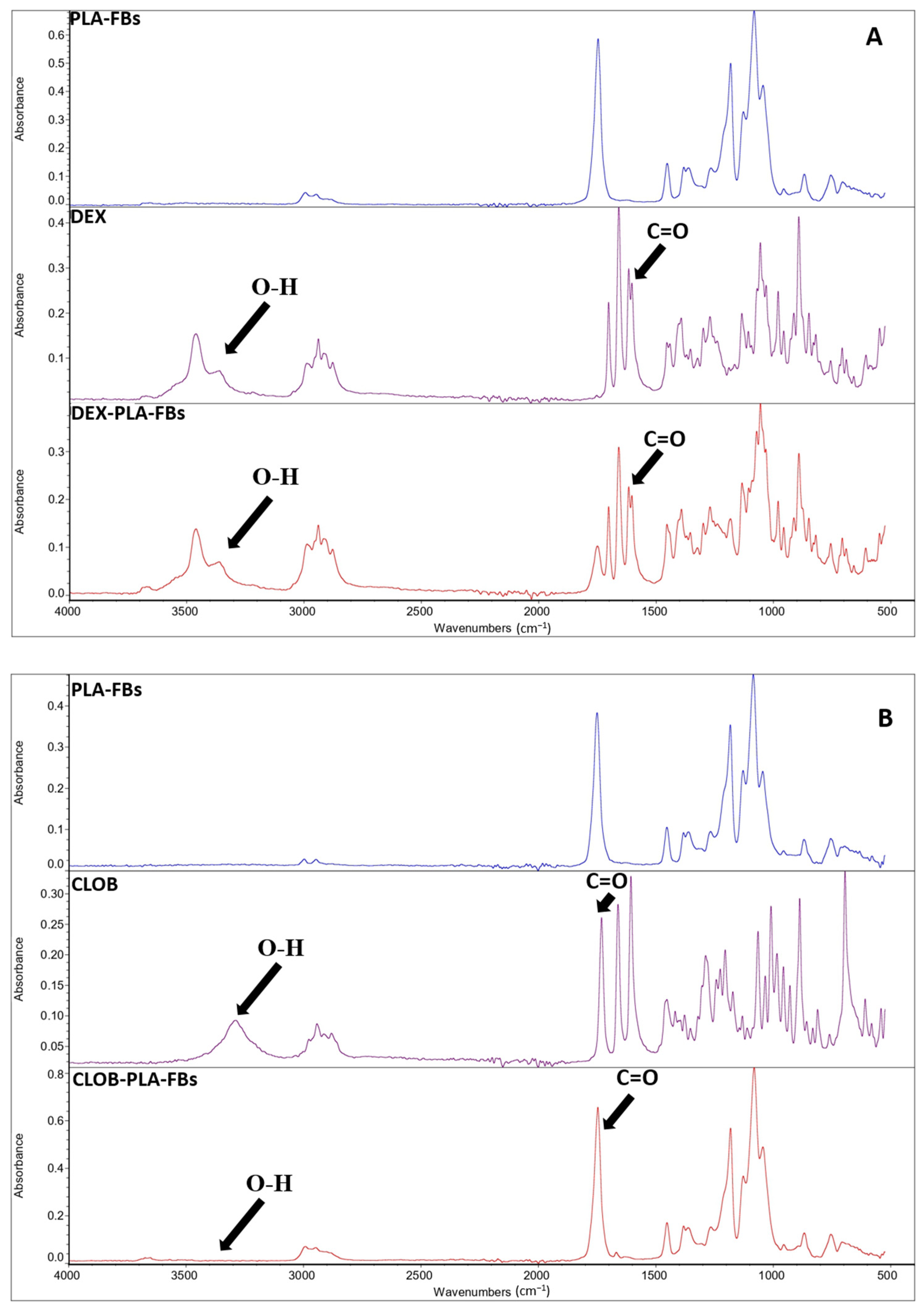
In order to validate the developed drug delivery systems, it is fundamental to evaluate and confirm the effective drug incorporation and preservation of the molecular structure that are critical prerequisites for subsequent evaluation of the controlled release behavior and biological activity of the fibers. The effective incorporation of the drug within the PLA polymer matrix was confirmed by FTIR measurements, as evident from the reported FTIR spectra of neat PLA fibers, free drugs (dexamethasone and clobetasol), and PLA fibers loaded with 5 wt% of each drug (
Figure 5A,B). In detail, in the FTIR spectra of the PLA fibers loaded with 5 wt% of each drug (
Figure 5A,B), an additional, albeit less intense, absorption band at around 1660 cm
−1 is present, in addition to the typical PLA peaks (the main signature of which appears at around 1750 cm
−1, associated with the ester carbonyl (C=O) group) [
20]. This extra band can be ascribed to the stretching vibration of the carbonyl group present in the used corticosteroids (i.e., dexamethasone and clobetasol) [
29], confirming the efficacy of the set-up incorporation procedure. The spectroscopic characterization suggests that the drugs are not only physically entrapped within the polymer matrix but also retain their chemical integrity during the incorporation process. Moreover, the absence of significant shifts in the main PLA peaks indicates that no evident chemical reactions occurred between the polymer chains and the active compounds during processing, suggesting a simple physical–mechanical interaction [
29].
DSC analyses were carried out on neat PLA fiber and on PLA fibers loaded with dexamethasone and clobetasol, in order to investigate the influence of the drugs on the thermal properties of the produced fibers. The glass transition (T
g), melting (T
m), cold crystallization temperature (T
cc), melting enthalpies (ΔH
m), cold crystallization enthalpies (ΔH
cc) and the crystallinity degree (χ), related to the first and second heating scans, are summarized in
Table 4. The first heating scan DSC curves are compared in
Figure 6.
From the comparison of the data reported in
Table 4, related to the first and second heating process, it was demonstrated that this initial scan highlights both the impact of the drug incorporation and the effect of the electrospinning process on the thermal properties of the PLA matrix (initial comparison figure). Neat PLA fibers exhibited a single melting peak (T
m) at 149 °C, with a shoulder at a lower temperature, in agreement with other studies [
30].
The addition of the drug affected the PLA thermal properties, leading to an increase in both T
g and T
cc, suggesting an interaction between the polymeric chain and the loaded corticosteroid. The increment in glass transition temperature (T
g) and cold crystallization temperature (T
cc) suggests that drug incorporation may confer greater rigidity to the polymer matrix, leading to crystallization at higher temperatures. However, all drug-loaded samples exhibited a marked decrease in both melting enthalpy and cold crystallization enthalpy (from 18.62 J/g for neat PLA to approximately 16 J/g for the drug-loaded PLA). This implies that the addition of the drug interferes with the crystallization process, reducing the material’s ability to form crystals at lower temperatures during cooling [
29].
As for the degree of crystallinity, it is generally very low in electrospun materials, indicating that most of the polymer chains are in the amorphous state due to the intrinsic characteristics of the electrospinning process (Cacciotti et al., 2014) [
31]. In fact, the rapid solvent evaporation and the high elongation rate of the jet during electrospinning usually lead to rapid solidification of the stretched chains in the final stages of the process, hindering the development of crystallinity. Consequently, the chains do not have sufficient time to organize into crystalline structures before solidifying [
32]. Comparing the recorded crystallinity degrees, lower values were obtained for the drug-loaded samples, which could indicate that the drug affects the behavior of the polymer chains, preventing them from arranging into a more ordered structure, in agreement with the increased T
cc.
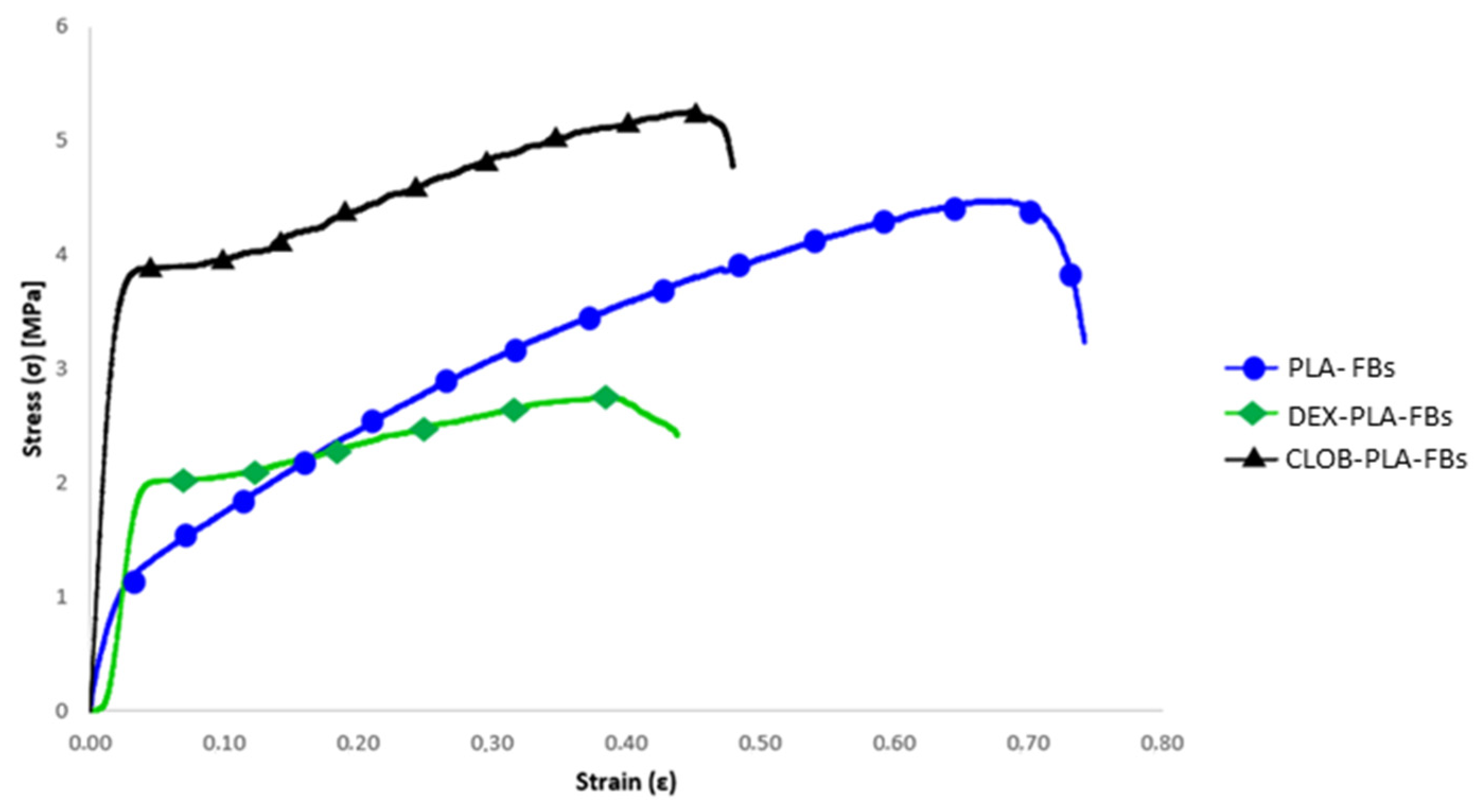
Concerning the mechanical behaviour, the stress–strain curves of the electrospun mats made of neat PLA and those loaded with drugs (dexamethasone and clobetasol) are compared in
Figure 7, and the related data (i.e., maximum tensile strength (σ
max), yield stress (σ
y), Young’s modulus (E), and maximum elongation) are summarized in
Table 5.
The results obtained from neat PLA samples and drug-loaded samples show significant differences in terms of mechanical performance, as evidenced by the values of maximum tensile strength (σmax), yield stress (σy), Young’s modulus (E), and maximum elongation. In particular, the neat PLA sample showed an average maximum tensile strength of 4.48 MPa, a yield stress of 1.01 MPa, a Young’s modulus of 60 MPa, and a maximum elongation of 70%. These results confirm the good mechanical performance of PLA, known for its relatively high tensile strength and its ability to deform without breaking (Alves et al., 2019) [
29].
The PLA sample containing 5% dexamethasone showed a marked reduction in maximum tensile strength (2.76 MPa), but a notable increase in yield stress (1.92 MPa), suggesting that DEX may have a positive effect on initial plastic deformation, while reducing the overall strength of the material. The Young’s modulus decreased to 42 MPa, indicating lower stiffness, while the maximum elongation dropped significantly to 43%, pointing to a less elastic behavior compared to the other samples. On the other hand, the PLA sample containing 5% clobetasol (CLOB) showed the best performance among all tested materials, with a significant increase in maximum tensile strength (5.29 MPa) and yield stress (3.56 MPa), accompanied by a substantial rise in Young’s modulus (188.89 MPa) and a reduced elongation at break, as expected.
As can be seen from the comparative stress–strain curves, the maximum elongation is significantly reduced in the drug-loaded samples, in agreement with the findings reported by [
33]. This behavior can be attributed to the interaction between the drug and the polymer matrix, which limits the fibers’ ability to undergo plastic deformation before breaking. Moreover, it is correlated with the DSC results, which evidence increased Tg values (
Table 4) for drug-loaded fibers with respect to the neat ones.