Sustainable Recovery of Vanadium from Stone Coal via Nitric Acid Oxygen Pressure Leaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments and Analytical Methods
2.3. Optimization of Experimental Design
3. Results and Discussion
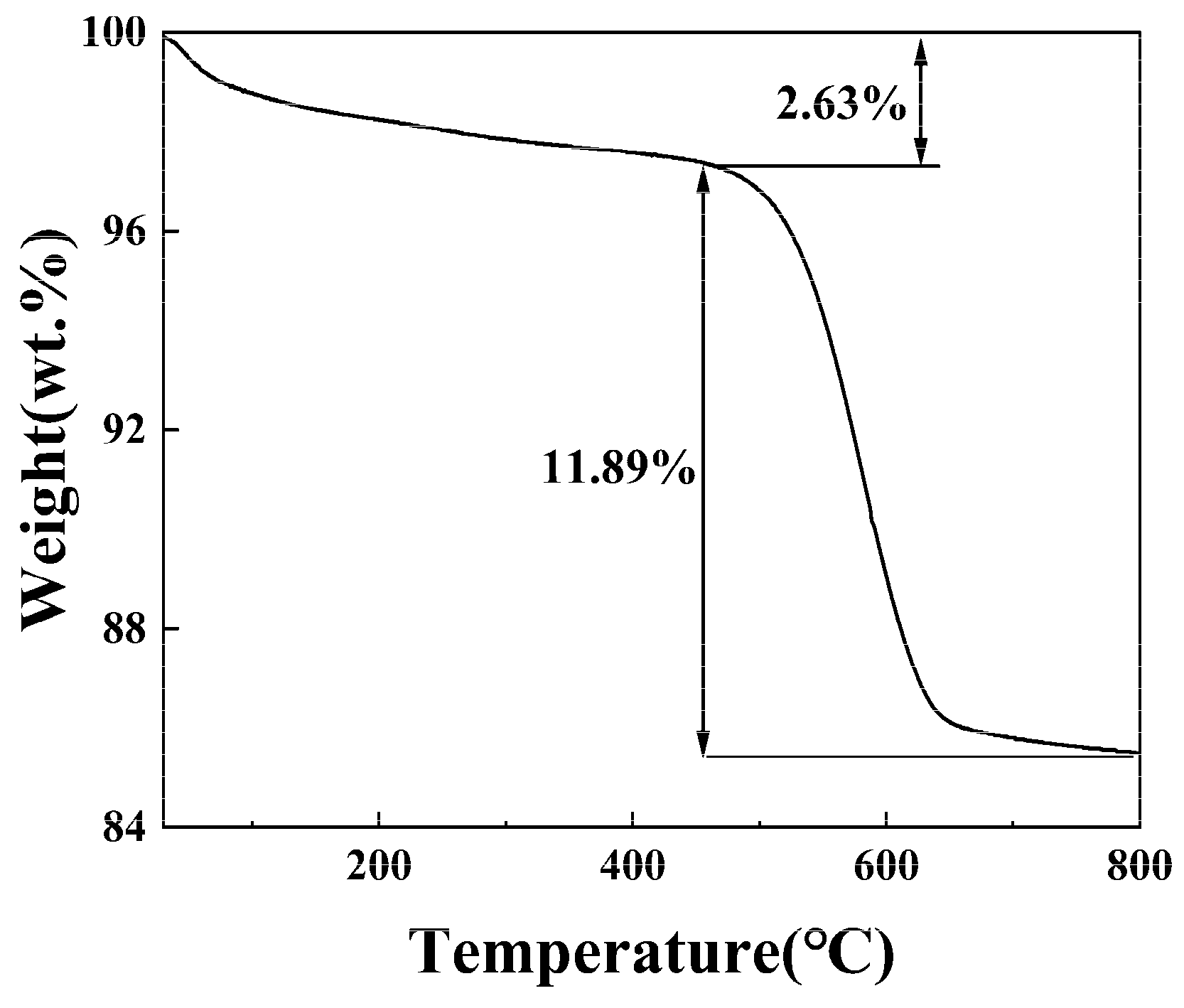
3.1. Thermal Decomposition Analysis of Stone Coal
3.2. Comparison of Leaching with Different Methods
3.2.1. Phase Transformation Analysis
3.2.2. Micromorphology of Stone Coal Surface
3.2.3. Parametric Analysis of Particle Pores
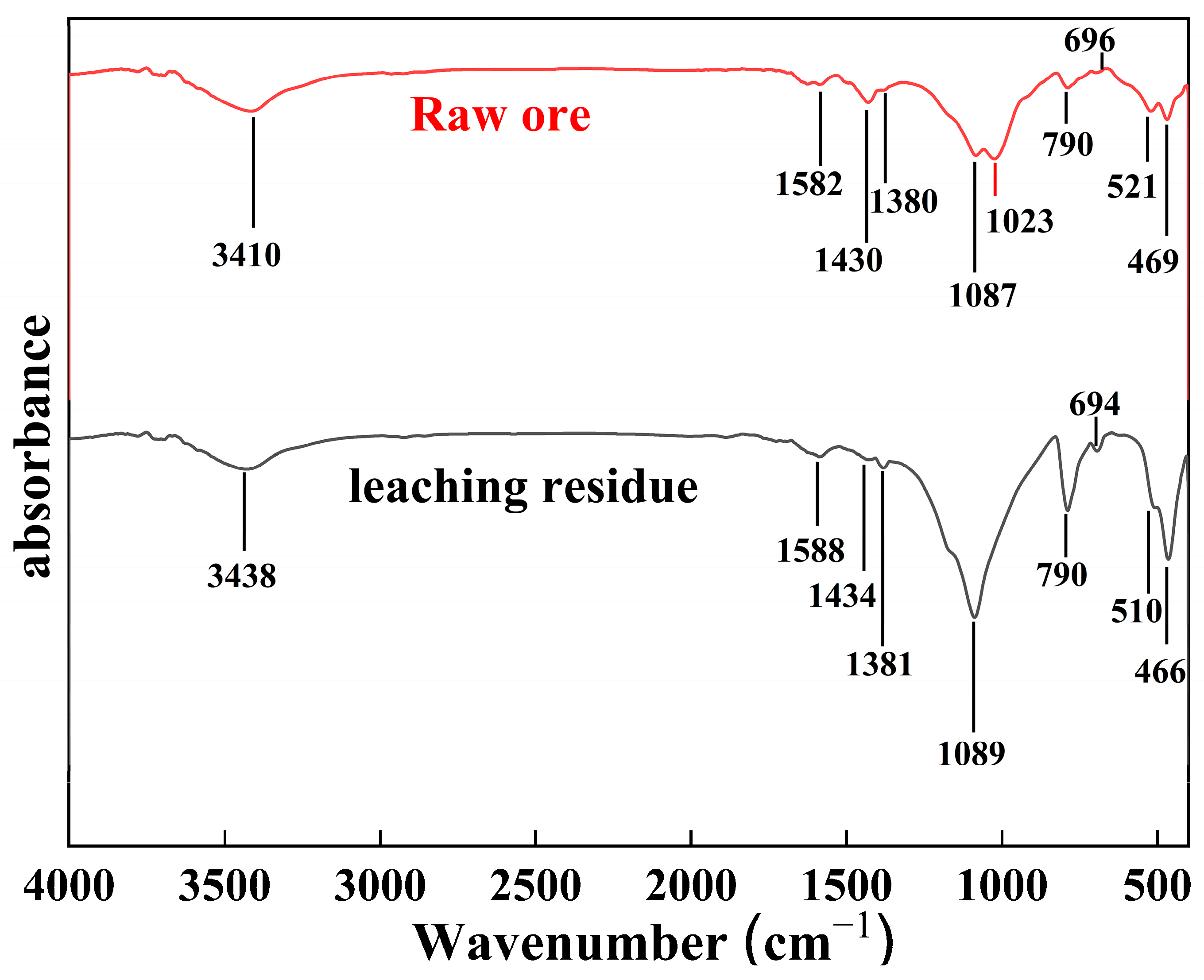
3.2.4. FTIR Analysis
3.3. Nitric Acid Oxygen Pressure Leaching
3.3.1. Effect of the Nitric Acid Concentration on the Leaching Rate
3.3.2. Effect of Temperature on the Leaching Rate
3.3.3. Effect of Liquid-to-Solid Ratio on the Leaching Rate
3.3.4. Effect of Time on the Leaching Rate
3.3.5. Effect of Total Pressure on the Leaching Rate
3.3.6. Effect of Stirring Speed on the Leaching Rate
3.3.7. Response Surface Optimization of Acid Leaching Systems
−0.003525AD − 0.000086BC − 0.000735BD − 0.00546667CD + 0.0557098A2 − 0.000056B2
−0.00319392C2 − 0.0632953D2
3.3.8. Cyclic Leaching Performance
3.4. Preliminary Estimation of Reagent Costs in the Nitrogen Recycling System
4. Conclusions
- Single-factor experiments revealed that nitric acid concentration, temperature, liquid-to-solid ratio, and total oxygen pressure markedly influence vanadium extraction efficiency. Through response surface optimization, the optimal conditions were identified as nitric acid concentration of 1.5 mol/L, temperature of 127.43 °C, liquid-to-solid ratio of 5 mL/g, and total pressure of 2 MPa, achieving a vanadium leaching efficiency of 73.1%.
- The cyclic leaching experiments demonstrated effective nitrate recycling by converting nitrogen oxide (NOx) generated during leaching back into nitric acid. This approach not only enhances vanadium leaching efficiency but also significantly reduces nitric acid consumption and wastewater discharge. Cyclic tests conducted under the optimized conditions identified by response surface methodology showed that the system maintains robust reactivity over multiple cycles, indicating strong process stability and sustainability. These findings further confirm the feasibility and industrial applicability of this method.
- Mineralogical analyses using SEM-EDS, BET, FTIR, and XRD confirmed that nitric acid oxygen pressure leaching effectively disrupts the muscovite structure. Its significant effects on mineral surface morphology, specific surface area, and crystal structure were clearly demonstrated. The oxidative action of nitric acid disrupts the muscovite lattice, forming a porous structure and enlarging the specific surface area, which in turn facilitates the efficient leaching of vanadium from stone coal.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.C.; Kim, E.Y.; Chung, K.W.; Kim, R.; Jeon, H.S. A review on the metallurgical recycling of vanadium from slags: Towards a sustainable vanadium production. J. Mater. Res. Technol. 2021, 12, 343–364. [Google Scholar] [CrossRef]
- Lee, D.; Joo, S.H.; Shin, D.J.; Shin, S.M. Recovery of vanadium and cesium from spent sulfuric acid catalysts by a hydrometallurgical process. Green Chem. 2022, 24, 790–799. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, M.; Qin, L.; Chen, M.; Chen, Z.; Guo, S.; Wang, L.; Fang, G.; Liang, S. Simultaneous regulation of cations and anions in an electrolyte for high-capacity, high-stability aqueous zinc–vanadium batteries. Escience 2022, 2, 209–218. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, R.; Xu, G.; Wang, W.; Liu, J. Laser additive manufacturing of bimetallic structure from Ti-6Al-4V to Ti-48Al-2Cr-2Nb via vanadium interlayer. Mater. Lett. 2020, 263, 127210. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y. Efficient vanadium extraction from shale with high silicon content using a short flow process by roasting-water leaching: Laboratory and industrial scale research. Silicon 2022, 14, 3775–3784. [Google Scholar] [CrossRef]
- Zou, K.; Xiao, J.; Liang, G.; Huang, W.; Xiong, W. Effective extraction of vanadium from bauxite-type vanadium ore using roasting and leaching. Metals 2021, 11, 1342. [Google Scholar] [CrossRef]
- Liu, S.; Xue, W.; Wang, L. Extraction of the rare element vanadium from vanadium-containing materials by chlorination method: A critical review. Metals 2021, 11, 1301. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, Y.F.; Li, Y.T.; Wu, C.; Han, X.L.; Zhao, N.N.; Zhang, Z.K.; Dai, L.; Wang, L.; He, Z.X. A review on vanadium extraction techniques from major vanadium-containing resources. Rare Met. 2024, 43, 4115–4131. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y. Phase transformations of vanadium recovery from refractory stone coal by novel NaOH molten roasting and water leaching technology. RSC Adv. 2017, 7, 36917–36922. [Google Scholar] [CrossRef]
- Bai, Z.; Sun, Y.; Xu, X.; Jin, J.; Han, Y. A novel process of gradient oxidation roasting-acid leaching for vanadium extraction from stone coal. Adv. Powder Technol. 2024, 35, 104296. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, K.; Hua, W.H.; Yang, Z.; Zhou, W.; Xie, B. Selective leaching of vanadium in calcification-roasted vanadium slag by ammonium carbonate. Hydrometallurgy 2016, 160, 18–25. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Liu, Y.; Zhao, Y. Blank roasting and bioleaching of stone coal for vanadium recycling. J. Clean. Prod. 2020, 243, 118625. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, L.; Li, Q.; Wang, X.; Xiang, X. Leaching of vanadium from stone coal with sulfuric acid. Rare Met. 2009, 28, 1–4. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Liu, T.; Huang, J.; Zhao, J.; Zhang, G.; Liu, J. A mechanism of calcium fluoride-enhanced vanadium leaching from stone coal. Int. J. Miner. Process. 2015, 145, 87–93. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, T.; Zhou, Y.; Xu, W.; Lin, H.; Yan, B. Optimization of oxidative leaching for vanadium extraction from low-grade stone coal using response surface methodology. Processes 2020, 8, 1534. [Google Scholar] [CrossRef]
- Dong, Y.; Zan, J.; Lin, H. Enhanced vanadium bioleaching from stone coal employing Aspergillus niger: Optimization of stone coal pretreatment method. Miner. Eng. 2024, 215, 108792. [Google Scholar] [CrossRef]
- Deng, Z.G.; Chang, W.E.I.; Gang, F.A.N.; Li, M.T.; Li, C.X.; Li, X.B. Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction. Trans. Nonferrous Met. Soc. China 2010, 20, s118–s122. [Google Scholar] [CrossRef]
- Xue, N.N.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, L.Y. Separation of impurities aluminum and iron during pressure acid leaching of vanadium from stone coal. J. Clean. Prod. 2017, 166, 1265–1273. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Huang, J.; Zhu, X.; Wang, Y. Separation and recovery of sulfuric acid from acidic vanadium leaching solution by diffusion dialysis. Sep. Purif. Technol. 2012, 96, 44–49. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Bao, S. Separation and recovery of sulfuric acid from acidic vanadium leaching solution of stone coal via solvent extraction. J. Environ. Chem. Eng. 2016, 4, 1399–1405. [Google Scholar] [CrossRef]
- Long, T.; Jin, Y.; Tang, W.; Du, J.; Lin, Q.; Fang, Z.; Chen, C.; Huo, Q. Recovery of manganese and lead from electrolytic manganese anode slime based on a roasting and acid leaching reduction system. Sep. Purif. Technol. 2025, 352, 128093. [Google Scholar] [CrossRef]
- Kolbadinejad, S.; Ghaemi, A. Optimization of atmospheric leaching parameters for cadmium and zinc recovery from low-grade waste by response surface methodology (RSM). Sci. Rep. 2024, 14, 1490. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Li, X.; Weng, Y.; Dong, X.; Zhao, X. Comparison on the effectiveness of Fourier transform infrared (FT-IR) and attenuated total reflection Fourier transform infrared (ATR-FT-IR) in characterizing plastics biodegradation by insect larvae. Sci. Total Environ. 2022, 839, 156289. [Google Scholar] [CrossRef]
- Ren, H.; Shu, X.; Liu, Z.; Zhou, J.; Ma, J.; Liu, Y.; Kong, L.B.; Min, F.; Shi, X.; Han, J.; et al. In-situ synthesis of layered porous coal-derived carbon/Ni magnetic composites with promising microwave absorption performance. J. Magn. Magn. Mater. 2020, 513, 167231. [Google Scholar] [CrossRef]
- dos Santos, V.H.J.M.; Pontin, D.; Ponzi, G.G.D.; e Stepanha, A.S.D.G.; Martel, R.B.; Schütz, M.K.; Einloft, S.M.O.; Dalla Vecchia, F. Application of Fourier Transform infrared spectroscopy (FTIR) coupled with multivariate regression for calcium carbonate (CaCO3) quantification in cement. Constr. Build. Mater. 2021, 313, 125413. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Li, Z.; Wang, G.; Li, C. Unveiling the promotion of Brønsted acid sites in Cs dispersion and consequential Si-O-Cs species formation for methyl acrylate synthesis from methyl acetate and formaldehyde over Cs/Beta zeolite catalyst. Chem. Eng. J. 2023, 474, 145655. [Google Scholar] [CrossRef]
- Bai, Z.; Han, Y.; Jin, J.; Sun, Y.; Zhou, Z. Extraction of vanadium from black shale by novel two-step fluidized roasting process. Powder Technol. 2022, 408, 117745. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Liu, T.; Hu, P.; Zheng, Q. Optimization of microwave roasting-acid leaching process for vanadium extraction from shale via response surface methodology. J. Clean. Prod. 2019, 234, 494–502. [Google Scholar] [CrossRef]
- Yuan, Y.Z.; Zhang, Y.M.; Liu, T.; Chen, T.J. Comparison of the mechanisms of microwave roasting and conventional roasting and of their effects on vanadium extraction from stone coal. Int. J. Miner. Metall. Mater. 2015, 22, 476–482. [Google Scholar] [CrossRef]
- Savari, C.; Barigou, M. Lagrangian wavelet analysis of turbulence modulation in particle–liquid mixing flows. Phys. Fluids 2022, 34, 115121. [Google Scholar] [CrossRef]










| Composition | SiO2 | Al2O3 | V2O5 | K2O | Na2O | MgO | TFe |
|---|---|---|---|---|---|---|---|
| Content/% | 66.34 | 2.87 | 0.82 | 0.37 | 0.86 | 0.55 | 0.93 |
| Composition | CaO | BaO | ZnO | Water | C | S | Loss |
| Content/% | 0.70 | 0.28 | 0.05 | 2.61 | 19.3 | 4.32 | 14.49 |
| No. | Leaching Condition | Acid Type | Gas Atmosphere, Total Pressure | Remarks |
|---|---|---|---|---|
| 1 | Nitric acid oxygen pressure leaching | 2 mol/L nitric acid | O2, 2 MPa | Reference condition |
| 2 | Non-assisted nitric acid leaching | 2 mol/L nitric acid | residual air | No gas injection |
| 3 | Nitric acid nitrogen pressure leaching | 2 mol/L nitric acid | N2, 2 MPa | Evaluates effect of gas type |
| 4 | Sulfuric acid oxygen pressure leaching | 1 mol/L sulfuric acid | O2, 2 MPa | Evaluates effect of acid type; constant initial H+ concentration |
| Samples | Specific Surface BET (m2/g) | Total Pore Volume (cm3/g) | BJH Average Aperture (nm) |
|---|---|---|---|
| Raw ore | 0.951 | 0.00873 | 3.092 |
| Leaching residue with H2SO4 | 4.274 | 0.08412 | 3.925 |
| Leaching residue with HNO3 | 32.297 | 0.05804 | 3.912 |
| Chemical Reaction | Gibbs Free Energy Change Relationship to Temperature (kJ/mol) | Equation |
|---|---|---|
| 3V2O3(s) + 2NO3−(aq) + 14H+(aq) = 6VO2+(aq) + 2NO(g) + 7H2O(l) | ΔG = −735.12 + 0.4978T | (3) |
| 2V2O3(s) + O2(g) + 8H+(aq) = 4VO2+(aq) + 4H2O(l) | ΔG = −652.12 + 0.6571T | (4) |
| 2H+(aq) + FeS2(s) = H2S(g) + Fe2+(aq) + S(s) | ΔG = 60.213 − 0.084T | (5) |
| H2S(g) + 2O2(g) = (aq) + 2H+(aq) | ΔG = −889.10 + 0.5972T | (6) |
| 3H2S(g) + (aq) + 2H+(aq) = 8NO(g) + 4H2O(l) + 3(aq) | ΔG = −1695.11 − 0.1598T | (7) |
| FeS2(s) + 5(aq) + 4H+(aq) = Fe3+(aq) + 5NO(g) + 2(aq) + 2H2O(l) | ΔG = −918.630 − 0.222T | (8) |
| 4FeS2(s) + 15O2(g) +2H2O(l) = 4Fe3+(aq) + 8 (aq) + 4H+(aq) | ΔG = −6115.45 + 4.029T | (9) |
| 4NO(g) + 3O2(g) + 2H2O(l) = 4H+(aq) + 4 (aq) | ΔG = −486.09 + 0.9757T | (10) |
| 2NO(g) + O2(g) = 2NO2(g) | ΔG = −114.40 + 0.1463T | (11) |
| 4NO2(g) + O2(g) + 2H2O(l) = 4H+(aq) + 4 (aq) | ΔG = −257.29 + 0.6831T | (12) |
| BaCO3(s) + 2H+(aq) = CO2(g) + Ba2+(aq) + H2O(l) | ΔG = −0.1353 − 0.1800T | (13) |
| KAl2Si3AlO10(OH)2(s) + 10H+(aq) = K+(aq) + 3Al3+(aq) + 3H2SiO3(s) + 3H2O(l) | ΔG = −307.57 + 0.5687T | (14) |
| Independent Factors | Range and Levels | ||||
|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | |
| A: HNO3 concentration (mol/L) | / | 0.5 | 1.5 | 2.5 | 3.5 |
| B: Leaching temperature (°C) | 70 | 90 | 120 | 150 | 170 |
| C: Liquid–Solid (mL/g) | / | 2 | 3.5 | 5 | 6.5 |
| D: Total pressure (MPa) | / | 1.5 | 2 | 2.5 | 3 |
| Std | Run | A: c | B: T | C: L/S | D: Total Pressure | Y: Leaching Rate |
|---|---|---|---|---|---|---|
| 1 | 25 | 0.5 | 90 | 2.0 | 1.5 | 0.5086 |
| 2 | 10 | 2.5 | 90 | 2.0 | 1.5 | 0.5845 |
| 3 | 6 | 0.5 | 150 | 2.0 | 1.5 | 0.6105 |
| 4 | 20 | 2.5 | 150 | 2.0 | 1.5 | 0.6649 |
| 5 | 19 | 0.5 | 90 | 5.0 | 1.5 | 0.5905 |
| 6 | 4 | 2.5 | 90 | 5.0 | 1.5 | 0.7210 |
| 7 | 11 | 0.5 | 150 | 5.0 | 1.5 | 0.6658 |
| 8 | 8 | 2.5 | 150 | 5.0 | 1.5 | 0.7913 |
| 9 | 7 | 0.5 | 90 | 2.0 | 2.5 | 0.6104 |
| 10 | 15 | 2.5 | 90 | 2.0 | 2.5 | 0.6613 |
| 11 | 27 | 0.5 | 150 | 2.0 | 2.5 | 0.6445 |
| 12 | 18 | 2.5 | 150 | 2.0 | 2.5 | 0.7154 |
| 13 | 1 | 0.5 | 90 | 5.0 | 2.5 | 0.6658 |
| 14 | 3 | 2.5 | 90 | 5.0 | 2.5 | 0.7856 |
| 15 | 24 | 0.5 | 150 | 5.0 | 2.5 | 0.6991 |
| 16 | 14 | 2.5 | 150 | 5.0 | 2.5 | 0.8156 |
| 17 | 26 | 3.1 | 120 | 3.5 | 2.0 | 0.9084 |
| 18 | 13 | 1.5 | 70 | 3.5 | 2.0 | 0.5074 |
| 19 | 21 | 1.5 | 170 | 3.5 | 2.0 | 0.5921 |
| 20 | 2 | 1.5 | 120 | 6.5 | 2.0 | 0.7537 |
| 21 | 17 | 1.5 | 120 | 3.5 | 3.0 | 0.6849 |
| 22 | 12 | 1.5 | 120 | 3.5 | 2.0 | 0.6856 |
| 23 | 16 | 1.5 | 120 | 3.5 | 2.0 | 0.6901 |
| 24 | 5 | 1.5 | 120 | 3.5 | 2.0 | 0.6820 |
| 25 | 22 | 1.5 | 120 | 3.5 | 2.0 | 0.6902 |
| 26 | 9 | 1.5 | 120 | 3.5 | 2.0 | 0.6945 |
| 27 | 23 | 1.5 | 120 | 3.5 | 2 | 0.6894 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.1988 | 14 | 0.0142 | 396.59 | <0.0001 | Significant |
| A-C | 0.0368 | 1 | 0.0368 | 1028.74 | <0.0001 | |
| B-T | 0.0179 | 1 | 0.0179 | 498.86 | <0.0001 | |
| C-L/S | 0.0351 | 1 | 0.0351 | 980.90 | <0.0001 | |
| D-P | 0.0139 | 1 | 0.0139 | 387.23 | <0.0001 | |
| AB | 0.000006 | 1 | 0.000006 | 0.1676 | 0.6895 | |
| AC | 0.0036 | 1 | 0.0036 | 100.69 | <0.0001 | |
| AD | 0.0000 | 1 | 0.0000 | 1.39 | 0.2616 | |
| BC | 0.0002 | 1 | 0.0002 | 6.62 | 0.0244 | |
| BD | 0.0019 | 1 | 0.0019 | 54.30 | <0.0001 | |
| CD | 0.0003 | 1 | 0.0003 | 7.51 | 0.0179 | |
| A2 | 0.0252 | 1 | 0.0252 | 704.04 | <0.0001 | |
| B2 | 0.0350 | 1 | 0.0350 | 976.03 | <0.0001 | |
| C2 | 0.0007 | 1 | 0.0007 | 20.06 | 0.0008 | |
| D2 | 0.0035 | 1 | 0.0035 | 97.26 | <0.0001 | |
| Residual | 0.0004 | 12 | 0.000033 | |||
| Lack-of-Fit | 0.0003 | 7 | 0.000043 | 2.46 | 0.1697 | Not significant |
| Pure Error | 0.0001 | 5 | 0.00002 | |||
| Cor Total | 0.1993 | 26 |
| Reagent | Amount of Substance (mol) | Mass (kg) | Estimated Total Cost (CNY) |
|---|---|---|---|
| Oxygen | 715 | 22.9 | 120 |
| Nitric acid | 1500 | 139.4 | 270 |
| Sulfuric acid | 2800 | 274.4 | 247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, K.; Li, F.; Long, Y.; Yang, Y.; Long, H.; Luo, R.; Ma, W.; Hua, J.; Yang, Z.; Zhuo, O.; et al. Sustainable Recovery of Vanadium from Stone Coal via Nitric Acid Oxygen Pressure Leaching. Materials 2025, 18, 2530. https://doi.org/10.3390/ma18112530
Shen K, Li F, Long Y, Yang Y, Long H, Luo R, Ma W, Hua J, Yang Z, Zhuo O, et al. Sustainable Recovery of Vanadium from Stone Coal via Nitric Acid Oxygen Pressure Leaching. Materials. 2025; 18(11):2530. https://doi.org/10.3390/ma18112530
Chicago/Turabian StyleShen, Keyu, Fei Li, Yuqin Long, Yang Yang, Huan Long, Ruixin Luo, Wenyuan Ma, Jun Hua, Zhaoxia Yang, Ou Zhuo, and et al. 2025. "Sustainable Recovery of Vanadium from Stone Coal via Nitric Acid Oxygen Pressure Leaching" Materials 18, no. 11: 2530. https://doi.org/10.3390/ma18112530
APA StyleShen, K., Li, F., Long, Y., Yang, Y., Long, H., Luo, R., Ma, W., Hua, J., Yang, Z., Zhuo, O., & Gao, F. (2025). Sustainable Recovery of Vanadium from Stone Coal via Nitric Acid Oxygen Pressure Leaching. Materials, 18(11), 2530. https://doi.org/10.3390/ma18112530






