Unveiling the Potential of Bioactive Glass in Volumetric Muscle Loss Regeneration
Abstract
1. Overview
- An overview of skeletal muscle structure and function;
- The natural muscle regeneration process and its limitations in VML;
- The potential of bioactive glasses (BG-) for skeletal muscle tissue engineering;
- BG properties, mechanisms of action, and bioactivity;
- Recent advances and studies on BG applications in muscle regeneration;
- Key findings and ongoing challenges in the field;
- Future perspectives on BG-based strategies for treating VML injuries.
2. Introduction
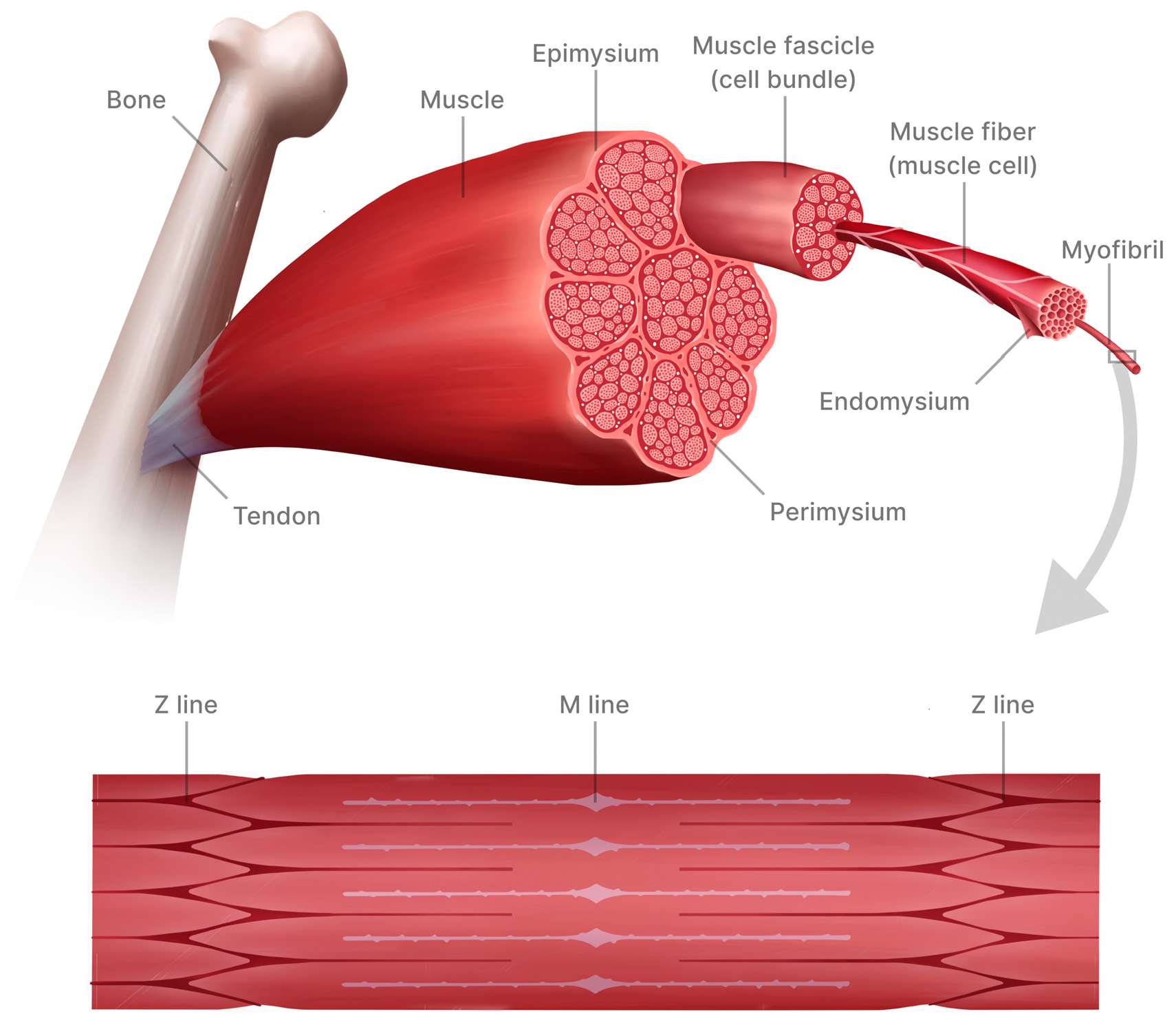
3. Skeletal Muscle Structure and Function
4. Skeletal Muscle Regeneration Process and Its Limitations
- Inflammatory phase: Damaged muscle fibers release cellular contents into the surrounding tissue, triggering an inflammatory response where immune cells are recruited to the injury site to remove cellular debris and begin the regeneration process. In response to injury, satellite cells (a population of muscle-specific stem cells) become activated.
- Proliferation phase: Cells proliferate and migrate to the site of injury to form a temporary extracellular cell matrix, guided by chemical signals [27]. This is followed by the regeneration phase, in which satellite cells differentiate into myoblasts that fuse into new myotubes, integrating with existing muscle fibers. Simultaneously, blood vessels regenerate (neoangiogenesis) to supply nutrients.
- Remodeling phase: The final phase, remodeling/maturation, involves the realignment of muscle fibers and the deposition of connective tissue to enhance structural integrity. The formation of functional neuromuscular junctions is essential for muscle contraction [3]. It is important to note that while this sequence of events generally characterizes the muscle repair process, the speed and efficacy of each phase can vary depending on factors such as the extent of injury, individual health, and age. However, this intrinsic regenerative response is often insufficient for substantial muscle loss, necessitating advanced approaches [28].
5. Bioactive Glasses as Potential Materials for Skeletal Muscle Tissue Regeneration
- -
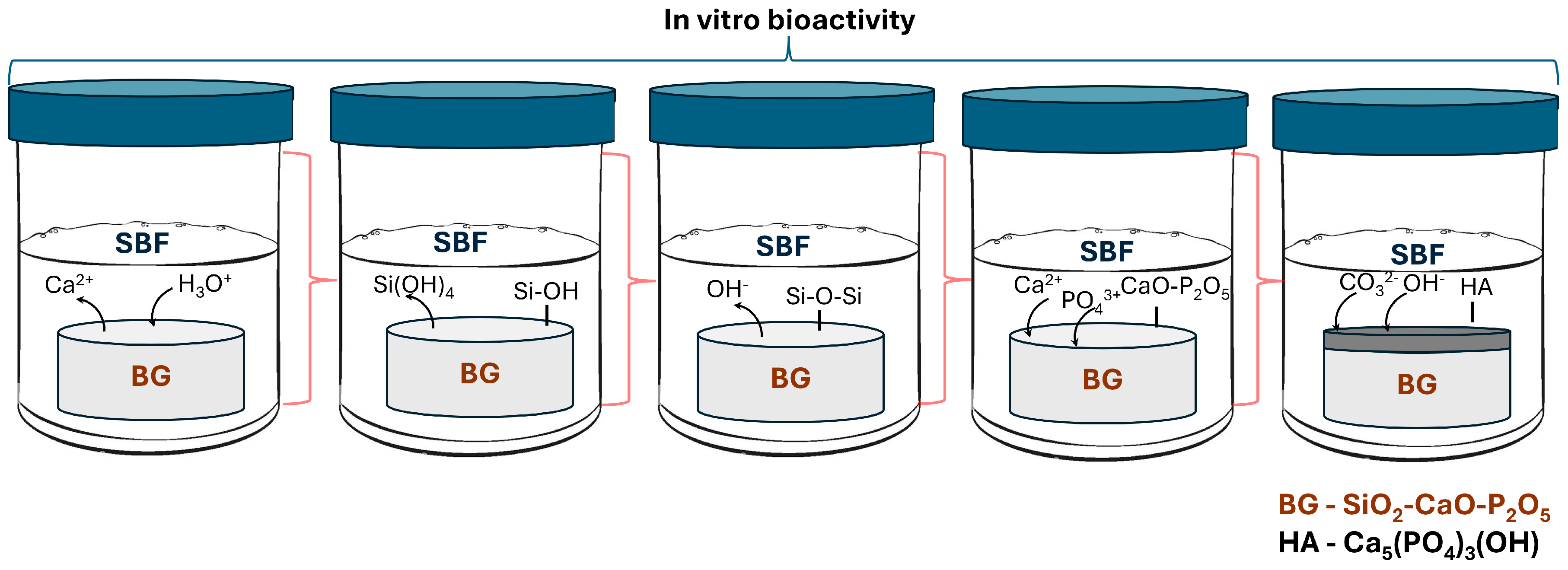
- In the first stage, Na+, K+, or Ca2+ ions from glass are exchanges with H+, H3O+ from solution. The leached ions depend on the glass composition. Vallet-Regi et al. [56] demonstrated that in the SiO2-CaO binary glass systems, the calcium content in the solution increases during the first hours, which is a result of the hydrolysis of Si-O-Ca groups.
- -
- In the second stage, silanization occurs; the soluble silica in the form of Si(OH)4 leaches into the solution by breaking Si-O-Si bonds, resulting in silanol (Si-OH).
- -
- The third stage is the condensation and repolymerization of SiO2 by forming Si-O-Si bonds.
- -
- In the fourth stage, an amorphous calcium phosphate layer forms through the migration of Ca2+ and PO43− ions to the silica-rich layer.
- -
- In the last stage, the hydroxyapatite (HA) layer crystallization occurs by including the CO32− and OH−.
6. Bioactive Glass’s Role in Muscle Regeneration
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VML | Volumetric Muscle Loss |
| BG | Bioactive glasses |
| VGEF | Vascular endothelial growth factor |
| bFGF | Linear dichroism basic fibroblast growth factor |
| PDGF | platelet-derived growth factor |
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
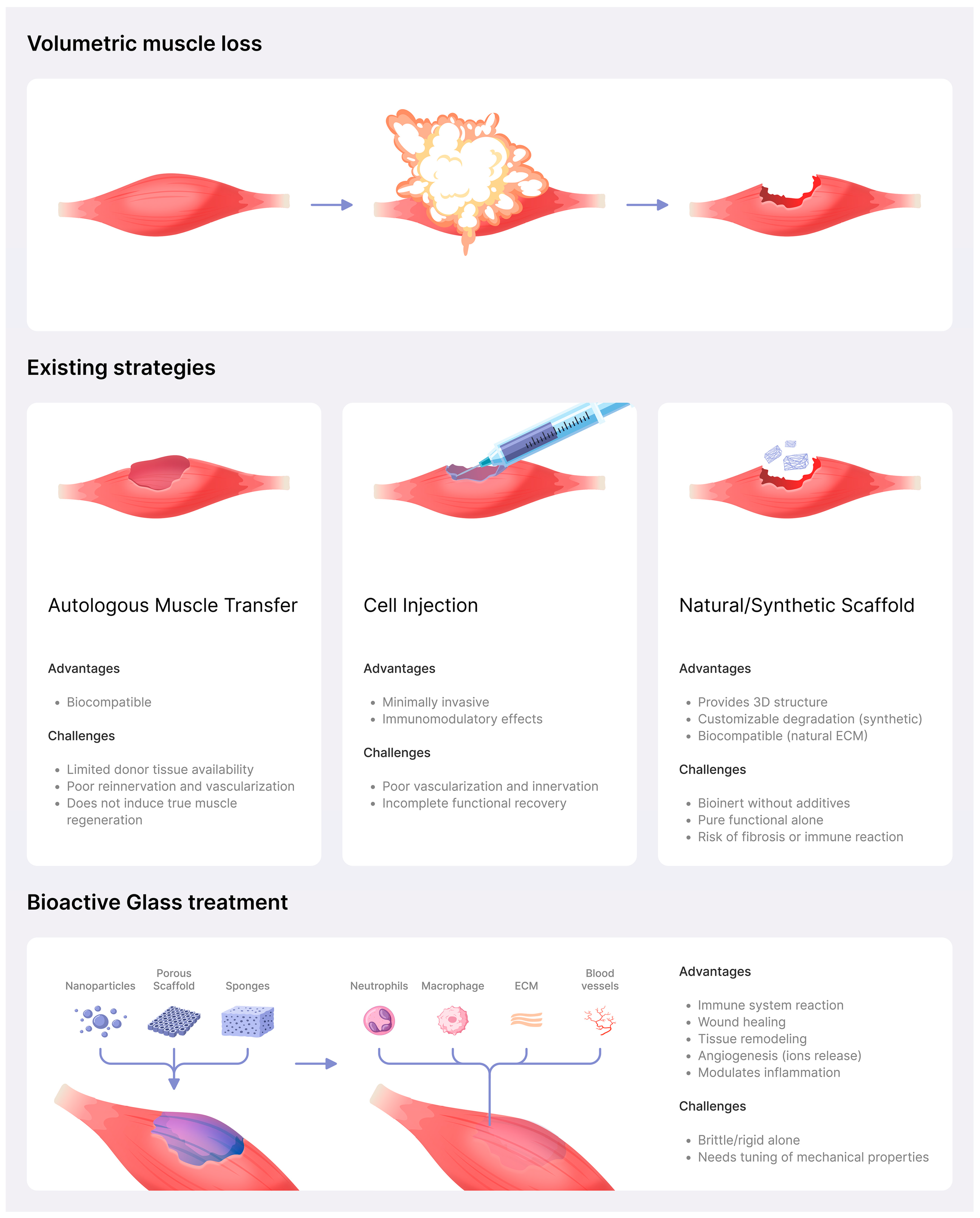
- Grogan, B.F.; Hsu, J.R. Volumetric Muscle Loss. J. Am. Acad. Orthop. Surg. 2011, 19, S35–S37. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Corona, B.T.; Walters, T.J. Therapeutic Strategies for Preventing Skeletal Muscle Fibrosis after Injury. Front. Pharmacol. 2015, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Downing, K.; Prisby, R.; Varanasi, V.; Zhou, J.; Pan, Z.; Brotto, M. Old and New Biomarkers for Volumetric Muscle Loss. Curr. Opin. Pharmacol. 2021, 59, 61–69. [Google Scholar] [CrossRef]
- Owens, B.D.; Kragh, J.F.; Macaitis, J.; Svoboda, S.J.; Wenke, J.C. Characterization of Extremity Wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J. Orthop. Trauma 2007, 21, 254–257. [Google Scholar] [CrossRef]
- Corona, B.T.; Rivera, J.C.; Owens, J.G.; Wenke, J.C.; Rathbone, C.R. Volumetric Muscle Loss Leads to Permanent Disability Following Extremity Trauma. J. Rehabil. Res. Dev. 2015, 52, 785–792. [Google Scholar] [CrossRef]
- Corona, B.T.; Machingal, M.A.; Criswell, T.; Vadhavkar, M.; Dannahower, A.C.; Bergman, C.; Zhao, W.; Christ, G.J. Further Development of a Tissue Engineered Muscle Repair Construct In Vitro for Enhanced Functional Recovery Following Implantation In Vivo in a Murine Model of Volumetric Muscle Loss Injury. Tissue Eng. Part A 2012, 18, 1213–1228. [Google Scholar] [CrossRef]
- Machingal, M.A.; Corona, B.T.; Walters, T.J.; Kesireddy, V.; Koval, C.N.; Dannahower, A.; Zhao, W.; Yoo, J.J.; Christ, G.J. A Tissue-Engineered Muscle Repair Construct for Functional Restoration of an Irrecoverable Muscle Injury in a Murine Model. Tissue Eng. Part A 2011, 17, 2291–2303. [Google Scholar] [CrossRef]
- Willett, N.J.; Li, M.-T.A.; Uhrig, B.A.; Boerckel, J.D.; Huebsch, N.; Lundgren, T.S.; Warren, G.L.; Guldberg, R.E. Attenuated Human Bone Morphogenetic Protein-2–Mediated Bone Regeneration in a Rat Model of Composite Bone and Muscle Injury. Tissue Eng. Part C Methods 2013, 19, 316–325. [Google Scholar] [CrossRef]
- Fortin, M.; Videman, T.; Gibbons, L.E.; Battié, M.C. Paraspinal Muscle Morphology and Composition. Med. Sci. Sports Exerc. 2014, 46, 893–901. [Google Scholar] [CrossRef]
- Javan, R.; Horvath, J.J.; Case, L.E.; Austin, S.; Corderi, J.; Dubrovsky, A.; Kishnani, P.S.; Bashir, M.R. Generating Color-Coded Anatomic Muscle Maps for Correlation of Quantitative Magnetic Resonance Imaging Analysis with Clinical Examination in Neuromuscular Disorders. Muscle Nerve 2013, 48, 293–295. [Google Scholar] [CrossRef]
- Hikida, R.S. Aging Changes in Satellite Cells and Their Functions. Curr. Aging Sci. 2011, 4, 279–297. [Google Scholar] [CrossRef]
- Wilkins, J.T.; Krivickas, L.S.; Goldstein, R.; Suh, D.; Frontera, W.R. Contractile Properties of Adjacent Segments of Single Human Muscle Fibers. Muscle Nerve 2001, 24, 1319–1326. [Google Scholar] [CrossRef]
- Macaluso, F.; Myburgh, K.H. Current Evidence That Exercise Can Increase the Number of Adult Stem Cells. J. Muscle Res. Cell Motil. 2012, 33, 187–198. [Google Scholar] [CrossRef]
- Bareja, A.; Holt, J.A.; Luo, G.; Chang, C.; Lin, J.; Hinken, A.C.; Freudenberg, J.M.; Kraus, W.E.; Evans, W.J.; Billin, A.N. Human and Mouse Skeletal Muscle Stem Cells: Convergent and Divergent Mechanisms of Myogenesis. PLoS ONE 2014, 9, e90398. [Google Scholar] [CrossRef]
- Thomas, G.D. Functional Muscle Ischemia in Duchenne and Becker Muscular Dystrophy. Front. Physiol. 2013, 4, 381. [Google Scholar] [CrossRef]
- Needham, D.M. Red and white muscle. Physiol. Rev. 1926, 6, 1–27. [Google Scholar] [CrossRef]
- Engel, W.K. The Essentiality of Histo- and Cytochemical Studies of Skeletal Muscle in the Investigation of Neuromuscular Disease. Neurology 1998, 51, 778–794. [Google Scholar] [CrossRef]
- Gollnick, P.D.; Armstrong, R.B.; Saubert, C.W.; Piehl, K.; Saltin, B. Enzyme Activity and Fiber Composition in Skeletal Muscle of Untrained and Trained Men. J. Appl. Physiol. 1972, 33, 312–319. [Google Scholar] [CrossRef]
- Mescher, A.L. Junqueira’s Basic Histology: Text and Atlas, 17th ed.; McGraw Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Periasamy, M.; Herrera, J.L.; Reis, F.C.G. Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism. Diabetes Metab. J. 2017, 41, 327. [Google Scholar] [CrossRef]
- Wolfe, R.R. The Underappreciated Role of Muscle in Health and Disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Nunes, J.S. Musculoskeletal System. In Atlas of Histology of the Juvenile Rat; Elsevier: Amsterdam, The Netherlands, 2016; pp. 29–44. [Google Scholar]
- Huard, J.; Li, Y.; Fu, F.H. Muscle Injuries and Repair: Current Trends in Research. J. Bone Jt. Surg. Am. 2002, 84, 822–832. [Google Scholar] [CrossRef]
- Morgan, J.E.; Partridge, T.A. Muscle Satellite Cells. Int. J. Biochem. Cell Biol. 2003, 35, 1151–1156. [Google Scholar] [CrossRef]
- Dhawan, J.; Rando, T.A. Stem Cells in Postnatal Myogenesis: Molecular Mechanisms of Satellite Cell Quiescence, Activation and Replenishment. Trends Cell Biol. 2005, 15, 666–673. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Järvinen, T.L.N.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle Injuries. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef]
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms Regulating Muscle Regeneration: Insights into the Interrelated and Time-Dependent Phases of Tissue Healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef]
- Verhelst, P.-J.; Dons, F.; Van Bever, P.-J.; Schoenaers, J.; Nanhekhan, L.; Politis, C. Fibula Free Flap in Head and Neck Reconstruction: Identifying Risk Factors for Flap Failure and Analysis of Postoperative Complications in a Low Volume Setting. Craniomaxillofac. Trauma Reconstr. 2019, 12, 183–192. [Google Scholar] [CrossRef]
- Novakovic, D.; Patel, R.S.; Goldstein, D.P.; Gullane, P.J. Salvage of Failed Free Flaps Used in Head and Neck Reconstruction. Head Neck Oncol. 2009, 1, 33. [Google Scholar] [CrossRef]
- Vogt, P.R.; Brunner-LaRocca, H.-P.; Lachat, M.; Ruef, C.; Turina, M.I. Technical Details with the Use of Cryopreserved Arterial Allografts for Aortic Infection: Influence on Early and Midterm Mortality. J. Vasc. Surg. 2002, 35, 80–86. [Google Scholar] [CrossRef]
- Sicari, B.M.; Dearth, C.L.; Badylak, S.F. Tissue Engineering and Regenerative Medicine Approaches to Enhance the Functional Response to Skeletal Muscle Injury. Anat. Rec. 2014, 297, 51–64. [Google Scholar] [CrossRef]
- Sicari, B.M.; Agrawal, V.; Siu, B.F.; Medberry, C.J.; Dearth, C.L.; Turner, N.J.; Badylak, S.F. A Murine Model of Volumetric Muscle Loss and a Regenerative Medicine Approach for Tissue Replacement. Tissue Eng. Part A 2012, 18, 1941–1948. [Google Scholar] [CrossRef]
- Yasuda, Y.; Koyama, H.; Tabata, Y.; Fujihara, Y.; Oba, M.; Uchinuma, E.; Takato, T. Controlled Delivery of BFGF Remodeled Vascular Network in Muscle Flap and Increased Perfusion Capacity Via Minor Pedicle. J. Surg. Res. 2008, 147, 132–137. [Google Scholar] [CrossRef]
- Matsui, M.; Tabata, Y. Enhanced Angiogenesis by Multiple Release of Platelet-Rich Plasma Contents and Basic Fibroblast Growth Factor from Gelatin Hydrogels. Acta Biomater. 2012, 8, 1792–1801. [Google Scholar] [CrossRef]
- Ju, Y.M.; Atala, A.; Yoo, J.J.; Lee, S.J. In Situ Regeneration of Skeletal Muscle Tissue through Host Cell Recruitment. Acta Biomater. 2014, 10, 4332–4339. [Google Scholar] [CrossRef]
- Hammers, D.W.; Sarathy, A.; Pham, C.B.; Drinnan, C.T.; Farrar, R.P.; Suggs, L.J. Controlled Release of IGF-I from a Biodegradable Matrix Improves Functional Recovery of Skeletal Muscle from Ischemia/Reperfusion. Biotechnol. Bioeng. 2012, 109, 1051–1059. [Google Scholar] [CrossRef]
- Grasman, J.M.; Do, D.M.; Page, R.L.; Pins, G.D. Rapid Release of Growth Factors Regenerates Force Output in Volumetric Muscle Loss Injuries. Biomaterials 2015, 72, 49–60. [Google Scholar] [CrossRef]
- Borselli, C.; Storrie, H.; Benesch-Lee, F.; Shvartsman, D.; Cezar, C.; Lichtman, J.W.; Vandenburgh, H.H.; Mooney, D.J. Functional Muscle Regeneration with Combined Delivery of Angiogenesis and Myogenesis Factors. Proc. Natl. Acad. Sci. USA 2010, 107, 3287–3292. [Google Scholar] [CrossRef]
- Dussoyer, M.; Michopoulou, A.; Rousselle, P. Decellularized Scaffolds for Skin Repair and Regeneration. Appl. Sci. 2020, 10, 3435. [Google Scholar] [CrossRef]
- Hench, L.L. The Story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Vulpoi, A.; Magyari, K.; Ştefan, R.; Baia, L. Overview of Properties of Bioactive Glasses and Glass Ceramics Induced by a Preparation Route; Nova Science Publisher: New York, NY, USA, 2016; ISBN 9781634859011. [Google Scholar]
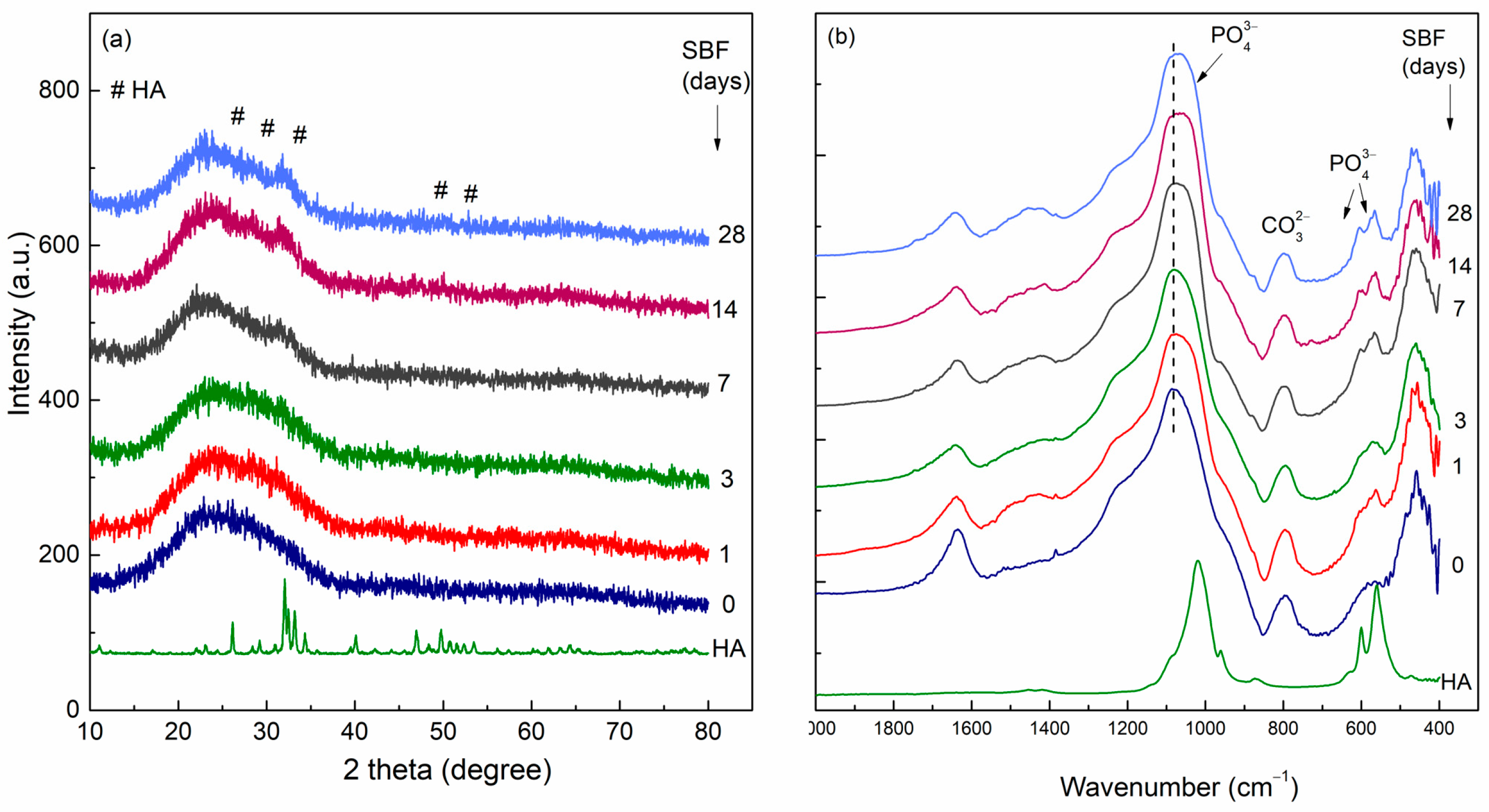
- Kokubo, T. Surface Chemistry of Bioactive Glass-Ceramics. J. Non-Cryst. Solids 1990, 120, 138–151. [Google Scholar] [CrossRef]
- Mârza, S.M.; Magyari, K.; Bogdan, S.; Moldovan, M.; Peştean, C.; Nagy, A.; Tǎbǎran, F.; Licarete, E.; Suarasan, S.; Dreanca, A.; et al. Skin Wound Regeneration with Bioactive Glass-Gold Nanoparticles Ointment. Biomed. Mater. 2019, 14, 025011. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce in Vivo Surface-structure Changes in Bioactive Glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Loh, Z.W.; Mohd Zaid, M.H.; Awang Kechik, M.M.; Fen, Y.W.; Amin, K.M.; Cheong, W.M. New Formulation Calcium-Based 45S5 Bioactive Glass: In Vitro Assessment in PBS Solution for Potential Dental Applications. J. Mater. Res. Technol. 2023, 24, 3815–3825. [Google Scholar] [CrossRef]
- Tóth, Z.R.; Feraru, A.; Debreczeni, D.; Todea, M.; Popescu, R.A.; Gyulavári, T.; Sesarman, A.; Negrea, G.; Vodnar, D.C.; Hernadi, K.; et al. Influence of Different Silver Species on the Structure of Bioactive Silicate Glasses. J. Non-Cryst. Solids 2022, 583, 121498. [Google Scholar] [CrossRef]
- Sinitsyna, P.; Engblom, M.; Hupa, L. Analysis and Modelling of in Vitro Bioactivity for Bioactive Glass Microspheres and Granules in Continuous Fluid Flow Conditions. J. Non-Cryst. Solids 2024, 637, 123029. [Google Scholar] [CrossRef]
- Côté, A.S.; Cormack, A.N.; Tilocca, A. Influence of Calcium on the Initial Stages of the Sol-Gel Synthesis of Bioactive Glasses. J. Phys. Chem. B 2016, 120, 11773–11780. [Google Scholar] [CrossRef]
- Salinas, A.J.; Martin, A.I.; Vallet-Regí, M. Bioactivity of Three CaO-P2O5-SiO2 Sol-Gel Glasses. J. Biomed. Mater. Res. 2002, 61, 524–532. [Google Scholar] [CrossRef]
- Shirtliff, V.J.; Hench, L.L. Bioactive Materials for Tissue Engineering, Regeneration and Repair. J. Mater. Sci. 2003, 38, 4697–4707. [Google Scholar] [CrossRef]
- Kovács, Z.; Fábián, M.; Szász, N.; Székács, I.; Kis, V.K. Tracking the Initial Stage of Bioactive Layer Formation on Si-Ca-Na-P Oxide Glasses by Nanoindentation. J. Non-Cryst. Solids 2022, 581, 121416. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Palamá, I.E.; Miranda Salvado, I.M.; Ferreira, J.M.F.; Pullar, R.C. The Role of Calcium (Source & Content) on the in Vitro Behaviour of Sol–Gel Quaternary Glass Series. Ceram. Int. 2020, 46, 1065–1075. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regí, M. Bioactive Ceramics: From Bone Grafts to Tissue Engineering. RSC Adv. 2013, 3, 11116. [Google Scholar] [CrossRef]
- Martínez, A.; Izquierdo-Barba, I.; Vallet-Regí, M. Bioactivity of a CaO−SiO2 Binary Glasses System. Chem. Mater. 2000, 12, 3080–3088. [Google Scholar] [CrossRef]
- Mavropoulos, E.; Costa, A.M.; Costa, L.T.; Achete, C.A.; Mello, A.; Granjeiro, J.M.; Rossi, A.M. Adsorption and Bioactivity Studies of Albumin onto Hydroxyapatite Surface. Colloids Surf. B Biointerfaces 2011, 83, 1–9. [Google Scholar] [CrossRef]
- Magyari, K.; Baia, L.; Vulpoi, A.; Simon, S.; Popescu, O.; Simon, V. Bioactivity Evolution of the Surface Functionalized Bioactive Glasses. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 261–272. [Google Scholar] [CrossRef]
- Shah Mohammadi, M.; Chicatun, F.; Stähli, C.; Muja, N.; Bureau, M.N.; Nazhat, S.N. Osteoblastic Differentiation under Controlled Bioactive Ion Release by Silica and Titania Doped Sodium-Free Calcium Phosphate-Based Glass. Colloids Surf. B Biointerfaces 2014, 121, 82–91. [Google Scholar] [CrossRef]
- Saffarian Tousi, N.; Velten, M.F.; Bishop, T.J.; Leong, K.K.; Barkhordar, N.S.; Marshall, G.W.; Loomer, P.M.; Aswath, P.B.; Varanasi, V.G. Combinatorial Effect of Si4+, Ca2+, and Mg2+ Released from Bioactive Glasses on Osteoblast Osteocalcin Expression and Biomineralization. Mater. Sci. Eng. C 2013, 33, 2757–2765. [Google Scholar] [CrossRef]
- Su, Y.; Cappock, M.; Dobres, S.; Kucine, A.J.; Waltzer, W.C.; Zhu, D. Supplemental Mineral Ions for Bone Regeneration and Osteoporosis Treatment. Eng. Regen. 2023, 4, 170–182. [Google Scholar] [CrossRef]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic Acid Stimulates Collagen Type 1 Synthesis and Osteoblastic Differentiation in Human Osteoblast-like Cells in Vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Finnson, K.W.; McLean, S.; Di Guglielmo, G.M.; Philip, A. Dynamics of Transforming Growth Factor Beta Signaling in Wound Healing and Scarring. Adv. Wound Care 2013, 2, 195–214. [Google Scholar] [CrossRef]
- Jones, J.R.; Brauer, D.S.; Hupa, L.; Greenspan, D.C. Bioglass and Bioactive Glasses and Their Impact on Healthcare. Int. J. Appl. Glass Sci. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Salih, V.; Franks, K.; James, M.; Hastings, G.W.; Knowles, J.C.; Olsen, I. Development of Soluble Glasses for Biomedical Use Part II: The Biological Response of Human Osteoblast Cell Lines to Phosphate-Based Soluble Glasses. J. Mater. Sci. Mater. Med. 2000, 11, 615–620. [Google Scholar] [CrossRef]
- Ege, D.; Nawaz, Q.; Beltrán, A.M.; Boccaccini, A.R. Effect of Boron-Doped Mesoporous Bioactive Glass Nanoparticles on C2C12 Cell Viability and Differentiation: Potential for Muscle Tissue Application. ACS Biomater. Sci. Eng. 2022, 8, 5273–5283. [Google Scholar] [CrossRef]
- Winston, D.D.; Li, T.; Lei, B. Bioactive Nanoglass Regulating the Myogenic Differentiation and Skeletal Muscle Regeneration. Regen. Biomater. 2023, 10, rbad059. [Google Scholar] [CrossRef]
- Jia, W.; Hu, H.; Li, A.; Deng, H.; Hogue, C.L.; Mauro, J.C.; Zhang, C.; Fu, Q. Glass-Activated Regeneration of Volumetric Muscle Loss. Acta Biomater. 2020, 103, 306–317. [Google Scholar] [CrossRef]
- Moura, D.; Souza, M.T.; Liverani, L.; Rella, G.; Luz, G.M.; Mano, J.F.; Boccaccini, A.R. Development of a Bioactive Glass-Polymer Composite for Wound Healing Applications. Mater. Sci. Eng. C 2017, 76, 224–232. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Popescu, R.A.; Magyari, K.; Taulescu, M.; Vulpoi, A.; Berce, C.; Bogdan, S.; Lelescu, C.; Dreancă, A.; Tudoran, O.; Papuc, I.; et al. New Alginate–Pullulan–Bioactive Glass Composites with Copper Oxide for Bone Tissue Regeneration Trials. J. Tissue Eng. Regen. Med. 2018, 12, 2112–2121. [Google Scholar] [CrossRef]
- Dreanca, A.; Muresan-Pop, M.; Taulescu, M.; Tóth, Z.R.; Bogdan, S.; Pestean, C.; Oren, S.; Toma, C.; Popescu, A.; Páll, E.; et al. Bioactive Glass-Biopolymers-gold Nanoparticle Based Composites for Tissue Engineering Applications. Mater. Sci. Eng. C 2021, 123, 112006. [Google Scholar] [CrossRef]
- Mao, C.; Chen, X.; Miao, G.; Lin, C. Angiogenesis Stimulated by Novel Nanoscale Bioactive Glasses. Biomed. Mater. 2015, 10, 025005. [Google Scholar] [CrossRef]
- Zahid, S.; Shah, A.T.; Jamal, A.; Chaudhry, A.A.; Khan, A.S.; Khan, A.F.; Muhammad, N.; Rehman, I. ur Biological Behavior of Bioactive Glasses and Their Composites. RSC Adv. 2016, 6, 70197–70214. [Google Scholar] [CrossRef]
- Kaou, M.H.; Furkó, M.; Balázsi, K.; Balázsi, C. Advanced Bioactive Glasses: The Newest Achievements and Breakthroughs in the Area. Nanomaterials 2023, 13, 2287. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, S.; Mathew, D.; Jo, J.; Tanaka, R.; Menon, D.; Ishimoto, T.; Nakano, T.; Nair, S.V.; Nair, M.B.; Tabata, Y. Dual Release of Growth Factor from Nanocomposite Fibrous Scaffold Promotes Vascularisation and Bone Regeneration in Rat Critical Sized Calvarial Defect. Acta Biomater. 2018, 78, 36–47. [Google Scholar] [CrossRef]
- Azizi, L.; Turkki, P.; Huynh, N.; Massera, J.M.; Hytönen, V.P. Surface Modification of Bioactive Glass Promotes Cell Attachment and Spreading. ACS Omega 2021, 6, 22635–22642. [Google Scholar] [CrossRef]
- Crush, J.; Hussain, A.; Seah, K.T.M.; Khan, W.S. Bioactive Glass: Methods for Assessing Angiogenesis and Osteogenesis. Front. Cell Dev. Biol. 2021, 9, 643781. [Google Scholar] [CrossRef]
- Sunderkötter, C.; Steinbrink, K.; Goebeler, M.; Bhardwaj, R.; Sorg, C. Macrophages and Angiogenesis. J. Leukoc. Biol. 1994, 55, 410–422. [Google Scholar] [CrossRef]
- Saygili, E.; Noor-Ebad, F.; Schröder, J.W.; Mischke, K.; Saygili, E.; Rackauskas, G.; Marx, N.; Kelm, M.; Rana, O.R. Autoantibodies in Dilated Cardiomyopathy Induce Vascular Endothelial Growth Factor Expression in Cardiomyocytes. Biochem. Biophys. Res. Commun. 2015, 465, 119–124. [Google Scholar] [CrossRef]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Tirziu, D.; Giordano, F.J.; Simons, M. Cell Communications in the Heart. Circulation 2010, 122, 928–937. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, F.; Sun, L.; Tang, F.; Gao, W.; Xie, W.; Cao, X.; Zhuang, J.; Chen, X. Bioactive Glass Activates VEGF Paracrine Signaling of Cardiomyocytes to Promote Cardiac Angiogenesis. Mater. Sci. Eng. C 2021, 124, 112077. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.M.; Scott, T.E.; Browe, D.P.; McGaughey, T.A.; Wood, C.; Wolyniak, M.J.; Freeman, J.W. Hydrogels for Skeletal Muscle Regeneration. Regen. Eng. Transl. Med. 2021, 7, 353–361. [Google Scholar] [CrossRef]
- Luz, G.M.; Mano, J.F. Chitosan/Bioactive Glass Nanoparticles Composites for Biomedical Applications. Biomed. Mater. 2012, 7, 054104. [Google Scholar] [CrossRef]
- Sarker, B.; Hum, J.; Nazhat, S.N.; Boccaccini, A.R. Combining Collagen and Bioactive Glasses for Bone Tissue Engineering: A Review. Adv. Healthc. Mater. 2015, 4, 176–194. [Google Scholar] [CrossRef]
- Barreto, M.E.V.; Medeiros, R.P.; Shearer, A.; Fook, M.V.L.; Montazerian, M.; Mauro, J.C. Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review. J. Funct. Biomater. 2022, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Sarmast Sh, M.; George, S.; Dayang Radiah, A.B.; Hoey, D.; Abdullah, N.; Kamarudin, S. Synthesis of Bioactive Glass Using Cellulose Nano Fibre Template. J. Mech. Behav. Biomed. Mater. 2022, 130, 105174. [Google Scholar] [CrossRef]
- Filipowska, J.; Pawlik, J.; Cholewa-Kowalska, K.; Tylko, G.; Pamula, E.; Niedzwiedzki, L.; Szuta, M.; Laczka, M.; Osyczka, A.M. Incorporation of Sol-Gel Bioactive Glass into PLGA Improves Mechanical Properties and Bioactivity of Composite Scaffolds and Results in Their Osteoinductive Properties. Biomed. Mater. 2014, 9, 065001. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, X.; Zhan, H.; Yan, F. Synthesis of Bioactive Poly(Ethylene Glycol)/SiO2-CaO-P2O5 Hybrids for Bone Regeneration. Mater. Sci. Eng. C 2012, 32, 707–711. [Google Scholar] [CrossRef]
- Atkinson, I.; Seciu-Grama, A.M.; Mocioiu, O.C.; Mocioiu, A.M.; Predoana, L.; Voicescu, M.; Cusu, J.P.; Grigorescu, R.M.; Ion, R.M.; Craciunescu, O. Preparation and Biocompatibility of Poly Methyl Methacrylate (PMMA)-Mesoporous Bioactive Glass (MBG) Composite Scaffolds. Gels 2021, 7, 180. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Flórez, M.; Cazón, P.; Vázquez, M. Selected Biopolymers’ Processing and Their Applications: A Review. Polymers 2023, 15, 641. [Google Scholar] [CrossRef]
- Shiva, S.; Asuwin Prabu, R.G.; Bajaj, G.; John, A.E.; Chandran, S.; Kumar, V.V.; Ramakrishna, S. A Review on the Recent Applications of Synthetic Biopolymers in 3D Printing for Biomedical Applications. J. Mater. Sci. Mater. Med. 2023, 34, 62. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ma, Z.; He, D.; Li, H. Modulating Degradation of Sodium Alginate/Bioglass Hydrogel for Improving Tissue Infiltration and Promoting Wound Healing. Bioact. Mater. 2021, 6, 3692–3704. [Google Scholar] [CrossRef]
- Zhang, W.; Hou, X.; Wang, H.; Kong, D.; Zhou, Y. Preparation of Chitosan-Sodium Alginate/Bioactive Glass Composite Cartilage Scaffolds with High Cell Activity and Bioactivity. Ceram. Int. 2023, 49, 1987–1996. [Google Scholar] [CrossRef]
- Magyari, K.; Dreancă, A.; Székely, I.; Popescu, A.; Feraru, A.; Páll, E.; Gyulavári, T.; Suciu, M.; Cenariu, M.; Bobu, E.; et al. How Does the Structure of Pullulan Alginate Composites Change in the Biological Environment? J. Mater. Sci. 2022, 57, 19050–19067. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Ahmed, I.; Blaker, J.J.; Bismarck, A.; Boccaccini, A.R.; Lewis, M.P.; Nazhat, S.N.; Knowles, J.C. Effect of Iron on the Surface, Degradation and Ion Release Properties of Phosphate-Based Glass Fibres. Acta Biomater. 2005, 1, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Collins, C.A.; Lewis, M.P.; Olsen, I.; Knowles, J.C. Processing, Characterisation and Biocompatibility of Iron-Phosphate Glass Fibres for Tissue Engineering. Biomaterials 2004, 25, 3223–3232. [Google Scholar] [CrossRef]
- Shah, R.; Ready, D.; Knowles, J.C.; Hunt, N.P.; Lewis, M.P. Sequential Identification of a Degradable Phosphate Glass Scaffold for Skeletal Muscle Regeneration. J. Tissue Eng. Regen. Med. 2014, 8, 801–810. [Google Scholar] [CrossRef]
- Shah, R.; Sinanan, A.C.M.; Knowles, J.C.; Hunt, N.P.; Lewis, M.P. Craniofacial Muscle Engineering Using a 3-Dimensional Phosphate Glass Fibre Construct. Biomaterials 2005, 26, 1497–1505. [Google Scholar] [CrossRef]
- Montarras, D.; Morgan, J.; Collins, C.; Relaix, F.; Zaffran, S.; Cumano, A.; Partridge, T.; Buckingham, M. Direct Isolation of Satellite Cells for Skeletal Muscle Regeneration. Science 2005, 309, 2064–2067. [Google Scholar] [CrossRef]
- Quarta, M.; Cromie, M.; Chacon, R.; Blonigan, J.; Garcia, V.; Akimenko, I.; Hamer, M.; Paine, P.; Stok, M.; Shrager, J.B.; et al. Bioengineered Constructs Combined with Exercise Enhance Stem Cell-Mediated Treatment of Volumetric Muscle Loss. Nat. Commun. 2017, 8, 15613. [Google Scholar] [CrossRef]
- Forbes, S.J.; Rosenthal, N. Preparing the Ground for Tissue Regeneration: From Mechanism to Therapy. Nat. Med. 2014, 20, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-H.; Froidevaux, C.; Biermann, I.; Kaňková, H.; Büchner, M.; Schubert, D.W.; Salehi, S.; Boccaccini, A.R. Printable ADA-GEL-Based Composite Inks Containing Zn-Doped Bioactive Inorganic Fillers for Skeletal Muscle Biofabrication. Biomater. Adv. 2025, 172, 214233. [Google Scholar] [CrossRef] [PubMed]
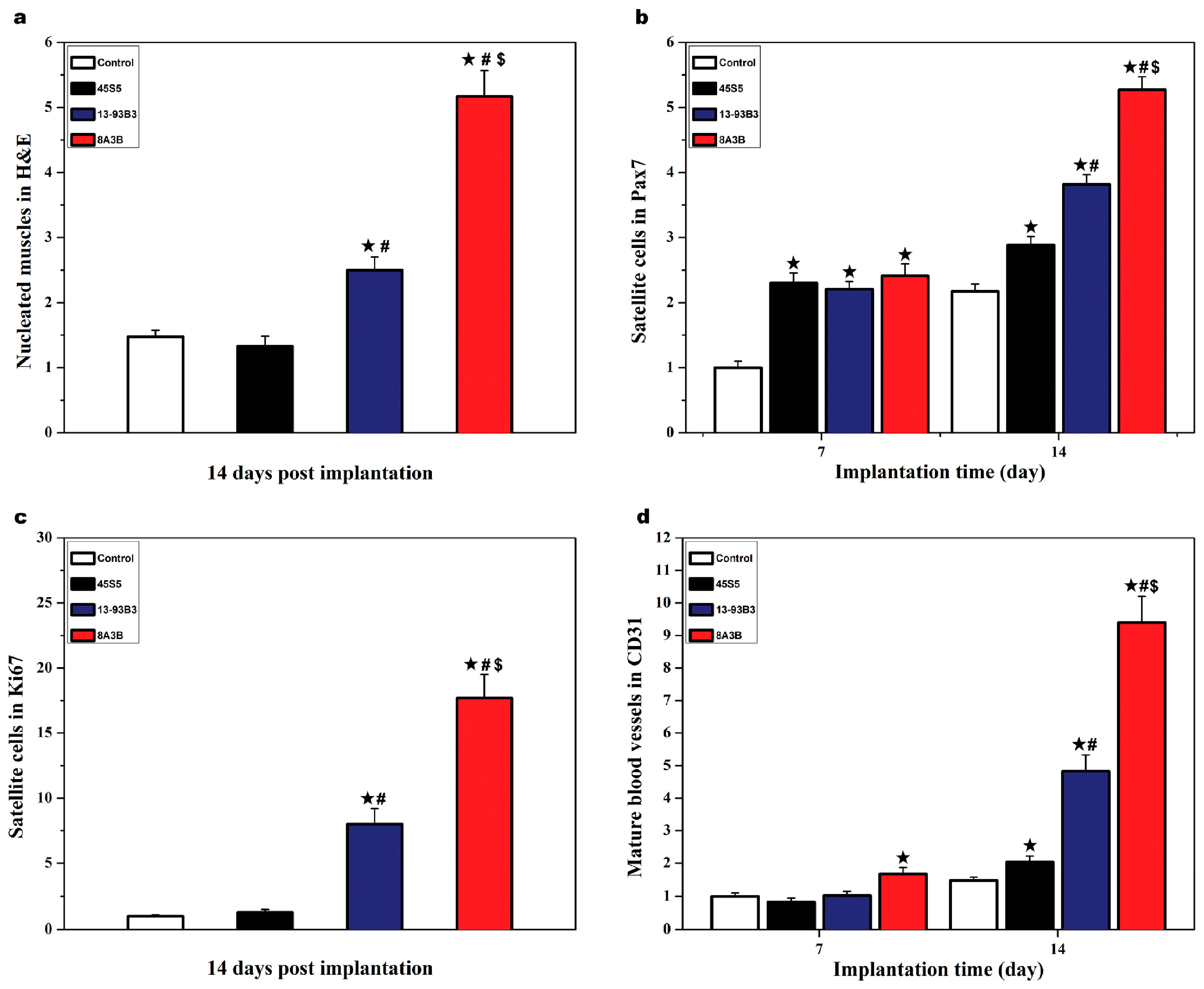

| Bioactive Glasses | Cell Type | Applied Form | Advantages/Disadvantages | Ref |
|---|---|---|---|---|
| 50P2O5∙30CaO∙(20-x)Na2O∙xFe2O3 (mol%) x = 0, 1, 2, 3, 4, 5 | Human craniofacial (masseter) muscle cell cultures | collagen coated glass fibers (in vitro) |
| [98,100] |
| 62.9P2O5 21.9Al2O3 15.2ZnO | Human masseter muscle-derived cell cultures | Glass fibres (in vitro) |
| [101] |
| 45S5 (45SiO2∙24.5Na2O∙24.5CaO∙6P2O5 wt%), 13-93B3 (56.7B2O3∙5.5Na2O∙11.1K2O∙4.6MgO∙18.4CaO∙3.4P2O5 wt%), 8A3B (50.7B2O3∙10.8Al2O3∙4.9Na2O∙9.9K2O∙4.1MgO∙16.4CaO∙3.2P2O5 wt%) | Mouse myoblast C2C12 | BG particles (in vivo) |
| [68] |
| MBG (58SiO2-42CaO mol%), 10B-MBG (50SiO2-40CaO-10B2O3 mol%), 18B-MBG (45SiO2-37CaO-18B2O3 mol%) | Myoblast C2C12 | Mesoporous BG (in vitro) |
| [66] |
| 100Si-BGN (SiO2), 80Si-BGN (80SiO2-16CaO-4P2O5 mol%), 60Si-BGN (60SiO2-30CaO-4P2O5 mol%), | Myoblast C2C12, Fibroblast L929 | BG-Pluronic F127 hydrogel (in vivo) |
| [67] |
| 8020 (80SiO2–20CaO mol%), 2Zn (80SiO2–18CaO-2ZnO mol%), 5Zn (80SiO2–15CaO-5ZnO mol%), 10Zn (80SiO2–10CaO-10ZnO mol%) | Myoblast cell line C2C12 cells | BG-alginate-gelatin hydrogels (in vitro) |
| [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zăvoi, A.-A.; Dreancă, A.; Magyari, K.; Baia, L.; Ober, C.; Oana, L. Unveiling the Potential of Bioactive Glass in Volumetric Muscle Loss Regeneration. Materials 2025, 18, 2529. https://doi.org/10.3390/ma18112529
Zăvoi A-A, Dreancă A, Magyari K, Baia L, Ober C, Oana L. Unveiling the Potential of Bioactive Glass in Volumetric Muscle Loss Regeneration. Materials. 2025; 18(11):2529. https://doi.org/10.3390/ma18112529
Chicago/Turabian StyleZăvoi, Andreea-Alina, Alexandra Dreancă, Klara Magyari, Lucian Baia, Ciprian Ober, and Liviu Oana. 2025. "Unveiling the Potential of Bioactive Glass in Volumetric Muscle Loss Regeneration" Materials 18, no. 11: 2529. https://doi.org/10.3390/ma18112529
APA StyleZăvoi, A.-A., Dreancă, A., Magyari, K., Baia, L., Ober, C., & Oana, L. (2025). Unveiling the Potential of Bioactive Glass in Volumetric Muscle Loss Regeneration. Materials, 18(11), 2529. https://doi.org/10.3390/ma18112529








