Synthesis and Photochromic Properties of Diarylethene Derivatives with Aggregation-Induced Emission (AIE) Behavior
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instruments
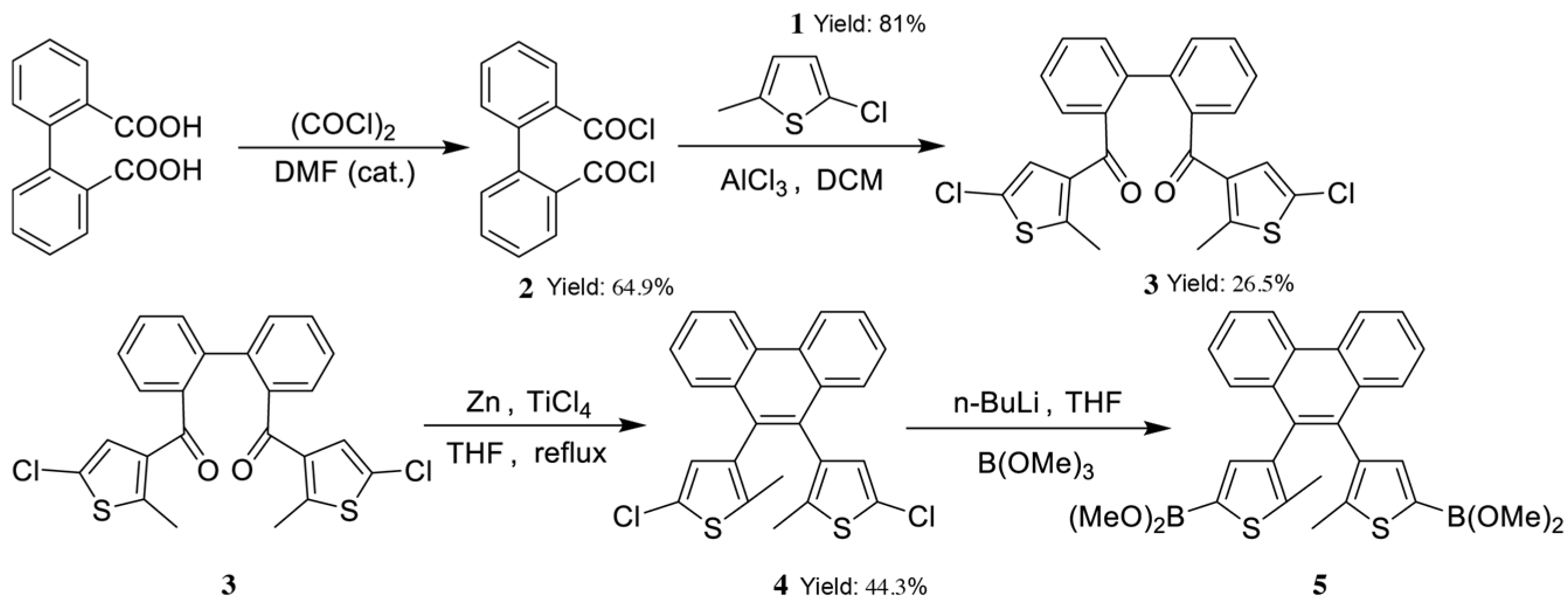
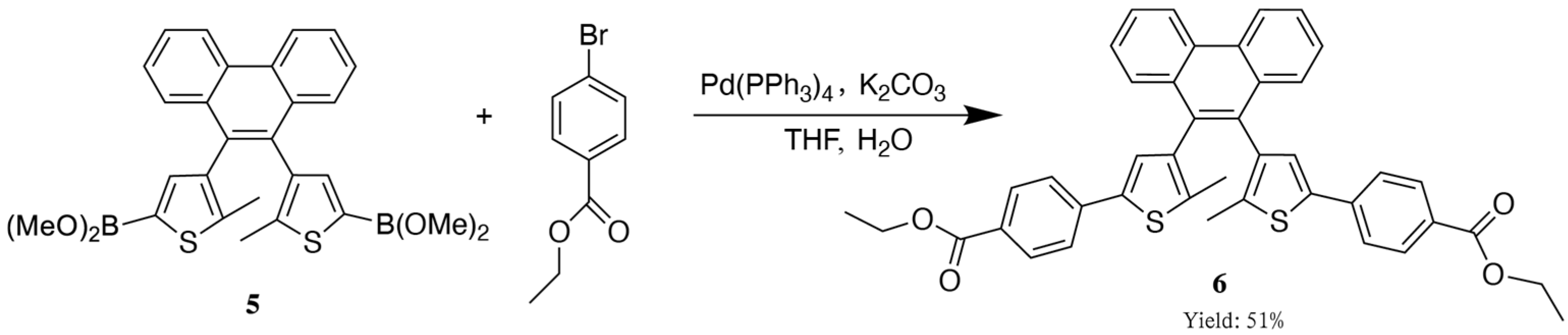
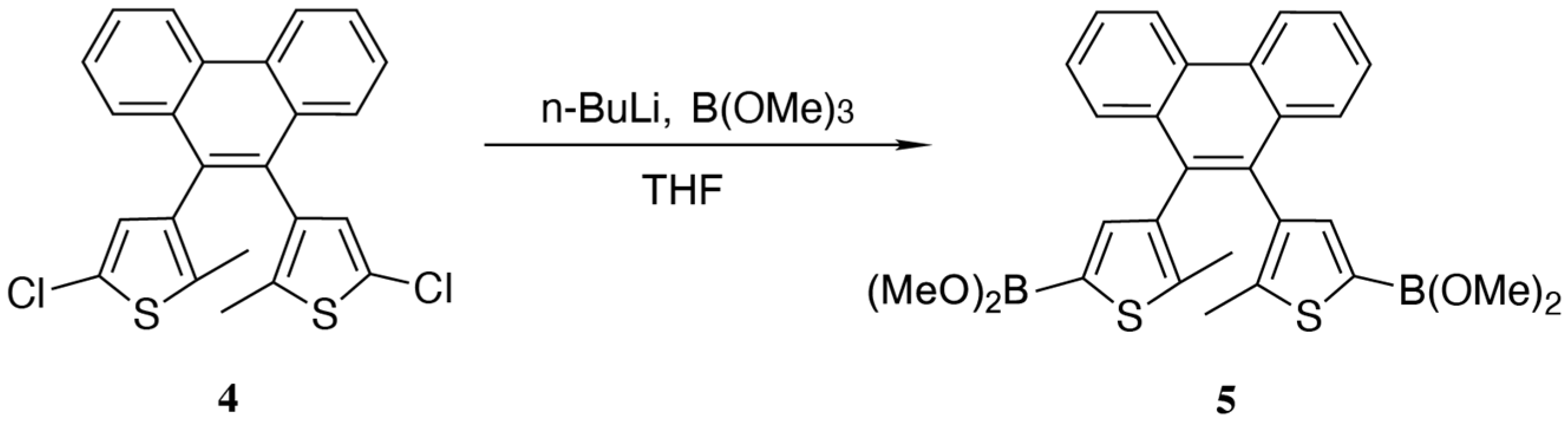
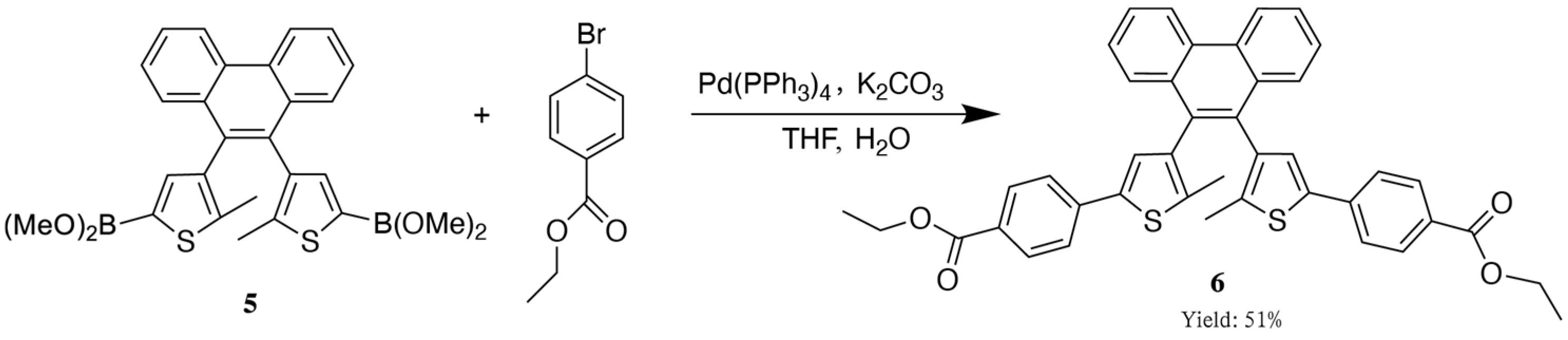
2.3. The Synthetic Route of the Target Compound, Diarylethene Ethyl Benzoate
2.4. Synthesis of Diarylethene Ethyl Benzoate
3. Results and Discussion
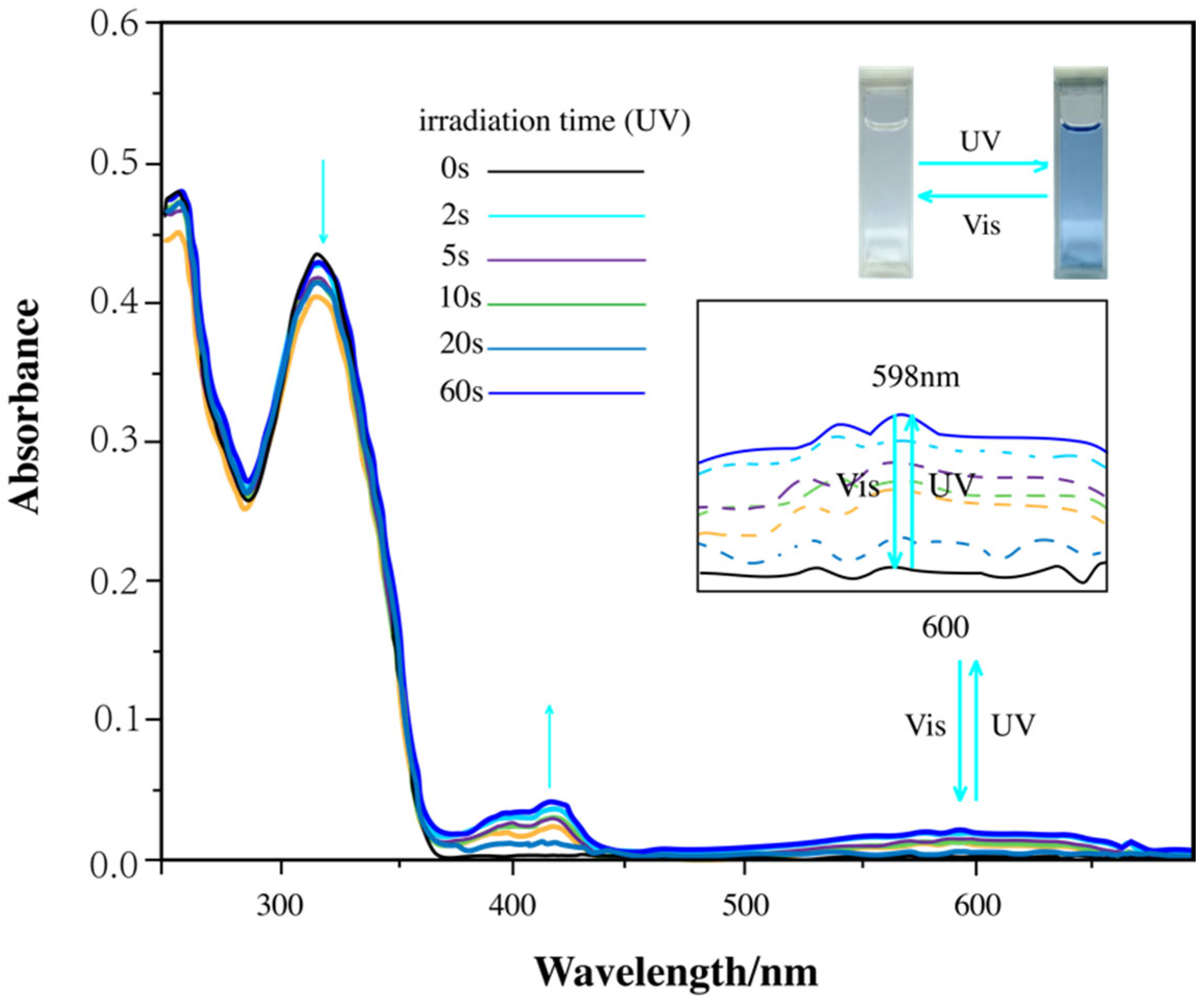
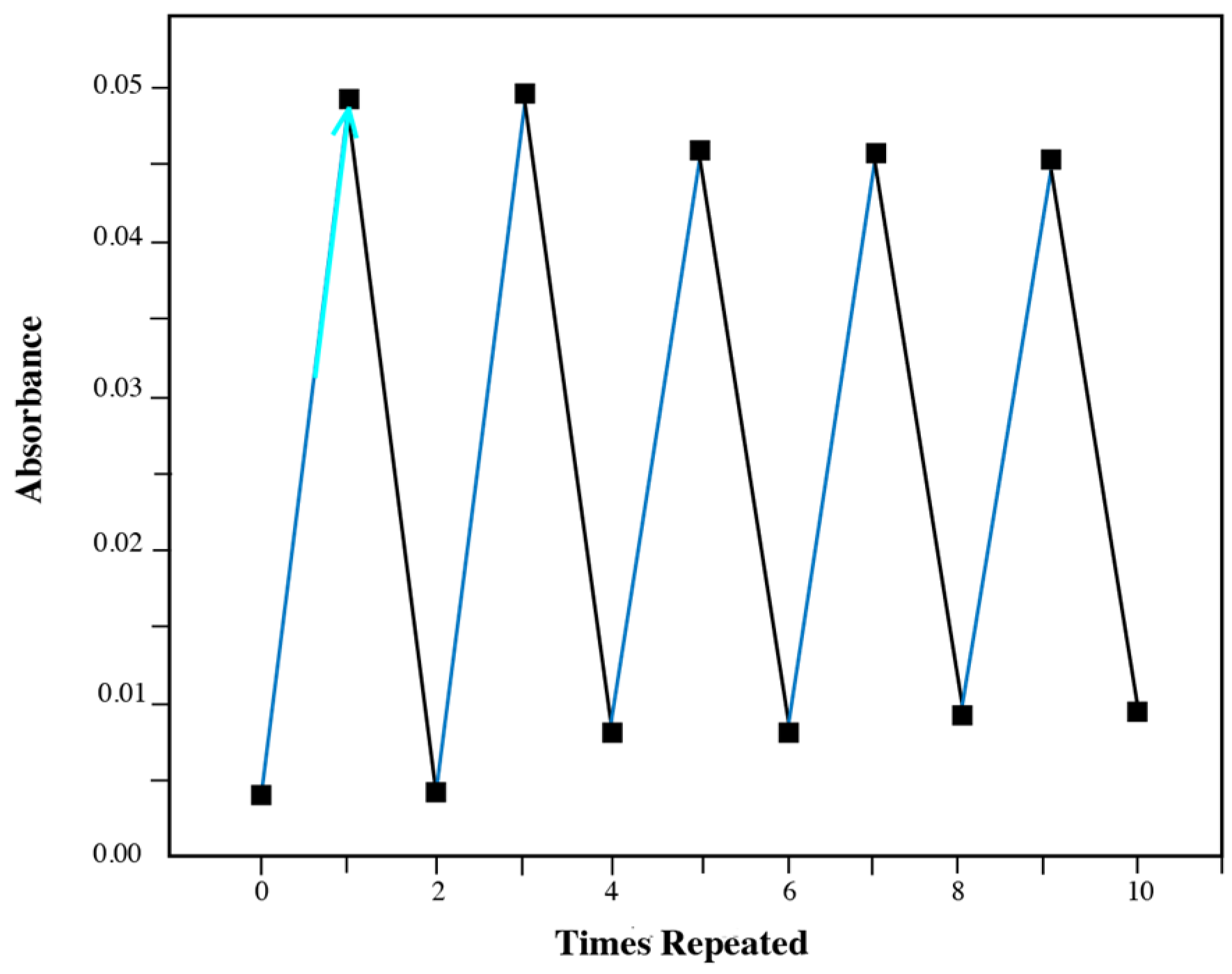
3.1. Photochromic Properties of Diarylethene Benzoate
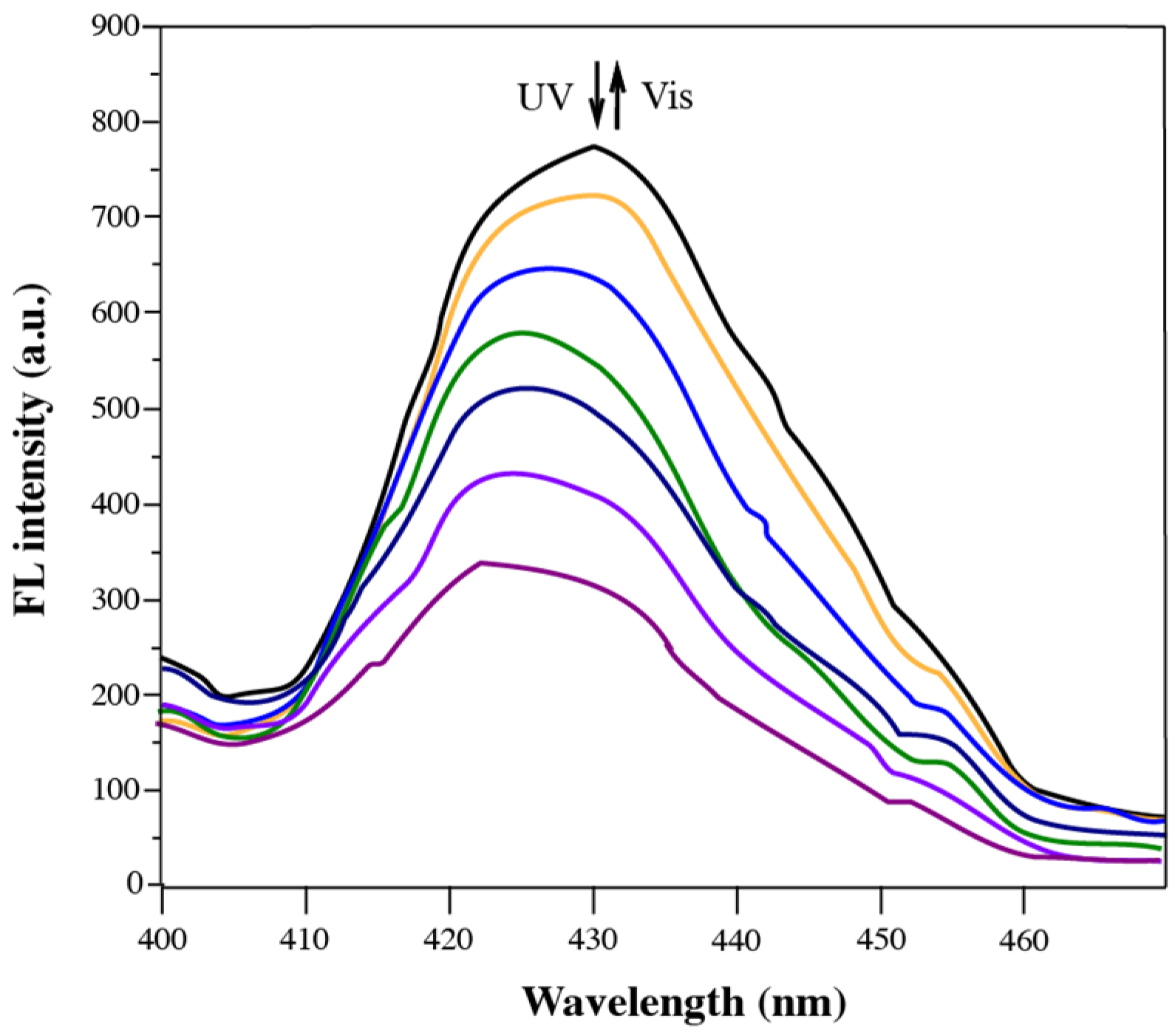
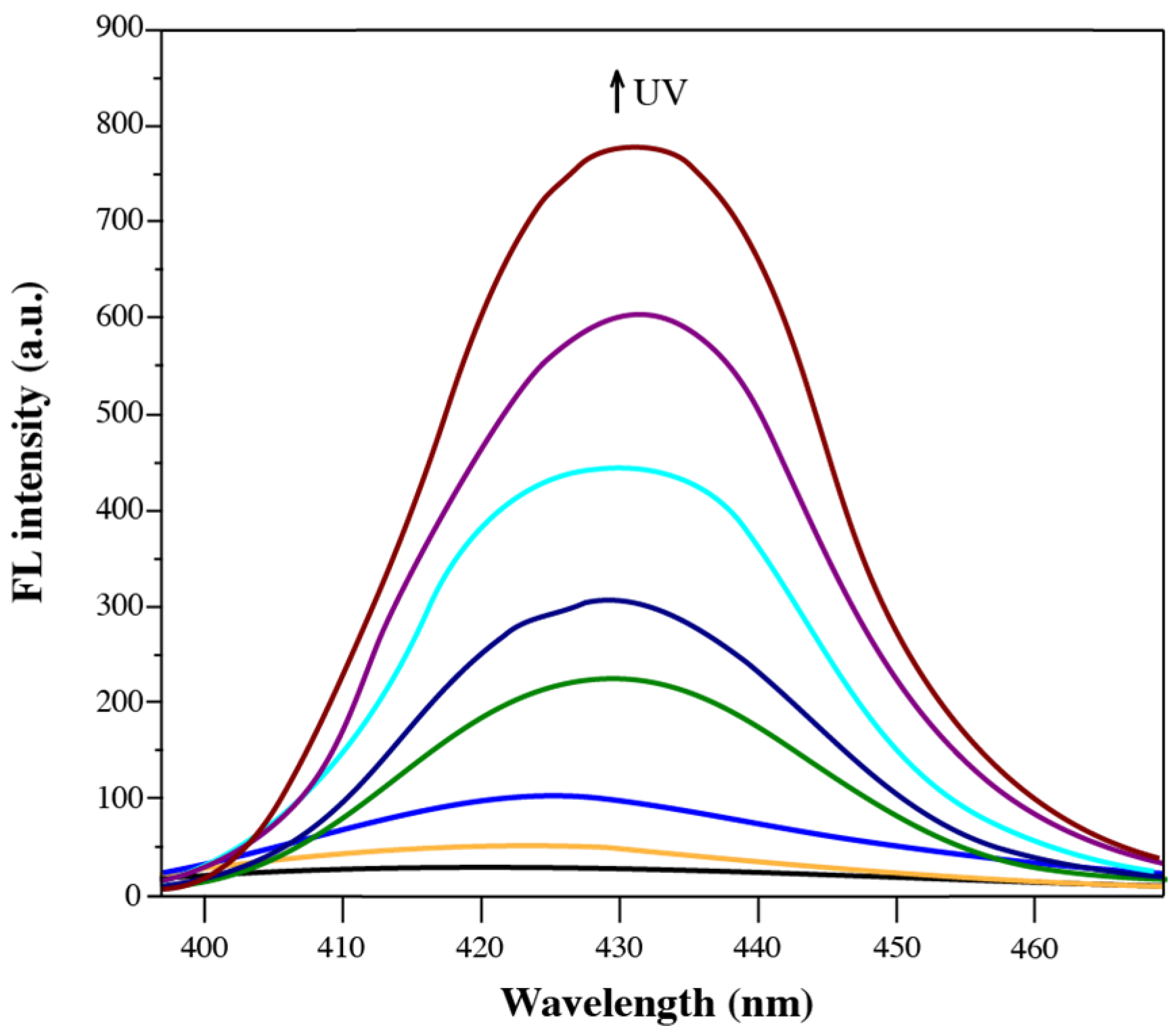
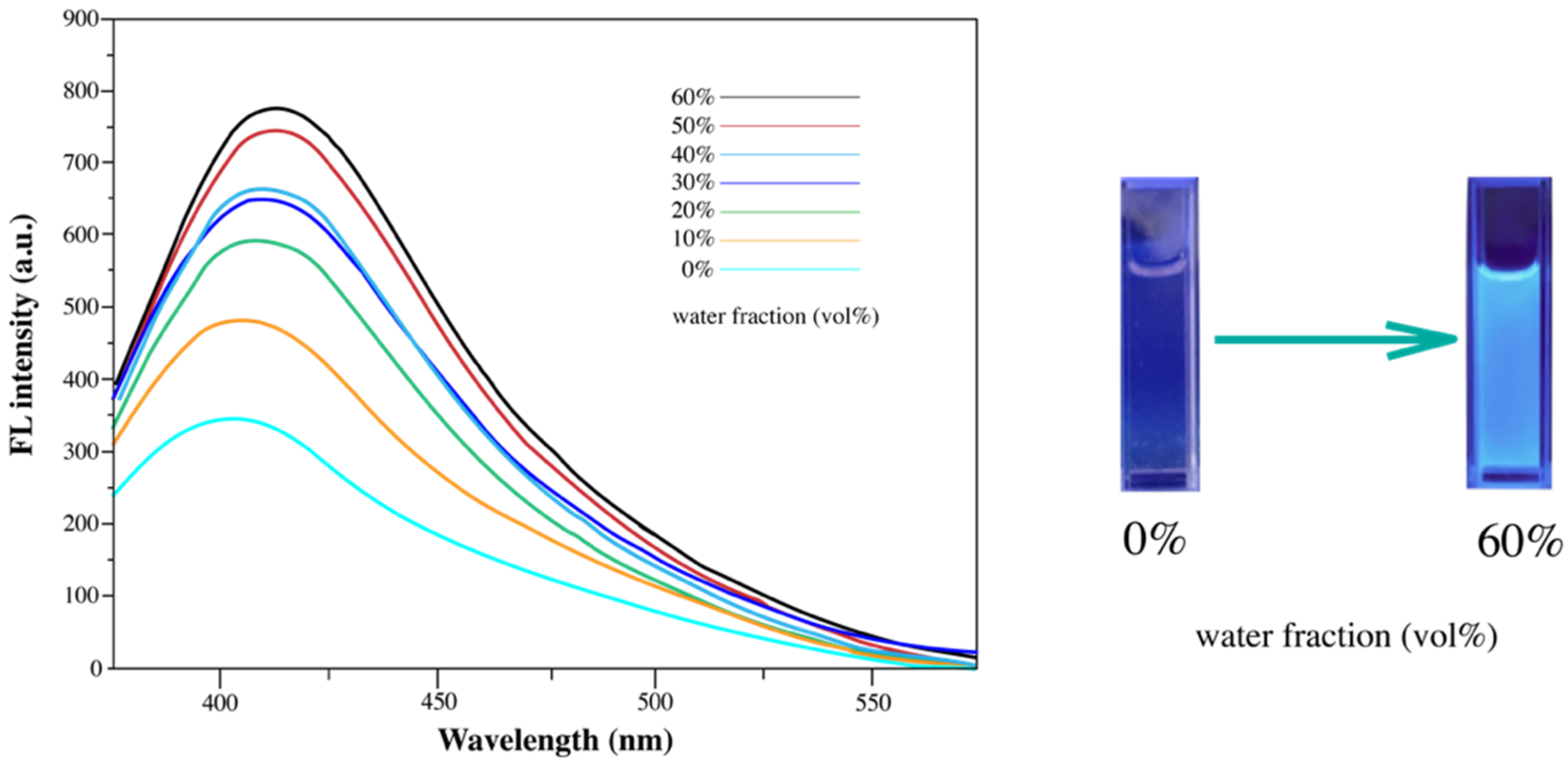
3.2. Fluorescence Properties of Diarylethene Benzoate
3.3. Potential Application Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irie, M. Photochromism of diarylethene single molecules and single crystals. Photochem. Photobiol. Sci. 2010, 9, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhao, J.; Liu, Y.; Mao, Z.; Wang, S.; Chi, Z. All-visible-light triggered photoswitch of dithienylethene derivatives with molecular conformation changes exceeding 5 Å. Adv. Funct. Mater. 2022, 33, 2211009. [Google Scholar] [CrossRef]
- Kobatake, S.; Takami, S.; Muto, H.; Irie, M. Rapid and reversible shape changes of molecular crystals on photoirradiation. Nature 2007, 446, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, X.; Zhang, H.; Ma, X.; Liu, Y.; Guo, H.; Yin, J. Efficient blue light-responsive dithienylethenes with exceptional photochromic performance. Chin. Chem. Lett. 2023, 34, 107645. [Google Scholar] [CrossRef]
- Li, R.-J.; Han, M.; Tessarolo, J.; Holstein, J.J.; Lübben, J.; Dittrich, B.; Volkmann, C.; Finze, M.; Jenne, C.; Clever, G.H. Successive photoswitching and derivatization effects in photochromic dithienylethene-based coordination cages. ChemPhotoChem 2019, 3, 378–383. [Google Scholar] [CrossRef]
- Mondal, P.P.; Neem, M.; Chand, R.; Pandit, A.; Neogi, S. Luminescent metal–organic frameworks as multimodal platforms for advanced anticounterfeiting and security applications. Chem. Mater. 2024, 36, 10451–10473. [Google Scholar] [CrossRef]
- Munendra, P.S.; Jubaraj, B.B. Dual modes and dual emissions of an amino-naphthoquinone derivative. J. Fluoresc. 2017, 27, 1923–1928. [Google Scholar] [CrossRef]
- Kawai, Y.; Oriki, T.; Sato, Y.; Nogami, J.; Kamiya, Y.; Suzuki, S.; Tanaka, K. Enantioselective synthesis, crystal structures, and stereoisomerism of substituted o,m,o,p-tetraphenylenes. Org. Lett. 2024, 26, 7869–7874. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, J.; Luo, M.; Zeng, X. Cr-catalyzed intramolecular arylative cross-coupling of unactivated C–H bonds with C–halide bonds. Org. Lett. 2024, 26, 4093–4097. [Google Scholar] [CrossRef]
- Chen, N.; Ning, Z.; Gong, Z.; Zhou, L.; Xu, L.; He, F.; Gao, Z.; Heng, J.Y.Y.; Du, S.; Ouyang, J. Realizing aggregation-induced emission improvement and multistimulus-responsive reversible fluorescence switching through multicomponent crystals. Cryst. Growth Des. 2025, 25, 2099–2109. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, H.; Liu, B.; Bai, J.; Peng, J.; Zhang, H.; Jia, J. AIE-active D-A-D(D′) dicyanodiaryl ethylene derivatives: Solvatohromism, aggregation-induced emission and high-contrast mechanofluorochromism. J. Lumin. 2025, 279, 121034. [Google Scholar] [CrossRef]
- Sherstiuk, A.; Lledós, A.; Lönnecke, P.; Hernando, J.; Sebastián, R.M.; Hey-Hawkins, E. Dithienylethene-based photoswitchable phosphines for the palladium-catalyzed Stille coupling reaction. Inorg. Chem. 2024, 63, 7652–7664. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Jeon, W.; Han, I.; Ahn, J.H.; Lansac, Y.; Jang, Y.H. Diarylethene-type photochromic electron traps extending narrow linear dynamic range of organic photodetectors. J. Phys. Chem. C 2024, 128, 7053–7062. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Hollister, K.K.; Molino, A.; Obi, A.D.; Deng, C.-L.; Tra, B.Y.E.; Stewart, B.M.; Dickie, D.A.; Wilson, D.J.D.; Gilliard, R.J., Jr. Bis(9-boraphenanthrene) and its stable biradical. J. Am. Chem. Soc. 2023, 145, 21475–21482. [Google Scholar] [CrossRef]
- Avramopoulos, A.; Reis, H.; Tzeli, D.; Zaleśny, R.; Papadopoulos, M.G. Photoswitchable molecular units with tunable nonlinear optical activity: A theoretical investigation. Molecules 2023, 28, 5646. [Google Scholar] [CrossRef]
- Barale, M.; Turcas, I.; Gousseau, V.; Escadeillas, M.; Caytan, E.; Taupier, G.; Molard, Y.; Fihey, A.; Boixel, J. Photo-modulation of the two-photon excited fluorescence of dithienylethene bis-(1-pyrenyl) compounds: An experimental and theoretical study. Dye. Pigment. 2025, 232, 112473. [Google Scholar] [CrossRef]
- Yan, Q.; Xu, J.; Luo, M.; Lu, J.; Ren, J.; Wang, S. Pyrene-dithienylethene-tetra(tri)phenylethylene triads: Photo-controlled intramolecular energy transfer and photochromic fluorescence switching. Dye. Pigment. 2023, 214, 111231. [Google Scholar] [CrossRef]
- Basak, U.; Chatterjee, D.P.; Mahapatra, G.; Nandi, A.K. Enhanced optoelectronic properties of polythio-phene-g-poly (dimethyl amino ethyl methacrylate)-b-poly(diethylene glycol methyl ether methacrylate) copolymers using “grafting onto” synthetic strategy. ACS Appl. Mater. Interfaces 2024, 16, 48854–48869. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.; Yuan, L. Recent advances of photoresponsive supramolecular switches. Asian J. Org. Chem. 2021, 10, 74–90. [Google Scholar] [CrossRef]
- Xing, Y.; Ma, Y.; Cong, L.; Li, W.; Wang, Y.; Guo, L. Preparation of spiropyran-based photochromic materials. Dye. Finish. 2021, 58, 18–19. [Google Scholar]
- Ding, A.; Tang, F.; Alsberg, E. 4D printing: A comprehensive review of technologies, materials, stimuli, design, and emerging applications. Chem. Rev. 2025, 125, 3663–3771. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Tang, S.; Li, L.; Fang, L.; Zeng, Q.; Sun, G.; Wu, S.; Naumov, P.; Gong, J. Flexible organic crystalline fibers and loops with strong second harmonic generation. J. Am. Chem. Soc. 2025, 147, 11346–11358. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Itoh, M.; Hirai, T. Colorimetric response of spiropyran derivative for anions in aqueous or organic media. Tetrahedron 2011, 67, 891–897. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sumiya, S.; Hirai, T. Highly sensitive cyanide anion detection with a coumarin-spiropyran conjugate as a fluorescent receptor. Chem. Commun. 2011, 47, 4953–4955. [Google Scholar] [CrossRef]
- Ding, N.; Hu, N.; Zhang, J.; Xu, H.; Meng, L.; Cai, X.; Müller-Buschbaum, P.; Qi, D.; Zhong, Q. Reversible and photochromic peony-shaped hairpin prepared from electrospun hybrid nanofibers for visualized solar UV radiation detector. ACS Appl. Nano Mater. 2024, 7, 8140–8150. [Google Scholar] [CrossRef]
- Liu, X.; Meng, J.; Wang, H. Preparation and performance of polyurethane-spiropyran photochromic micro-capsules. Print Dye 2021, 47, 1–5. [Google Scholar]
- Wang, X.; Zhang, M.; Li, M. Preparation of tungsten-based polyvinyl alcohol waterborne coating and devel-opment of photochromic composite fabric. Macromol. Mater. Eng. 2021, 306, 2100540. [Google Scholar] [CrossRef]
- Ganguly, T.; Das, S.; Maity, D.; Baitalik, S. Luminescent ruthenium–terpyridine complexes coupled with stil-bene-appended naphthalene, anthracene, and pyrene motifs demonstrate fluoride ion sensing and reversible trans–cis pho-toisomerization. Inorg. Chem. 2024, 63, 6883–6897. [Google Scholar] [CrossRef]
- Yang, L.; Gridnev, I.D.; Terada, M.; Jin, T. Intermolecular oxidative Friedel–Crafts reaction triggered ring ex-pansion affording 9,10-diarylphenanthrenes. Org. Lett. 2020, 22, 8920–8924. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hase, A.; Shiromae, R.; Nishimura, R.; Morimoto, M.; Hattori, Y.; Mayama, H.; Yokojima, S.; Nakamura, S.; Uchida, K. Straightforward fabrication of double roughness structures on a microcrystalline film of a diarylethene derivative. Langmuir 2024, 40, 7661–7668. [Google Scholar] [CrossRef]
- Usuki, A.; Kim, S.; Sakanishi, A.; Nabetani, S.; Maehashi, R.; Kamikubo, M.; Sato, F.; Ohta, Y.; Kurihara, S.; Fukaminato, T. Design and synthesis of a black photochromic diarylethene for bioinspired display applications. ACS Appl. Eng. Mater. 2024, 2, 1122–1130. [Google Scholar] [CrossRef]
- Vasilyuk, G.T.; Askirka, V.F.; German, A.E.; Sveklo, J.F.; Yasinskii, V.M.; Yaroshevich, A.A.; Kobeleva, O.I.; Valova, T.M.; Ayt, A.O.; Barachevsky, V.A.; et al. Photochromism of diarylethene composite organometallic nanostructures. J. Appl. Spectrosc. 2017, 84, 588–595. [Google Scholar] [CrossRef]
- Gu, F.; Ma, X. Photo-responsive supramolecular polymers based on host-guest interactions. In Handbook of Macrocyclic Supramolecular Assembly; Springer Nature: Singapore, 2020; pp. 1–32. [Google Scholar] [CrossRef]
- Usui, I. Development of novel chiral phosphine ligands utilizing helical space and their application to asymmetric catalytic reactions. Yakugaku Zasshi 2017, 137, 1381–1390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rybczyński, P.; Muzioł, T.; Kaczmarek-Kędziera, A.; Ośmiałowski, B. Topology switch between AIE and ACQ: A balance of substituents. J. Phys. Chem. C 2024, 128, 5651–5658. [Google Scholar] [CrossRef]
- Gong, Z.; Yue, L.; Tan, Z.; Wu, Y.; Huang, Y.; Ding, H.; Fan, C.; Liu, G.; Pu, S. Development of a novel AIEE fluorescent probe for hydrazine based on diarylethene and its applications in invisible two-dimensional code. Microchem. J. 2025, 209, 112598. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, H.; Zeng, X. Aggregation-induced emission: A new strategy for optoelectronic materials. J. Mater. Chem. C 2013, 1, 4627–4635. [Google Scholar]
- Shen, J.; Okamoto, Y. Autoamplification of molecular chirality through the induction of supramolecular chirality. Angew. Chem. Int. Ed. 2014, 53, 6000–6003. [Google Scholar] [CrossRef]
- Liu, X.; Ma, X. Photo-responsive supramolecular polymer hosts and guests. J. East China Univ. Sci. Technol. (Nat. Sci. Ed.) 2019, 45, 517–527. [Google Scholar] [CrossRef]
- Luo, Q.; Fan, Q.; Huang, W. Synthesis overview of diarylethene-based photochromic materials. Org. Chem. 2007, 02, 175–187. [Google Scholar]
- Nishimura, R.; Nagakawa, Y.; Morimoto, M. Multicolor photochromism of two-component diarylethene crystals containing oxidized and unoxidized benzothiophene groups. Crystals 2022, 12, 1730. [Google Scholar] [CrossRef]
- Zhai, S.; Zhu, X.; Zeng, H. Synthesis and properties of 1,3,4-oxadiazole-linked diarylethene compounds. J. Chin. Univ. Chem. 2011, 32, 2316–2320. [Google Scholar]
- Li, H.; Lu, D.; Qian, B.-Y.; Lin, J.; Zhang, H.-J. Direct synthesis of K-region functionalized polycyclic aromatic hydrocarbons via twofold intramolecular C–H/C–H arylation. Org. Lett. 2024, 26, 11140–11144. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, G.; Cheng, R.; Li, Z.F.; Luo, Q.F. Synthesis and properties of multi-unit diarylethene photochromic compounds. Chin. J. Appl. Chem. 2011, 28, 1–5. [Google Scholar]
- Spangenberg, A.; Métivier, R.; Gonzalez, J.; Nakatani, K.; Yu, P.; Giraud, M.; Léaustic, A.; Guillot, R.; Uwada, T.; Asahi, T. Multiscale approach of photochromism: Synthesis and photo-chromic properties of a diarylethene in solution, in nanoparticles, and in bulk crystals. Adv. Mater. 2009, 21, 309–313. [Google Scholar] [CrossRef]
- Gao, Y. Preparation and Performance Study of Aryl Vinyl-Based Photochromic Materials. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2023. [Google Scholar]
- Roubinet, B.; Weber, M.; Shojaei, H.; Bates, M.; Bossi, M.L.; Belov, V.N.; Irie, M.; Hell, S.W. Fluorescent photo-switchable diarylethene for biolabeling and sin-gle-molecule localization microscopies with optical super resolution. J. Am. Chem. Soc. 2017, 139, 6611–6620. [Google Scholar] [CrossRef]
- Luo, Q.; Cao, F.; Xiong, C.; Dou, Q.; Qu, D.-H. Hybrid cis/trans Tetra-arylethenes with Switchable Aggregation-Induced Emission (AIE) and Reversible Photochromism in the Solution, PMMA Film, Solid Powder, and Single Crystal. J. Org. Chem. 2017, 82, 11242–11347. [Google Scholar] [CrossRef]
- Peng, Q.; Shuai, Z. Molecular mechanism of aggregation-induced emission. Aggregate 2021, 2, e91. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes based on AIE fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, C.; Tang, B.Z. Aggregation-induced emission: New vistas at the aggregate level. Angew. Chem. Int. Ed. 2021, 60, 2070–2098. [Google Scholar] [CrossRef]
- Xue, P.; Lu, R. Aggregation-Induced Emission in Supramolecular π-Organogels. In Aggregation-Induced Emission; Qin, A., Tang, B.Z., Eds.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Gao, B.; Wang, H.; Chen, Q.; Sun, H. Time-Resolved Spectroscopic Study of the Aggregation-Induced Emission Mechanism. In Aggregation-Induced Emission; Qin, A., Tang, B.Z., Eds.; Wiley: Hoboken, NJ, USA, 2013; Chapter 16. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- He, J.J. Photophysical and Chemical Properties of Phenanthrene- and Perylene-Based Diarylethene Photochromic compounds. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2014. [Google Scholar]
- Hu, Z.; Zhang, H.K.; Chen, Y.; Wang, Q.S.; Elsegood, M.R.J.; Teat, S.J.; Feng, X.; Islam, M.M.; Wu, F.G.; Tang, B.Z. Tetraphenylethylene-based color-tunable AIE-ESIPT chromophores. Dyes Pigm. 2020, 175, 108175. [Google Scholar] [CrossRef]
- Patel, D.G.; Walton, I.M.; Cox, J.M.; Gleason, C.J.; Butzer, D.R.; Benedict, J.B. Photoresponsive porous materials: The design and synthesis of photochromic diarylethene-based linkers and a metal–organic framework. Chem. Commun. 2014, 50, 2653–2656. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Xu, L.; Yan, W.; Shi, L.; Qiu, M. High-speed laser writing of structural colors for full-color inkless printing. Nat. Commun. 2023, 14, 653. [Google Scholar] [CrossRef]
- Wessely, M.; Jin, Y.; Nuengsigkapian, C.; Kashapov, A.; Qamar, I.P.S.; Tsetserukou, D.; Mueller, S. ChromoUpdate: Fast design iteration of photochromic color textures using grayscale previews and local color updates. In CHI ‘21 Proceedings; Association for Computing Machinery: New York, NY, USA, 2021; pp. 1–13. [Google Scholar] [CrossRef]
- Shen, P.; Liu, Y.; Qu, X.; Zhu, M.; Huang, T.; Sun, Q. An optical keypad lock with high resettability based on a quantum dot–porphyrin FRET nanodevice. J. Mater. Chem. C 2023, 5, 2986–2993. [Google Scholar] [CrossRef]
- Lu, L.; Wang, K.; Wu, H.; Qin, A.; Tang, B.Z. Simultaneously achieving high capacity storage and multilevel anti-counterfeiting using electrochromic and electrofluorochromic dual-functional AIE polymers. J. Mater. Chem. C 2021, 12, 7058–7065. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, P.; Tang, B.Z. Aggregation-induced emission (AIE) luminescent materials boosting optical storage into the new era of petabit-level capacity. Aggregate 2024, 5, e605. [Google Scholar] [CrossRef]
- Dolai, J.; Ray, R.; Ghosh, S.; Maity, A.; Jana, N.R. Optical nanomaterials for advanced bioimaging applications. Nanoscale 2023, 15, 23579–23597. [Google Scholar] [CrossRef]
- Duo, Y.; Han, L.; Yang, Y.; Wang, Z.; Wang, L.; Chen, J.; Xiang, Z.; Yoon, J.; Luo, G.; Tang, B.Z. Aggregation-Induced Emission Luminogen: Role in Biopsy for Precision Medicine. Chem. Rev. 2024, 124, 11242–11347. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Yu, H.; Jin, Y. Synthesis and Photochromic Properties of Diarylethene Derivatives with Aggregation-Induced Emission (AIE) Behavior. Materials 2025, 18, 2520. https://doi.org/10.3390/ma18112520
Guo J, Yu H, Jin Y. Synthesis and Photochromic Properties of Diarylethene Derivatives with Aggregation-Induced Emission (AIE) Behavior. Materials. 2025; 18(11):2520. https://doi.org/10.3390/ma18112520
Chicago/Turabian StyleGuo, Jiaxin, Haoyuan Yu, and Yuhua Jin. 2025. "Synthesis and Photochromic Properties of Diarylethene Derivatives with Aggregation-Induced Emission (AIE) Behavior" Materials 18, no. 11: 2520. https://doi.org/10.3390/ma18112520
APA StyleGuo, J., Yu, H., & Jin, Y. (2025). Synthesis and Photochromic Properties of Diarylethene Derivatives with Aggregation-Induced Emission (AIE) Behavior. Materials, 18(11), 2520. https://doi.org/10.3390/ma18112520





