Effect of Surface Aluminizing on the Zinc Corrosion Resistance of Fe-20Cr-5B-3Al Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Aluminizing Treatment
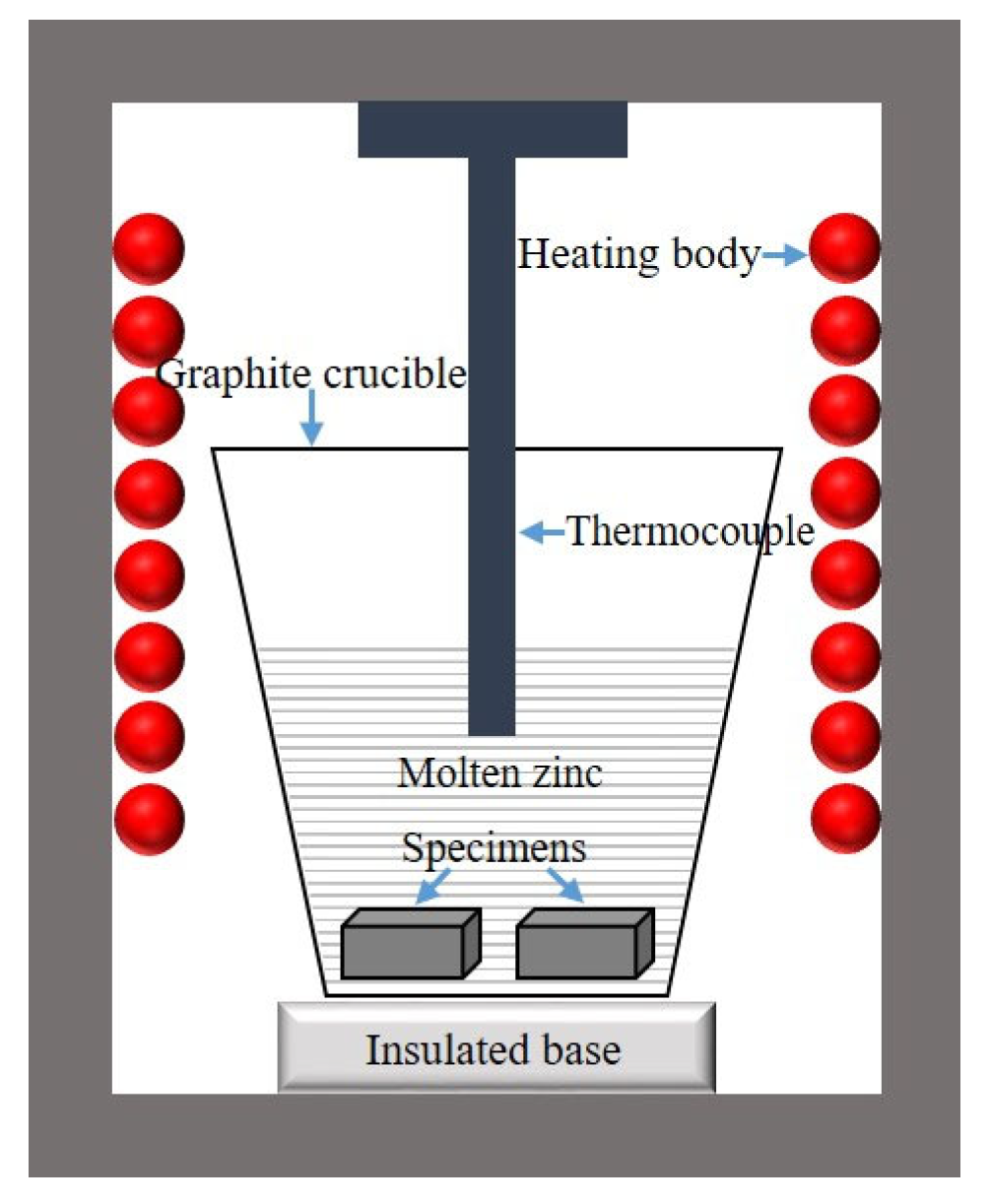
2.3. Corrosion Test
2.4. Characterization
3. Results and Discussion
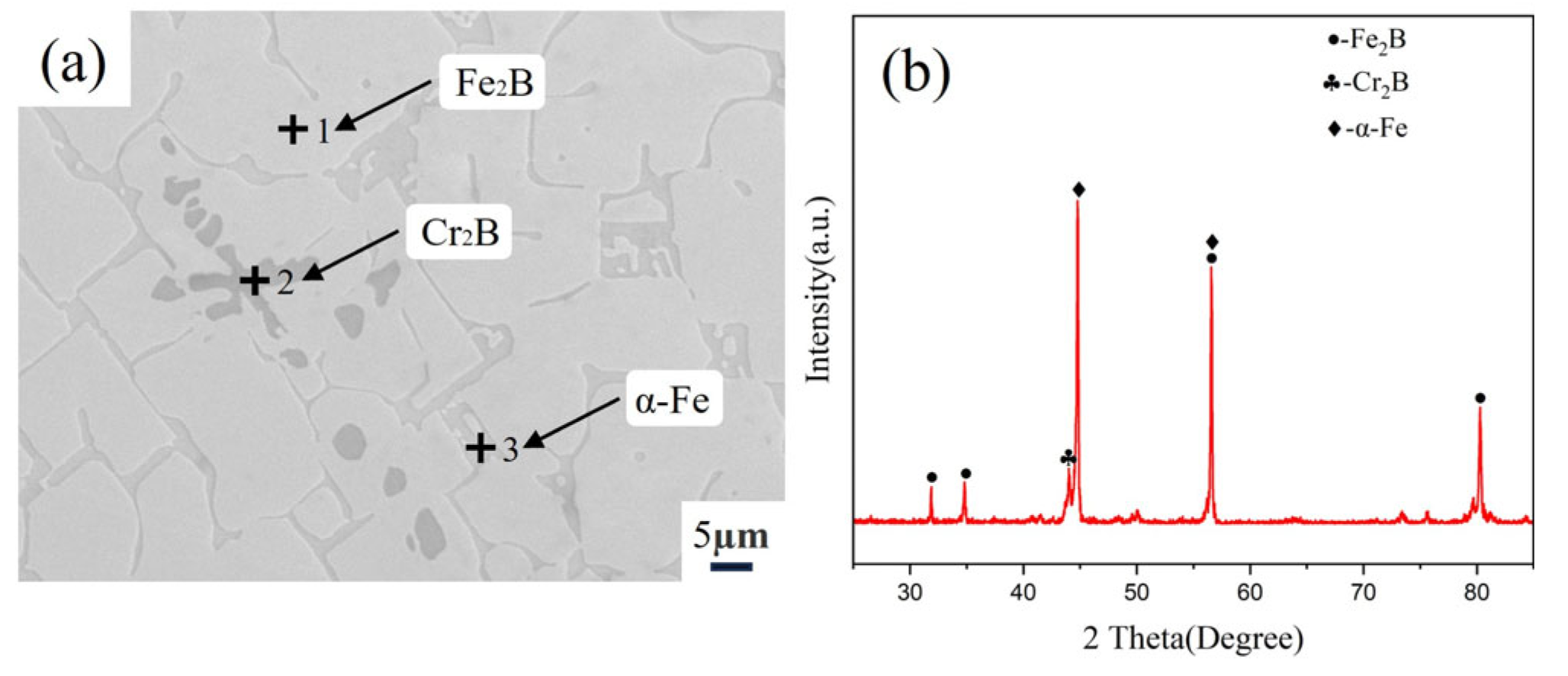
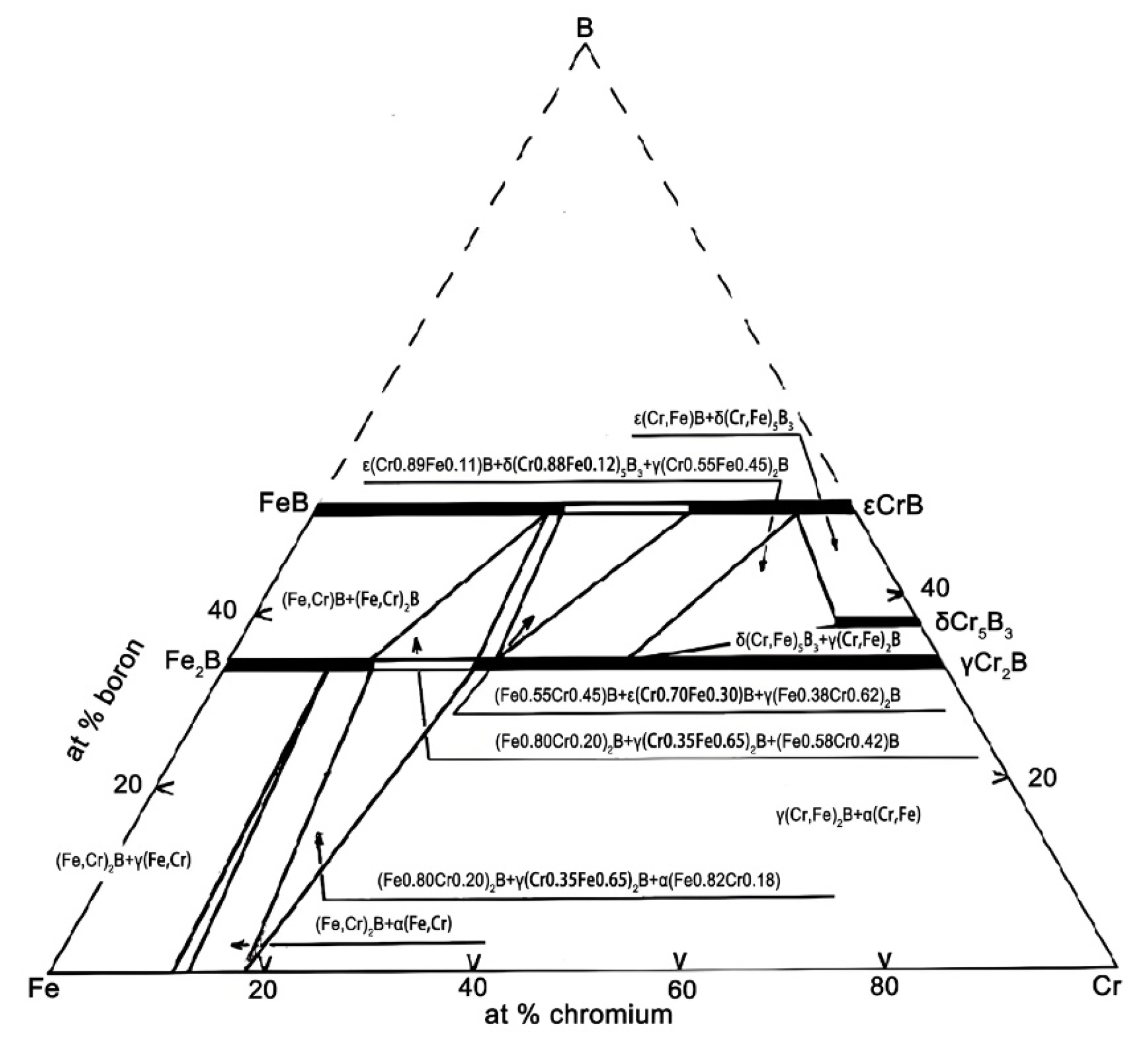
3.1. Microstructure of Fe-20Cr-5B-3Al Alloy
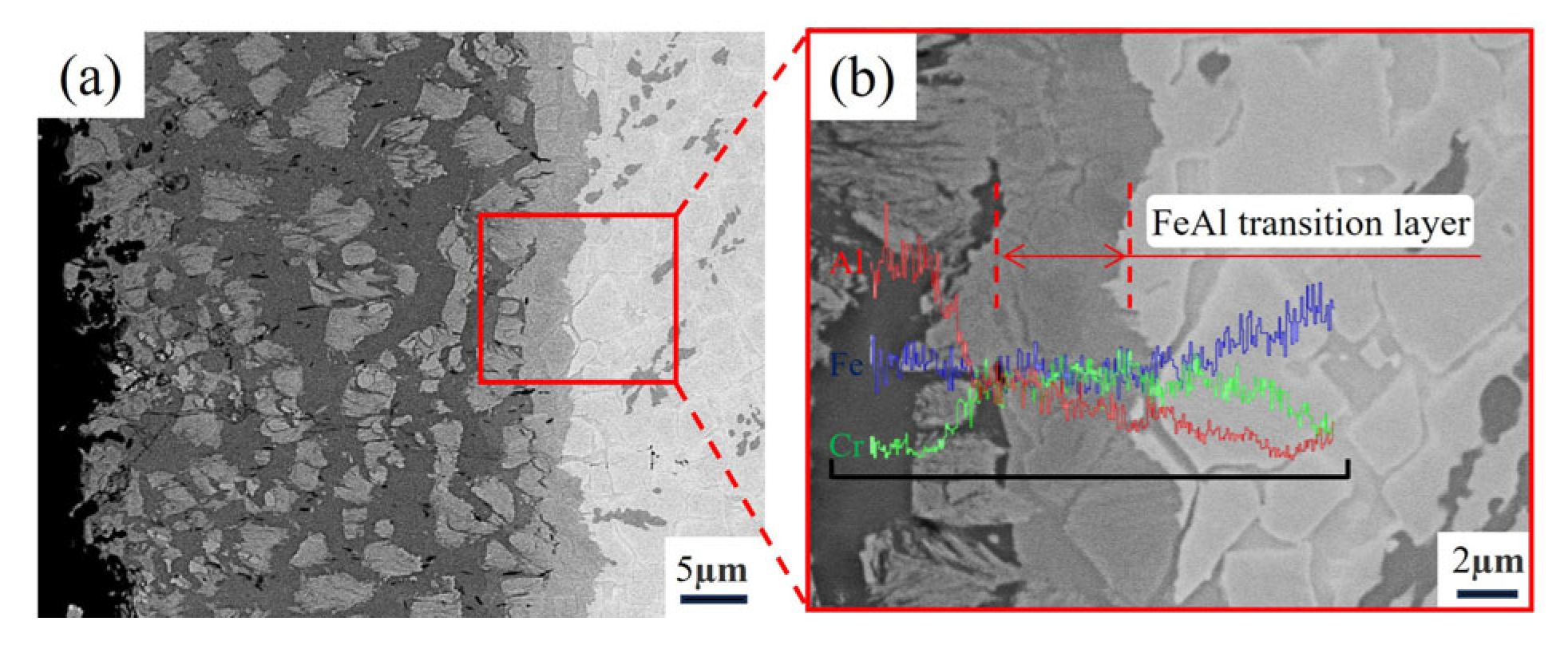
3.2. Microstructure of Fe-20Cr-5B-3Al Alloy Aluminizing
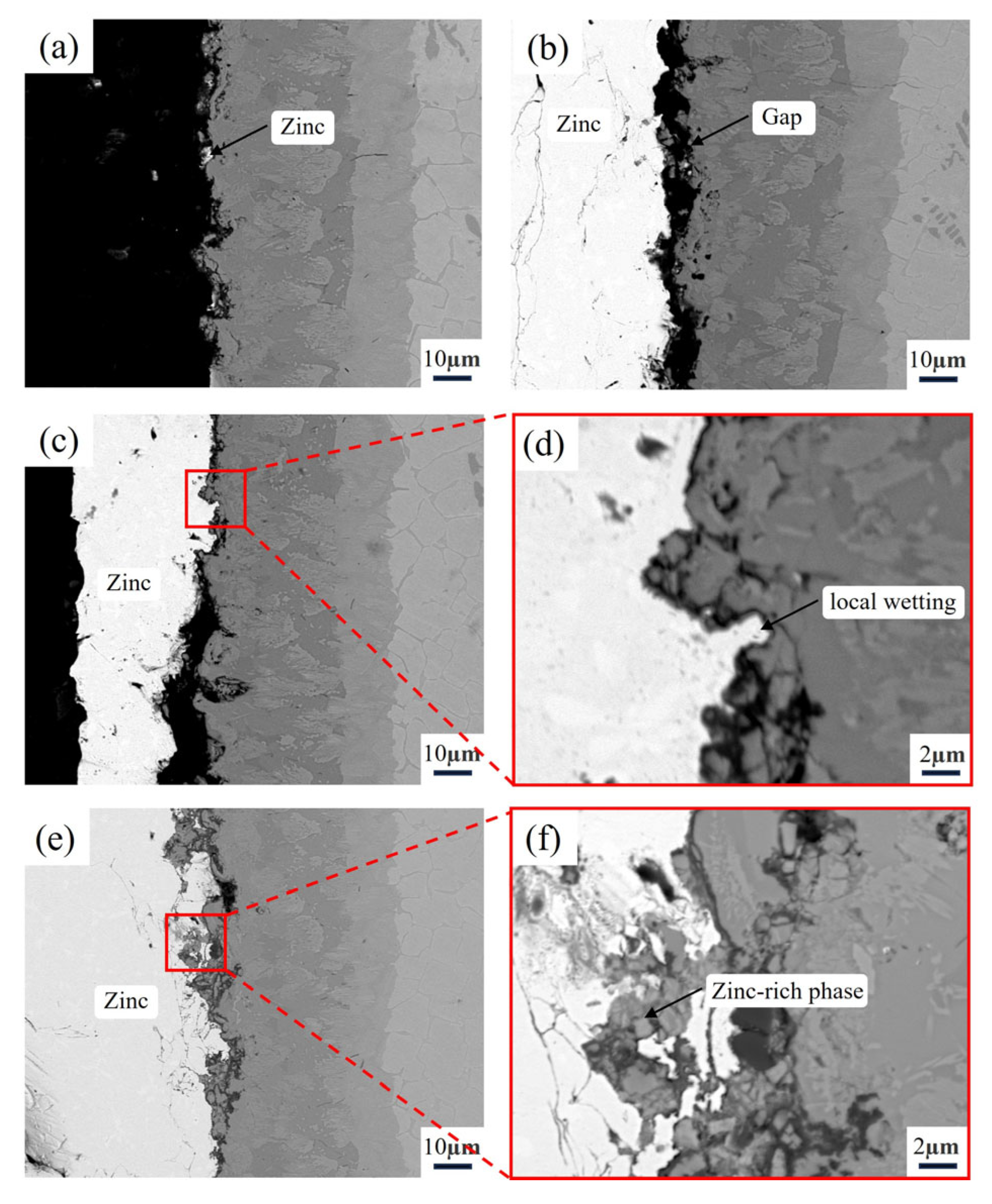
3.3. Microstructure of the Alloy After Zinc Liquid Corrosion
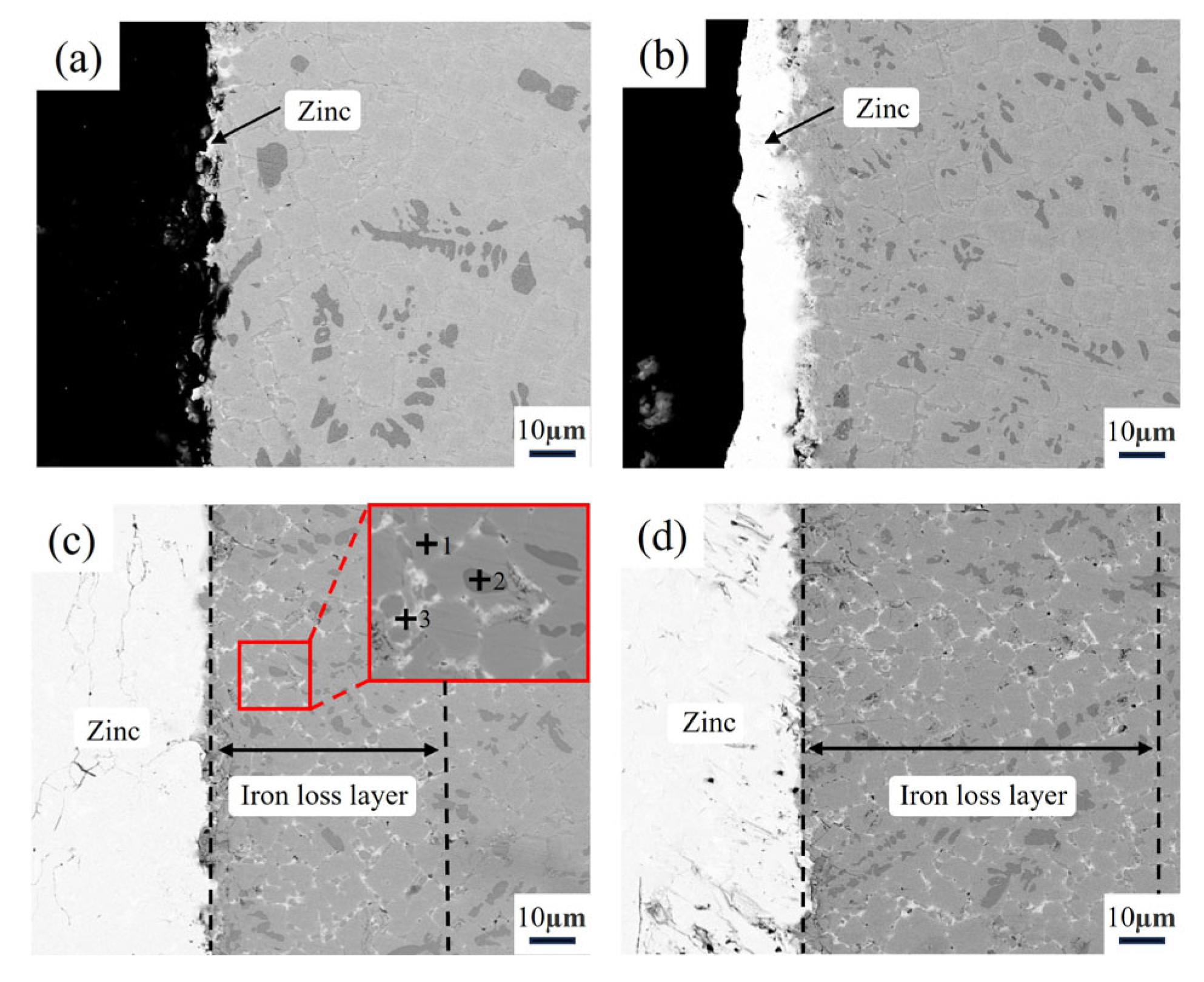
3.4. Microstructure of the Aluminizing Alloy After Zinc Liquid Corrosion
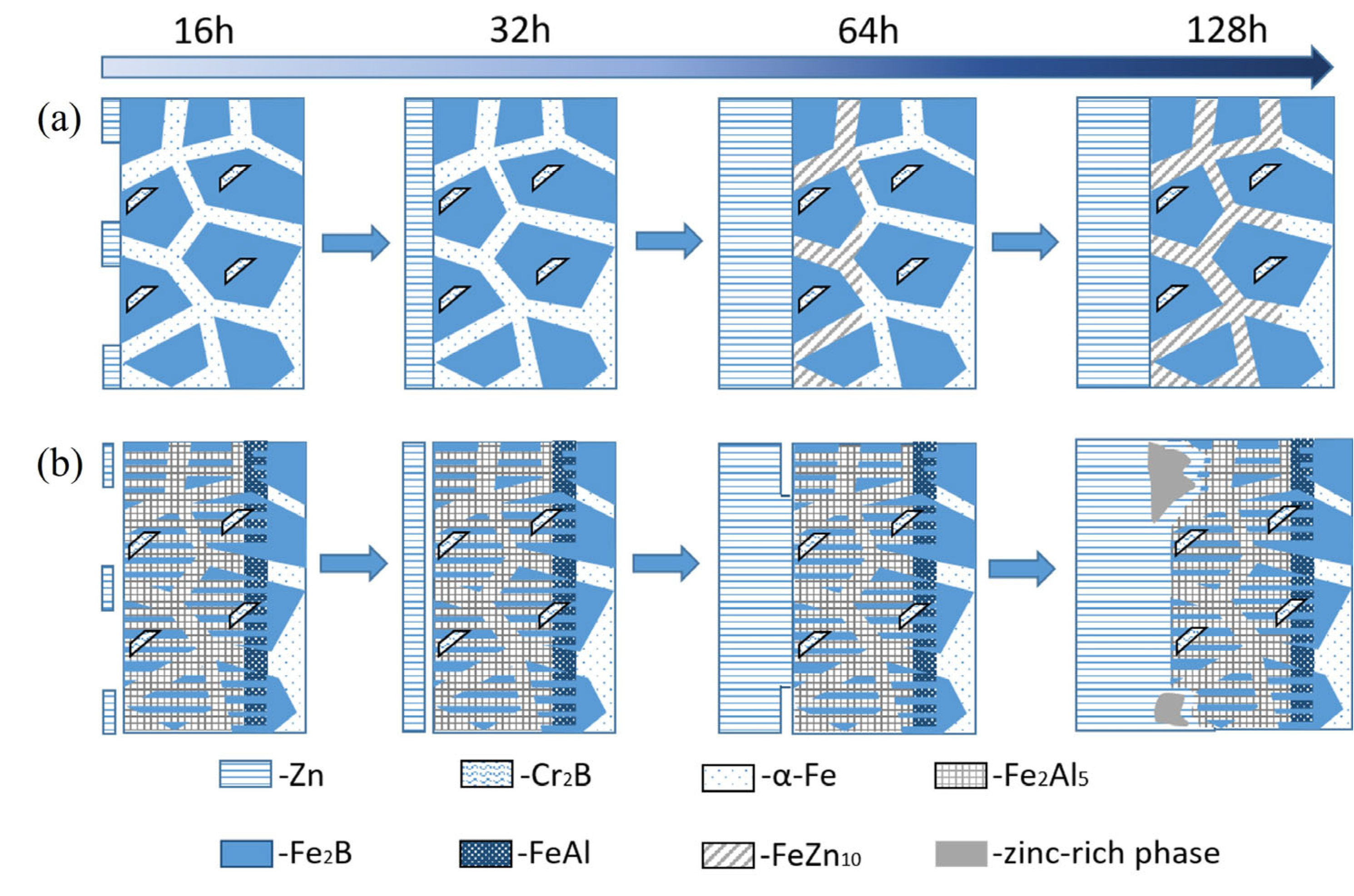
3.5. Zinc Liquid Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Z.; Hu, J.; Meng, H. A review of recent developments in coating systems for hot-dip galvanized steel. Front. Mater. 2020, 7, 00074. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Meena, B.N.; Remya, R. A review on recent approaches in the field of hot dip zinc galvanizing process. Surf. Coat. Technol. 2015, 262, 210–215. [Google Scholar] [CrossRef]
- Shreyas, P.; Panda, B.; Vishwanatha, A.D. Embrittlement of hot-dip galvanized steel: A review. AIP Conf. Proc. 2021, 2371, 020038. [Google Scholar] [CrossRef]
- Ren, X.; Mei, X.; She, J.; Ma, J. Materials resistance to liquid zinc corrosion on surface of sink roll. J. Iron Steel Res. Int. 2007, 14, 130–136. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, M.; Li, F.; Zhu, S.; Wang, F. Synergistic effect of corrosion and wear of the 316 stainless steel in molten zinc alloy at 460 °C. Corr. Sci. 2020, 165, 108411. [Google Scholar] [CrossRef]
- Cao, X.; Yi, L.; Run, W. Mechanism of liquid zinc corrosion on metals. J. Univ. Sci. Technol. Beijing 1997, 4, 22–26. [Google Scholar]
- Wang, W.; Lin, J.; Wang, Y.; Chen, G. Isothermal corrosion Fe3Si alloy in liquid zinc. Int. J. Miner. Metall. Mater. 2007, 14, 52–55. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, D.; He, J.; Zhang, J.; Li, X. Degradation behaviour of ZrO2-Ni/Al gradient coatings in molten Zn. Surf. Coat. Technol. 2006, 201, 2455–2459. [Google Scholar] [CrossRef]
- Mehrabian, M.; Nayebi, B.; Dietrich, D.; Lampke, T.; Shokouhimehr, M. Characteristics of dynamically-formed surface oxide layers on molten zinc-aluminum alloys: A multimodality approach. Thin Solid Films 2018, 667, 34–39. [Google Scholar] [CrossRef]
- Ma, S.; Xing, J.; Fu, H.; He, Y.; Bai, Y.; Li, Y. Interface characteristics and corrosion behaviour of oriented bulk Fe2B alloy in liquid zinc. Corr. Sci. 2014, 78, 71–80. [Google Scholar] [CrossRef]
- Ma, S.; Xing, J.; He, Y.; Fu, H.; Li, Y.; Liu, G. Effect of orientation and lamellar spacing of Fe2B on interfaces and corrosion behavior of Fe-B alloy in hot-dip galvanization. Acta Mater. 2016, 115, 392–402. [Google Scholar] [CrossRef]
- Shah, I.; Lv, P.; Ma, S.; Luo, Y.; Liu, Y.; Cui, X.; Guo, P.; Xing, J. Effect of Si and prolonged time on interfacial morphologies and cavitation erosion behavior of directionally solidified Fe-B alloy in flowing liquid zinc. Corros. Sci. 2024, 236, 112281. [Google Scholar] [CrossRef]
- Liu, G.; Ma, S.; Xing, J.; Fu, H.; Gao, Y.; Bai, Y.; Wang, Y. Investigation of flowing liquid zinc erosion and corrosion properties of the Fe–B alloy at various times. J. Mater. Res. 2015, 30, 727–735. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; Feng, L.; Kang, Y.; Yue, D. Effect of silicon on corrosion of directional Fe-B-Si alloy in liquid zinc. Metall. Mater. Trans. A. 2021, 52, 691–699. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, J.; Ma, S.; Zheng, B.; Liu, G.; Yang, D.; Bai, Y. Interface characterization and erosion–corrosion behavior of directional Fe-3.5wt.% B steel in flowing liquid zinc at various temperatures. Corr. Sci. 2016, 104, 260–268. [Google Scholar] [CrossRef]
- Kocaman, E. Effect of the molybdenum content on wear and corrosion behavior of Fe-B-based surface-alloyed layer. Coatings 2023, 13, 2050. [Google Scholar] [CrossRef]
- Ouyang, X.; Chen, G.; Yin, F.; Liu, Y.; Zhao, M. Effect of molybdenum on the microstructures of As-Cast Fe-B alloys and their corrosion resistance in molten zinc. Corrosion 2017, 73, 942–952. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, Y.; Li, W.; Yi, Y.; Liu, Y.; Cui, S.; Song, S. Effect of Fe2B orientation morphology on high temperature erosion-wear behavior of Fe–B alloy in liquid zinc. Wear 2021, 484, 204038. [Google Scholar] [CrossRef]
- Baron, C.; Springer, H.; Raabe, D. Development of high modulus steels based on the Fe–Cr–B system. Mater. Sci. Eng. A 2018, 724, 142–147. [Google Scholar] [CrossRef]
- Guo, C.; Kelly, P.M. Boron solubility in Fe–Cr–B cast irons. Mater. Sci. Eng. A 2003, 352, 40–45. [Google Scholar] [CrossRef]
- Sundawa, R.Y.; Aryanto, D.; Wismogroho, A.S.; Sudiro, T. Microstructure and phase composition of Fe-B-Al coatings on low carbon steel prepared by using mechanical alloying technique. J. Phys. Conf. Ser. 2017, 817, 012071. [Google Scholar] [CrossRef]
- Lin, S.; Wu, Y.; Li, C.; Cai, Z.; Nie, L.; Ren, Q.; Luo, B.; Yang, H.; Huang, H. Preparation of Fe-Al coating on 316LSS by low-temperature and high-activation pack aluminizing. Fusion Energy 2025, 215, 114978. [Google Scholar] [CrossRef]
- Shigematsu, I.; Saitou, N.; Shimojima, K.; Nakamura, M. Surface treatment of AZ91D magnesium alloy by aluminum diffusion coating. J. Mater. Sci. Lett. 2000, 19, 473–475. [Google Scholar] [CrossRef]
- Owolabi, A.I.; Seman, A.A.; Abdullah, T.K. Low-temperature slurry aluminizing: Investigating the influence of aluminizing time on the corrosion performance of aluminide coating on 304 SS. J. Phys. Conf. Ser. 2024, 2907, 012003. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, T.; Liu, Y.; Kolawole, S.K.; Su, X. Effect of pre-oxidation at 850 °C under low oxygen pressure on the zinc liquid corrosion resistance of Fe-20Cr-5B–3Al alloy. Mater. Today Commun. 2023, 36, 106709. [Google Scholar] [CrossRef]
- Cheng, X.; Ju, J.; Qu, Y.; Cao, L.; Fu, H. Microstructure and properties of Fe–Cr–B–Al alloy after heat treatment. Trans. Indian Inst. Met. 2018, 71, 2261–2268. [Google Scholar] [CrossRef]
- Carbucicchio, M.; Grazzi, C.; Palombarini, G.; Rateo, M.; Sambogna, G. On the phase transformations in Cr–FeB and Fe–CrB systems at high temperature. Hyperfine Interact. 2002, 139, 393–398. [Google Scholar] [CrossRef]
- Yan, D.; He, J.; Tian, B.; Dong, Y.; Li, X.; Zhang, J.; Xiao, L.; Jing, W. The corrosion behavior of plasma sprayed Fe2Al5 coating in molten Zn. Surf. Coat. Technol. 2006, 201, 2662–2666. [Google Scholar] [CrossRef]


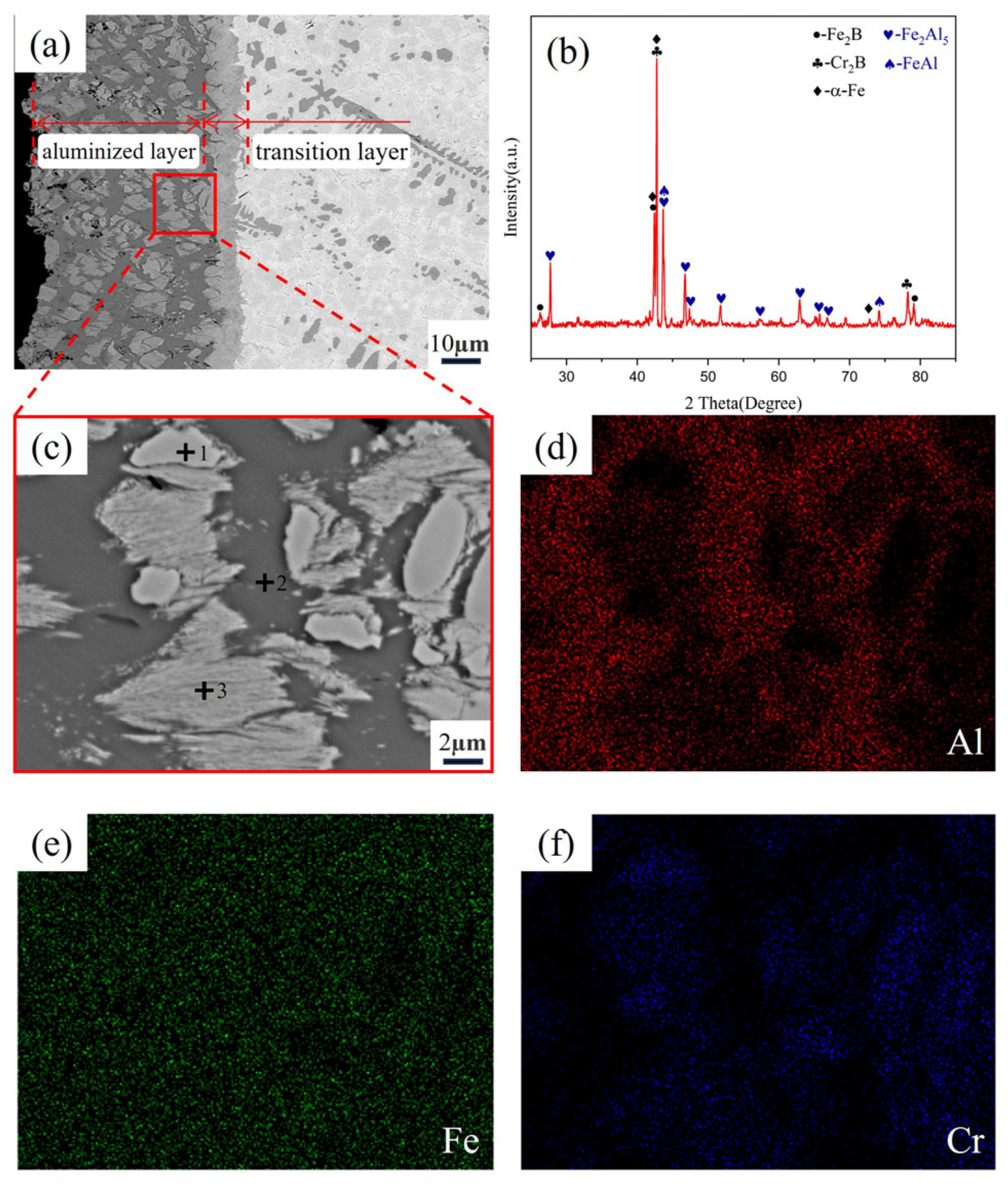
| Elements | Al | Cr | Fe | Phase |
|---|---|---|---|---|
| Point 1 | 1.27 | 29.65 | 69.08 | Fe2B |
| Point 2 | 0.16 | 60.36 | 39.48 | Cr2B |
| Point 3 | 12.91 | 2.05 | 85.04 | α-Fe |
| Elements | Al | Cr | Fe |
|---|---|---|---|
| Point 1 | 0.33 | 61.98 | 37.69 |
| Point 2 | 69.15 | 0.89 | 29.96 |
| Point 3 | 35.28 | 12.55 | 52.17 |
| Elements | Al | Cr | Fe | Zn |
|---|---|---|---|---|
| Point 1 | 1.35 | 24.14 | 74.28 | 0.23 |
| Point 2 | 0.14 | 61.38 | 38.36 | 0.12 |
| Point 3 | 1.97 | 4.12 | 8.63 | 85.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Liu, Y.; Wu, C.; Zhu, X.; Su, X. Effect of Surface Aluminizing on the Zinc Corrosion Resistance of Fe-20Cr-5B-3Al Alloy. Materials 2025, 18, 2493. https://doi.org/10.3390/ma18112493
Zhu S, Liu Y, Wu C, Zhu X, Su X. Effect of Surface Aluminizing on the Zinc Corrosion Resistance of Fe-20Cr-5B-3Al Alloy. Materials. 2025; 18(11):2493. https://doi.org/10.3390/ma18112493
Chicago/Turabian StyleZhu, Shanlong, Ya Liu, Changjun Wu, Xiangying Zhu, and Xuping Su. 2025. "Effect of Surface Aluminizing on the Zinc Corrosion Resistance of Fe-20Cr-5B-3Al Alloy" Materials 18, no. 11: 2493. https://doi.org/10.3390/ma18112493
APA StyleZhu, S., Liu, Y., Wu, C., Zhu, X., & Su, X. (2025). Effect of Surface Aluminizing on the Zinc Corrosion Resistance of Fe-20Cr-5B-3Al Alloy. Materials, 18(11), 2493. https://doi.org/10.3390/ma18112493







