N/S Co-Doped Carbon-Coated Micro-Expanded Graphite for High-Performance Lithium-Ion Battery Anodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
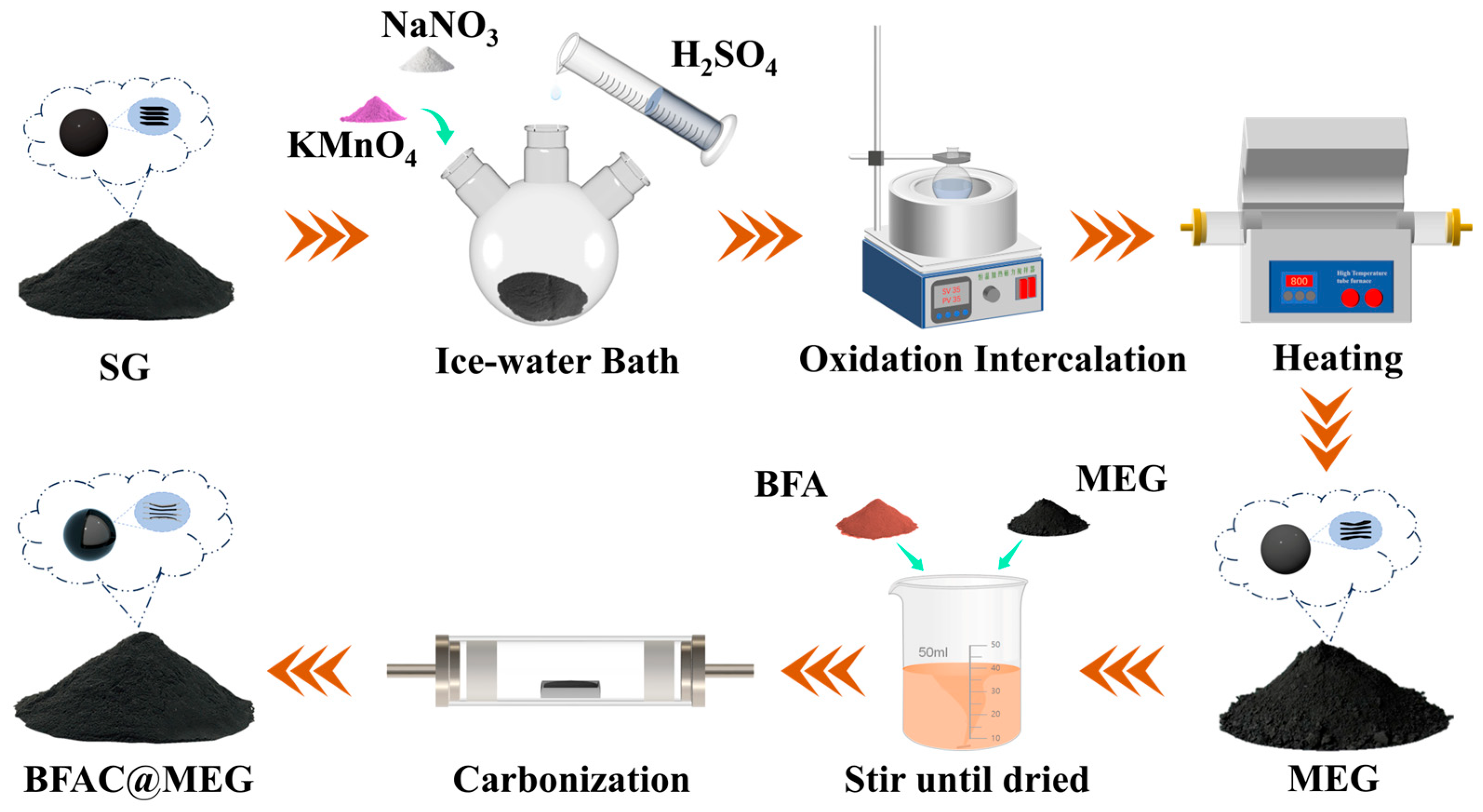
2.2. Synthesis Process
2.2.1. Preparation of Micro-Expanded Graphite (MEG)
2.2.2. Preparation of BFAC@MEG
2.3. Materials Characterization
2.4. Electrochemical Measurements
3. Results
3.1. Preparation Route and Morphological Characterization of BFAC@MEG
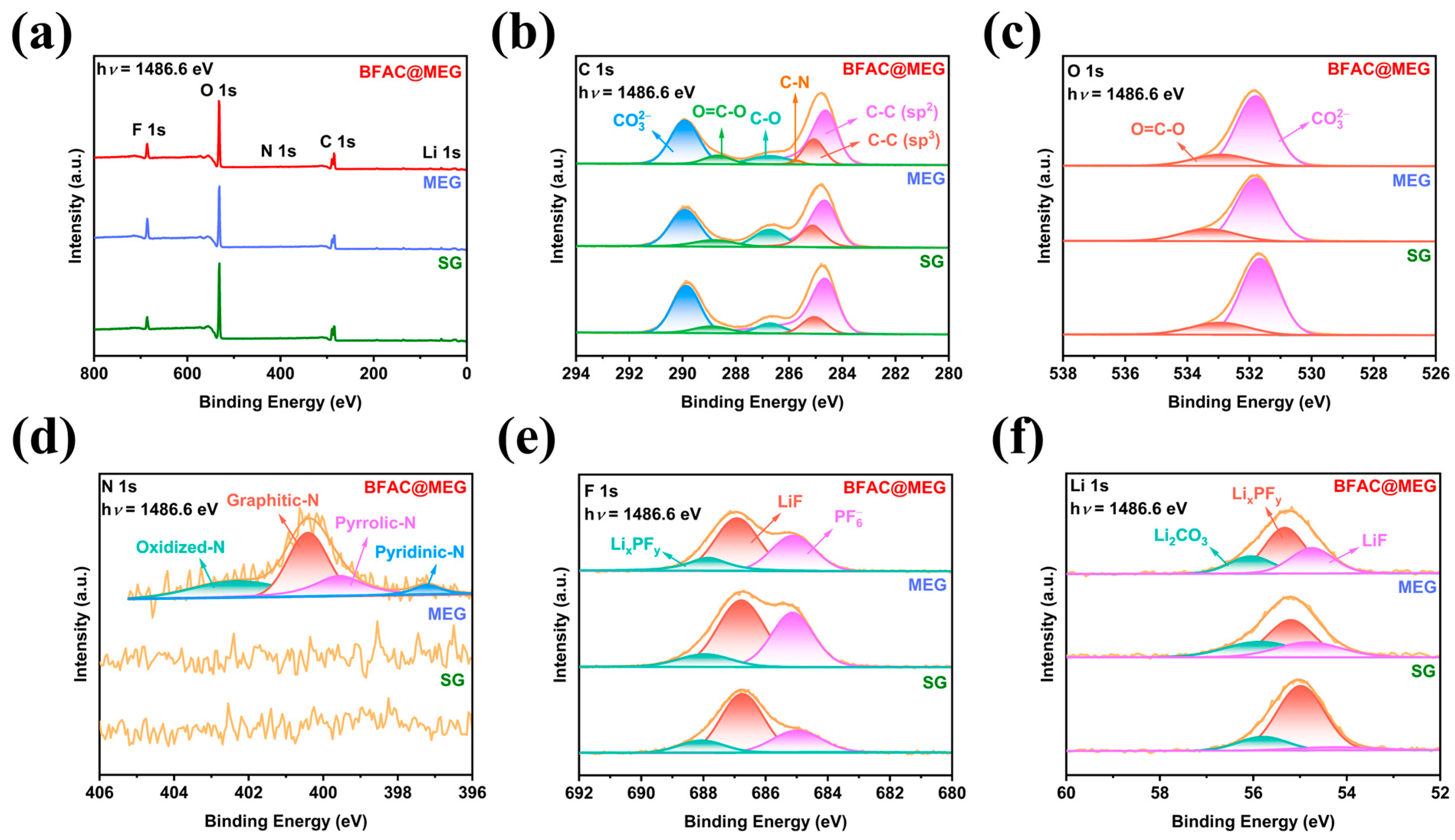
3.2. Physicochemical Properties of BFAC@MEG
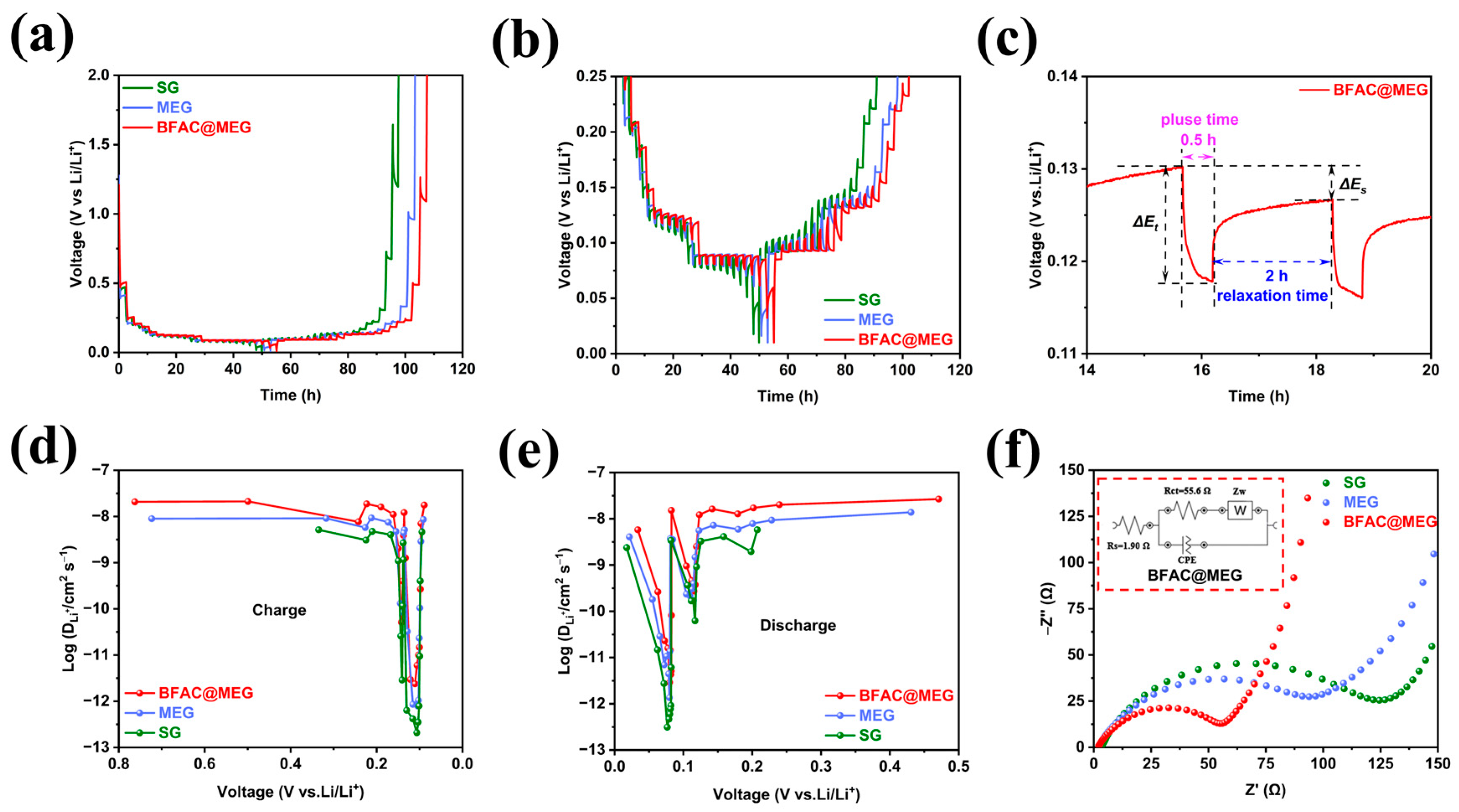
3.3. Electrochemical Performance of BFAC@MEG Anode
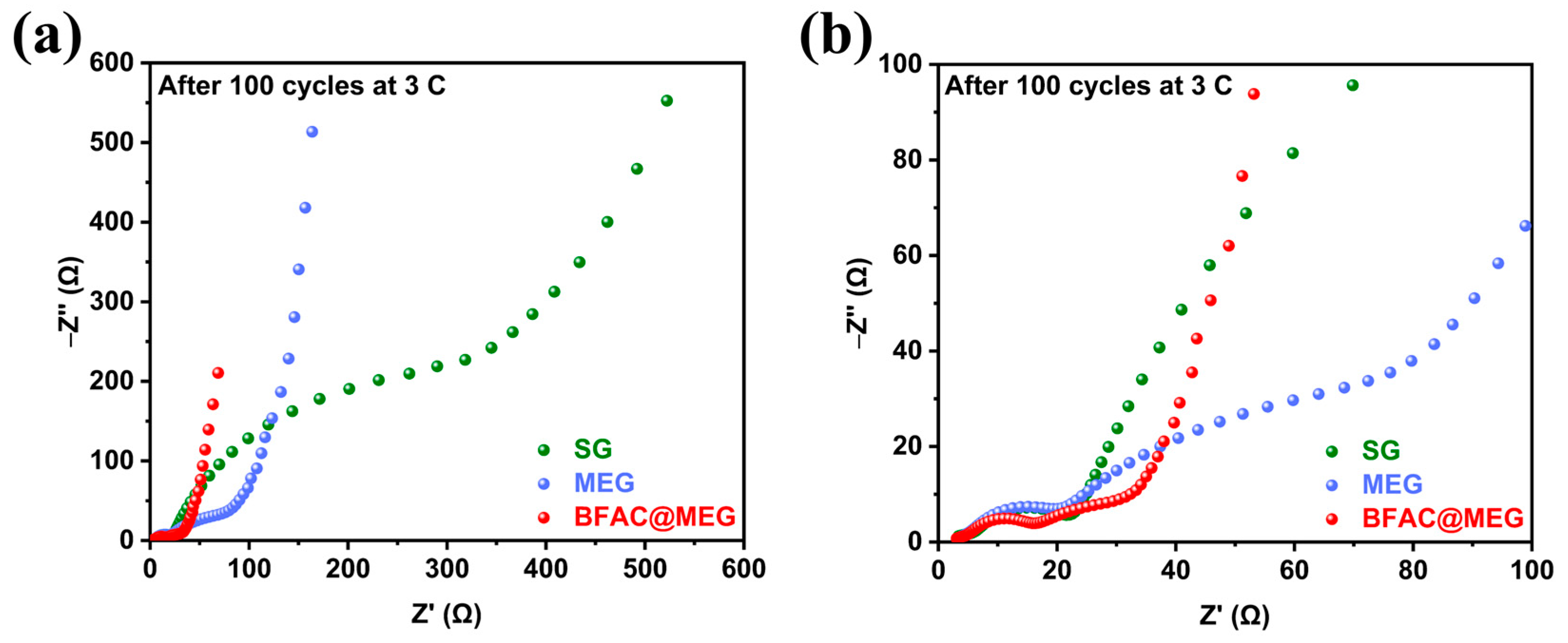
3.4. The Influence of Electrochemical Cycling on the AC Impedance and SEI Film of Materials
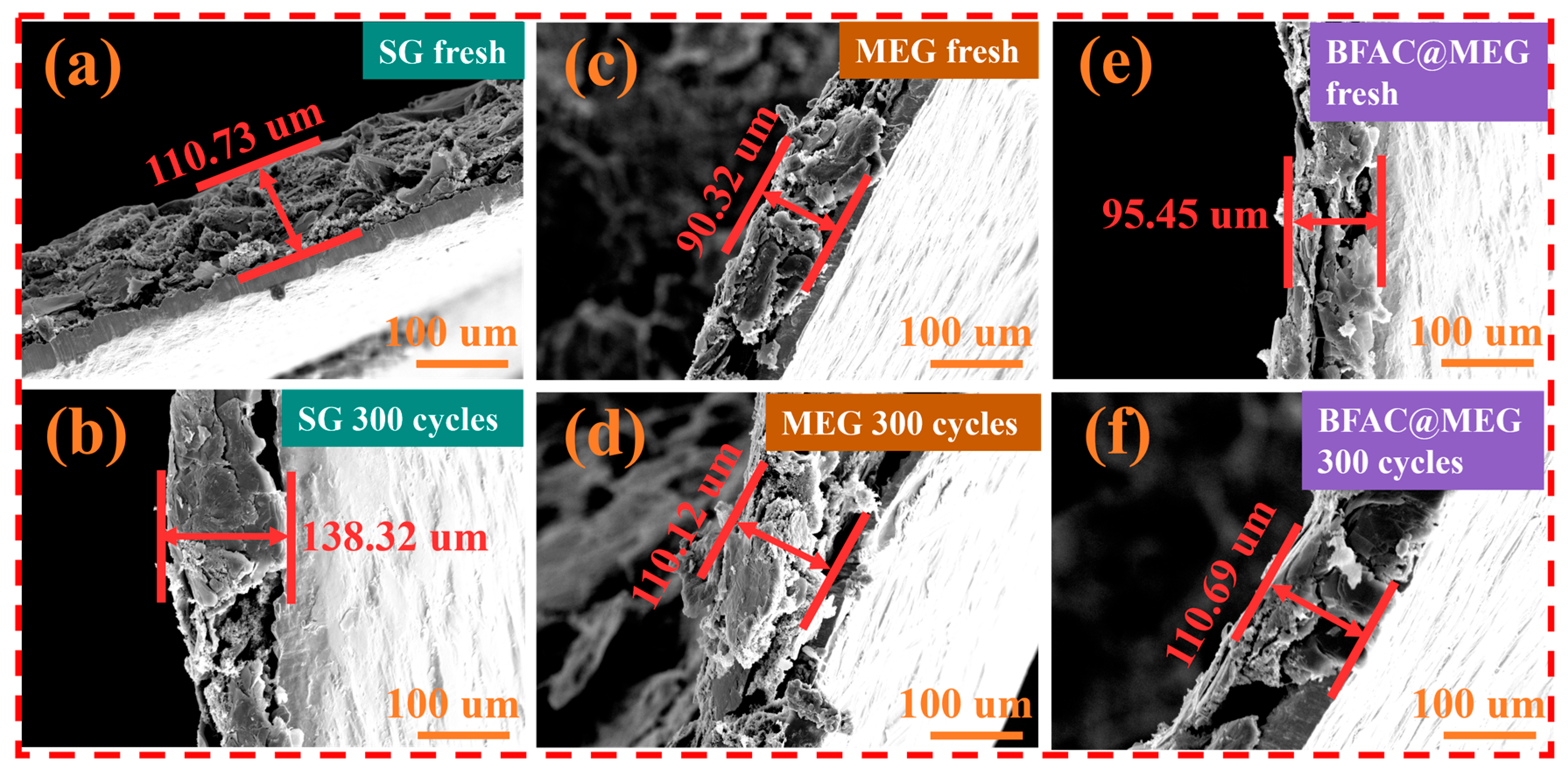
3.5. Analysis of the Structural Stability of Electrodes After Cycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, C.; Fu, Y.; Yu, Y. Designed Nanoarchitectures by Electrostatic Spray Deposition for Energy Storage. Adv. Mater. 2019, 31, e1803408. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qian, X.; Hou, S.; Xia, X.; He, D.; Xia, J.; He, G.; Chen, H. Recent Progress of Self-Supported Anode Materials for Li-Ion Batteries. J. Energy Storage 2024, 99, 113188. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Liu, R.; Li, X.; Wu, Y.; Ran, F. “Fast-Charging” Anode Materials for Lithium-Ion Batteries from Perspective of Ion Diffusion in Crystal Structure. ACS Nano 2024, 18, 2611–2648. [Google Scholar] [CrossRef]
- Xia, X.; Qian, X.; Chen, C.; Li, W.; He, D.; He, G.; Chen, H. Recent Progress of Si-Based Anodes in the Application of Lithium-Ion Batteries. J. Energy Storage 2023, 72, 108715. [Google Scholar] [CrossRef]
- Gao, X.; Liu, L.; Zu, L.; Lian, H.; Cui, X.; Wang, X. Facile Fabrication of Fibrous Phosphorus as Anode Material for Lithium-Ion Batteries with Long Cycle Life and High-Rate Performance. J. Power Sources 2024, 595, 234077. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Zhao, J.; Zhang, B.; Liu, L.; Bao, R.; Jing, Z. Sn-Based Anode Materials for Lithium-Ion Batteries: From Mechanism to Modification. J. Energy Storage 2024, 80, 109862. [Google Scholar] [CrossRef]
- Bartoli, M.; Piovano, A.; Elia, G.A.; Meligrana, G.; Pedraza, R.; Pianta, N.; Tealdi, C.; Pagot, G.; Negro, E.; Triolo, C.; et al. Pristine and Engineered Biochar as Na-Ion Batteries Anode Material: A Comprehensive Overview. Renew. Sustain. Energy Rev. 2024, 194, 114304. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Li, Z.; Beyene, T.T.; Zheng, T.; Liu, Y.; Zhu, K. Review and Perspectives on Preparation Strategies and Applications of Ti3C2 MXene for Li Metal Batteries/Li-S Batteries. Energy Fuels 2024, 38, 14866–14890. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, Y.; Meng, Y.; Wang, Z.; Liu, J.; Gong, X. Challenges and Strategies of Lithium-Ion Mass Transfer in Natural Graphite Anode. Chem. Eng. J. 2024, 480, 148047. [Google Scholar] [CrossRef]
- Zhao, L.; Ding, B.; Qin, X.Y.; Wang, Z.; Lv, W.; He, Y.B.; Yang, Q.H.; Kang, F. Revisiting the Roles of Natural Graphite in Ongoing Lithium-Ion Batteries. Adv. Mater. 2022, 34, e2106704. [Google Scholar] [CrossRef]
- Yan, W.; Chen, Z.; Ma, S.; Chen, S.; Lu, Y.; Wang, M.; Chen, L.; Huang, Q.; Wang, B.; Su, Y.; et al. Unraveling the Relationship between the Mineralogical Characteristics and Lithium Storage Performance of Natural Graphite Anode Materials. Carbon 2024, 227, 119270. [Google Scholar] [CrossRef]
- Mundszinger, M.; Farsi, S.; Rapp, M.; Golla-Schindler, U.; Kaiser, U.; Wachtler, M. Morphology and Texture of Spheroidized Natural and Synthetic Graphites. Carbon 2017, 111, 764–773. [Google Scholar] [CrossRef]
- Ruggeri, I.; Martin, J.; Wohlfahrt-Mehrens, M.; Mancini, M. Interfacial Kinetics and Low-Temperature Behavior of Spheroidized Natural Graphite Particles as Anode for Li-Ion Batteries. J. Solid-State Electrochem. 2021, 26, 73–83. [Google Scholar] [CrossRef]
- Son, D.-K.; Kim, J.; Raj, M.R.; Lee, G. Elucidating the Structural Redox Behaviors of Nanostructured Expanded Graphite Anodes toward Fast-Charging and High-Performance Lithium-Ion Batteries. Carbon 2021, 175, 187–201. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Zhang, G.; Li, S.; Xu, Y.; Zhang, X.; Zhang, X.; Zheng, S.; Sun, X.; Ma, Y. Fast Charging Anode Materials for Lithium-Ion Batteries: Current Status and Perspectives. Adv. Funct. Mater. 2022, 32, 2200796. [Google Scholar] [CrossRef]
- Xiang, F.; Hou, W.; Gu, X.; Wen, L.; Sun, Y.; Lu, W. One-Pot Synthesis of MnO-Loaded Mildly Expanded Graphite Composites as High-Performance Lithium-Ion Battery Anode Materials. J. Alloys Compd. 2022, 897, 163202. [Google Scholar] [CrossRef]
- Zhao, J.; Dumont, J.H.; Martinez, U.; Macossay, J.; Artyushkova, K.; Atanassov, P.; Gupta, G. Graphite Intercalation Compounds Derived by Green Chemistry as Oxygen Reduction Reaction Catalysts. ACS Appl Mater Interfaces 2020, 12, 42678–42685. [Google Scholar] [CrossRef]
- Li, X.; Lei, Y.; Qin, L.; Han, D.; Wang, H.; Zhai, D.; Li, B.; Kang, F. Mildly-Expanded Graphite with Adjustable Interlayer Distance as High-Performance Anode for Potassium-Ion Batteries. Carbon 2021, 172, 200–206. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, Z.-H.; Yu, X.; Shen, W.; Zheng, Y.; Kang, F. Mildly Expanded Graphite for Anode Materials of Lithium-Ion Battery Synthesized with Perchloric Acid. Electrochim. Acta 2014, 116, 170–174. [Google Scholar] [CrossRef]
- Seo, S.W.; Ahn, W.J.; Kang, S.C.; Im, J.S. Effect of Pitch Crystallinity on Electrochemical Performance of Graphite Carbon Coatings. J. Energy Storage 2024, 81, 110489. [Google Scholar] [CrossRef]
- Huang, R.; Xu, C.; Feng, Z.; He, M.; Wen, K.; Chen, L.; Liang, T.; Xiong, D. Comparison of Carbon Coating and MCMB Structures Used in Graphite Anodes for Potassium-Ion Batteries. Carbon Lett. 2024, 34, 1693–1706. [Google Scholar] [CrossRef]
- Zheng, H.; Park, D.-Y.; Kim, M.-S. Preparation and Characterization of Anode Materials Using Expanded Graphite/Pitch Composite for High-Power Li-Ion Secondary Batteries. Res. Chem. Intermed. 2014, 40, 2501–2507. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Guo, S.; Pinna, N. Graphene/N-Doped Carbon Sandwiched Nanosheets with Ultrahigh Nitrogen Doping for Boosting Lithium-Ion Batteries. J. Mater. Chem. A 2016, 4, 1423–1431. [Google Scholar] [CrossRef]
- Zhuo, P.; Jiang, J.; Jiang, Y.; Hao, Y.; He, Q.; Chen, T.; Ding, E.; Zhang, Y.; Han, Y.; Si, W.; et al. Biochemical Fulvic Acid Derived Amorphous Carbon Modified Microcrystalline Graphite as Low-Cost Anode for Potassium-Ion Storage. J. Colloid Interface Sci. 2023, 648, 108–116. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, N.; Li, H.; Dong, Q.; Wang, Y.; Li, H.; Wang, S.; Zhang, X.; Qiu, J. Nitrogen-Doped Soft Carbon Frameworks Built of Well-Interconnected Nano-capsules Enabling a Superior Potassium-Ion Batteries Anode. Chem. Eng. J. 2020, 382, 121759. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Lei, C.; Qiu, H.; Jiang, W.; Sun, X.; Zhang, Y.; He, W. Ammonium Polyphosphate Assisted Thermal Polymerization of Pre-Oxidized Asphalt to Prepare N/P Double-Doped Hard Carbon for Improved Sodium Ion Storage. J. Energy Storage 2024, 85, 111045. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, S.; Yan, X.; Li, Y.; Zhu, L.; Qiao, Y.; Wang, L.; Xie, H. Acid-Base Encapsulation Prepared N/P Co-Doped Carbon-Coated Natural Graphite for High-Performance Lithium-Ion Batteries. J. Electroanal. Chem. 2024, 952, 117990. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Wu, D.; Zhao, X.; Zhou, Z. S-Doped N-Rich Carbon Nanosheets with Expanded Interlayer Distance as Anode Materials for Sodium-Ion Batteries. Adv. Mater. 2017, 29, 27885733. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Wang, L.; Qiao, Y.; Xu, J.; Li, J.; Zhang, S. PVA Generated Carbon-Coated Natural Graphite Anode Material for Enhanced Performances of Lithium-Ion Batteries. Ionics 2024, 30, 6845–6853. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman Spectroscopy as a Versatile Tool for Studying the Properties of Graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Kononenko, O.; Brzhezinskaya, M.; Zotov, A.; Korepanov, V.; Levashov, V.; Matveev, V.; Roshchupkin, D. Influence of Numerous Moiré Superlattices on Transport Properties of Twisted Multilayer Graphene. Carbon 2022, 194, 52–61. [Google Scholar] [CrossRef]
- An, X.; Ni, Z.; Shen, Z. Raman Imaging of Two Dimensional Materials. In Raman Spectroscopy of Two-Dimensional Materials; Springer: Berlin/Heidelberg, Germany, 2019; pp. 231–261. [Google Scholar]
- Wu, S.; Yan, X.; Sun, X.; Tian, S.; Wang, J.; Liu, C.; Sun, S.; Wu, L.; Zhao, X.; Yang, Q. S, N Co-Doped Porous Carbon Materials for High Performance Supercapacitor. J. Energy Storage 2023, 71, 108152. [Google Scholar] [CrossRef]
- Xiu, S.; Kim, D.K.; Kang, Y.; Duan, S.; Wang, Q.; Chen, T.; Piao, Y.; Suk, J.; Jin, X.; Quan, B. One-Step Synthesis of Nitrogen and Sulfur Co-Doped Hierarchical Porous Carbon Derived from Acesulfame Potassium as a Dual-Function Agent for Supercapacitors and Lithium-Sulfur Batteries. J. Energy Storage 2023, 66, 107214. [Google Scholar] [CrossRef]
- Sun, Q.; Mu, J.; Ma, F.; Li, Y.; Zhou, P.; Zhou, T.; Wu, X.; Zhou, J. Sulfur-Doped Hollow Porous Carbon Spheres as High-Performance Anode Materials for Potassium Ion Batteries. J. Energy Storage 2023, 72, 108297. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bauman, Y.I.; Brzhezinskaya, M.; Netskina, O.V.; Shubin, Y.V.; Kibis, L.S.; Stoyanovskii, V.O.; Larionov, K.B.; Serkova, A.N.; Vedyagin, A.A. Water Purification from Chlorobenzenes Using Heteroatom-Functionalized Carbon Nanofibers Produced on Self-Organizing Ni-Pd Catalyst. J. Environ. Chem. Eng. 2022, 10, 107873. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Belenkov, E.A.; Greshnyakov, V.A.; Yalovega, G.E.; Bashkin, I.O. New Aspects in the Study of Carbon-Hydrogen Interaction in Hydrogenated Carbon Nanotubes for Energy Storage Applications. J. Alloys Compd. 2019, 792, 713–720. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, B.; Zhu, J.; Li, K.; Tian, S. Hierarchical ZnO/MXene (Nb2C and V2C) Heterostructure with Efficient Electron Transfer for Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2022, 590, 153095. [Google Scholar] [CrossRef]
- Bao, C.; Liu, Q.; Chen, H.; Zeng, Q.; Peng, K.; Wu, W.; Liu, H.; Li, B. Catalytic Decomposition of Ethylene Carbonate by Pyrrolic-N to Stabilize Solid Electrolyte Interphase on Graphite Anode under Extreme Conditions. Nano Energy 2024, 127, 109775. [Google Scholar] [CrossRef]
- Pérez-Poyatos, L.T.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Maldonado-Hódar, F.J. Sulfur-Doped Carbon/TiO2 Composites for Ethylene Photo-Oxidation. Enhanced Performance by Doping TiO2 Phases with Sulfur by Mobile Species Inserted on the Carbon Support. Catal. Today 2025, 446, 115115. [Google Scholar] [CrossRef]
- Sobaszek, M.; Brzhezinskaya, M.; Olejnik, A.; Mortet, V.; Alam, M.; Sawczak, M.; Ficek, M.; Gazda, M.; Weiss, Z.; Bogdanowicz, R. Highly Occupied Surface States at Deuterium-Grown Boron-Doped Diamond Interfaces for Efficient Photoelectrochemistry. Small 2023, 19, 2208265. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Liu, T.; Han, C.; Guo, C.; Zhao, H.; Li, Q.; Lu, J.; Amine, K.; Qiu, X. Impacts of Dissolved Ni2+ on the Solid Electrolyte Interphase on a Graphite Anode. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202894. [Google Scholar] [CrossRef]
- Niu, M.; Dong, L.; Yue, J.; Li, Y.; Dong, Y.; Cheng, S.; Lv, S.; Zhu, Y.H.; Lei, Z.; Liang, J.Y.; et al. A Fast-Charge Graphite Anode with a Li-Ion-Conductive, Electron/Solvent-Repelling Interface. Angew. Chem. Int. Ed. Engl. 2024, 63, e202318663. [Google Scholar] [CrossRef] [PubMed]
- Sharova, V.; Moretti, A.; Giffin, G.; Carvalho, D.; Passerini, S. Evaluation of Carbon-Coated Graphite as a Negative Electrode Material for Li-Ion Batteries. C 2017, 3, 22. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.; Qu, Q.; Liu, G.; Battglia, V.S.; Zheng, H. A Novel Maleic Acid/Graphite Composite Anode for Lithium-Ion Batteries with High Energy and Power Density. Carbon 2018, 132, 420–429. [Google Scholar] [CrossRef]
- Guo, R.; Huang, W.; Wang, Y.; Wang, W.; Lv, L.; Zheng, H. A High-Performance Maleic Acid/Graphene/Graphite Composite Anode under the Effect of Synergistic Lithium Storage Mechanism. J. Power Sources 2020, 479, 229112. [Google Scholar] [CrossRef]
- Dan, J.; Jin, C.; Wen, L.; Xu, G.; Li, X.; Sun, F.; Zhou, L.; Yue, Z. A Double-Layer-Coated Graphite Anode Material for High-Rate Lithium-Ion Batteries. Solid State Sci. 2023, 141, 107220. [Google Scholar] [CrossRef]
- Zou, L.; Kang, F.; Zheng, Y.-P.; Shen, W. Modified Natural Flake Graphite with High Cycle Performance as Anode Material in Lithium-Ion Batteries. Electrochim. Acta 2009, 54, 3930–3934. [Google Scholar] [CrossRef]
- Xu, C.; Ma, G.; Yang, W.; Wang, Y.; Jia, Y.; Sun, Y.; Kong, X.; Yang, J.; Liu, H.; Zhang, X.; et al. Ultrahigh Edge-Nitrogen-Doped Porous Carbon Anode Materials for High-Capacity and Fast-Charging Lithium-Ion Batteries. J. Energy Storage 2023, 65, 107256. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, J.; Yi, Z.; Xie, L.; Yu, Y.; Su, F.; Zhao, Y.; Sun, G.; Chen, C.; Li, X. Light Fraction of Pitch Realizes Robust Coating on Silicon Anodes: The Inhibition of Volume Expansion by Self-Delithiation. Carbon 2024, 224, 119074. [Google Scholar] [CrossRef]
- Pham, T.K.; Snook, G.A.; Glass, D.; Ellis, A.V. Lithium-Ion Diffusion Behaviour in Silicon Nanoparticle/Graphite Blended Anodes. J. Power Sources 2025, 638, 236623. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Dai, H.; Yu, C.; Wei, X. Impedance Investigation of Silicon/Graphite Anode during Cycling. Batteries 2023, 9, 242. [Google Scholar] [CrossRef]
- Pritzl, D.; Landesfeind, J.; Solchenbach, S.; Gasteiger, H.A. An Analysis Protocol for Three-Electrode Li-Ion Battery Impedance Spectra: Part II. Analysis of a Graphite Anode Cycled vs. LNMO. J. Electrochem. Soc. 2018, 165, A2145–A2153. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Fan, K.; Zhao, D.; Liu, B.; Jiang, J.; Qian, D.; Yang, C.; Li, J. N-Doped Carbon Coated Anatase TiO2 Nanoparticles as Superior Na-Ion Battery Anodes. J. Colloid Interface Sci. 2018, 517, 134–143. [Google Scholar] [CrossRef]






| Materials | Specific Capacity (mAh g−1) | |||||
|---|---|---|---|---|---|---|
| 0.1 C | 0.3 C | 0.5 C | 1 C | 3 C | 5 C | |
| BFAC@MEG | 370 | 359 | 351 | 333 | 205 | 124 |
| MEG | 369 | 355 | 340 | 296 | 149 | 74 |
| SG | 352 | 331 | 314 | 261 | 95 | 36 |
| Materials | Specific Capacity (mAh g−1) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.1 C | 0.2 C | 0.3 C | 0.5 C | 1 C | 3 C | 5 C | |||
| 1 | BFAC@MEG | 370 | 359 | 351 | 333 | 205 | 124 | This work | |
| 2 | MnO@MEG-13.2 | 450 | 350 | 270 | 100 | [16] | |||
| 3 | NG-PN@C | 330 | 325 | 200 | 52 | 20 | [27] | ||
| 4 | P1G5 | 400 | 350 | 250 | 120 | 40 | [29] | ||
| 5 | GMIB-2P | 350 | 340 | 325 | 275 | 100 | [39] | ||
| 6 | AG@HG@Al2O3 | 350 | 340 | 330 | 175 | 90 | [47] | ||
| 7 | CMEG | 361 | [48] | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, X.; Wang, X.; Gao, C.; Yin, J.; Wen, Q.; Wang, G. N/S Co-Doped Carbon-Coated Micro-Expanded Graphite for High-Performance Lithium-Ion Battery Anodes. Materials 2025, 18, 2477. https://doi.org/10.3390/ma18112477
Wang W, Zhang X, Wang X, Gao C, Yin J, Wen Q, Wang G. N/S Co-Doped Carbon-Coated Micro-Expanded Graphite for High-Performance Lithium-Ion Battery Anodes. Materials. 2025; 18(11):2477. https://doi.org/10.3390/ma18112477
Chicago/Turabian StyleWang, Wenjie, Xuan Zhang, Xianchao Wang, Chengwei Gao, Jinling Yin, Qing Wen, and Guiling Wang. 2025. "N/S Co-Doped Carbon-Coated Micro-Expanded Graphite for High-Performance Lithium-Ion Battery Anodes" Materials 18, no. 11: 2477. https://doi.org/10.3390/ma18112477
APA StyleWang, W., Zhang, X., Wang, X., Gao, C., Yin, J., Wen, Q., & Wang, G. (2025). N/S Co-Doped Carbon-Coated Micro-Expanded Graphite for High-Performance Lithium-Ion Battery Anodes. Materials, 18(11), 2477. https://doi.org/10.3390/ma18112477







