Relaxation and Devitrification of Mg66Zn30Ca4 Metallic Glass
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
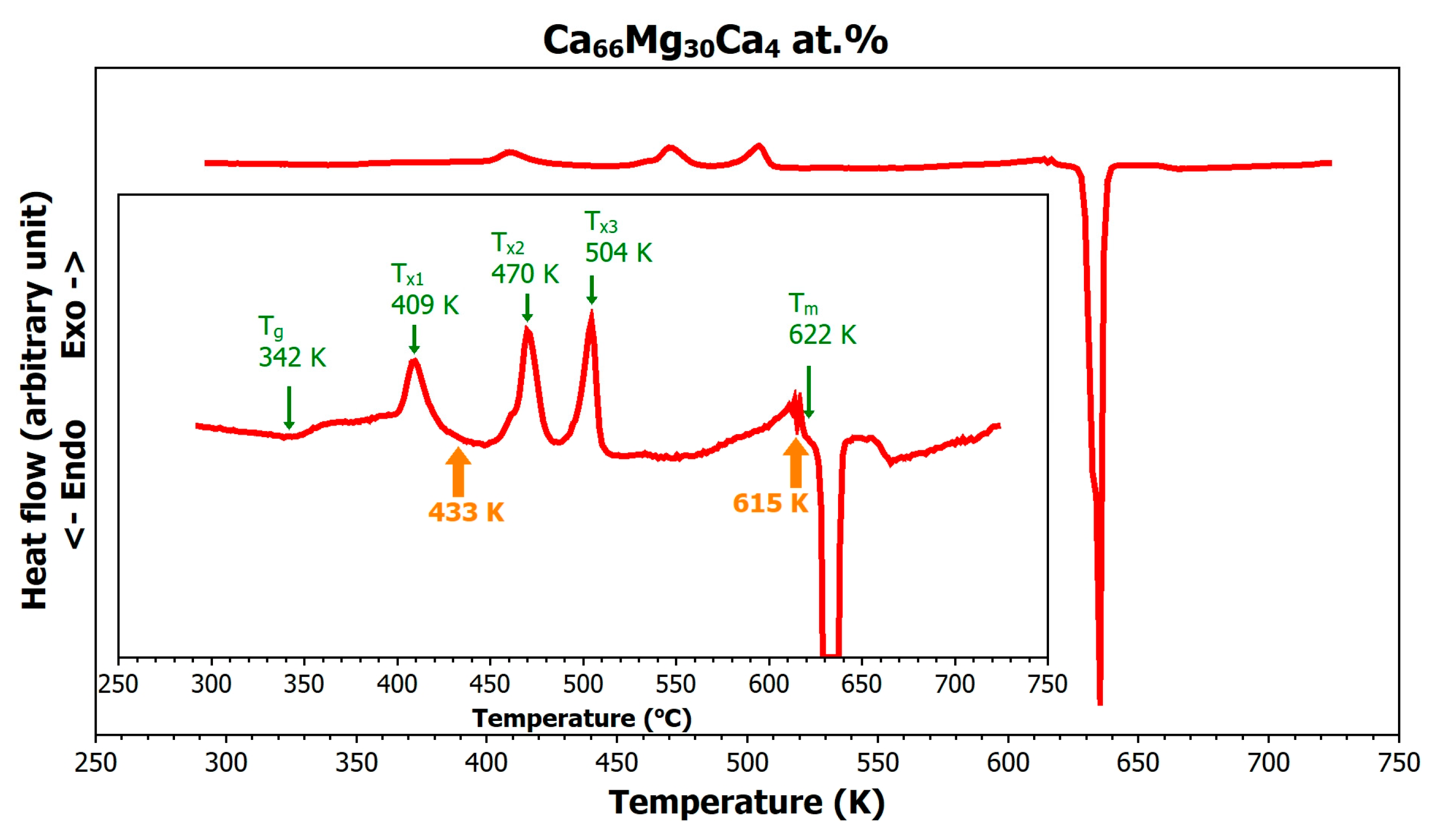
3.1. Thermal Stability of the Mg66Zn30Ca4 Metallic Glass
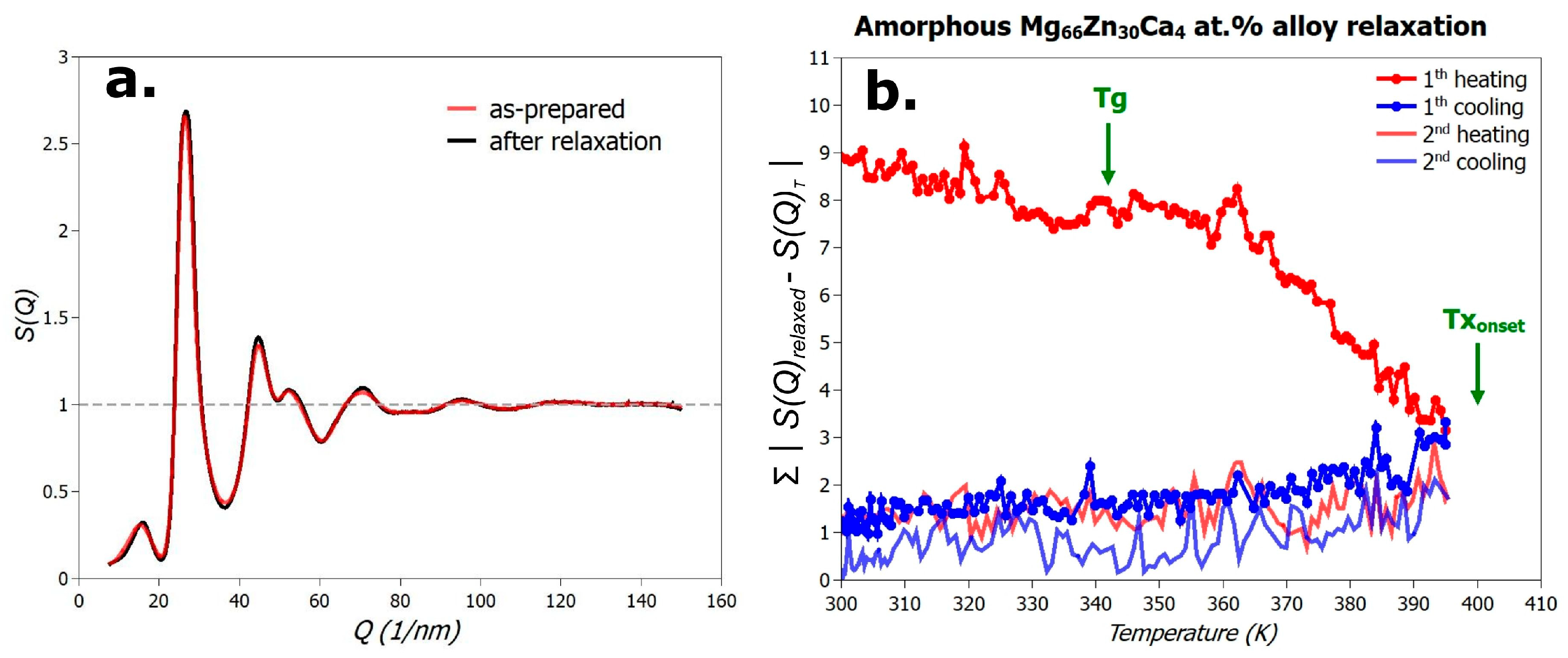
3.2. Relaxation of the Mg66Zn30Ca4 Metallic Glass
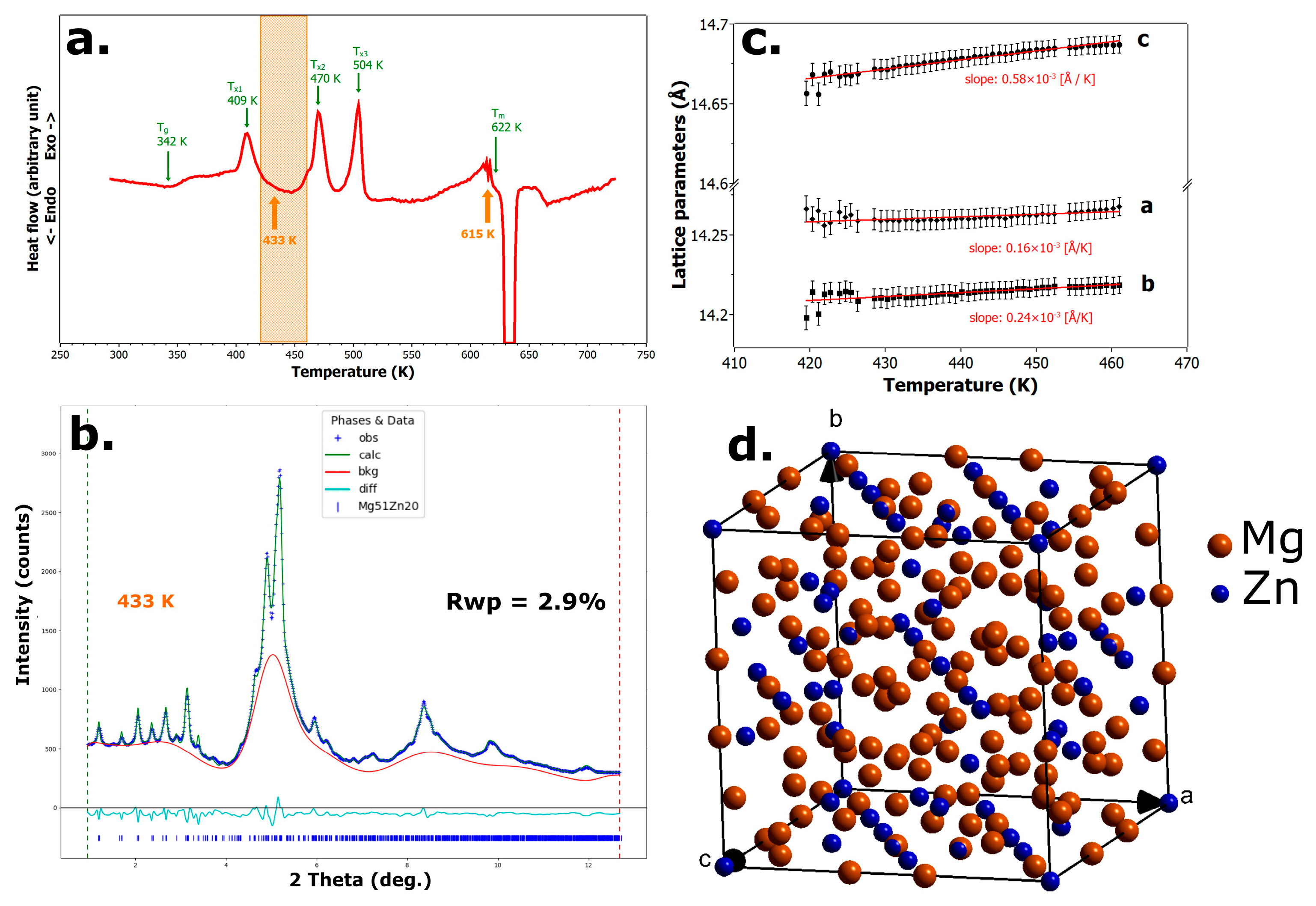
3.3. Crystallization of the Mg66Zn30Ca4 Metallic Glass
- −
- During the first phase transformation, the binary orthorhombic phase Mg51Zn20 crystallizes from the amorphous precursor. This phase belongs to space group Immm, with lattice parameters a = 14.224 Å, b = 14.195 Å, and c = 14.667 Å. A significant fraction of the amorphous matrix remains untransformed at this stage.
- −
- The second transformation involves the crystallization of the residual amorphous phase into the hexagonal Mg phase (P63/mmc, a = 3.205 Å, c = 5.210 Å) and an intermetallic phase denoted as IM1, also with a hexagonal structure (P63/mmc, a = 9.459 Å, c = 9.926 Å). This IM1 phase was identified by Schäublin et al. [18] as a solid solution with the general formula Ca3MgxZn15−x, with 4.6 ≤ x ≤ 12.
- −
- The third transformation leads to the coexistence of the Mg phase, the IM1 phase, and a new intermetallic phase designated as IM4. According to Schäublin et al., the chemical composition of IM4 is approximately Ca1 5Mg55 3Zn43 2; however, its crystal structure has not yet been resolved.
- −
- The fourth transformation results in the formation of Mg, IM1, and yet another new intermetallic phase, Ca8Mg26 1Zn57 9 IM3. Similar to the IM1, the IM3 phase exhibits a hexagonal lattice (P63/mmc) with lattice parameters a = 14.748 Å and c = 8.783 Å.
3.4. First Crystallization
3.5. Fourth Crystallization
4. Conclusions
- Mg66Zn30Ca4 metallic glass shows a distinct supercooled liquid region of 58 K, enabling thermoplastic processing before crystallization.
- Structural relaxation is nearly complete in the first heating cycle, and repeated heating–cooling cycles up to 396 K induce no signs of devitrification.
- Crystallization proceeds in four distinct steps. During the first crystallization stage, the alloy partially transforms into the orthorhombic Mg51Zn20 phase, while a significant fraction of the amorphous matrix remains untransformed. In the final (fourth) crystallization stage, two crystalline phases were identified: nearly pure hexagonal Mg and the intermetallic Ca8Mg26 1Zn57 9 phase (IM3). The presence of the hexagonal Ca3MgxZn15−x phase (IM1, with 4.6 ≤ x ≤ 12), which was reported in a previous study [17,18] to appear during the last stage of crystallization, was not confirmed in our measurements.
- The orthorhombic Mg51Zn20 phase exhibits anisotropic thermal expansion, with the highest relative increase along the c-axis.
- The combination of in situ synchrotron X-ray diffraction and Rietveld refinement is effective for identifying phase evolution and lattice parameter changes in multicomponent metallic glasses.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BN | Boron nitride |
| DSC | Differential scanning calorimetry |
| T | Temperature in K |
| Tg | Glass transition temperature |
| Tx | Crystallization peak |
| XRD | X-ray diffraction |
| I(2θ) | Intensity—2θ space |
| S(Q) | X-ray structure factor |
| Qmax | Maximum scattering vector |
| IM | Intermetallic compound |
References
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Ma, E.; Xu, J. The glass window of opportunities. Nat. Mater. 2009, 8, 855–857. [Google Scholar] [CrossRef]
- Chen, M. A brief overview of bulk metallic glasses. NPG Asia Mater. 2011, 3, 82–90. [Google Scholar] [CrossRef]
- Bin, S.J.B.; Fong, K.S.; Chua, B.W.; Gupta, M. Mg-based bulk metallic glasses: A review of recent developments. J. Magnes. Alloys 2022, 10, 899–914. [Google Scholar] [CrossRef]
- Shamlaye, K.F.; Löffler, J.F. Synthesis and characterization of Mg-based bulk metallic glasses in the Mg–Ag–Y–(Cu) system. J. Alloys Compd. 2021, 859, 157803. [Google Scholar] [CrossRef]
- Lee, J.I.; Ryu, W.H.; Yoon, K.N.; Park, E.S. In-situ synthesis of Mg-based bulk metallic glass matrix composites with primary α-Mg phases. J. Alloys Compd. 2021, 879, 160417. [Google Scholar] [CrossRef]
- Gu, X.; Shiflet, G.; Guo, F.; Poon, S. Mg–Ca–Zn Bulk Metallic Glasses with High Strength and Significant Ductility. J. Mater. Res. 2005, 20, 1935–1938. [Google Scholar] [CrossRef]
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef]
- Zberg, B.; Arata, E.R.; Uggowitzer, P.J.; Löffler, J.F. Tensile properties of glassy MgZnCa wires and reliability analysis using Weibull statistics. Acta Mater. 2009, 57, 3223–3231. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, W.; Wang, X.; Yang, S. Fabrication of Mg65Zn30Ca5 amorphous coating by laser remelting. J. Non-Cryst. Solids 2018, 500, 205–209. [Google Scholar] [CrossRef]
- Luo, A. Recent magnesium alloy development for elevated temperature applications. Int. Mater. Rev. 2004, 49, 13–30. [Google Scholar] [CrossRef]
- Nie, J.; Muddle, B. Precipitation hardening of Mg-Ca(-Zn) alloys. Scr. Mater. 1997, 37, 1475–1481. [Google Scholar] [CrossRef]
- Oh-Ishi, K.; Watanabe, R.; Mendis, C.; Hono, K. Age-hardening response of Mg–0.3at.%Ca alloys with different Zn contents. Mater. Sci. Eng. A 2009, 526, 177–184. [Google Scholar] [CrossRef]
- Li, K.; Xie, G.; Li, K.; Xie, G. Development of Mg-Based Bulk Metallic Glasses and Applications in Biomedical Field. In Magnesium Alloys—Processing, Potential and Applications; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Ma, E.; Xu, J. Reliability of compressive fracture strength of Mg–Zn–Ca bulk metallic glasses: Flaw sensitivity and Weibull statistics. Scr. Mater. 2008, 58, 496–499. [Google Scholar] [CrossRef]
- Saksl, K.; Pethes, I.; Jóvári, P.; Molčanová, Z.; Ďurišin, J.; Ballóková, B.; Temleitner, L.; Michalik, Š.; Šuliková, M.; Šuľová, K.; et al. Atomic structure of the Mg66Zn30Ca4 metallic glass. J. Non-Cryst. Solids 2021, 558, 120660. [Google Scholar] [CrossRef]
- Zhang, Y.; Rocher, G.; Briccoli, B.; Kevorkov, D.; Liu, X.; Altounian, Z.; Medraj, M. Crystallization characteristics of the Mg-rich metallic glasses in the Ca–Mg–Zn system. J. Alloys Compd. 2013, 552, 88–97. [Google Scholar] [CrossRef]
- Schäublin, R.; Becker, M.; Cihova, M.; Gerstl, S.; Deiana, D.; Hébert, C.; Pogatscher, S.; Uggowitzer, P.; Löffler, J. Precipitation in lean Mg–Zn–Ca alloys. Acta Mater. 2022, 239, 118223. [Google Scholar] [CrossRef]
- Dippel, A.-C.; Liermann, H.-P.; Delitz, J.T.; Walter, P.; Schulte-Schrepping, H.; Seeck, O.H.; Franz, H. Beamline P02.1 at PETRA III for high-resolution and high-energy powder diffraction. J. Synchrotron Radiat. 2015, 22, 675–687. [Google Scholar] [CrossRef]
- Franz, H.; Leupold, O.; Röhlsberger, R.; Roth, S.; Seeck, O.; Spengler, J.; Strempfer, J.; Tischer, M.; Viefhaus, J.; Weckert, E.; et al. Technical Report: PETRA III: DESY’s New High Brilliance Third Generation Synchrotron Radiation Source. Synchrotron Radiat. News 2006, 19, 25–29. [Google Scholar] [CrossRef]
- Skinner, L.B.; Benmore, C.J.; Parise, J.B. Area detector corrections for high quality synchrotron X-ray structure factor measurements. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2012, 662, 61–70. [Google Scholar] [CrossRef]
- Bednarcik, J.; Gamcova, J.; Liermann, H.P. New Heating/Cooling Stage Available to Users at P02. 1. In DESY Annual Report; DESY: Hamburg, Germany, 2012. [Google Scholar]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Hausermann, D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Faber, T.E.; Ziman, J.M. A Theory of the Electrical Properties of Liquid Metals: III. The Resistivity of Binary Alloys. Philos. Mag. 1965, 11, 153–173. [Google Scholar] [CrossRef]
- Qiu, X.; Thompson, J.W.; Billinge, S.J.L. PDFgetX2: A GUI-driven program to obtain the pair distribution function from X-ray powder diffraction data. J. Appl. Crystallogr. 2004, 37, 678. [Google Scholar] [CrossRef]
- Saksl, K.; Molčanová, Z.; Ďurišin, J.; Jóvári, P.; Michalik, Š.; Temleitner, L.; Ballóková, B.; Girman, V.; Katuna, Y.; Šuliková, M.; et al. Atomic structure of Ca–Mg biodegradable metallic glass. J. Alloys Compd. 2019, 801, 651–657. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Greer, A.L. Metallic glasses…on the threshold. Mater. Today 2009, 12, 14–22. [Google Scholar] [CrossRef]
- Saksl, K.; Ďurišin, J.; Balga, D.; Milkovič, O.; Brestovič, T.; Jasminská, N.; Ďurišin, M.; Girman, V.; Balko, J.; Katuna, Y.; et al. Devitrification and hydrogen storage capacity of the eutectic Ca72Mg28 metallic glass. J. Alloys Compd. 2017, 725, 916–922. [Google Scholar] [CrossRef]
- Lisnichuk, M.; Katuna, Y.; Saksl, K.; Fejerčak, M.; Šulikova, M.; Michalik, Š.; Čižmár, E.; Kliuikov, A.; Girman, V.; Vorobiov, S.; et al. Magnetic Characterization and Thermal Stability of Gd50Co48Fe2 Metallic Glass. Acta Phys. Pol. A 2020, 137, 914–917. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saksl, K.; Ďurišin, J.; Fujda, M.; Molčanová, Z.; Ballóková, B.; Matvija, M.; Gáborová, K. Relaxation and Devitrification of Mg66Zn30Ca4 Metallic Glass. Materials 2025, 18, 2464. https://doi.org/10.3390/ma18112464
Saksl K, Ďurišin J, Fujda M, Molčanová Z, Ballóková B, Matvija M, Gáborová K. Relaxation and Devitrification of Mg66Zn30Ca4 Metallic Glass. Materials. 2025; 18(11):2464. https://doi.org/10.3390/ma18112464
Chicago/Turabian StyleSaksl, Karel, Juraj Ďurišin, Martin Fujda, Zuzana Molčanová, Beáta Ballóková, Miloš Matvija, and Katarína Gáborová. 2025. "Relaxation and Devitrification of Mg66Zn30Ca4 Metallic Glass" Materials 18, no. 11: 2464. https://doi.org/10.3390/ma18112464
APA StyleSaksl, K., Ďurišin, J., Fujda, M., Molčanová, Z., Ballóková, B., Matvija, M., & Gáborová, K. (2025). Relaxation and Devitrification of Mg66Zn30Ca4 Metallic Glass. Materials, 18(11), 2464. https://doi.org/10.3390/ma18112464





