Efficacy of Biomimetic Hydroxyapatite in the Treatment of Extrinsic Dental Stains in Smokers and Non-Smokers
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Exclusion Criteria
2.4. Data Collection
2.5. Intervention
2.6. Adverse Events and Safety Monitoring
2.7. Clinical Observations
2.8. Statistical Analysis
3. Results
3.1. Oral Health Indices at Baseline (Smokers vs. Non-Smokers)
3.2. Oral Health Indices After Trial (Smokers vs. Non-Smokers)
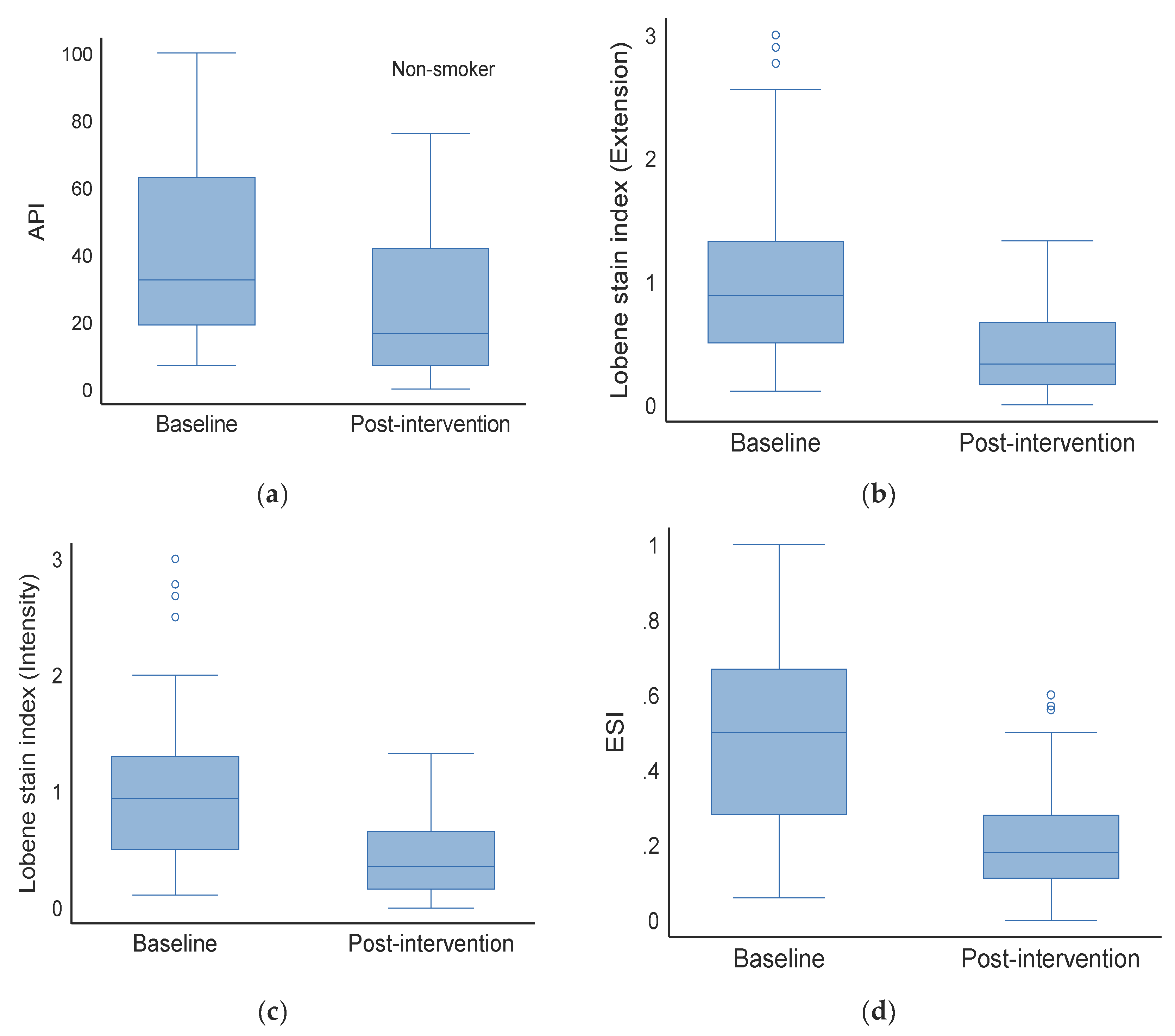
3.3. Effect on Non-Smokers (Baseline vs. Post-Intervention)
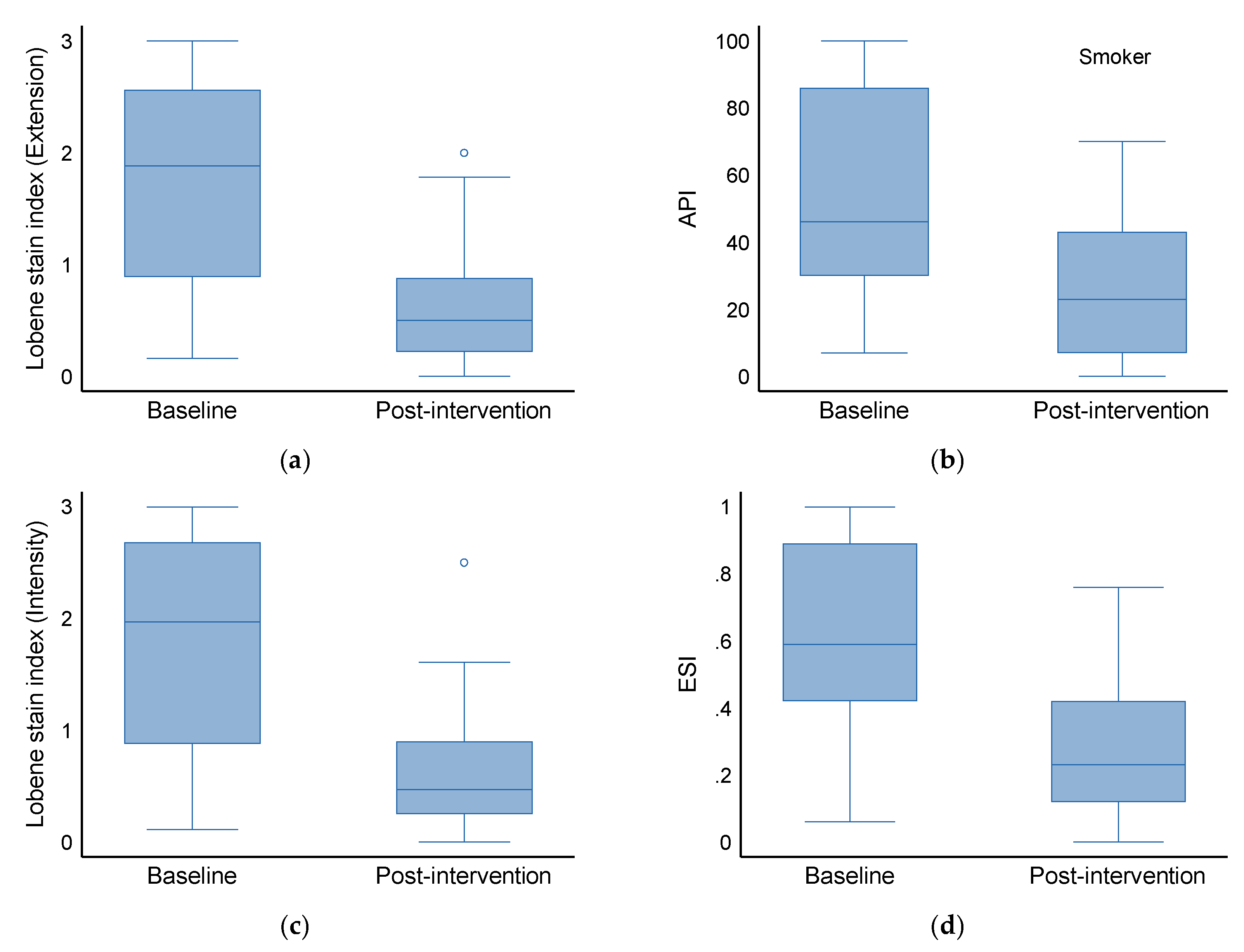
3.4. Effect on Smokers (Baseline vs. Post-Intervention)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- More, A.B.; Rodrigues, A.; Sadhu, B.J. Effects of smoking on oral health: Awareness among dental patients and their attitude towards its cessation. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2021, 32, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Ford, P.J.; Rich, A.M. Tobacco Use and Oral Health. Addiction 2021, 116, 3531–3540. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.V.; Guilardi, I.J.; Pavan, L.M.C.; Greggianin, B.F. Oral health-related quality of life and periodontal status according to smoking status. Int. J. Dent. Hyg. 2024, 22, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Battancs, E.; Gheorghita, D.; Nyiraty, S.; Lengyel, C.; Eördegh, G.; Baráth, Z.; Várkonyi, T.; Antal, M. Periodontal Disease in Diabetes Mellitus: A Case-Control Study in Smokers and Non-Smokers. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2020, 11, 2715–2728. [Google Scholar] [CrossRef]
- Wirth, R.; Maróti, G.; Mihók, R.; Simon-Fiala, D.; Antal, M.; Pap, B.; Demcsák, A.; Minarovits, J.; Kovács, K.L. A case study of salivary microbiome in smokers and non-smokers in Hungary: Analysis by shotgun metagenome sequencing. J. Oral Microbiol. 2020, 12, 1773067. [Google Scholar] [CrossRef]
- Karanjkar, R.R.; Preshaw, P.M.; Ellis, J.S.; Holliday, R. Effect of tobacco and nicotine in causing staining of dental hard tissues and dental materials: A systematic review and meta-analysis. Clin. Exp. Dent. Res. 2023, 9, 150–164. [Google Scholar] [CrossRef]
- Kaur, T.; Ramadoss, R.; Krishnasamy, N.; Sundar, S.; Panneer Selvam, S.; Shree K, H. Comprehensive characterization of tobacco-induced changes in enamel surface topography. J. Oral Biol. Craniofacial Res. 2025, 15, 97–102. [Google Scholar] [CrossRef]
- Sigwart, L.; Wiesmüller, V.; Kapferer-Seebacher, I. Colour Changes and Surface Roughness After Air-Polishing for Tobacco Stain Removal. Int. Dent. J. 2025, 75, 1409–1419. [Google Scholar] [CrossRef]
- Whelton, H.; Kingston, R.; O’Mullane, D.; Nilsson, F. Randomized controlled trial to evaluate tooth stain reduction with nicotine replacement gum during a smoking cessation program. BMC Oral Health 2012, 12, 13. [Google Scholar] [CrossRef]
- Newton, J.T.; Subramanian, S.S.; Westland, S.; Gupta, A.K.; Luo, W.; Joiner, A. The impact of tooth colour on the perceptions of age and social judgements. J. Dent. 2021, 112, 103771. [Google Scholar] [CrossRef]
- Subramanian, S.S.; Asimakopoulou, K.; Newton, T.; Chopra, A.; Luo, W.; Joiner, A. The impact of priming on dentally induced social judgements: An experimental study. J. Dent. 2022, 127, 104347. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, M.N.; Holt, R.D.; Bedi, R. Smoking and tooth discolouration: Findings from a national cross-sectional study. BMC Public Health 2005, 5, 27. [Google Scholar] [CrossRef]
- Joiner, A. Whitening toothpastes: A review of the literature. J. Dent. 2010, 38 (Suppl. S2), e17–e24. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yrastorza, J.T.; Matis, M.; Cusick, J.; Zhao, S.; Wang, G.; Xie, J. Biofilms: Formation, Research Models, Potential Targets, and Methods for Prevention and Treatment. Adv. Sci. 2022, 9, e2203291. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 28. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Nanda, A.; Walsh, L.J.; Xu, C. Microbial Decontamination and Antibacterial Activity of Nanostructured Titanium Dental Implants: A Narrative Review. Nanomaterials 2021, 11, 2336. [Google Scholar] [CrossRef]
- Łasica, A.; Golec, P.; Laskus, A.; Zalewska, M.; Gędaj, M.; Popowska, M. Periodontitis: Etiology, conventional treatments, and emerging bacteriophage and predatory bacteria therapies. Front. Microbiol. 2024, 15, 1469414. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, Y.W.; Zhu, J.X.; Li, Q.L. Occluding dentin tubules with monetite paste in vitro. West China J. Stomatol. 2021, 39, 667–674. [Google Scholar] [CrossRef]
- Nathoo, S.; Singh, S.; Petrone, D.M.; Wachs, G.N.; Chaknis, P.; DeVizio, W.; Proskin, H.M. Clinical studies to assess the extrinsic stain prevention and stain removal efficacy of a variant of a commercially available dentifrice containing a new dual silica system. J. Clin. Dent. 2008, 19, 95–101. [Google Scholar]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Li, Y.; Weng, W.; Cheng, K. Preparation of amorphous calcium phosphate in the presence of poly(ethylene glycol). J. Mater. Sci. Lett. 2003, 22, 1015. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- Habibovic, P.; de Groot, K. Osteoinductive biomaterials—Properties and relevance in bone regeneration. J. Tissue Eng. Regen. Med. 2007, 1, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Milleman, K.R.; Creeth, J.E.; Burnett, G.R.; Milleman, J.L. A randomized clinical trial to evaluate the stain removal efficacy of a sodium phytate dentifrice formulation. J. Esthet. Restor. Dent. 2018, 30, E45–E51. [Google Scholar] [CrossRef]
- Farrell, S.; Grender, J.M.; Terézhalmy, G.; Archila, L.R. Stain Removal Assessment of Two Manual Toothbrushes with an Interproximal Tooth Stain Index. J. Clin. Dent. 2015, 26, 39–43. [Google Scholar]
- Parkinson, C.R.; Burnett, G.R.; Smith, G.; Pradhan, M.; Gallob, J.; Qaqish, J. A Randomized Clinical Study Investigating the Stain Removal Efficacy of Two Experimental Dentifrices. J. Esthet. Restor. Dent. 2025, 1267–1608. [Google Scholar] [CrossRef] [PubMed]
- Brading, M.G.; Marsh, P.D. The oral environment: The challenge for antimicrobials in oral care products. Int. Dent. J. 2003, 53 (Suppl. S1), 353–362. [Google Scholar] [CrossRef]
- ten Cate, J.M. The need for antibacterial approaches to improve caries control. Adv. Dent. Res. 2009, 21, 8–12. [Google Scholar] [CrossRef]
- Marsh, P.D. Controlling the oral biofilm with antimicrobials. J. Dent. 2010, 38 (Suppl. S1), S11–S15. [Google Scholar] [CrossRef] [PubMed]
- Latimer, J.; Munday, J.L.; Buzza, K.M.; Forbes, S.; Sreenivasan, P.K.; McBain, A.J. Antibacterial and anti-biofilm activity of mouthrinses containing cetylpyridinium chloride and sodium fluoride. BMC Microbiol. 2015, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Enax, J. Hydroxyapatite in Oral Biofilm Management. Eur. J. Dent. 2019, 13, 287–290. [Google Scholar] [CrossRef]
- Antal, M.A.; Kiscsatári, R.; Braunitzer, G.; Piffkó, J.; Varga, E.; Eliaz, N. Assessment of a novel electrochemically deposited smart bioactive trabecular coating (SBTC®): A randomized controlled clinical trial. Head Face Med. 2024, 20, 24. [Google Scholar] [CrossRef]
- Villaseñor-Cerón, L.S.; Mendoza-Anaya, D.; López-Ortiz, S.; Rosales-Ibañez, R.; Rodríguez-Martínez, J.J.; Reyes-Valderrama, M.I.; Rodríguez-Lugo, V. Biocompatibility analysis and chemical characterization of Mn-doped hydroxyapatite. J. Mater. Sci. Mater. Med. 2023, 34, 40. [Google Scholar] [CrossRef]
- Fratzl, P. Biomimetic materials research: What can we really learn from nature’s structural materials? J. R. Soc. Interface 2007, 4, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Enax, J.; Epple, M. Synthetic Hydroxyapatite as a Biomimetic Oral Care Agent. Oral Health Prev. Dent. 2018, 16, 7–19. [Google Scholar]
- Meyer, F.; Amaechi, B.T.; Fabritius, H.-O.; Enax, J. Overview of Calcium Phosphates used in Biomimetic Oral Care. Open Dent. J. 2018, 12, 406–423. [Google Scholar] [CrossRef]
- Lobene, R.R.; Mankodi, S.M.; Ciancio, S.G.; Lamm, R.A.; Charles, C.H.; Ross, N.M. Correlations among gingival indices: A methodology study. J. Periodontol. 1989, 60, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Carlos, J.P.; Wolfe, M.D.; Kingman, A. The extent and severity index: A simple method for use in epidemiologic studies of periodontal disease. J. Clin. Periodontol. 1986, 13, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, M.B.; Oliveira, C.S.; Tavaria, F.K. Novel Strategies for Preventing Dysbiosis in the Oral Cavity. Front. Biosci. (Elite Ed.) 2023, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Xu, X.; Zhou, X.D. Sichuan da xue xue bao. Yi Xue Ban J. Sichuan Univ. (Med. Sci. Ed.) 2022, 53, 220–225. [Google Scholar] [CrossRef]
- Lange, D.E.; Plagmann, H.C.; Eenboom, A.; Promesberger, A. Clinical methods for the objective evaluation of oral hygiene. Dtsch. Zahnarztl. Z. 1977, 32, 44–47. [Google Scholar] [PubMed]
- Generlich, A. Remineralisation, whitening and prevention. Vital 2013, 11, 32. [Google Scholar] [CrossRef]
- Macpherson, L.M.; Stephen, K.W.; Joiner, A.; Schäfer, F.; Huntington, E. Comparison of a conventional and modified tooth stain index. J. Clin. Periodontol. 2000, 27, 854–859. [Google Scholar] [CrossRef]
- Ellingsen, J.E.; Eriksen, H.M.; Rölla, G. Extrinsic dental stain caused by stannous fluoride. Eur. J. Oral Sci. 1982, 90, 9–13. [Google Scholar] [CrossRef]
- Watts, A.M.; Addy, M. Tooth discolouration and staining: A review of the literature. Br. Dent. J. 2001, 190, 309–319. [Google Scholar] [CrossRef]
- Lippert, F. An introduction to toothpaste-its purpose, history and ingredients. Toothpastes 2013, 23, 1–14. [Google Scholar]
- Demarco, F.F.; Meireles, S.S.; Masotti, A.S. Over-the-counter whitening agents: A concise review. Braz. Oral Res. 2009, 23, 64–70. [Google Scholar] [CrossRef]
- Addy, M.; Moran, J. Mechanisms of Stain Formation on Teeth, in Particular Associated with Metal Ions and Antiseptics. Adv. Dent. Res. 1995, 9, 450–456. [Google Scholar] [CrossRef]
- Soparkar, P.; Rustogi, K.; Zhang, Y.P.; Petrone, M.E.; DeVizio, W.; Proskin, H.M. Comparative tooth whitening and extrinsic tooth stain removal efficacy of two tooth whitening dentifrices: Six-week clinical trial. J. Clin. Dent. 2004, 15, 46–51. [Google Scholar] [PubMed]
- Liu, H.; Tu, J. Reduction of extrinsic tooth stain by a toothpaste containing 10% high cleaning silica, 0.5% sodium phytate and 0.5% sodium pyrophosphate: An 8-week randomised clinical trial. BMC Oral Health 2021, 21, 113. [Google Scholar] [CrossRef]
- Vaz, V.T.P.; Jubilato, D.P.; de Oliveira, M.R.M.; Bortolatto, J.F.; Floros, M.C.; Dantas, A.A.R.; de Oliveira, O.B., Jr. Whitening toothpaste containing activated charcoal, blue covarine, hydrogen peroxide or microbeads: Which one is the most effective? J. Appl. Oral Sci. 2019, 27, e20180051. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, R.W.; Barker, M.L.; Tucker, H.L. Clinical response of three whitening products having different peroxide delivery: Comparison of tray, paint-on gel, and dentifrice. J. Clin. Dent. 2004, 15, 112–117. [Google Scholar]
- Silva, E.M.D.; Maia, J.; Mitraud, C.G.; Russo, J.; Poskus, L.T.; Guimarães, J.G.A. Can whitening toothpastes maintain the optical stability of enamel over time? J. Appl. Oral Sci. 2018, 26, e20160460. [Google Scholar] [CrossRef]
- Soeteman, G.D.; Valkenburg, C.; Van der Weijden, G.A.; Van Loveren, C.; Bakker, E.; Slot, D.E. Whitening dentifrice and tooth surface discoloration—A systematic review and meta-analysis. Int. J. Dent. Hyg. 2018, 16, 24–35. [Google Scholar] [CrossRef]
- Singh, R.D.; Ram, S.M.; Shetty, O.; Chand, P.; Yadav, R. Efficacy of casein phosphopeptide-amorphous calcium phosphate to prevent stain absorption on freshly bleached enamel: An in vitro study. J. Conserv. Dent. JCD 2010, 13, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kwon, H.K.; Kim, B.I. Effect of nano-carbonate apatite to prevent re-stain after dental bleaching in vitro. J. Dent. 2011, 39, 636–642. [Google Scholar] [CrossRef]
- Pedreira De Freitas, A.C.; Botta, S.B.; Teixeira, F.D.S.; Salvadori, M.C.B.S.; Garone-Netto, N. Effects of fluoride or nanohydroxiapatite on roughness and gloss of bleached teeth. Microsc. Res. Tech. 2011, 74, 1069–1075. [Google Scholar] [CrossRef]
- Rezvani, M.B.; Atai, M.; Rouhollahi, M.R.; Malekhoseini, K.; Rezai, H.; Hamze, F. Effect of Nano-Tricalcium Phosphate and Nanohydroxyapatite on the Staining Susceptibility of Bleached Enamel. Int. Sch. Res. Not. 2015, 2015, 935264. [Google Scholar] [CrossRef]
- Porciani, P.F.; Grandini, S.; Perra, C.; Grandini, R. Whitening effect by stain inhibition from a chewing gum with sodium hexametaphosphate in a controlled twelve-week single-blind trial. J. Clin. Dent. 2006, 17, 14–16. [Google Scholar] [PubMed]
- Collins, L.Z.; Naeeni, M.; Schäfer, F.; Brignoli, C.; Schiavi, A.; Roberts, J.; Colgan, P. The effect of a calcium carbonate/perlite toothpaste on the removal of extrinsic tooth stain in two weeks. Int. Dent. J. 2005, 55 (Suppl. S3), 179–182. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, R.W.; White, D.J. Removal of extrinsic stain using a tartar control whitening dentifrice: A randomized clinical trial. J. Clin. Dent. 2001, 12, 42–46. [Google Scholar]
- Ziebolz, D.; Helms, K.; Hannig, C.; Attin, T. Efficacy and oral side effects of two highly concentrated tray-based bleaching systems. Clin. Oral Investig. 2007, 11, 267–275. [Google Scholar] [CrossRef]
- Hughes, N.; Maggio, B.; Sufi, F.; Mason, S.; Kleber, C.J. A comparative clinical study evaluating stain removal efficacy of a new sensitivity whitening dentifrice compared to commercially available whitening dentifrices. J. Clin. Dent. 2009, 20, 218–222. [Google Scholar]
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Rupp, C.M.; Hirsch, S.; Muehler, D.; Enax, J.; Meyer, F.; Hiller, K.A.; Buchalla, W. Ca2+ release and buffering effects of synthetic hydroxyapatite following bacterial acid challenge. BMC Oral Health 2020, 20, 85. [Google Scholar] [CrossRef]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-hydroxyapatite in oral care cosmetics: Characterization and cytotoxicity assessment. Sci. Rep. 2019, 9, 11050. [Google Scholar] [CrossRef]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef]
- Bersezio, C.; Martín, J.; Mayer, C.; Rivera, O.; Estay, J.; Vernal, R.; Haidar, Z.S.; Angel, P.; Oliveira, O.B., Jr.; Fernández, E. Quality of life and stability of tooth color change at three months after dental bleaching. Qual. Life Res. 2018, 27, 3199–3207. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Gawriolek, M.; Kaminska, I.; Otulakowska-Skrzynska, J.; Marczuk-Kolada, G.; Rzatowski, S.; Sokolowska, K.; Olszewska, A.; Schlagenhauf, U.; et al. Impact of a toothpaste with microcrystalline hydroxyapatite on the occurrence of early childhood caries: A 1-year randomized clinical trial. Sci. Rep. 2021, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T.; AbdulAzees, P.A.; Alshareif, D.O.; Shehata, M.A.; Lima, P.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Kapoor, A.; Grover, V.; Kaushal, S. Nicotine and periodontal tissues. J. Indian Soc. Periodontol. 2010, 14, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, M.; Ramesh, A.; Dwarakanath, C. Periodontal Status in Smokers and Nonsmokers: A Clinical, Microbiological, and Histopathological Study. Int. J. Dent. 2012, 2012, 571590. [Google Scholar] [CrossRef]
| Non-Smoker | Smoker | Total | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 50) | (N = 50) | (N = 100) | |||||||||
| Median | Q1 | Q3 | Median | Q1 | Q3 | Median | Q1 | Q3 | |||
| Baseline | Approximal plaque index (API) | 32.5 | 19.0 | 63.0 | 46.0 | 30.0 | 86.0 | 41.0 | 21.0 | 71.0 | 0.098 |
| Lobene stain index (extension) | 0.9 | 0.5 | 1.3 | 1.9 | 0.9 | 2.6 | 1.1 | 0.7 | 2.3 | 0.001 | |
| Lobene stain index (intensity) | 0.9 | 0.5 | 1.3 | 2.0 | 0.9 | 2.7 | 1.1 | 0.6 | 2.1 | 0.001 | |
| The extent and severity index (ESI) | 0.5 | 0.3 | 0.7 | 0.6 | 0.4 | 0.9 | 0.5 | 0.3 | 0.8 | 0.043 | |
| Post-intervention | Approximal plaque index (API) | 16.5 | 7.0 | 42.0 | 23.0 | 7.0 | 43.0 | 20.5 | 7.0 | 42.5 | 0.937 |
| Lobene stain index (extension) | 0.3 | 0.2 | 0.7 | 0.5 | 0.2 | 0.9 | 0.4 | 0.2 | 0.9 | 0.142 | |
| Lobene stain index (intensity) | 0.4 | 0.2 | 0.7 | 0.5 | 0.3 | 0.9 | 0.4 | 0.2 | 0.8 | 0.149 | |
| The extent and severity index (ESI) | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0.4 | 0.2 | 0.1 | 0.3 | 0.091 | |
| Sex | 0.548 | ||||||||||
| Male | 23 (46.0%) | 26 (52.0%) | 49 (49.0%) | ||||||||
| Female | 27 (54.0%) | 24 (48.0%) | 51 (51.0%) | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sozkes, S.; Chomyszyn-Gajewska, M.; Dudzik, A.; Olszewska-Czyz, I. Efficacy of Biomimetic Hydroxyapatite in the Treatment of Extrinsic Dental Stains in Smokers and Non-Smokers. Materials 2025, 18, 2441. https://doi.org/10.3390/ma18112441
Sozkes S, Chomyszyn-Gajewska M, Dudzik A, Olszewska-Czyz I. Efficacy of Biomimetic Hydroxyapatite in the Treatment of Extrinsic Dental Stains in Smokers and Non-Smokers. Materials. 2025; 18(11):2441. https://doi.org/10.3390/ma18112441
Chicago/Turabian StyleSozkes, Sarkis, Maria Chomyszyn-Gajewska, Agata Dudzik, and Iwona Olszewska-Czyz. 2025. "Efficacy of Biomimetic Hydroxyapatite in the Treatment of Extrinsic Dental Stains in Smokers and Non-Smokers" Materials 18, no. 11: 2441. https://doi.org/10.3390/ma18112441
APA StyleSozkes, S., Chomyszyn-Gajewska, M., Dudzik, A., & Olszewska-Czyz, I. (2025). Efficacy of Biomimetic Hydroxyapatite in the Treatment of Extrinsic Dental Stains in Smokers and Non-Smokers. Materials, 18(11), 2441. https://doi.org/10.3390/ma18112441







