Analysis of the Wear and Corrosion Resistance on Cu-Ni-Al Composites Reinforced with CeO2 Nanoparticles
Abstract
1. Introduction
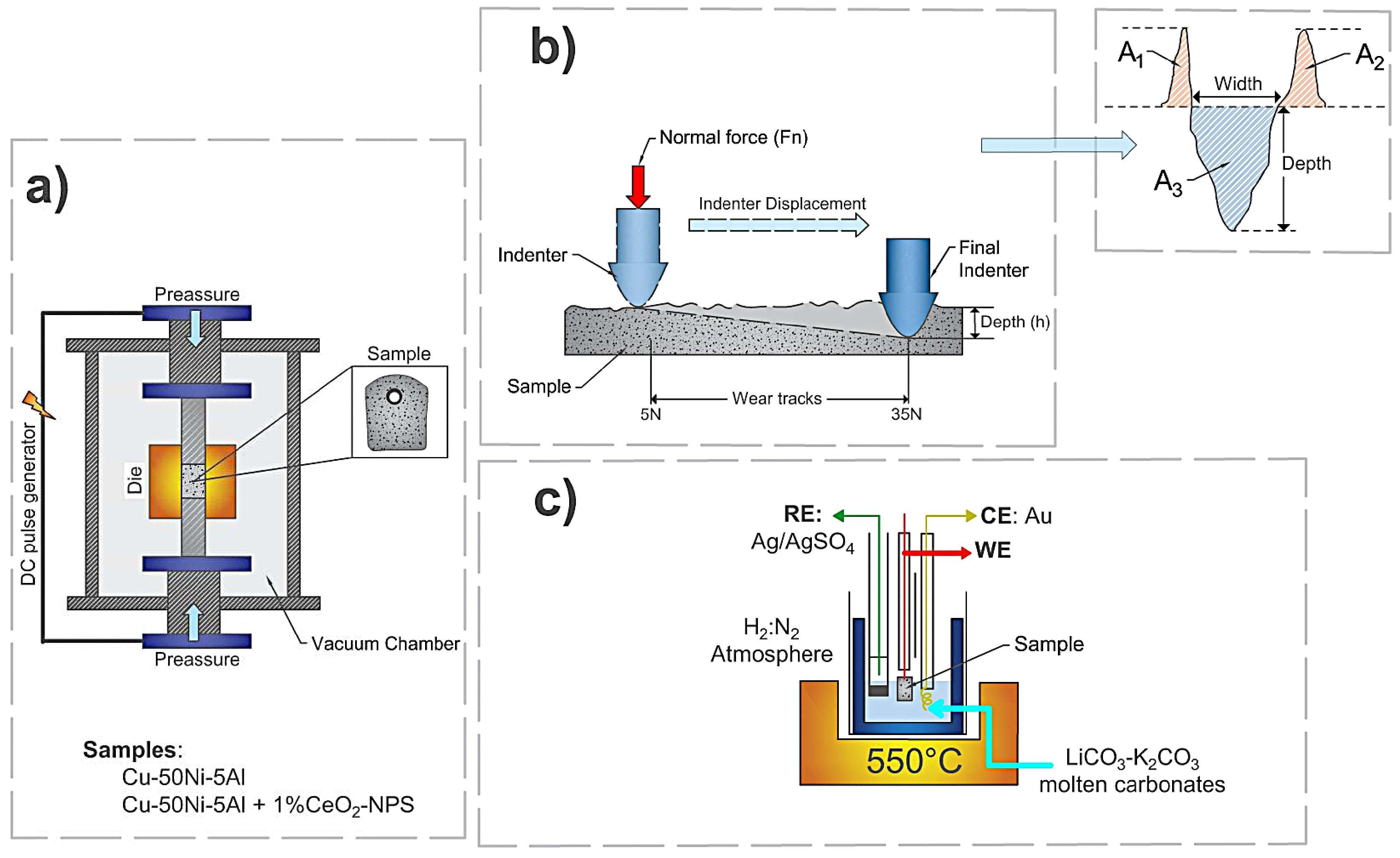
2. Materials and Methods
2.1. Samples Manufacturing
2.2. Singular Scratch Test
2.3. Electrochemical Measurements
2.4. Morphological and Microstructural Analysis
3. Results
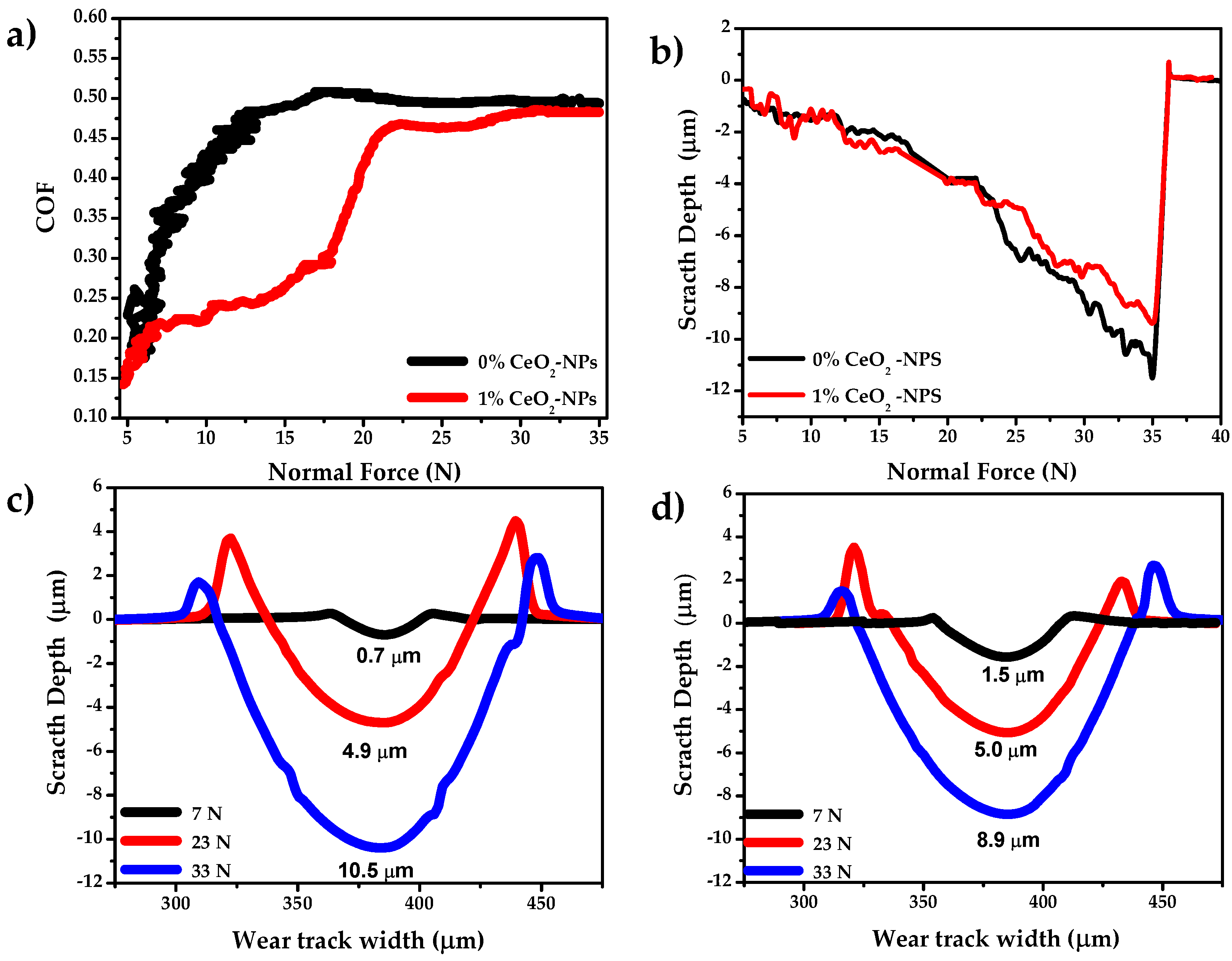
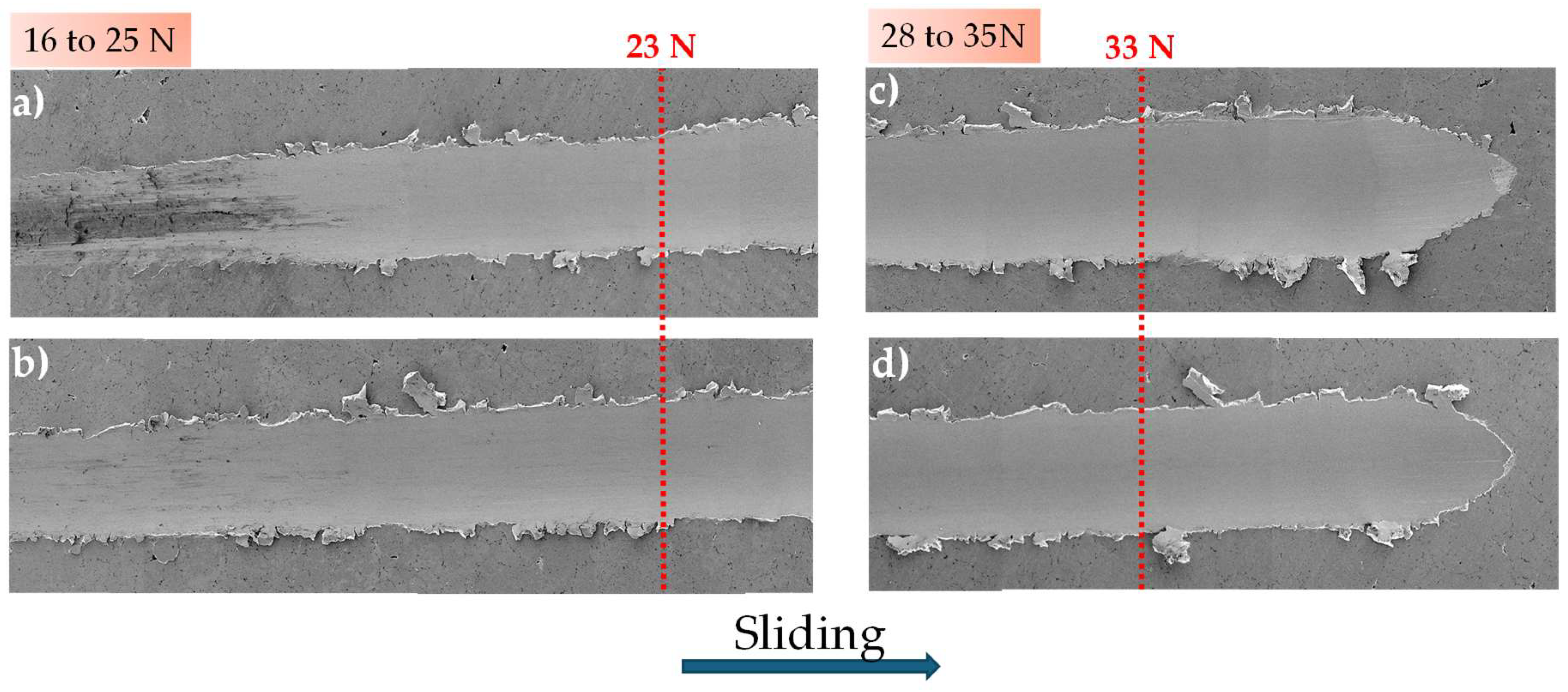
3.1. Scratch Test with Progressive Normal Load
3.2. Electrochemical Measurements
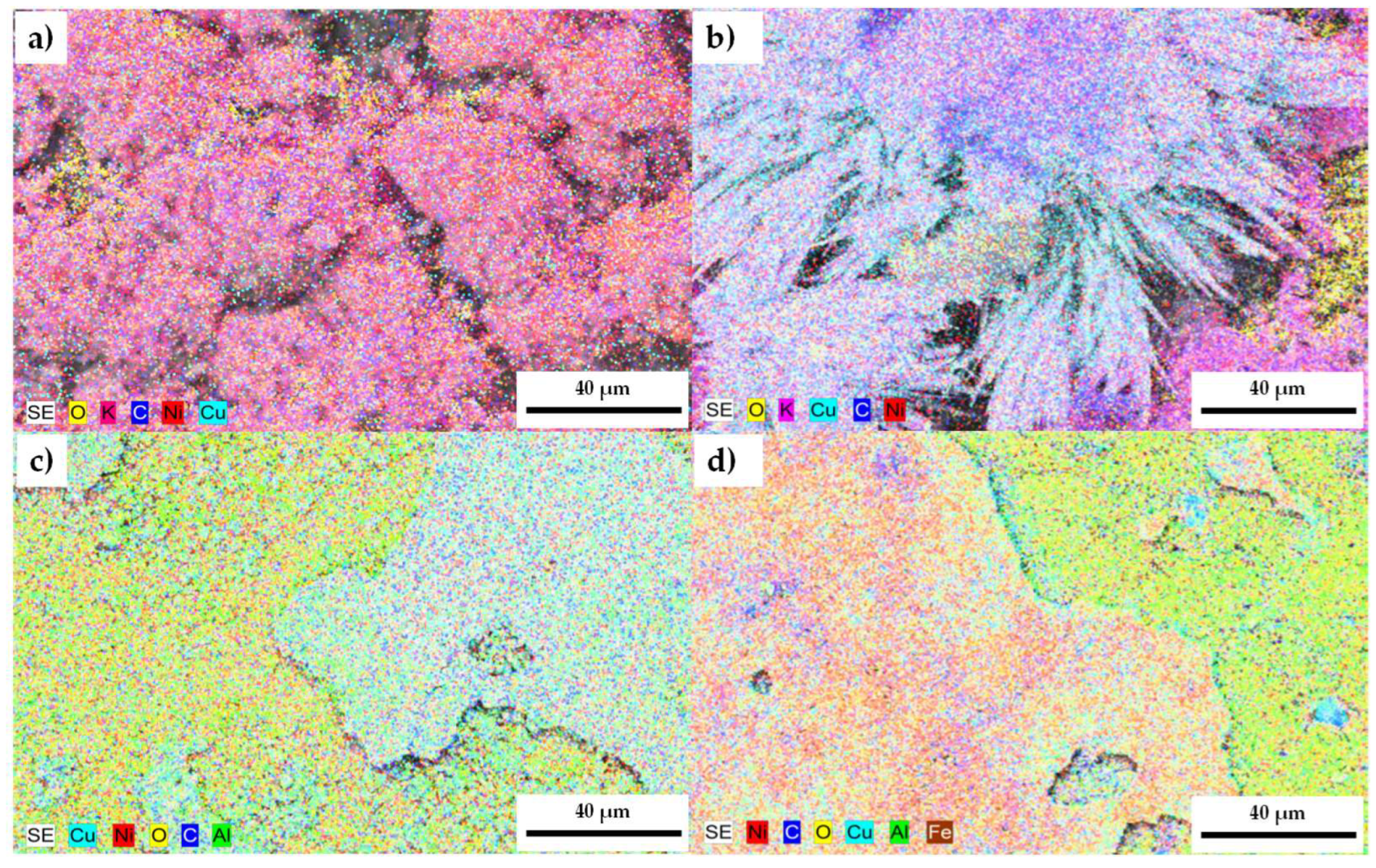
3.3. Morphological and Microstructural Analysis
3.3.1. Scanning Electron Microscopy Analysis
3.3.2. X-Ray Diffraction Analysis
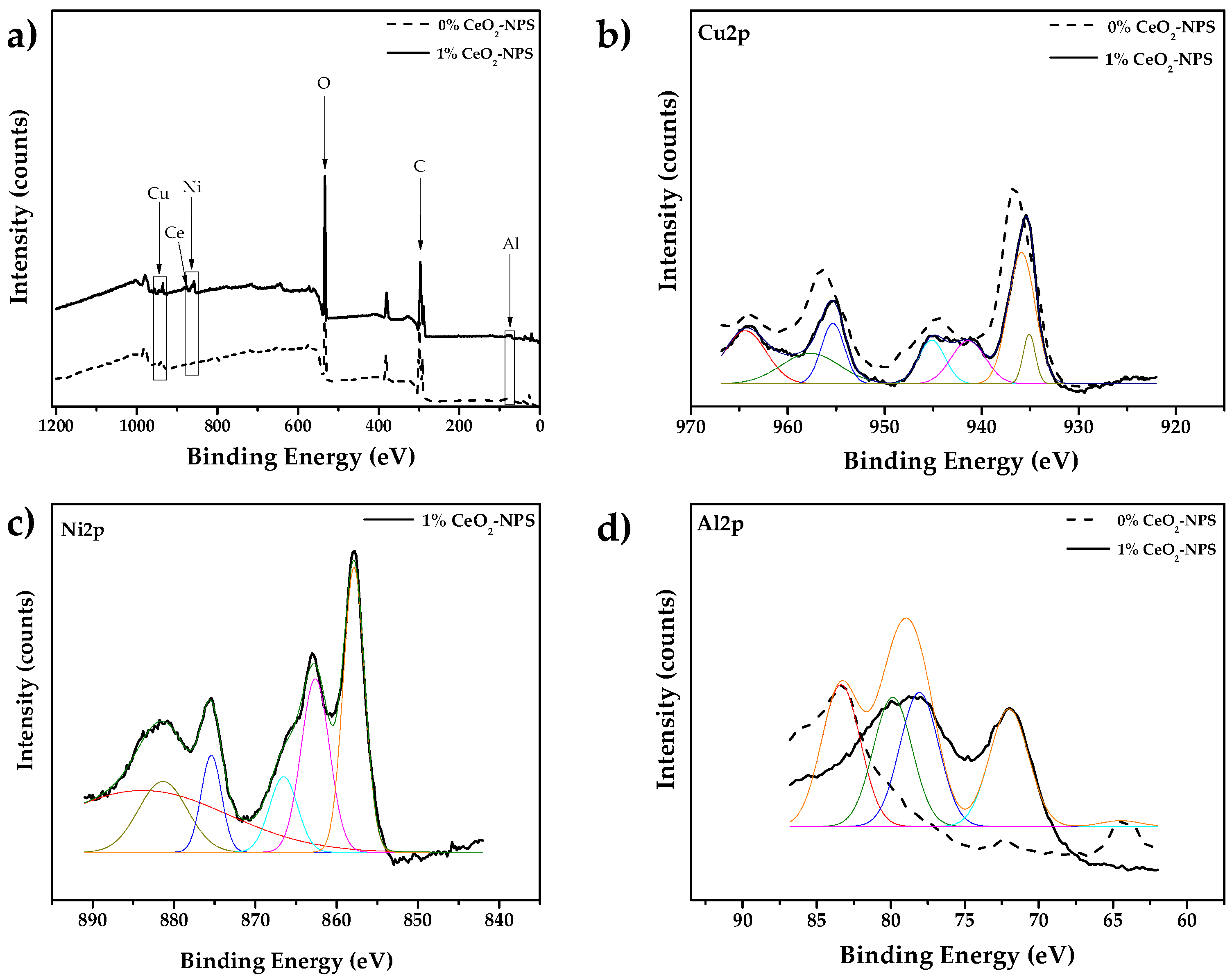
3.3.3. X-Ray Photoelectron Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SPS | Spark plasma sintering |
| EIS | Electrochemical impedance spectroscopy |
| NP | Nanoparticles |
| SEM | Scanning electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| MCFCs | Molten carbonate fuel cells |
| SOFCs | Solid oxide fuel cells |
| Cu | Copper |
| Al | Aluminum |
| Ni | Nickel |
| CeO2 | Cerium oxide |
| ICR | Interface contact resistance |
| BPR | Ball-to-powder ratio |
| Ft | Tangential force |
| Fn | Normal force |
| COF | Coefficient of friction |
| EOC | Open circuit potential |
| HF | High frequency |
| LF | Low frequency |
| Cdl | Capacitance of the electric double-layer |
| CPE | Constant phase element |
| PLM | Power low model |
| Re | Electrolytic resistance |
References
- Manzo, D.; Thai, R.; Le, H.T.; Venayagamoorthy, G.K. Fuel cell technology review: Types, economy, applications, and vehicle-to-grid scheme. Sustain. Energy Technol. Assess. 2025, 75, 104229. [Google Scholar] [CrossRef]
- Wejrzanowski, T.; Cwieka, K.; Skibinski, J.; Lysik, A.; Ibrahim, S.H.; Milewski, J.; Xing, W.; Lee, C.-G. Microstructure driven design of porous electrodes for molten carbonate fuel cell application: Recent progress. Int. J. Hydrogen Energy 2020, 45, 25719–25732. [Google Scholar] [CrossRef]
- Ang, S.M.C.; Fraga, E.S.; Brandon, N.P.; Samsatli, N.J.; Brett, D.J. Fuel cell systems optimisation—Methods and strategies. Int. J. Hydrogen Energy 2011, 36, 14678–14703. [Google Scholar] [CrossRef]
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive Review on Fuel Cell Technology for Stationary Applications as Sustainable and Efficient Poly-Generation Energy Systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Abdulrahman, G.A.Q. A Recent Comprehensive Review of Fuel Cells: History, Types, and Applications. Int. J. Energy Res. 2024, 2024, 7271748. [Google Scholar] [CrossRef]
- Rana, M.; Uddin, M.; Sarkar, R.; Meraj, S.T.; Shafiullah, G.; Muyeen, S.; Islam, A.; Jamal, T. Applications of energy storage systems in power grids with and without renewable energy integration—A comprehensive review. J. Energy Storage 2023, 68, 107811. [Google Scholar] [CrossRef]
- Tomczyk, P. MCFC versus other fuel cells—Characteristics, technologies and prospects. J. Power Sources 2006, 160, 858–862. [Google Scholar] [CrossRef]
- Mehr, A.; Lanzini, A.; Santarelli, M.; Rosen, M.A. Polygeneration systems based on high temperature fuel cell (MCFC and SOFC) technology: System design, fuel types, modeling and analysis approaches. Energy 2021, 228, 120613. [Google Scholar] [CrossRef]
- Jang, S.-C.; Lee, B.Y.; Nam, S.W.; Ham, H.C.; Han, J.; Yoon, S.P.; Oh, S.-G. New method for low temperature fabrication of Ni–Al alloy powder for molten carbonate fuel cell applications. Int. J. Hydrogen Energy 2014, 39, 12259–12265. [Google Scholar] [CrossRef]
- Li, G.; Thomas, B.G.; Stubbins, J.F. Modeling creep and fatigue of copper alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2000, 31, 2491–2502. [Google Scholar] [CrossRef]
- Klassert, A.; Tikana, L. Copper and copper-nickel alloys—An overview. In Corrosion Behaviour and Protection of Copper and Aluminium Alloys in Seawater; CRC Press: Boca Raton, FL, USA, 2007; pp. 47–61. [Google Scholar] [CrossRef]
- Abdollahipour, A.; Sayyaadi, H. Thermal energy recovery of molten carbonate fuel cells by thermally regenerative electrochemical cycles. Energy 2021, 227, 120489. [Google Scholar] [CrossRef]
- Appleby, A.J.; Blomen, L.J.M.J.; Mugerwa, M.N. (Eds.) Fuel Cell Systems; Plenum Press: New York, NY, USA, 1993; pp. 157–197. [Google Scholar]
- Czelej, K.; Cwieka, K.; Colmenares, J.C.; Kurzydlowski, K.J. Catalytic activity of NiO cathode in molten carbonate fuel cells. Appl. Catal. B Environ. 2018, 222, 73–75. [Google Scholar] [CrossRef]
- Wee, J.-H.; Song, D.-J.; Jun, C.-S.; Lim, T.-H.; Hong, S.-A.; Lim, H.-C.; Lee, K.-Y. Evaluation of Ni-Ni3Al(5 wt.%)-Al(3 wt.%) as an anode electrode for molten carbonate fuel cell: Part I: Creep and sintering resistance. J. Alloys Compd. 2005, 390, 155–160. [Google Scholar] [CrossRef]
- Hwang, E.; Park, J.; Kim, Y.; Kim, S.; Kang, S. Effect of alloying elements on the copper-base anode for molten carbonate fuel cells. J. Power Sources 1997, 69, 55–60. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.; Li, W.; Hu, Y.; Ren, Y.; Qiu, W.; He, J.; Chen, J. Investigation on compressive behavior of Cu-35Ni-15Al alloy at high temperatures. Mater. Sci. 2014, 32, 341–349. [Google Scholar] [CrossRef]
- Martínez, C.; Arcos, C.; Briones, F.; Machado, I.; Sancy, M.; Bustamante, M. The Effect of Adding CeO2 Nanoparticles to Cu-Ni-Al Alloy for High Temperatures Applications. Nanomaterials 2024, 14, 143. [Google Scholar] [CrossRef]
- Hume-Rothery, W. The Structure of Metals and Alloys. Nature 1936, 138, 7–8. [Google Scholar]
- Elorz, J.A.P.-S.; Hernández, M.J.Q.; González, L.F.V.; Hernández, Q.; González, L.F.V. Total Insolubility and Solubility in Alloys. In Solidification and Solid-State Transformations of Metals and Alloys; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–92. [Google Scholar] [CrossRef]
- Accardo, G.; Frattini, D.; Moreno, A.; Yoon, S.P.; Han, J.H.; Nam, S.W. Influence of nano zirconia on NiAl anodes for molten carbonate fuel cell: Characterization, cell tests and post-analysis. J. Power Sources 2017, 338, 74–81. [Google Scholar] [CrossRef]
- Accardo, G.; Frattini, D.; Yoon, S.P.; Ham, H.C.; Nam, S.W. Performance and properties of anodes reinforced with metal oxide nanoparticles for molten carbonate fuel cells. J. Power Sources 2017, 370, 52–60. [Google Scholar] [CrossRef]
- Jomjaree, T.; Sintuya, P.; Srifa, A.; Koo-Amornpattana, W.; Kiatphuengporn, S.; Assabumrungrat, S.; Sudoh, M.; Watanabe, R.; Fukuhara, C.; Ratchahat, S. Catalytic performance of Ni catalysts supported on CeO2 with different morphologies for low-temperature CO2 methanation. Catal. Today 2021, 375, 234–244. [Google Scholar] [CrossRef]
- Mustafa, S.Z.; Qureshi, M.D.; Jabeen, I.; Khalid, S.; Hussain, G.; Riaz, J.; Akram, M.N.; Maqsood, F. Physiochemical Role of Nanoparticles in Solid Fuel Cells, Production and Applications in Physics and Chemical Sciences. Sch. Bull. 2022, 8, 66–70. [Google Scholar] [CrossRef]
- Gong, B.; Ma, X.; Wang, T.; Hou, J.; Ji, S.; Xu, Q.; Cao, H. Emerging two dimensional MXene for corrosion protection in new energy systems: Design and mechanisms. Adv. Colloid Interface Sci. 2024, 336, 103373. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.G.; Jun, J.; Keum, J.K.; Su, Y.-F.; Pole, M.; Niverty, S.; Joshi, V.V. Lithium nitrate salt-assisted CO2 absorption for the formation of corrosion barrier layer on AZ91D magnesium alloy. RSC Adv. 2024, 14, 17696–17709. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, G.; Gnanamoorthy, R.; Okazaki, M. Fretting wear behaviour of nickel foam struts used in fuel cell applications. Proceedings of the Institution of Mechanical Engineers. Part J J. Eng. Tribol. 2021, 236, 144–155. [Google Scholar] [CrossRef]
- Pukha, V.E.; Glukhov, A.; Belmesov, A.A.; Kabachkov, E.N.; Khodos, I.I.; Khadem, M.; Kim, D.E.; Karaseov, P.A. Corrosion-resistant nanostructured carbon-based coatings for applications in fuel cells based on bipolar plates. Vacuum 2023, 218, 112643. [Google Scholar] [CrossRef]
- Xu, N.; Ma, J.; Liu, Q.; Han, W.; Shan, Z. Size Effect of CeO2 Particle on Nanoscale Single-Asperity Sliding Friction. Tribol. Lett. 2022, 70, 4. [Google Scholar] [CrossRef]
- Mishra, T.K.; Kumar, A.; Sinha, S.K.; Gupta, B. Wear behavior and XRD analysis of reinforced copper matrix composite re-inforced with Cerium Oxide (CeO2). Mater. Today Proc. 2018, 5, 27786–27794. [Google Scholar] [CrossRef]
- Xu, N.; Han, W.; Wang, Y.; Li, J.; Shan, Z. Nanoscratching of copper surface by CeO2. Acta Mater. 2017, 124, 343–350. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, M.; Wang, Q.; Wang, F. A CeO2-Doped Copper Matrix Self-Lubricating Composite with Well-Balanced Mechanical and Lubricating Properties Fabricated by Spark Plasma Sintering. Met. Mater. Trans. A 2022, 53, 2665–2678. [Google Scholar] [CrossRef]
- Martínez, C.; Briones, F.; Araya, N.; Aguilar, C.; Machado, I.; Guerra, C.; Medina, A.; Sancy, M. Influence of the synthesis technique on tribological behavior of a Ti-6Al-4V alloy. Mater. Lett. 2020, 281, 128627. [Google Scholar] [CrossRef]
- Bergami, L.B.; Lima, A.O.; Venturelli, B.N.; Machado, I.F.; Albertin, E.; Souza, R.M. Effect of carbide orientation during single scratch test in directionally solidified and heat-treated high chromium cast irons. Wear 2023, 523, 204823. [Google Scholar] [CrossRef]
- Mangam, V.; Bhattacharya, S.; Das, K.; Das, S. Friction and wear behavior of Cu-CeO2 nanocomposite coatings synthesized by pulsed electrodeposition. Surf. Coat. Technol. 2010, 205, 801–805. [Google Scholar] [CrossRef]
- Shi, Z.; Meng, Y. Effects of indentation depth and grain size on scratching behavior of nanograin FCC Fe polycrystalline substrate. Tribol. Int. 2024, 193, 109464. [Google Scholar] [CrossRef]
- Holmberg, K.; Matthews, A.; Ronkainen, H. Coatings tribology: Properties, techniques and applications in surface engineering. Tribol. Int. 1998, 31, 107–120. [Google Scholar] [CrossRef]
- Roy, M.; Singh, S.K.; Das, S. Influence of hardness and toughness on wear resistance of materials. Tribol. Int. 2018, 119, 10–20. [Google Scholar]
- Zum Gahr, K.H. Microstructure and Wear of Materials; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Chen, J.; Shen, Y.; Liu, W.; Beake, B.D.; Shi, X.; Wang, Z.; Zhang, Y.; Guo, X. Effects of loading rate on development of pile-up during indentation creep of polycrystalline copper. Mater. Sci. Eng. A 2016, 656, 216–221. [Google Scholar] [CrossRef]
- Setti, D.; Kirsch, B.; Aurich, J.C. Experimental investigations and kinematic simulation of single grit scratched surfaces considering pile-up behaviour: Grinding perspective. Int. J. Adv. Manuf. Technol. 2019, 103, 471–485. [Google Scholar] [CrossRef]
- Franco, L.A.; Sinatora, A. Material removal factor (f ab): A critical assessment of its role in theoretical and practical approaches to abrasive wear of ductile materials. Wear 2017, 382–383, 51–61. [Google Scholar] [CrossRef]
- Hokkirigawa, K.; Kato, K. An experimental and theoretical investigation of ploughing, cutting and wedge formation during abrasive wear. Tribol. Int. 1988, 21, 51–57. [Google Scholar] [CrossRef]
- Boidi, G.; Fukumasu, N.K.; Machado, I.F. Wear and friction performance under lubricated reciprocating tests of steel powder mixtures sintered by Spark Plasma Sintering. Tribol. Int. 2018, 121, 139–147. [Google Scholar] [CrossRef]
- Fukumasu, N.K.; Boidi, G.; Seriacopi, V.; Machado, G.A.A.; Souza, R.M.; Machado, I.F. Numerical analyses of stress induced damage during a reciprocating lubricated test of fecmo SPS sintered alloy. Tribol. Int. 2017, 113, 443–447. [Google Scholar] [CrossRef]
- Meléndez-Ceballos, A.; Albin, V.; Fernández-Valverde, S.M.; Ringuedé, A.; Cassir, M. Electrochemical Properties of Atomic Layer Deposition Processed CeO2 as a Protective Layer for the Molten Carbonate Fuel Cell Cathode. Electrochim. Acta 2014, 140, 174–181. [Google Scholar] [CrossRef]
- Fang, Y.; Shu, X.; Dong, S. High Temperature Oxidation Behavior of Nano-Alumina–Modified NiAl Coating. Front. Mater. 2022, 9, 934215. [Google Scholar] [CrossRef]
- Appleby, A.J.; Nicholson, S.B. Reduction of oxygen in lithium-potassium carbonate melt. J. Electroanal. Chem. Interfacial Electrochem. 1980, 112, 71–76. [Google Scholar] [CrossRef]
- Nishina, T.; Masuda, Y.; Uchida, I.; Saboungi, M.-L.; Kojima, H. Química y Tecnología de Sales Fundidas; Elec-trochemical Society Proceeding Series; Saboungi, M.-L., Kojima, H., Eds.; Electrochemical Society: Pennington, NJ, USA, 1993; p. 424. [Google Scholar]
- Valverde, B.; Sancy, M.; Martínez, C.; Botello, E.; Pío, E.; Aguilar, C. Effect of Sn Content on the Passivity of Ti-Ta-Sn Alloys. Corros. Sci. 2023, 211, 110887. [Google Scholar] [CrossRef]
- Pineda, F.; Walczak, M.; Vilchez, F.; Guerra, C.; Escobar, R.; Sancy, M. Evolution of Corrosion Products on ASTM A36 and AISI 304L Steels Formed in Exposure to Molten NaNO3-KNO3 Eutectic Salt: Electrochemical Study. Corros. Sci. 2022, 196, 110047. [Google Scholar] [CrossRef]
- Martínez, C.; Guerra, C.; Silva, D.; Cubillos, M.; Briones, F.; Muñoz, L.; Páez, M.A.; Aguilar, C.; Sancy, M. Effect of Porosity on Mechanical and Electrochemical Properties of Ti-6Al-4V Alloy. Electrochim. Acta 2020, 338, 135858. [Google Scholar] [CrossRef]
- Lee, C.-G. Influence of temperature on the anode reaction in a molten carbonate fuel cell. J. Electroanal. Chem. 2017, 785, 152–158. [Google Scholar] [CrossRef]
- Lee, C.-G. Analysis of impedance in a molten carbonate fuel cell. J. Electroanal. Chem. 2016, 776, 162–169. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Constant-Phase Elements. In Electrochemical Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2017; pp. 395–419. ISBN 9781118527399. [Google Scholar]
- Tribollet, B.; Vivier, V.; Orazem, M.E. EIS Technique in Passivity Studies: Determination of the Dielectric Properties of Passive Films; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128098943. [Google Scholar]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-Phase-Element Behavior Caused by Resistivity Distributions in Films: II. Applications. J. Electrochem. Soc. 2010, 157, C458. [Google Scholar] [CrossRef]
- Turchanin, M.A.; Agraval, P.G.; Abdulov, A.R. Phase equilibria and thermodynamics of binary copper systems with 3d-metals. VI. Copper-nickel system. Powder Metall. Met. Ceram. 2007, 46, 467–477. [Google Scholar] [CrossRef]
- Patel, K.B.; Mariyaselvakumar, M.; Vyas, G.; Chaudhari, J.C.; Patidar, R.; Srinivasan, K.; Srivastava, D.N.; Bhadu, G.R. Nickel oxide doped ceria nanoparticles (NiO@CeO2) for boosting oxygen evolution reaction and enhancing stability. Appl. Surf. Sci. 2024, 649, 159212. [Google Scholar] [CrossRef]
- He, D.; Wang, G.; Liu, G.; Bai, J.; Suo, H.; Zhao, C. Facile route to achieve mesoporous Cu(OH)2 nanorods on copper foam for high-performance supercapacitor electrode. J. Alloys Compd. 2017, 699, 706–712. [Google Scholar] [CrossRef]
- Tang, J.; Han, Z.; Zuo, Y.; Tang, Y. A corrosion resistant cerium oxide based coating on aluminum alloy 2024 prepared by brush plating. Appl. Surf. Sci. 2010, 257, 2806–2812. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, W.; Li, N.; Liu, X.; Han, L.; Dai, W. Surface Characterization and Corrosion Behavior of 90/10 Copper-Nickel Alloy in Marine Environment. Materials 2019, 12, 1869. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Yamada, Y.; Kobayashi, T.; Loridant, S.; Volta, J.-C. Surface Characterization of CeO2/SiO2 and V2O5/CeO2/SiO2 Catalysts by Raman, XPS, and Other Techniques. J. Phys. Chem. B 2003, 106, 1520–6106. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; He, H.; Zhang, Y.; Qin, X.; Wang, B. Oxygen vacancy clusters essential for the catalytic activity of CeO2 nanocubes for o-xylene oxidation. Sci. Rep. 2017, 7, 12845. [Google Scholar] [CrossRef]
- Kumar, M.; Yun, J.H.; Bhatt, V.; Singh, B.; Kim, J.; Kim, J.S.; Kim, B.S.; Lee, C.Y. Role of Ce3+ valence state and surface oxygen vacancies on enhanced electrochemical performance of single step solvothermally synthesized CeO2 nanoparticles. Electro-Chim. Acta 2018, 284, 709–720. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Y.; Hou, F.; Wu, C.; Pan, L.; Zou, J.; Wang, L.; Zhang, X.; Liu, G.; Li, G. Engineering oxygen vacancies and nickel dispersion on CeO2 by Pr doping for highly stable ethanol steam reforming. Appl. Catal. B 2019, 258, 117940. [Google Scholar] [CrossRef]
- Brisse, A.-L.; Stevens, P.; Toussaint, G.; Crosnier, O.; Brousse, T. Ni(OH)2 and NiO Based Composites: Battery Type Electrode Materials for Hybrid Supercapacitor Devices. Materials 2018, 11, 1178. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G. Nickel Hydroxide Powder for NiO·OH/Ni(OH)2 Electrodes of the Alkaline Batteries. J. Power Sources 2001, 93, 163–173. [Google Scholar] [CrossRef]
- Cheng, T.-L.; Lei, Y.; Chen, Y.; Fan, Y.; Abernathy, H.; Song, X.; Wen, Y.-H. Oxidation of Nickel in Solid Oxide Cells during Electrochemical Operation: Experimental Evidence, Theoretical Analysis, and an Alternative Hypothesis on the Nickel Migration. J. Power Sources 2023, 569, 232991. [Google Scholar] [CrossRef]

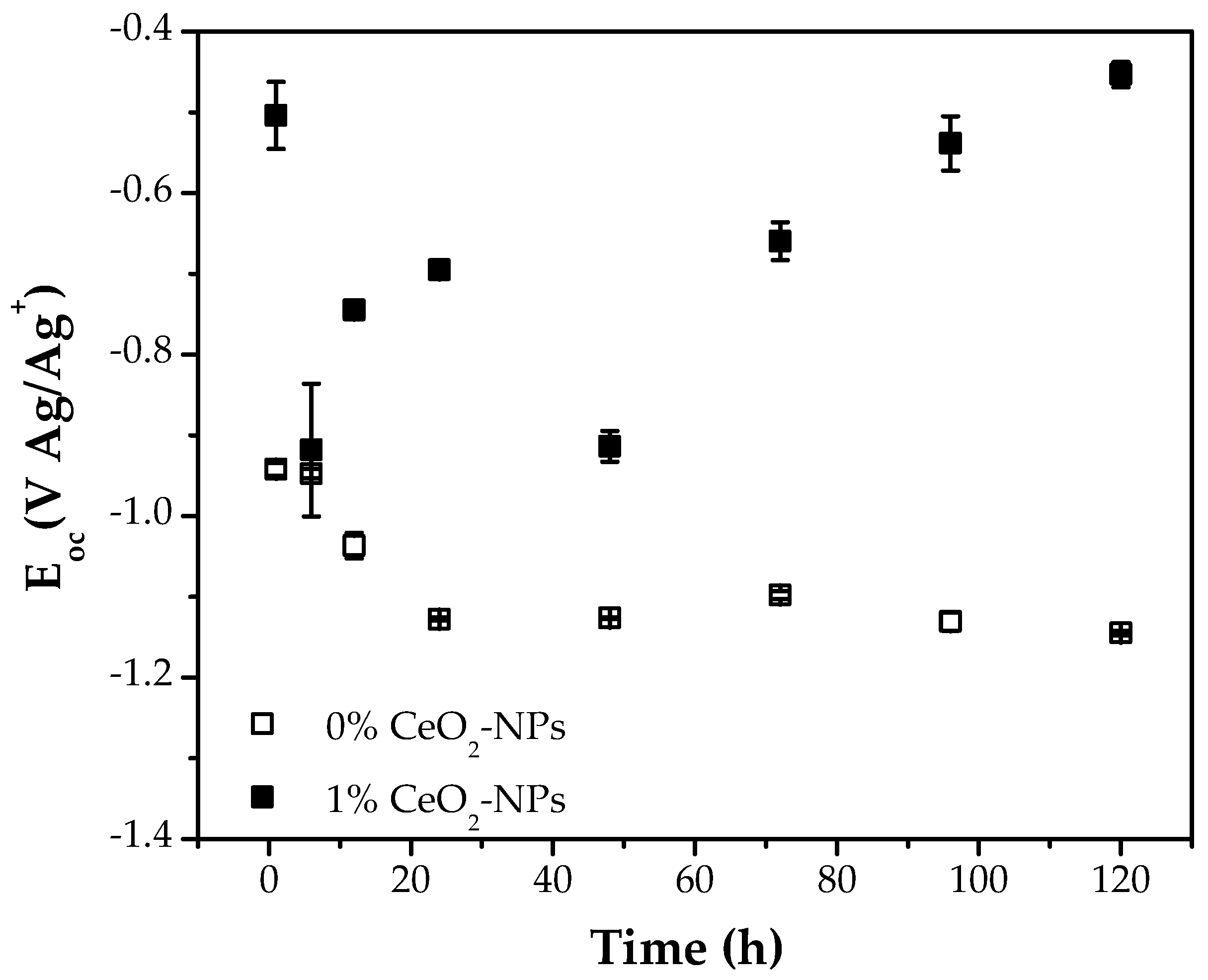
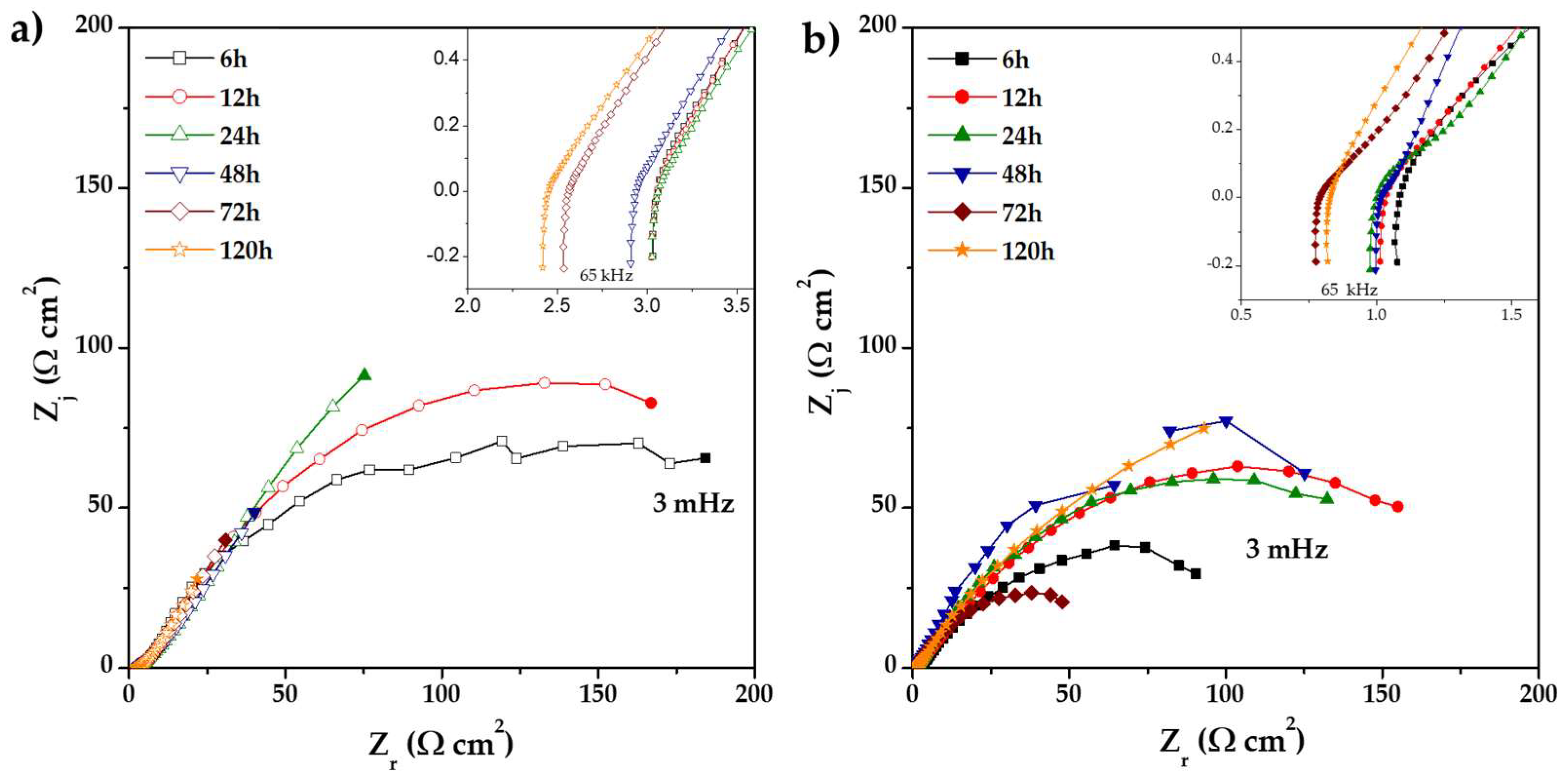
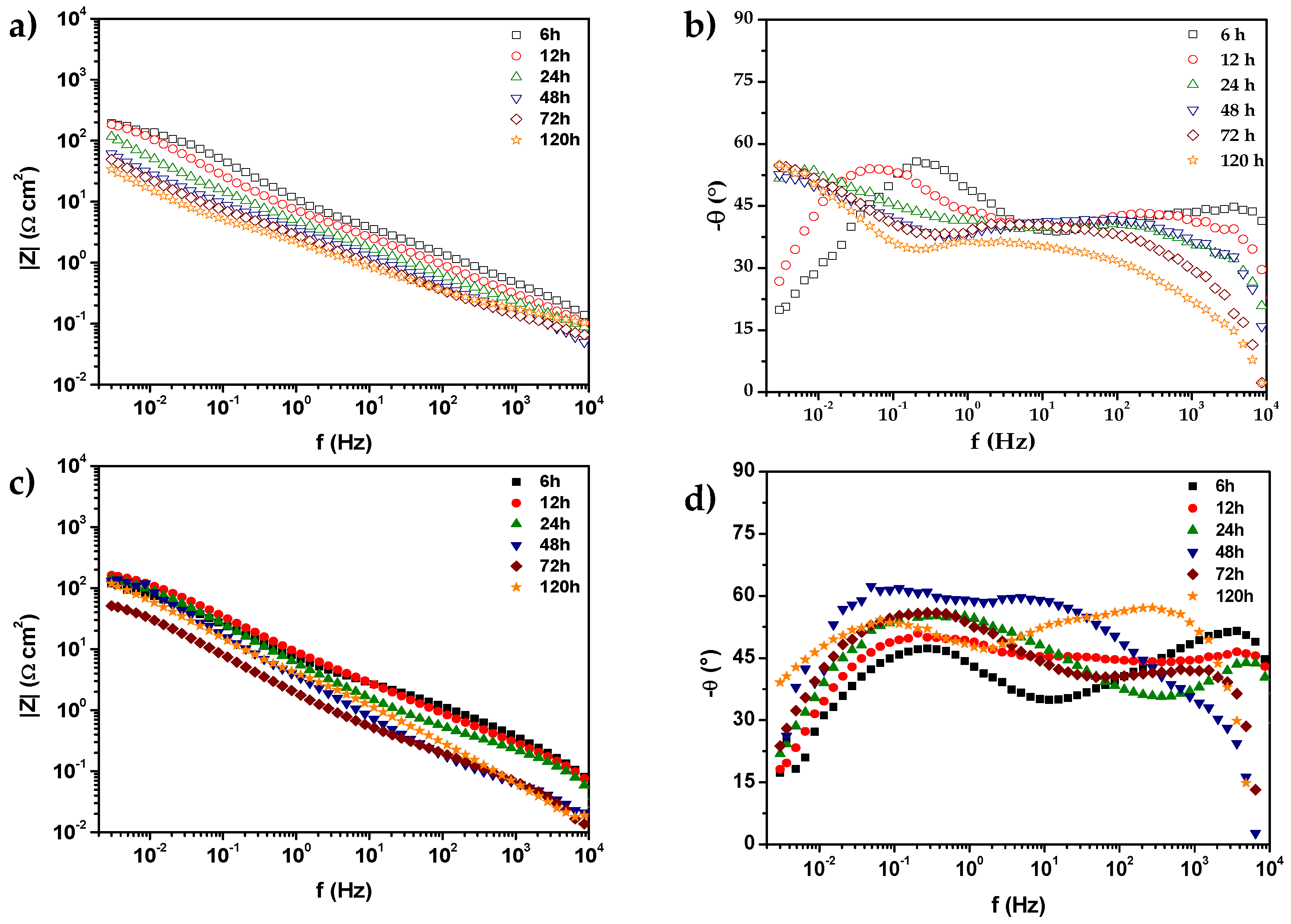
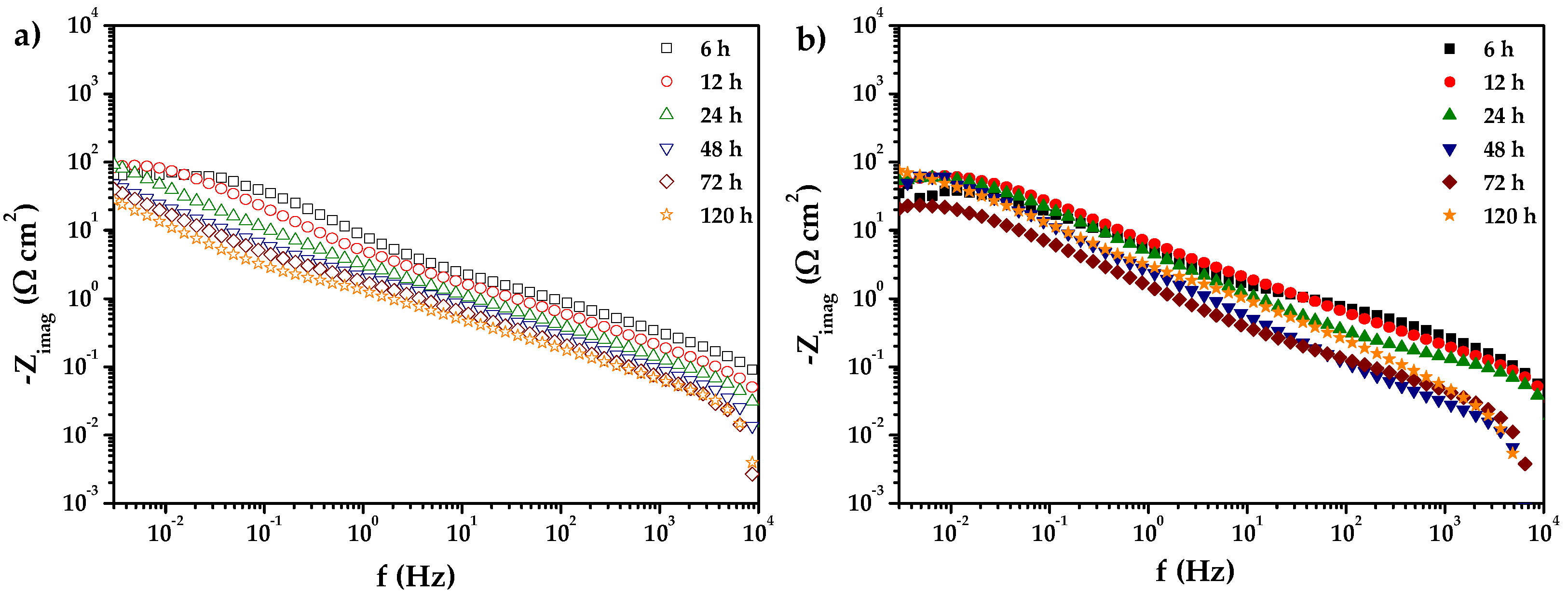
 ) 0% CeO2–NPs, (
) 0% CeO2–NPs, ( ) 1% CeO2–NPs) experimental, and (—) fitting adjusting of impedance data after 120 h of exposure in Li2CO3-K2CO3 at 550 °C under an H2/N2 atmosphere.
) 1% CeO2–NPs) experimental, and (—) fitting adjusting of impedance data after 120 h of exposure in Li2CO3-K2CO3 at 550 °C under an H2/N2 atmosphere.
 ) 0% CeO2–NPs, (
) 0% CeO2–NPs, ( ) 1% CeO2–NPs) experimental, and (—) fitting adjusting of impedance data after 120 h of exposure in Li2CO3-K2CO3 at 550 °C under an H2/N2 atmosphere.
) 1% CeO2–NPs) experimental, and (—) fitting adjusting of impedance data after 120 h of exposure in Li2CO3-K2CO3 at 550 °C under an H2/N2 atmosphere.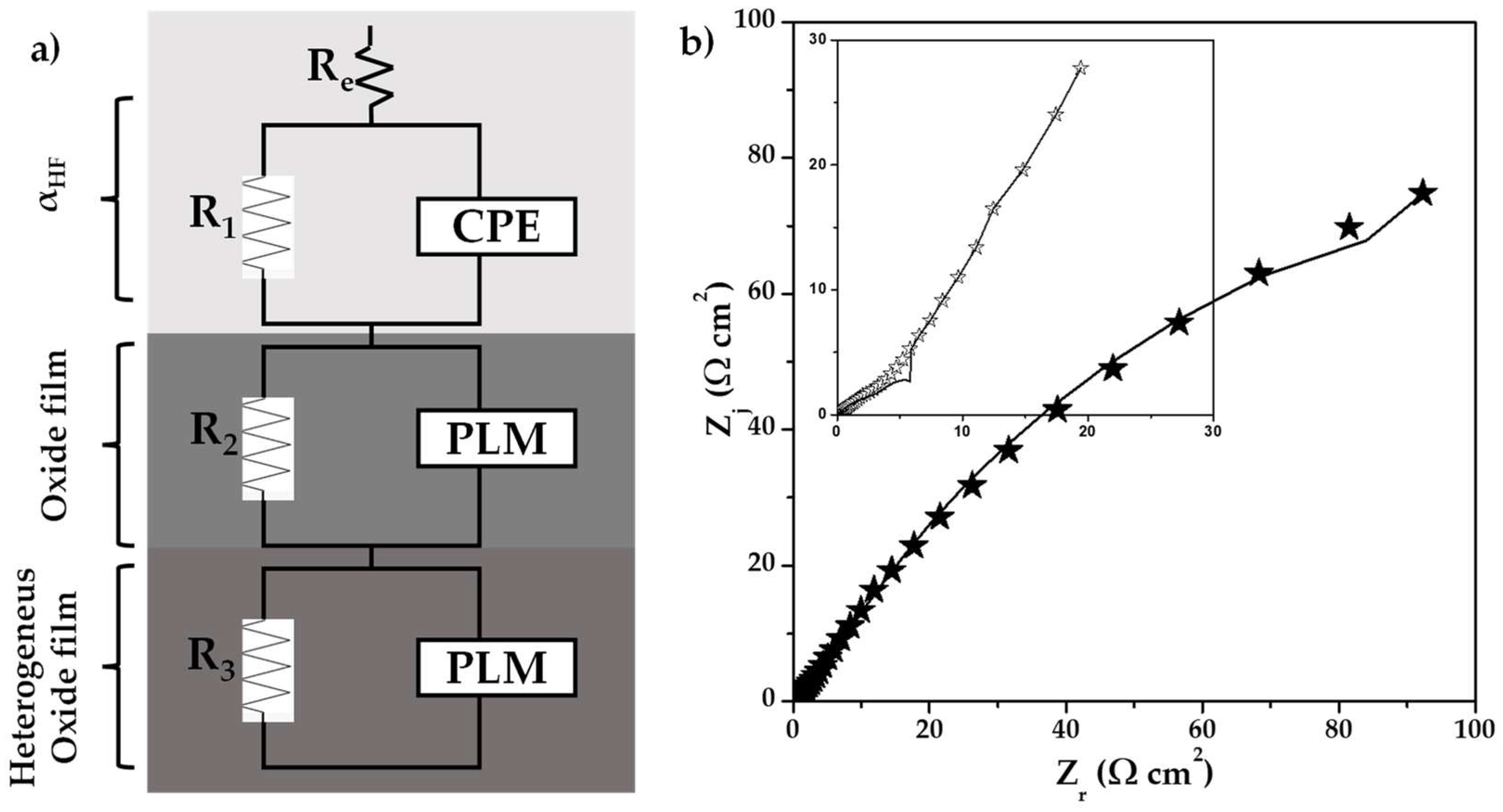


| Sample | Load (N) | Depth (μm) | Width (μm) | A1+A2 (μm2) | A3 (μm2) | Pile-Up Ratio |
|---|---|---|---|---|---|---|
| 0% CeO2-NPs | 7 | 0.7 ± 0.10 | 31.6 | 9.7 | 13.9 | 0.70 |
| 23 | 4.9 ± 0.06 | 84.3 | 111.5 | 277.0 | 0.42 | |
| 33 | 10.5 ± 0.02 | 124.7 | 45.3 | 842.0 | 0.05 | |
| 1% CeO2-NPs | 7 | 1.5 ± 0.12 | 50.8 | 7.5 | 50.9 | 0.15 |
| 23 | 5.0 ± 0.04 | 87.7 | 53.23 | 280.3 | 0.18 | |
| 33 | 8.9 ± 0.03 | 116.2 | 49.5 | 673.8 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, C.; Valverde, B.; Del Valle-Rodríguez, A.; Bustos-De La Fuente, B.; Machado, I.F.; Briones, F. Analysis of the Wear and Corrosion Resistance on Cu-Ni-Al Composites Reinforced with CeO2 Nanoparticles. Materials 2025, 18, 2438. https://doi.org/10.3390/ma18112438
Martínez C, Valverde B, Del Valle-Rodríguez A, Bustos-De La Fuente B, Machado IF, Briones F. Analysis of the Wear and Corrosion Resistance on Cu-Ni-Al Composites Reinforced with CeO2 Nanoparticles. Materials. 2025; 18(11):2438. https://doi.org/10.3390/ma18112438
Chicago/Turabian StyleMartínez, Carola, Bárbara Valverde, Aurora Del Valle-Rodríguez, Brennie Bustos-De La Fuente, Izabel Fernanda Machado, and Francisco Briones. 2025. "Analysis of the Wear and Corrosion Resistance on Cu-Ni-Al Composites Reinforced with CeO2 Nanoparticles" Materials 18, no. 11: 2438. https://doi.org/10.3390/ma18112438
APA StyleMartínez, C., Valverde, B., Del Valle-Rodríguez, A., Bustos-De La Fuente, B., Machado, I. F., & Briones, F. (2025). Analysis of the Wear and Corrosion Resistance on Cu-Ni-Al Composites Reinforced with CeO2 Nanoparticles. Materials, 18(11), 2438. https://doi.org/10.3390/ma18112438







