Studies of Raman-Scattered Technology on S-Shaped Dinaphtho[2,1-b:2′,1′-f]thieno[3,2-b]thiophene-10 (S-DNTT-10)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of S-DNTT-10 Thin Films
2.2. Fabrication of Gold (Au) Nanostructures
2.3. Computational and Test Details
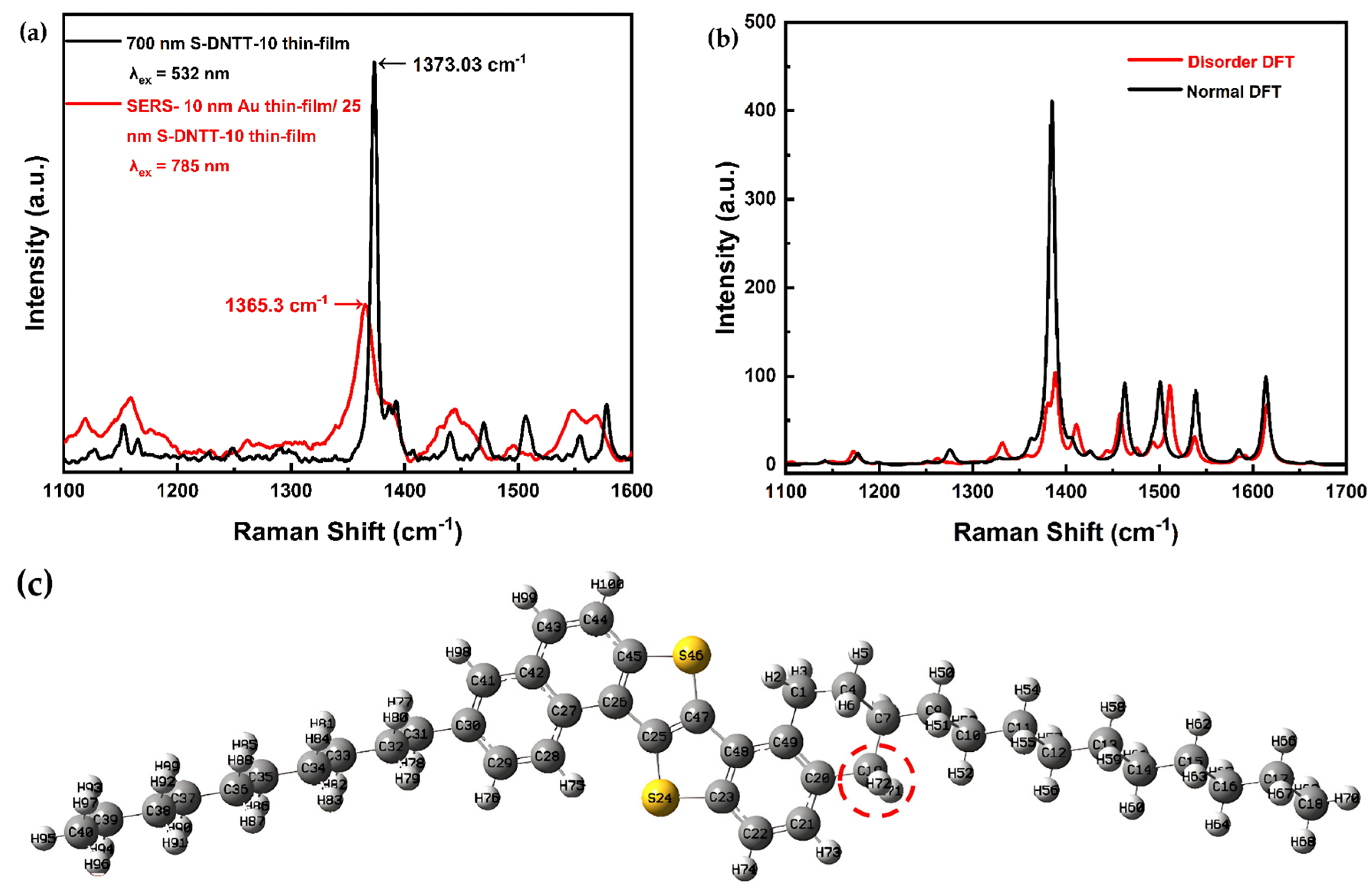
3. Results and Discussion
3.1. Theoretical Calculation and Measured Raman Spectra
3.2. The SERS Effect on S-DNTT-10 Thin Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Lee, Y.H.; Jang, M.; Lee, M.Y.; Kweon, O.Y.; Oh, J.H. Flexible Field-Effect Transistor-Type Sensors Based on Conjugated Molecules. Chem 2017, 3, 724–763. [Google Scholar] [CrossRef]
- Torikai, K.; Furlan de Oliveira, R.; Starnini de Camargo, D.H.; Bof Bufon, C.C. Low-Voltage, Flexible, and Self-Encapsulated Ultracompact Organic Thin-Film Transistors Based on Nanomembranes. Nano Lett. 2018, 18, 5552–5561. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T. Next-Generation Organic Semiconductors Driven by Bent-Shaped π-Electron Cores. Polym. J. 2019, 51, 825–833. [Google Scholar] [CrossRef]
- Waldrip, M.; Jurchescu, O.D.; Gundlach, D.J.; Bittle, E.G. Contact Resistance in Organic Field-Effect Transistors: Conquering the Barrier. Adv. Funct. Mater. 2020, 30, 1904576. [Google Scholar] [CrossRef]
- Kim, C.-H. Contact Resistance in Organic Transistors: Use It or Remove It. Appl. Phys. Rev. 2020, 7, 031306. [Google Scholar] [CrossRef]
- Romero, A.; Jiménez-Tejada, J.A.; González, J.; Deen, M.J. Unified Electrical Model for the Contact Regions of Staggered Thin Film Transistors. Org. Electron. 2021, 92, 106129. [Google Scholar] [CrossRef]
- Ma, Y.-X.; Lai, P.-T.; Tang, W.-M. Low-Temperature-Processed High-Performance Pentacene OTFTs with Optimal Nd-Ti Oxynitride Mixture as Gate Dielectric. Materials 2022, 15, 2255. [Google Scholar] [CrossRef]
- Borthakur, T.; Sarma, R. Performance Enhancement of Top Contact Pentacene-Based Organic Thin-Film Transistor (OTFT) Using Perylene Interlayer between Organic/Electrode Interface. Appl. Phys. A 2017, 123, 207. [Google Scholar] [CrossRef]
- Lamport, Z.A.; Barth, K.J.; Lee, H.; Gann, E.; Engmann, S.; Chen, H.; Guthold, M.; McCulloch, I.; Anthony, J.E.; Richter, L.J.; et al. A Simple and Robust Approach to Reducing Contact Resistance in Organic Transistors. Nat. Commun. 2018, 9, 5130. [Google Scholar] [CrossRef]
- Ana, F.; Din, N. Design and Performance Investigation of Short Channel Bottom-Contact Organic Thin-Film Transistors. J. Comput. Electron. 2018, 17, 1315–1323. [Google Scholar] [CrossRef]
- Simonetti, O.; Giraudet, L. Transport Models in Disordered Organic Semiconductors and Their Application to the Simulation of Thin-film Transistors. Polym. Int. 2019, 68, 620–636. [Google Scholar] [CrossRef]
- Borchert, J.W.; Weitz, R.T.; Ludwigs, S.; Klauk, H. A Critical Outlook for the Pursuit of Lower Contact Resistance in Organic Transistors. Adv. Mater. 2022, 34, 2104075. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Wu, Y.-E.; Kuo, C.-W.; Chang, T.-C.; Chen, L.-Y.; Chen, F.-C.; Kuo, H.-C. Exploring Light Stability and Trapping Mechanisms in Organic Thin-Film Transistors for High-Brightness MicroLED Integration. Materials 2024, 17, 5643. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, Y.; Jang, Y.; Yang, H.; Kim, Y.; Han, J.; Moon, D.; Park, S.; Chang, T.; Chang, C. Enhancement of Field-Effect Mobility Due to Surface-Mediated Molecular Ordering in Regioregular Polythiophene Thin Film Transistors. Adv. Funct. Mater. 2005, 15, 77–82. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, S.; Qiu, L.; Qian, J.; Ono, L.K.; Leyden, M.R.; Wang, X.; Shi, Y.; Zheng, Y.; Qi, Y.; et al. Interfacial Flat-Lying Molecular Monolayers for Performance Enhancement in Organic Field-Effect Transistors. Acs Appl. Mater. Interfaces 2018, 10, 22513–22519. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, K.; Okudaira, K.K. Effect of Chemical Interaction at Modification Layer/Substrate Interface on Molecular Orientation of Dinaphtho[2,3-b:2′,3′-f]Thieno[3,2-b]Thiophene Thin Films. Jpn. J. Appl. Phys. 2020, 59, 091004. [Google Scholar] [CrossRef]
- Bhardwaj, B.; Sugiyama, T.; Namba, N.; Umakoshi, T.; Uemura, T.; Sekitani, T.; Verma, P. Raman Spectroscopic Studies of Dinaphthothienothiophene (DNTT). Materials 2019, 12, 615. [Google Scholar] [CrossRef]
- Katz, H.E.; Bao, Z.; Gilat, S.L. Synthetic Chemistry for Ultrapure, Processable, and High-Mobility Organic Transistor Semiconductors. Acc. Chem. Res. 2001, 34, 359–369. [Google Scholar] [CrossRef]
- Anthony, J.E. Functionalized Acene & Heteroacene for Organic Electronics. Chem. Rev. 2006, 106, 5028–5048. [Google Scholar] [CrossRef]
- Benor, A.; Hoppe, A.; Wagner, V.; Knipp, D. Electrical Stability of Pentacene Thin Film Transistors. Org. Electron. 2007, 8, 749–758. [Google Scholar] [CrossRef]
- Facchetti, A. Semiconductors for Organic Transistors. Mater. Today 2007, 10, 28–37. [Google Scholar] [CrossRef]
- Scholz, R.; Lehmann, D.; Müller, A.-D.; Müller, F.; Zahn, D.R.T. Potentiometry on Pentacene OFETs: Charge Carrier Mobilities and Injection Barriers in Bottom and Top Contact Configurations. Phys. Status Solidi 2008, 205, 591–599. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takimiya, K. Facile Synthesis of Highly Pi-Extended Heteroarenes, Dinaphtho[2,3-B:2′,3′-f]Chalcogenopheno[3,2-b]Chalcogenophenes, and Their Application to Field-Effect Transistors. J. Am. Chem. Soc. 2007, 129, 2224–2225. [Google Scholar] [CrossRef] [PubMed]
- Zschieschang, U.; Ante, F.; Kälblein, D.; Yamamoto, T.; Takimiya, K.; Kuwabara, H.; Ikeda, M.; Sekitani, T.; Someya, T.; Nimoth, J.B.; et al. Dinaphtho[2,3-b:2′,3′-f]Thieno[3,2-b]Thiophene (DNTT) Thin-Film Transistors with Improved Performance and Stability. Org. Electron. 2011, 12, 1370–1375. [Google Scholar] [CrossRef]
- Kraft, U.; Takimiya, K.; Kang, M.J.; Rödel, R.; Letzkus, F.; Burghartz, J.N.; Weber, E.; Klauk, H. Detailed Analysis and Contact Properties of Low-Voltage Organic Thin-Film Transistors Based on Dinaphtho[2,3-b:2′,3′-f]Thieno[3,2-b]Thiophene (DNTT) and Its Didecyl and Diphenyl Derivatives. Org. Electron. 2016, 35, 33–40. [Google Scholar] [CrossRef]
- Za’aba, N.K.; Taylor, D.M. Bias and Related Stress Effects in Organic Thin Film Transistors Based on Dinaphtho [2,3-b:2′,3′-f] Thieno[3,2-b] Thiophene (DNTT). Org. Electron. 2018, 62, 382–393. [Google Scholar] [CrossRef]
- Acharya, R.; Günder, D.; Breuer, T.; Schmitz, G.; Klauk, H.; Witte, G. Stability of Organic Thin-Film Transistors Based on Ultrathin Films of Dinaphtho[2,3-b:2′,3′-f]Thieno[3,2-b]Thiophene (DNTT). J. Mater. Chem. C 2021, 9, 270–280. [Google Scholar] [CrossRef]
- Lin, P.-P.; Qin, G.-Y.; Guo, J.-F.; Zhang, B.-H.; Li, H.-Y.; Zou, L.-Y.; Ren, A.-M. Unveiling the Effects of Substituents on the Packing Motif and the Carrier Transport of Dinaphtho-Thieno-Thiophene (DNTT)-Based Materials. New J. Chem. 2021, 45, 11552–11565. [Google Scholar] [CrossRef]
- Becharguia, H.; Mahdouani, M.; Bourguiga, R.; Branchini, P.; Fabbri, A.; De Rosa, S.; Calvi, S.; Mariucci, L.; Valletta, A.; Tortora, L. Effects of Illumination on the Electrical Characteristics in Organic Thin-Film Transistors Based on Dinaphtho [2,3-b:2′,3′-f] Thieno[3,2-b] Thiophene (DNTT): Experiment and Modeling. Synth. Met. 2022, 283, 116985. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kojiguchi, Y.; Kawata, S.; Mori, T.; Okamoto, K.; Tsutsui, M.; Koganezawa, T.; Katagiri, H.; Yasuda, T. Solution-Processable Organic Semiconductors Featuring S-Shaped Dinaphthothienothiophene (S-DNTT): Effects of Alkyl Chain Length on Self-Organization and Carrier Transport Properties. Chem. Mater. 2020, 32, 5350–5360. [Google Scholar] [CrossRef]
- Wood, S.; Hollis, J.R.; Kim, J.-S. Raman Spectroscopy as an Advanced Structural Nanoprobe for Conjugated Molecular Semiconductors. J. Phys. D Appl. Phys. 2017, 50, 073001. [Google Scholar] [CrossRef]
- Ma, H.; Pan, S.-Q.; Wang, W.-L.; Yue, X.; Xi, X.-H.; Yan, S.; Wu, D.-Y.; Wang, X.; Liu, G.; Ren, B. Surface-Enhanced Raman Spectroscopy: Current Understanding, Challenges, and Opportunities. ACS Nano 2024, 18, 14000–14019. [Google Scholar] [CrossRef]
- Cheng, H.-L.; Mai, Y.-S.; Chou, W.-Y.; Chang, L.-R.; Liang, X.-W. Thickness-Dependent Structural Evolutions and Growth Models in Relation to Carrier Transport Properties in Polycrystalline Pentacene Thin Films. Adv. Funct. Mater. 2007, 17, 3639–3649. [Google Scholar] [CrossRef]
- Jentzsch, T.; Juepner, H.J.; Brzezinka, K.-W.; Lau, A. Efficiency of Optical Second Harmonic Generation from Pentacene Films of Different Morphology and Structure. Thin Solid Films 1998, 315, 273–280. [Google Scholar] [CrossRef]
- He, R.; Dujovne, I.; Chen, L.; Miao, Q.; Hirjibehedin, C.F.; Pinczuk, A.; Nuckolls, C.; Kloc, C.; Ron, A. Resonant Raman Scattering in Nanoscale Pentacene Films. Appl. Phys. Lett. 2004, 84, 987–989. [Google Scholar] [CrossRef]
- Adil, D.; Guha, S. Surface-Enhanced Raman Spectroscopic Studies of the Au-Pentacene Interface: A Combined Experimental and Theoretical Investigation. J. Chem. Phys. 2013, 139, 044715. [Google Scholar] [CrossRef]
- Richards, T.J.; Sirringhaus, H. Analysis of the Contact Resistance in Staggered, Top-Gate Organic Field-Effect Transistors. J. Appl. Phys. 2007, 102, 094510. [Google Scholar] [CrossRef]
- Li, J.; Ou-Yang, W.; Weis, M. Electric-Field Enhanced Thermionic Emission Model for Carrier Injection Mechanism of Organic Field-Effect Transistors: Understanding of Contact Resistance. J. Phys. D Appl. Phys. 2017, 50, 035101. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, S.; Zhang, B.; Shin, E.-Y.; Noh, Y.-Y.; Xu, Y.; Shi, Y.; Li, Y. Role of Schottky Barrier and Access Resistance in Organic Field-Effect Transistors. J. Phys. Chem. Lett. 2020, 11, 1466–1472. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, Applications, and the Future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Yamamoto, Y.S.; Ozaki, Y.; Itoh, T. Recent Progress and Frontiers in the Electromagnetic Mechanism of Surface-Enhanced Raman Scattering. J. Photochem. Photobiol. C Photochem. Rev. 2014, 21, 81–104. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, C.; Shou, M.; Lin, Y.; Hou, X.; Xie, Z.; Liu, L. Introducing a Surface-Enhanced-Raman-Scattering Enhancer for Experimental Estimation of the Debye Screening Length in Organic Field-Effect Transistors. ACS Appl. Electron. Mater. 2021, 3, 1920–1930. [Google Scholar] [CrossRef]
- Adil, D.; Guha, S. Surface-Enhanced Raman Spectroscopic Studies of Metal–Semiconductor Interfaces in Organic Field-Effect Transistors. J. Phys. Chem. C 2012, 116, 12779–12785. [Google Scholar] [CrossRef]
- Basiuk, V.A.; Borys-Sosa, P.A. Interaction of Au Atom with Fullerene C60: Performance of DFT Functionals Incorporated into the DMol3 Module. J. Comput. Theor. Nanosci. 2013, 10, 328–333. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Xie, J.; Shen, Z. [Au(CN)2]—Adsorption on a Graphite (0001) Surface: A First Principles Study. Minerals 2018, 8, 425. [Google Scholar] [CrossRef]
- Womack, J.C.; Mardirossian, N.; Head-Gordon, M.; Skylaris, C.-K. Self-Consistent Implementation of Meta-GGA Functionals for the ONETEP Linear-Scaling Electronic Structure Package. J. Chem. Phys. 2016, 145, 204114. [Google Scholar] [CrossRef]
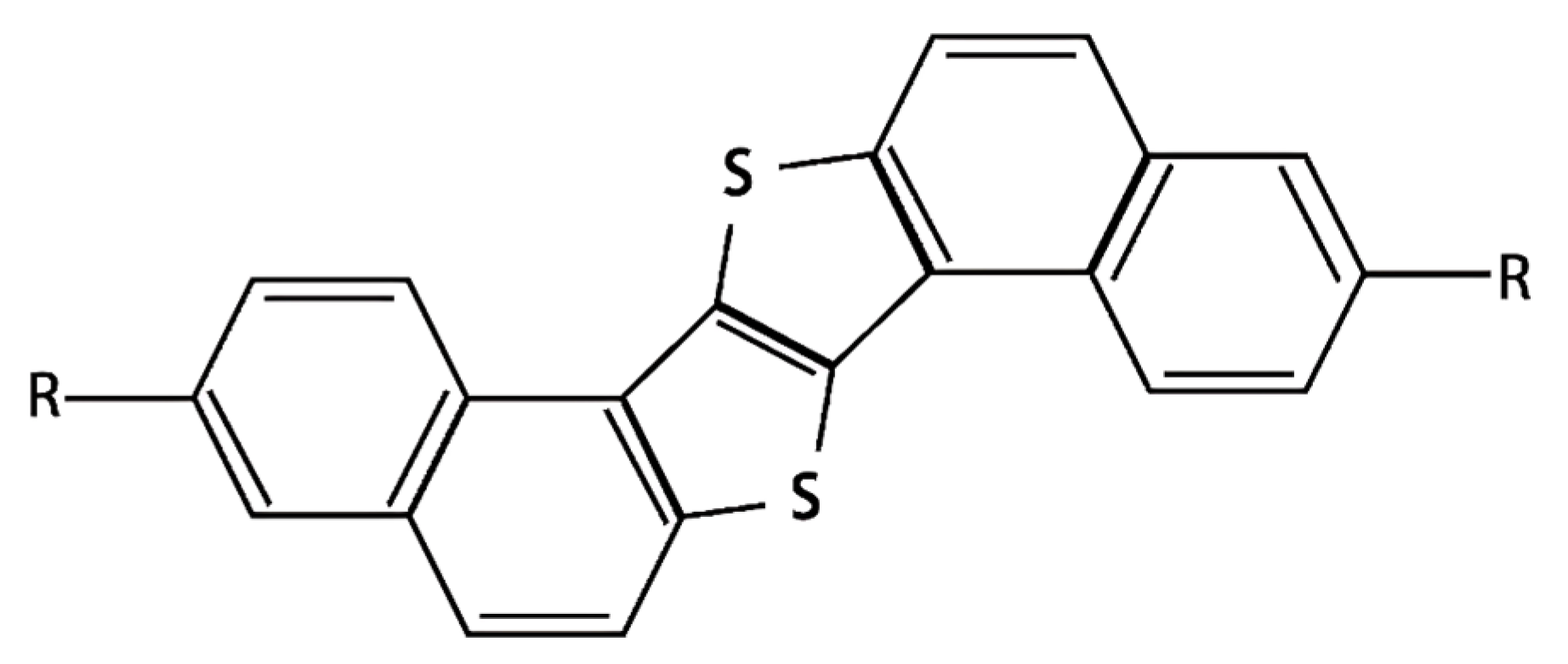

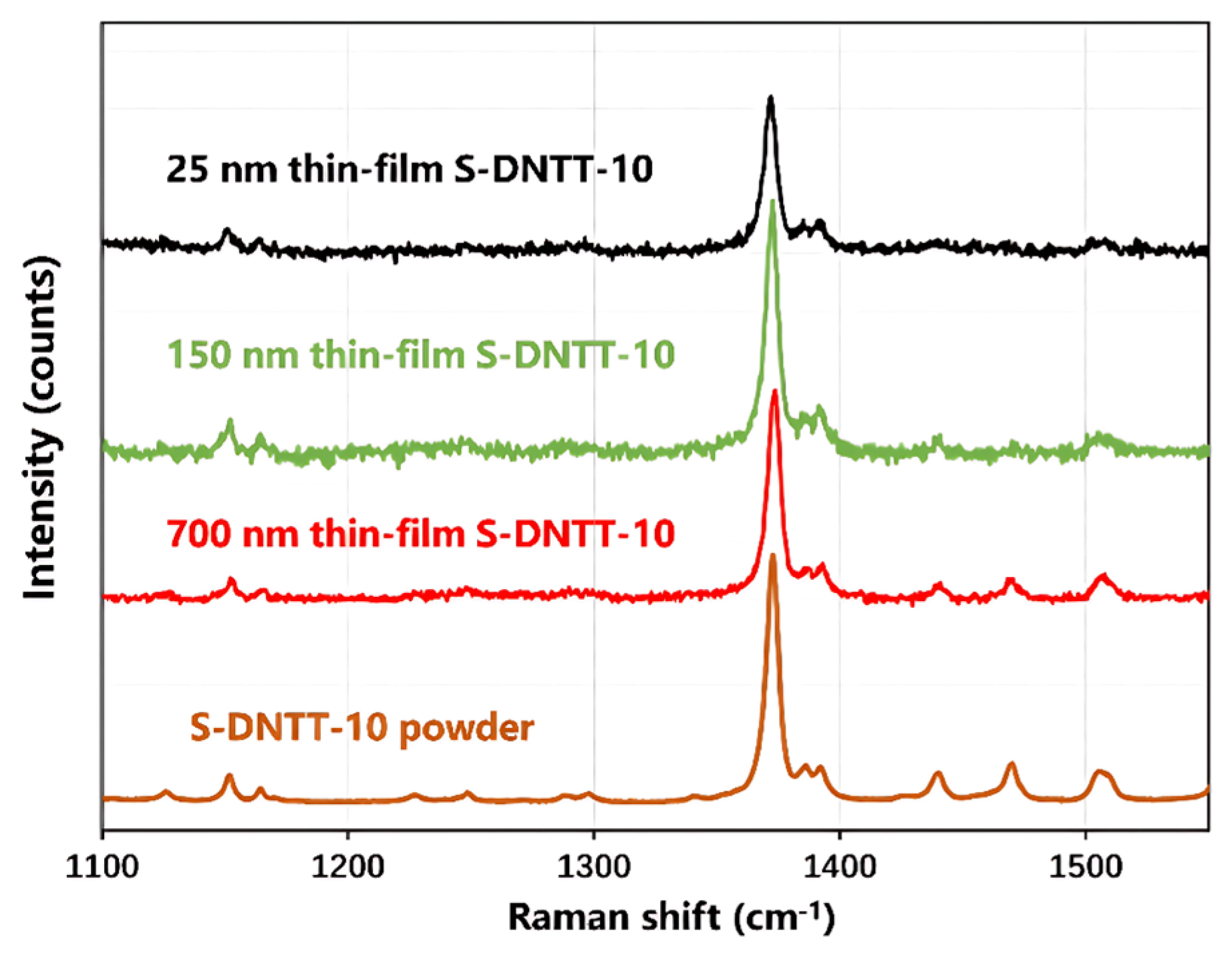

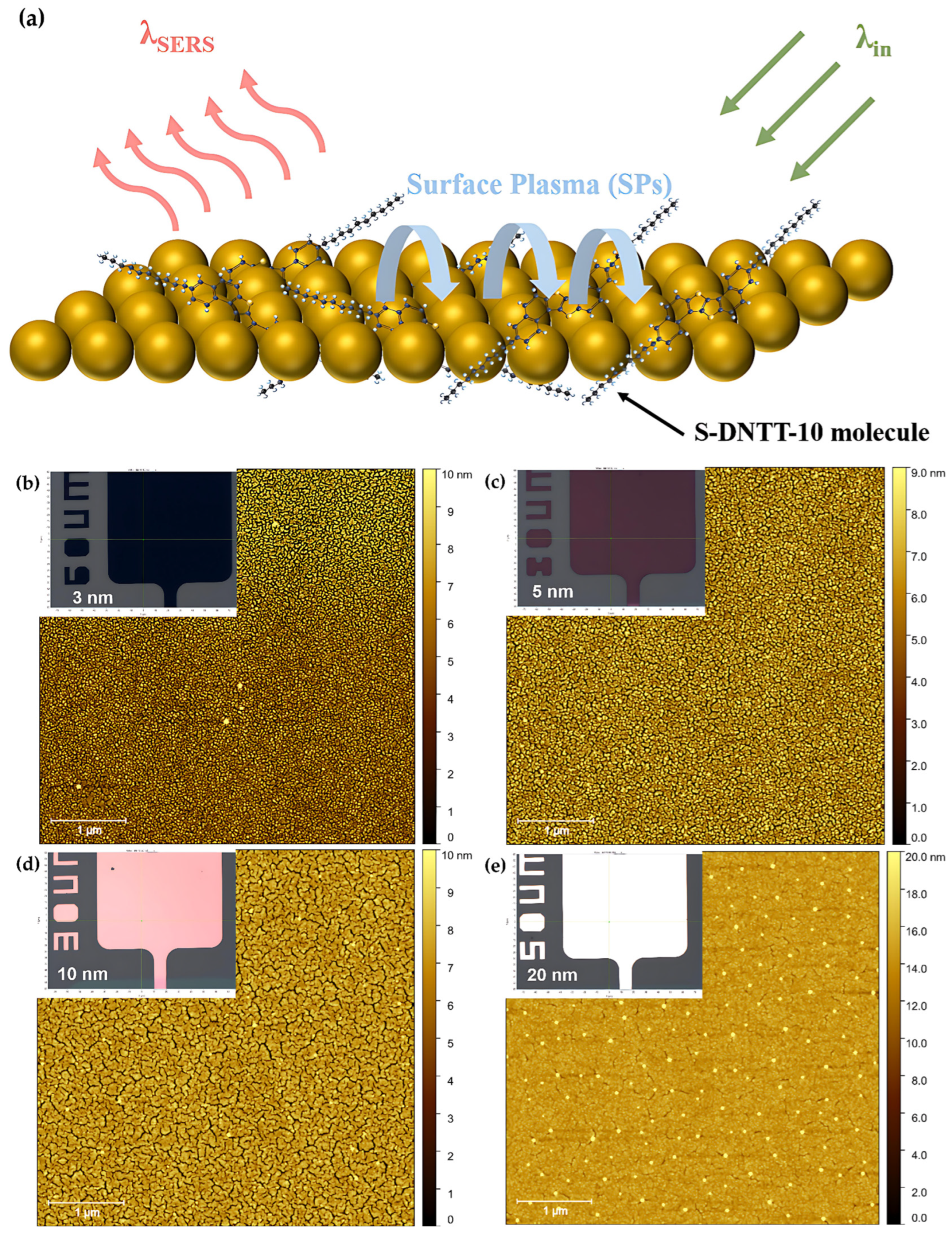
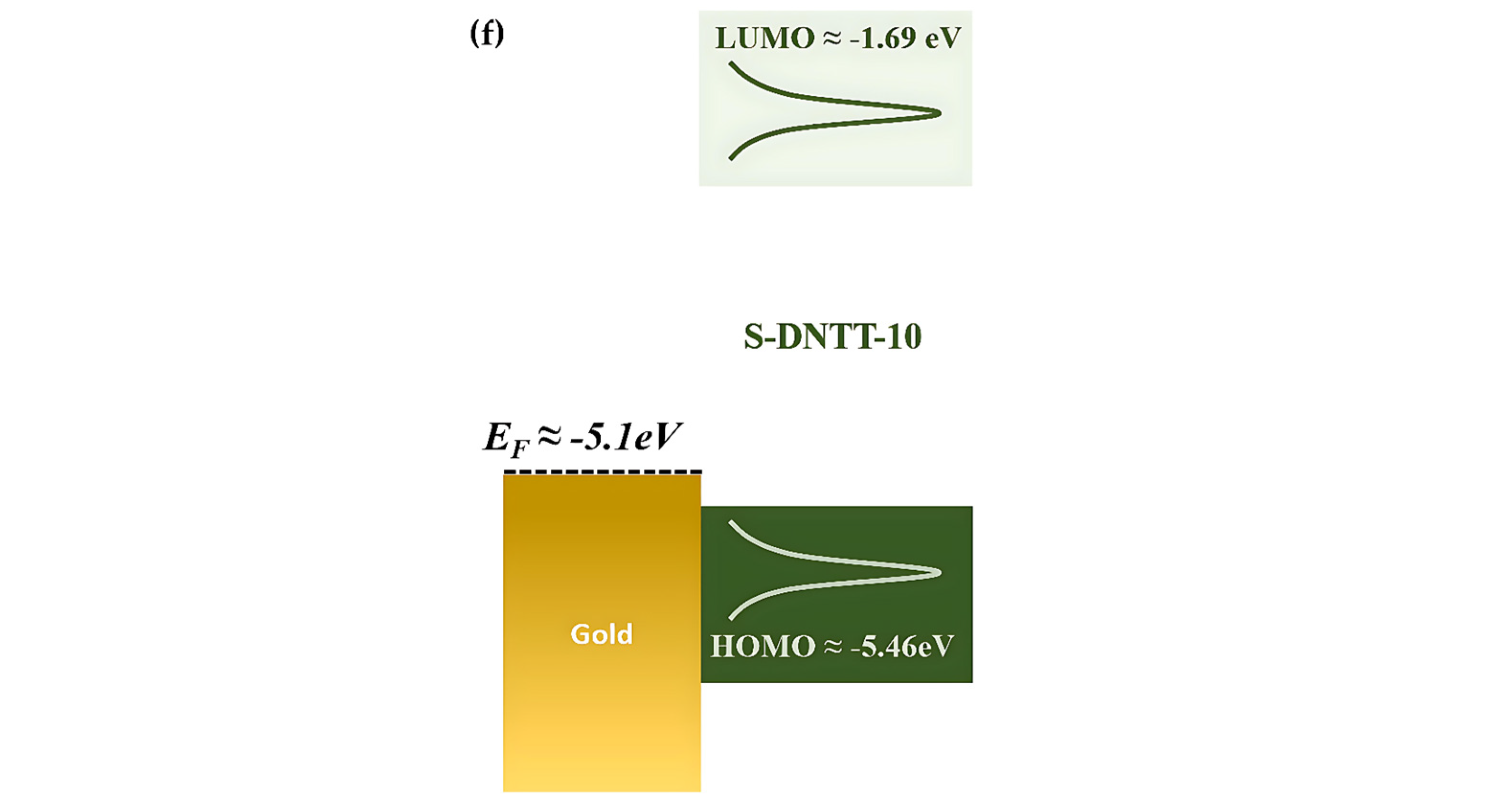

| Mode | Raman Shift/cm−1 | Vibrational Modes Descriptions |
|---|---|---|
| 1 | 1141.79 | C–H bond bending at alkyl chains |
| 2 | 1177.11 | C–H bond bending located at the aromatic rings and coupled with few slight deformations of thiophene rings |
| 3 | 1251.53 | C–H bond bending at alkyl chains; C–H bond bending coupled with slight breathing stretching aromatic rings and thiophene rings deformed |
| 4 | 1274.84 | C–H bond bending at alkyl chains; some serious C–H bond bending located at C(38), C(33), C(93), C(86), C(81) and C(45), and coupled with few slight deformation of aromatic and thiophene rings |
| 5 | 1328.95 | C–H bond anti-symmetry bending at alkyl chains coupled with C–C bond movement at aromatic and thiophene rings |
| 6 | 1362.20 | C–H bond bending at alkyl chains; deformation of thiophene rings and linked aromatic rings; seriously C–H bond bending located at C(36), C(95), C(84) and C(47); C–C bond stretched at aromatic and thiophene rings |
| 7 | 1384.79 | C–H bond slightly bending at alkyl chains; strongly C–H bond bending located at C(38), C(33), C(86) and C(81) coupled with deformation of aromatic and thiophene |
| 8 | 1406.24 | C–H bond slightly bending at alkyl chains; strongly C–H bond bending located at C(36), C(93), C(84) and C(85) and C(42)=C(90) double-bond stretching coupled with deformation of aromatic and thiophene |
| 9 | 1426.09 | slight C–H bond bending at the end of alkyl chains; strong C–H bond bending at C(36), C(33), C(95), C(86), C(81) and C(47) coupled with deformation of aromatic and thiophene rings. |
| 10 | 1462.96 | C(42)–C(43) and C(90)–C(91) strongly stretching coupled with deformation of aromatic and thiophene rings |
| 11 | 1500.85 | C–H slightly bending at the end of alkyl chains; deformation of aromatic and thiophene rings |
| 12 | 1539.00 | strongly C(42)=C(90) double-bond stretching coupled with deformation of aromatic and thiophene rings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Simonetti, O.; Et-Thakafy, O.; Bercu, N.; Etienne, F.; Potiron, S.; Adam, P.-M.; Giraudet, L. Studies of Raman-Scattered Technology on S-Shaped Dinaphtho[2,1-b:2′,1′-f]thieno[3,2-b]thiophene-10 (S-DNTT-10). Materials 2025, 18, 2389. https://doi.org/10.3390/ma18102389
Wang H, Simonetti O, Et-Thakafy O, Bercu N, Etienne F, Potiron S, Adam P-M, Giraudet L. Studies of Raman-Scattered Technology on S-Shaped Dinaphtho[2,1-b:2′,1′-f]thieno[3,2-b]thiophene-10 (S-DNTT-10). Materials. 2025; 18(10):2389. https://doi.org/10.3390/ma18102389
Chicago/Turabian StyleWang, Haobing, Olivier Simonetti, Oumaima Et-Thakafy, Nicolas Bercu, Florence Etienne, Sylvain Potiron, Pierre-Michel Adam, and Louis Giraudet. 2025. "Studies of Raman-Scattered Technology on S-Shaped Dinaphtho[2,1-b:2′,1′-f]thieno[3,2-b]thiophene-10 (S-DNTT-10)" Materials 18, no. 10: 2389. https://doi.org/10.3390/ma18102389
APA StyleWang, H., Simonetti, O., Et-Thakafy, O., Bercu, N., Etienne, F., Potiron, S., Adam, P.-M., & Giraudet, L. (2025). Studies of Raman-Scattered Technology on S-Shaped Dinaphtho[2,1-b:2′,1′-f]thieno[3,2-b]thiophene-10 (S-DNTT-10). Materials, 18(10), 2389. https://doi.org/10.3390/ma18102389





