Studying the Sintering Behavior of H2-Reduced Bauxite Residue Pellets Using High-Temperature Thermal Analysis
Abstract
1. Introduction
1.1. Bayer Process and Bauxite Residue
1.2. Reduction of Bauxite Residue
2. Materials and Methods
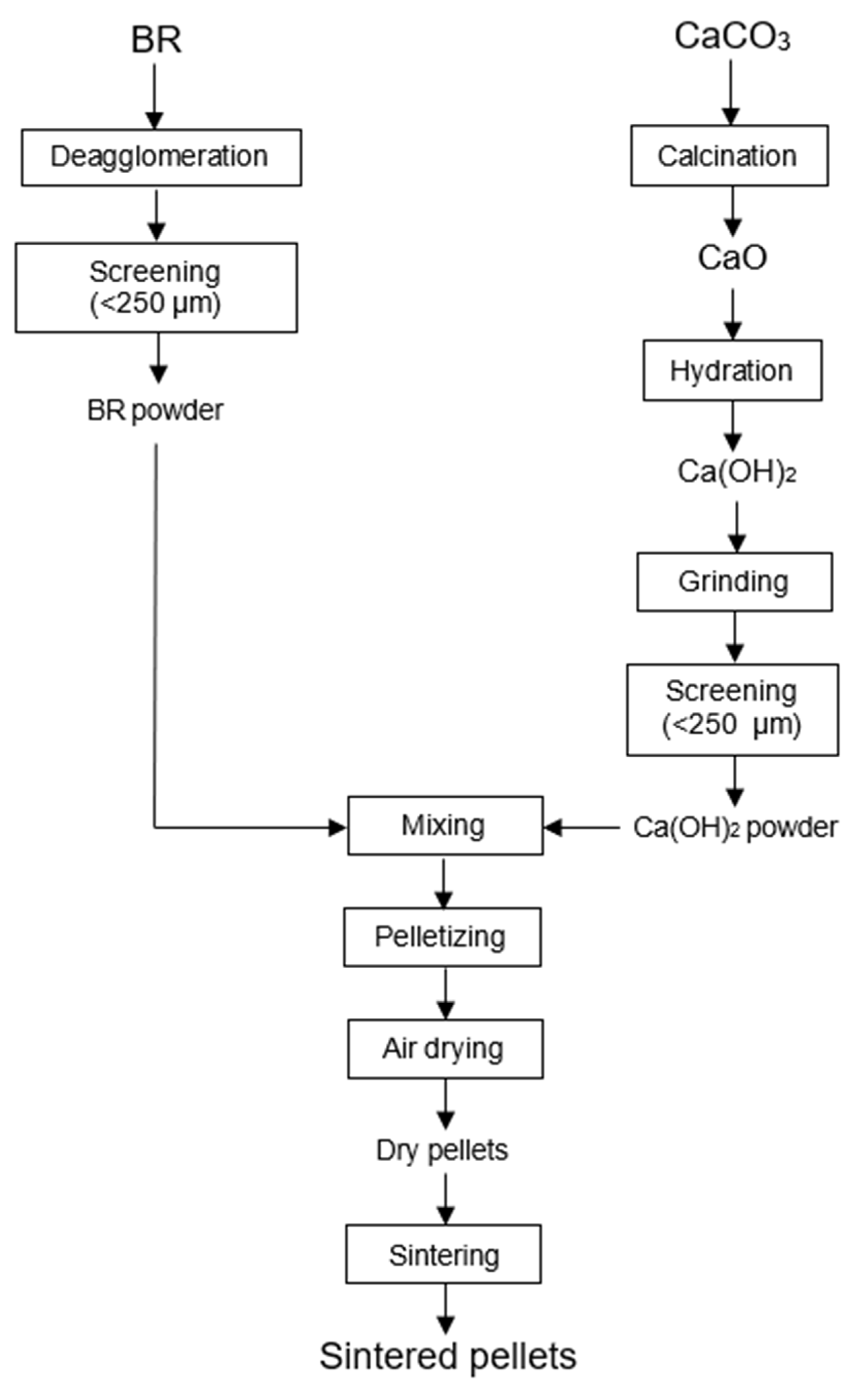
2.1. Pelletizing and Sintering
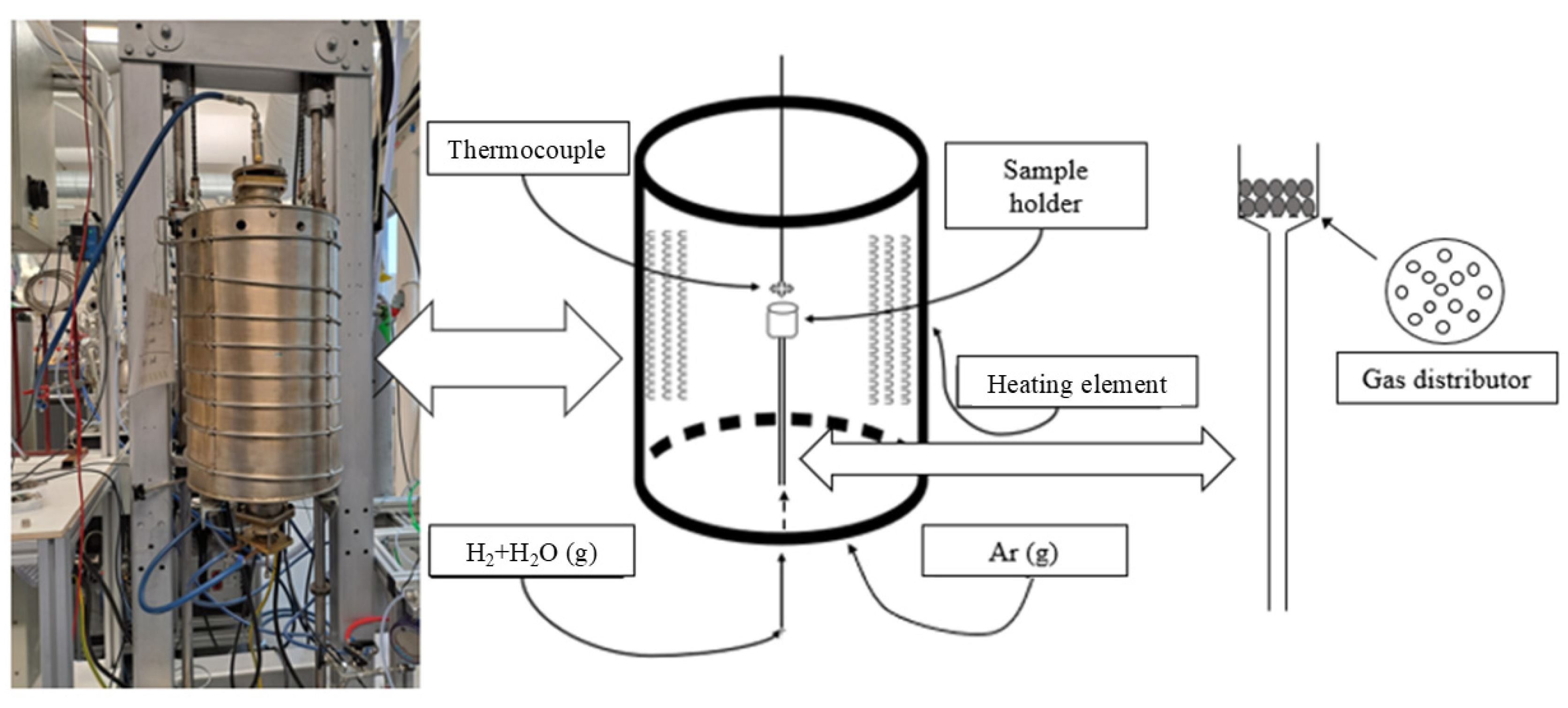
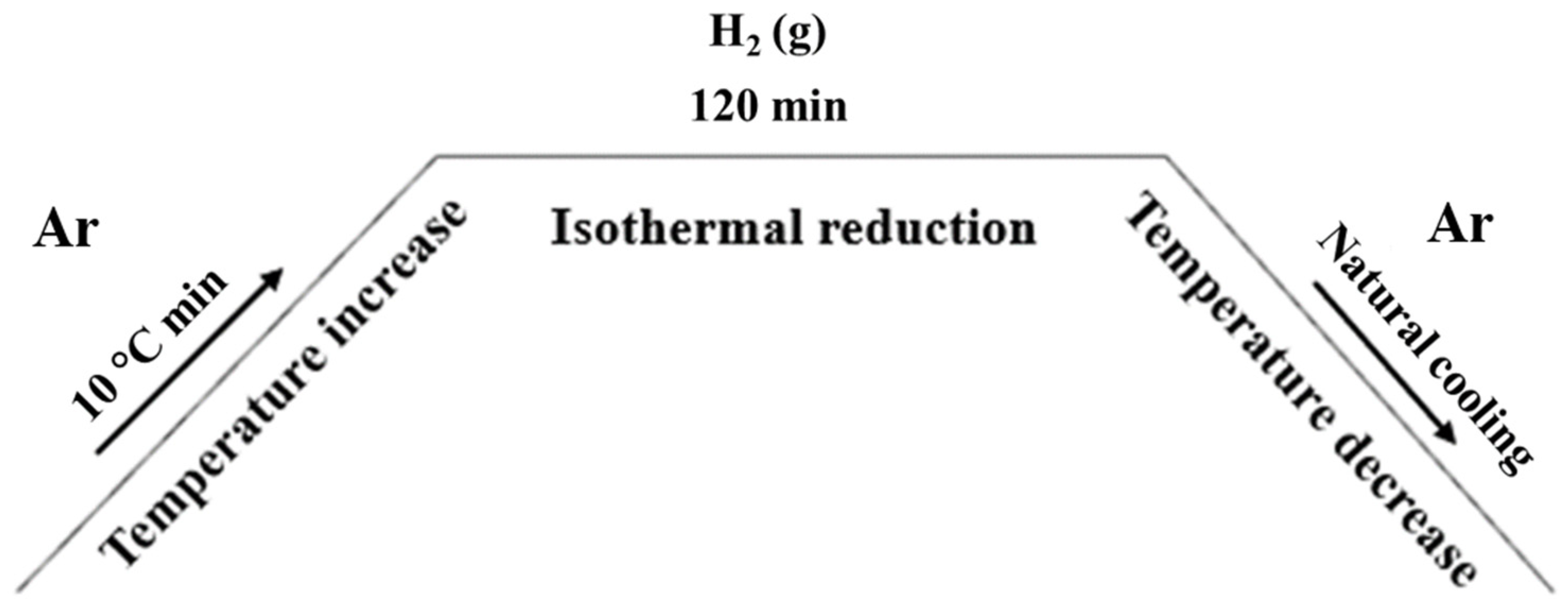
2.2. H2 Reduction
2.3. Thermal Analysis
3. Results and Discussion
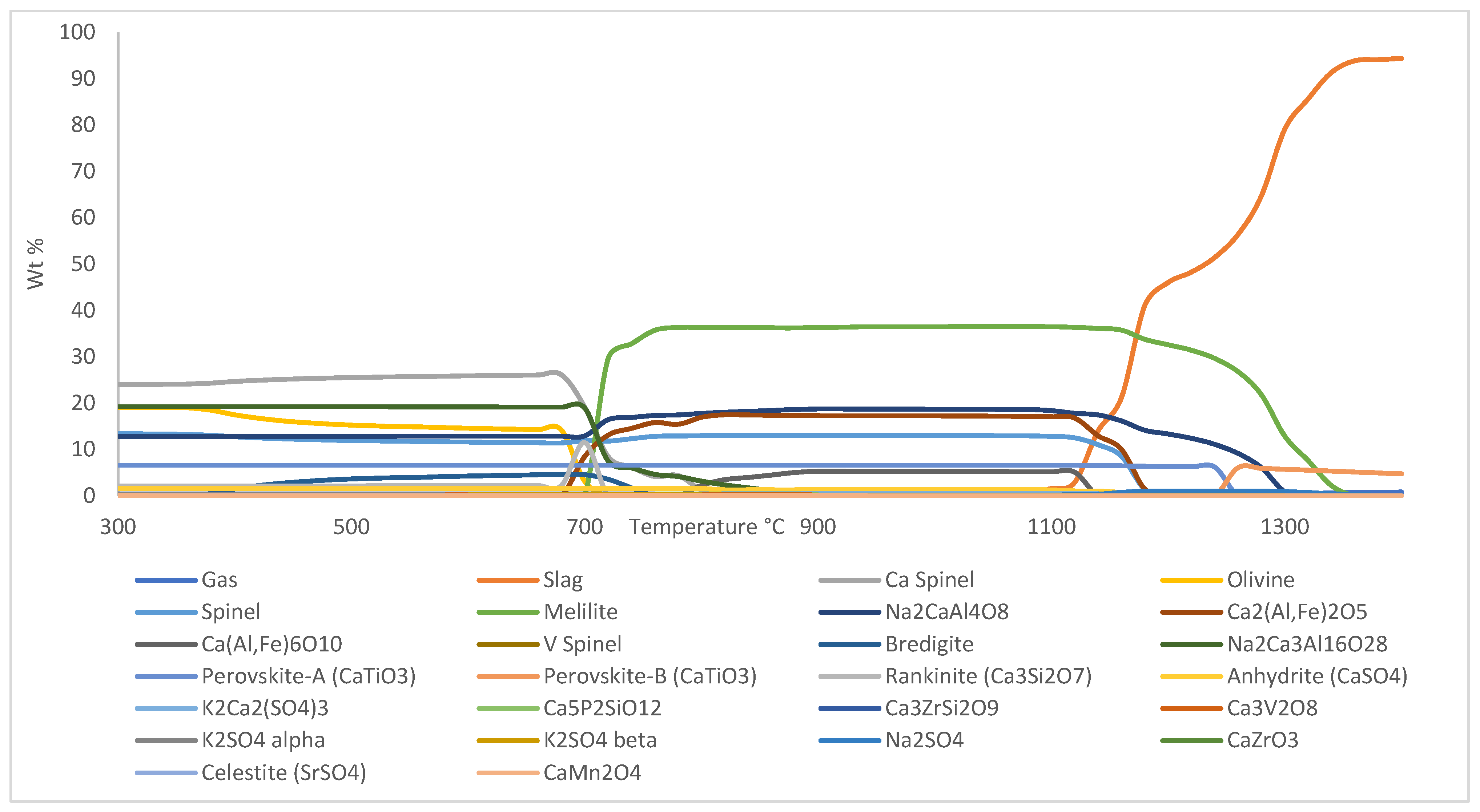
3.1. XRD Analysis
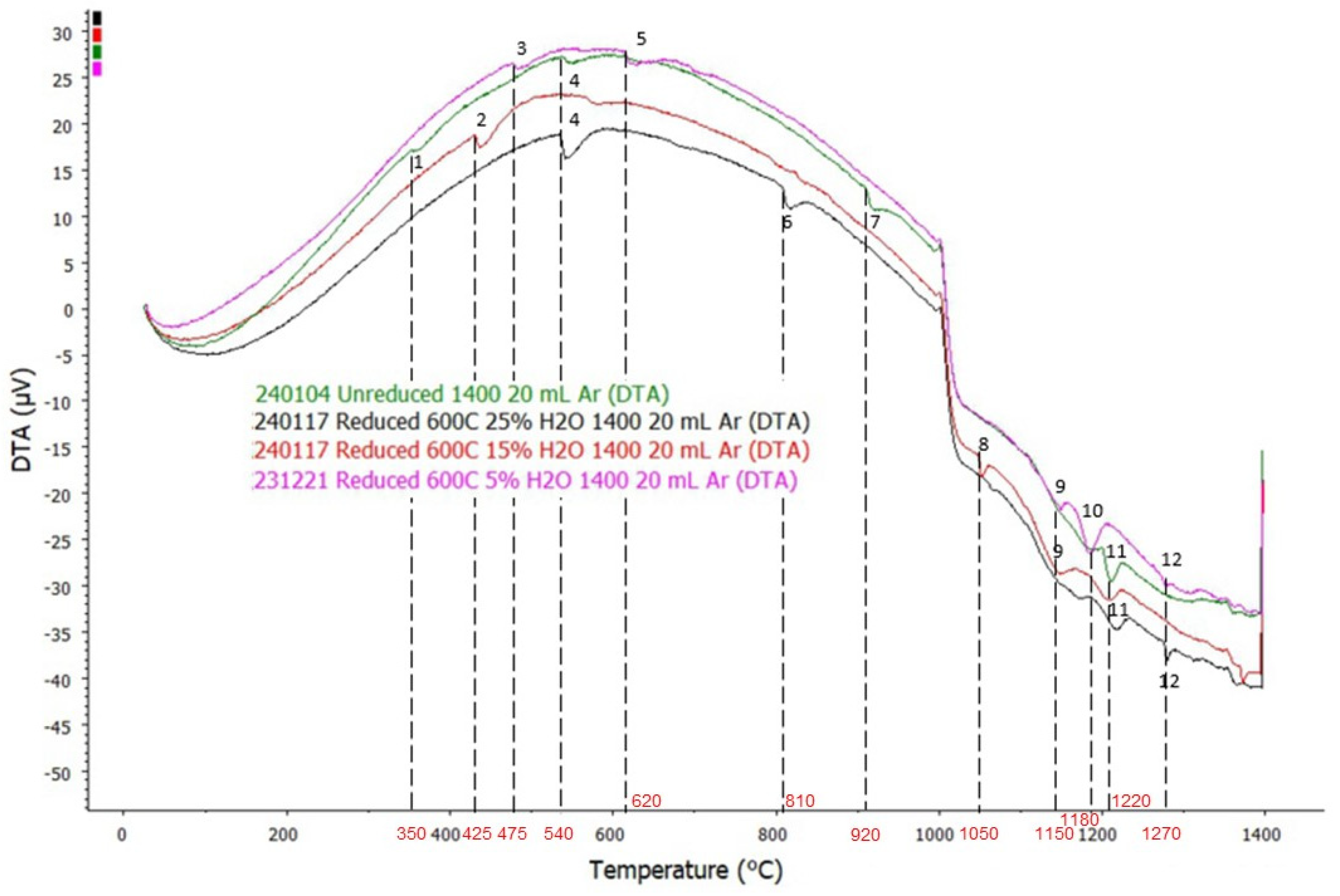
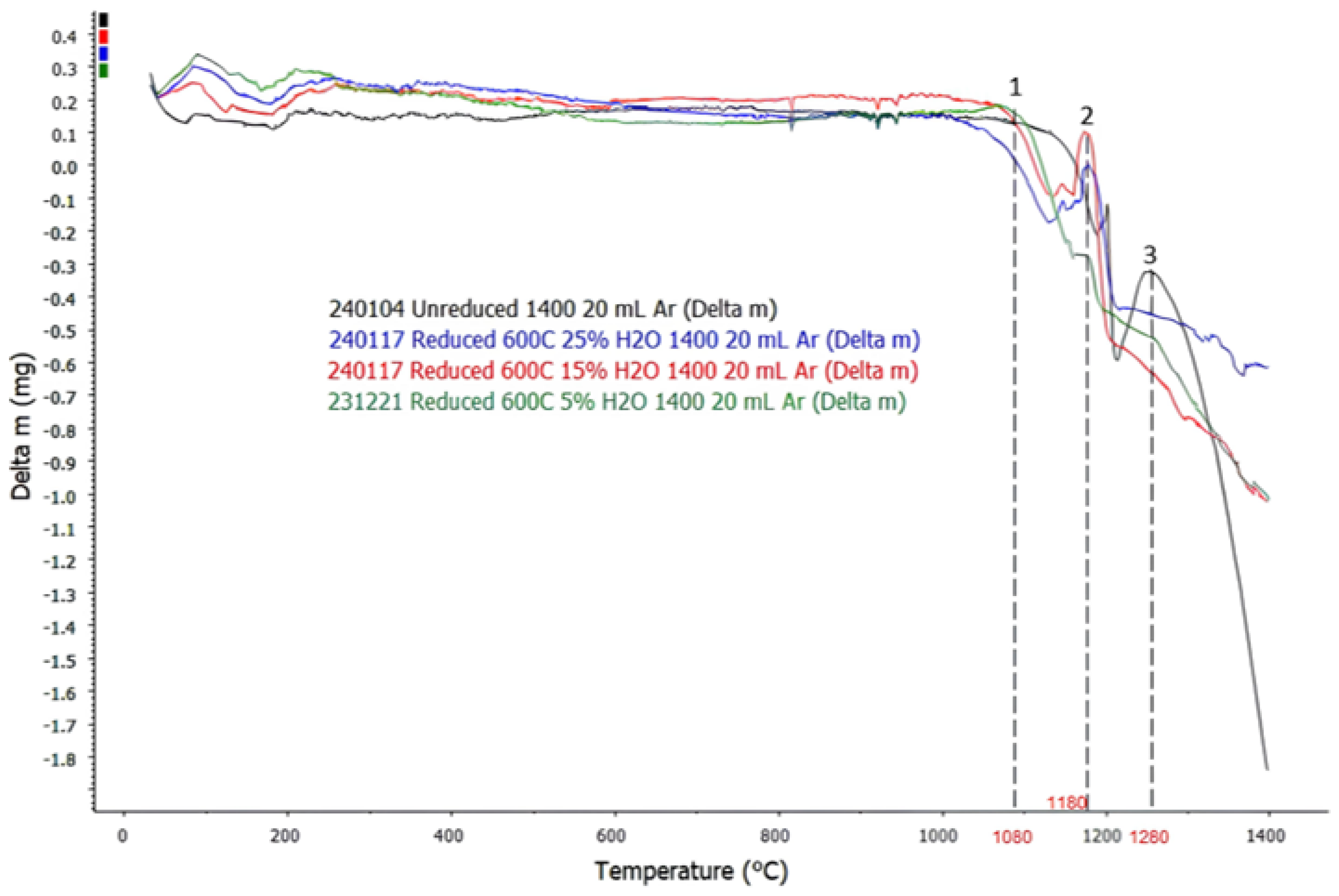
3.2. DTA Analysis
3.3. TGA Analysis
4. Conclusions
- Transformation of brownmillerite into srebrodolskite and dissolution of calcium silicate-containing phases into gehlenite occurred during H2 reduction of BR pellets.
- The following reactions were successfully detected during TG–DTA analysis, which was signified by occurrences of crests and troughs in the DTA curves:
- Ca5P2SiO12 breaks down into tricalcium phosphate-β, rankinite, and CaO at T ≈ 350 °C; however, since tricalcium phosphate-β is an unstable phase, it reverts into Ca5P2SiO12 at T ≈ 460 °C.
- Formation of bredigite occurred at T ≈ 425 °C, where olivine reacted with CaO to form bredigite. Significant amounts of mayenite and rankinite were formed at T ≈ 810 °C, which originates from the Ca-rich spinel phase in the system. Na2CaAl4O8 in the pellet yields molten slag and reacts with free SO3 in the system to form sodium sulphate at T ≈ 1050 °C.
- Transformation of potassium sulphate-α (K2SO4-α) into potassium sulphate-β (K2SO4-β) occurred at T ≈ 540 °C and transformation of perovskite-A into perovskite-B occurred at T ≈ 1270 °C.
- Formation of liquid (slag and gas) phases were successfully detected during TG–DTA analysis, which was signified by significant increases in the gradients of TGA curves:
- Initial slag formation for both sintered and reduced pellets occurred at T = 900 °C, with a small liquid slag formation that contains mostly CaO, CaS, SrO, and SrS. The amount of slag phase start to increase significantly with increasing temperature at T ≈ 1100 °C, where it starts to pick up iron oxides in the system, which was then followed by other oxides dissolution/melting in the system.
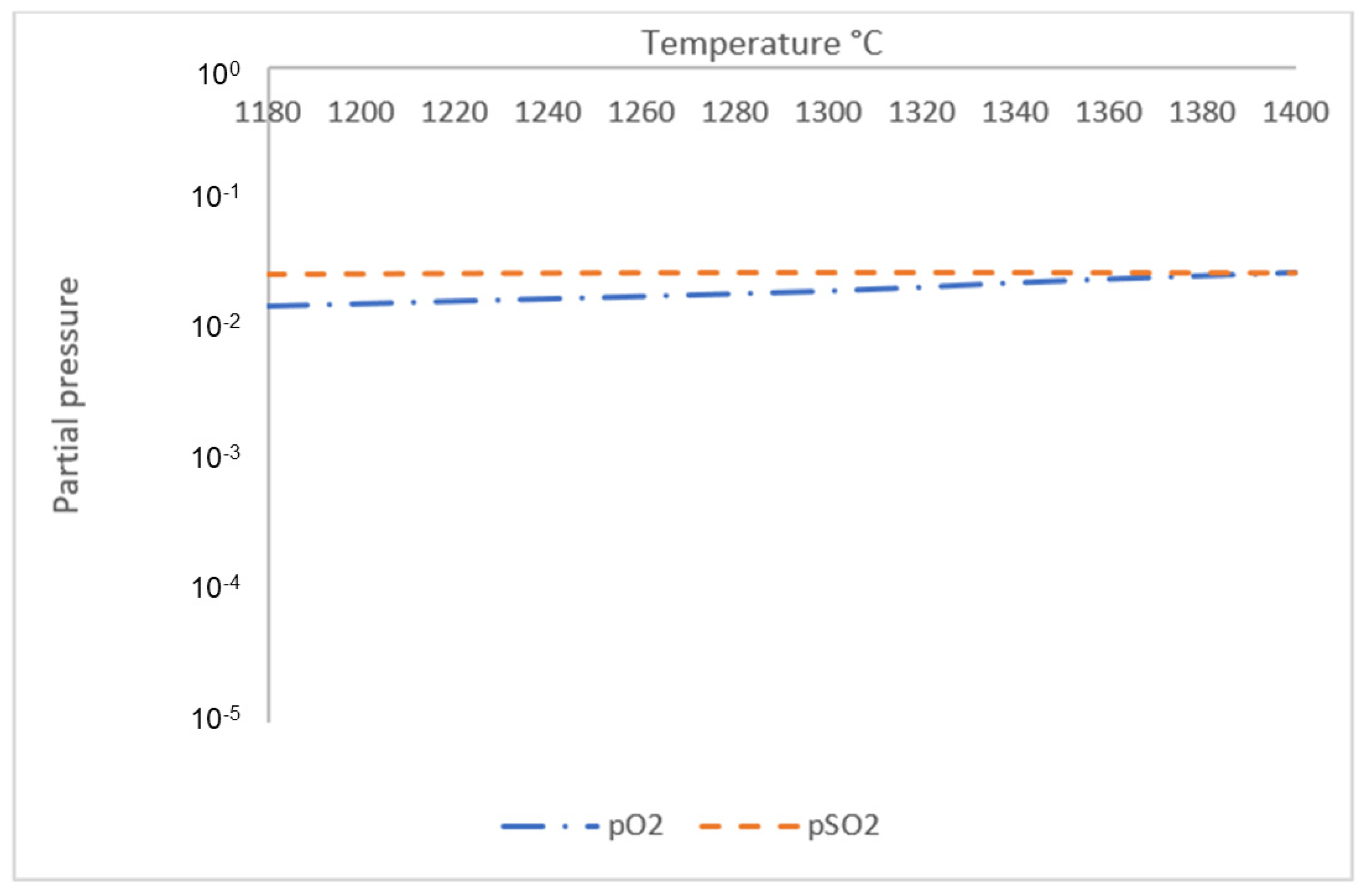
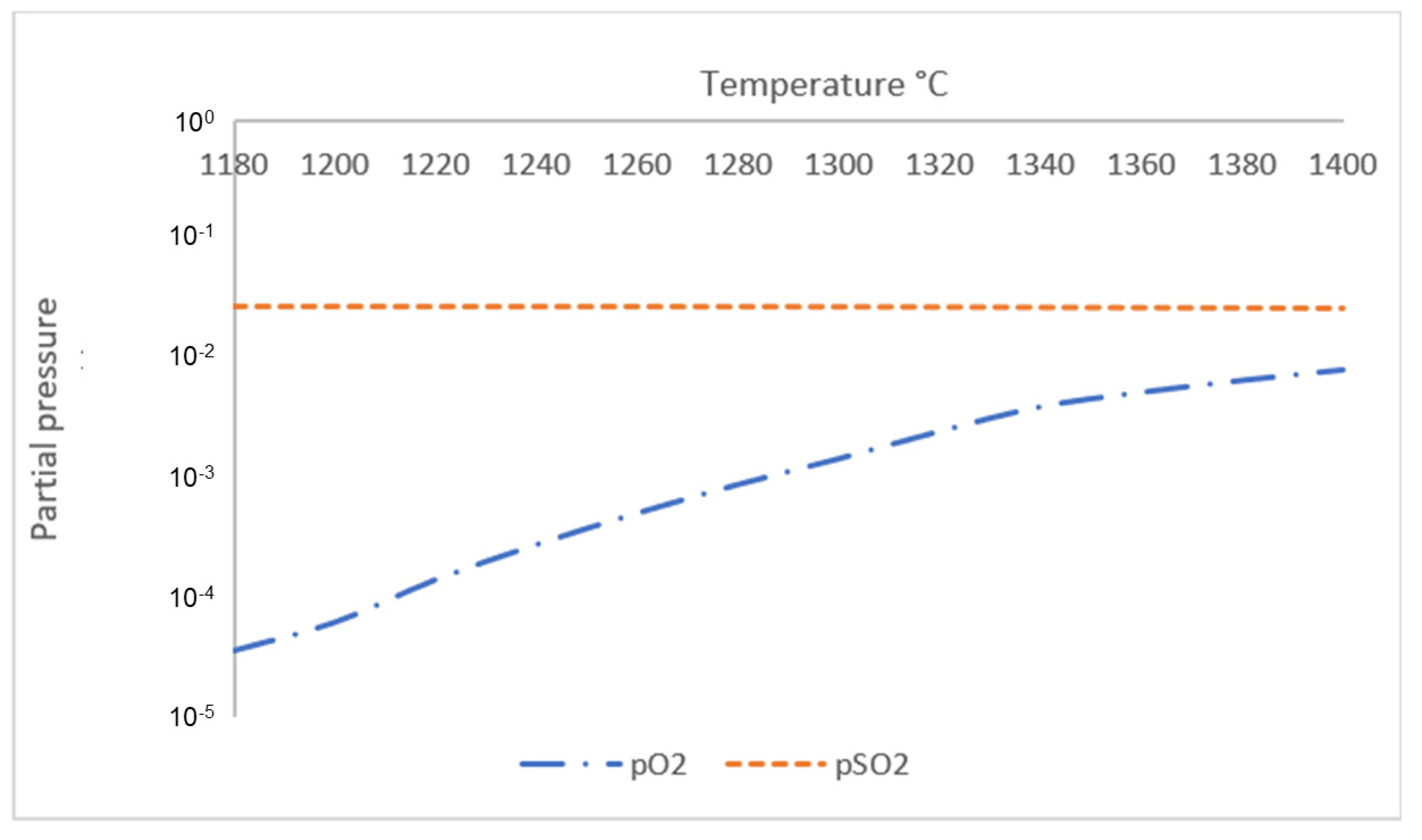
- Initial discharge of gas phase from both sintered and reduced pellets occurred at T ≈ 1180 °C, where sintered samples started to release both O2 and SO2 gas in the system and reduced samples released SO2 gas. The amount of gas phase for both samples started to increase significantly with increasing temperature starting from T ≈ 1280 °C, when increased reaction kinetics forces remaining O2 in the sample to be released into the atmosphere.
- Formation of mayenite, which was observed at T ≈ 810 °C, provides an opportunity for alumina recovery from H2-reduced BR pellets due to its leachability.
- Partial sintering is expected to happen during H2 reduction of BR pellets starting from T ≈ 770 °C (0.7 × Tmelt), which ultimately may affect reactivity and physical properties of reduced pellets for further processing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smallman, R.E.; Bishop, R.J. Chapter 10—Ceramics and Glasses. In Modern Physical Metallurgy and Materials Engineering, 6th ed.; Butterworth-Heinemann: Oxford, UK, 1999; pp. 320–350. [Google Scholar]
- Pandey, A.K.; Prakash, R. Opportunities for Sustainability Improvement in Aluminum Industry. Eng. Rep. 2020, 2, e12160. [Google Scholar] [CrossRef]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Brunori, C.; Cremisini, C.; Massanisso, P.; Pinto, V.; Torricelli, L. Reuse of a Treated Red Mud Bauxite Waste: Studies on Environmental Compatibility. J. Hazard. Mater. 2005, 117, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.S.; Suri, N.M.; Kant, S. Applications of bauxite residue: A mini-review. Waste Manag. Res. 2017, 35, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Y.; He, F.; Gao, P.; Yuan, S. Characteristic, Hazard and Iron Recovery Technology of Red Mud—A Critical Review. J. Hazard. Mater. 2021, 420, 126542. [Google Scholar] [CrossRef] [PubMed]
- Hoseinpur, A.; Friborg, P.I.; Van Der Eijk, C.; Safarian, J. High temperature hydrogen reduction of bauxite residue for iron recovery. In Proceedings of the 61st Conference of Metallurgists, COM 2022, Montreal, QC, Canada, 21–24 August 2022; Springer: Cham, Switzerland, 2022; pp. 119–127. [Google Scholar] [CrossRef]
- Lazou, A.; van der Eijk, C.; Tang, K.; Balomenos, E.; Kolbeinsen, L.; Safarian, J. The Utilization of Bauxite Residue with a Calcite-Rich Bauxite Ore in the Pedersen Process for Iron and Alumina Extraction. Metall. Mater. Trans. B 2021, 52B, 1265. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The Composition, Recycling and Utilisation of Bayer Red Mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Borra, R.C.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of Bauxite Residue (Red Mud) in View of Iron and Selective Rare Earths Recovery. J. Sustain. Metall. 2016, 2, 28–37. [Google Scholar] [CrossRef]
- Ekstroem, K.E.; Voll Bugten, A.; van der Eijk, C.; Lazou, A.; Balomenos, E.; Tranell, G. Recovery of Iron and Aluminum from Bauxite Residue by Carbothermic Reduction and Slag Leaching. J. Sustain. Metall. 2021, 7, 1314–1326. [Google Scholar] [CrossRef]
- Kar, M.K.; van der Eijk, C.; Safarian, J. Hydrogen Reduction of High Temperature Sintered and Self-Hardened Pellets of Bauxite Residue produced via the Addition of Limestone and Quicklime. In Proceedings of the 40th International Conference of ICSOBA, Athens, Greece, 10–14 October 2022; pp. 823–833. [Google Scholar]
- Skibelid, O.B.; Velle, S.O.; Vollan, F.; van der Eijk, C.; Hosseinpur-Kermani, A.; Safarian, J. Isothermal Hydrogen Reduction of a Lime-Added Bauxite Residue Agglomerate at Elevated Temperatures for Iron and Alumina Recovery. Materials 2022, 15, 6012. [Google Scholar] [CrossRef] [PubMed]
- Samouhos, M.; Taxiarchou, M.; Pilatos, G.; Tsakiridis, P.E.; Devlin, E.; Pissas, M. Controlled Reduction of Red Mud by H2 followed by Magnetic Separation. Miner. Eng. 2017, 105, 36–43. [Google Scholar] [CrossRef]
- Pilla, G.; Kapelari, S.V.; Hertel, T.; Blanpain, B.; Pontikes, Y. Hydrogen reduction of bauxite residue and selective metal recovery. Mater. Today Proc. 2022, 57, 705–710. [Google Scholar] [CrossRef]
- Kapelari, S.; Gamaletsos, P.N.; Pilla, G.; Pontikes, Y.; Blanpain, B. Developing a low-temperature, carbon-lean hybrid valorisation process for bauxite residue (red mud) towards metallic Fe and Al recovery. J. Sustain. Metall. 2023, 9, 578–587. [Google Scholar] [CrossRef]
- Kar, M.K.; van der Eijk, C.; Safarian, J. The Effect of Composition and Temperature on the Hydrogen Reduction Behavior of Sintered Pellets of Bauxite Residue-Lime Mixtures. J. Sustain. Metall. 2024, 10, 1393–1414. [Google Scholar] [CrossRef]
- Evans, K. The history, challenges, and new developments in the management and use of bauxite residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef]
- Kar, M.K.; Safarian, J. Characteristics of bauxite residue limestone pellets as feedstock for Fe and Al2O3 recovery. Processes 2023, 11, 137. [Google Scholar] [CrossRef]
- Kar, M.K.; Zhu, M.; Safarian, J. Hydrogen Reduction of Hazardous Bauxite Residue for Green Steel and Sustainable Alumina Production. J. Sustain. Metall. 2025. [Google Scholar] [CrossRef]
- Gound, T.U.; Ramachandran, V.; Kulkarni, S. Various methods to reduce SO2 emission-a review. Int. J. Ethics Eng. Manag. Educ. 2014, 1, 1–6. [Google Scholar]


| Composition | Wt% | Composition | Wt% |
|---|---|---|---|
| CaO | 9.98 | TiO2 | 5.67 |
| MgO | 0.26 | Na2O | 3.51 |
| SiO2 | 8.05 | K2O | 0.10 |
| Al2O3 | 24.94 | P2O5 | 0.13 |
| Fe2O3 | 46.20 | SO3 | 1.07 |
| MnO | 0.09 | Total | 100 |
| Composition | Wt% | Composition | Wt% |
|---|---|---|---|
| CaO | 98.632 | TiO2 | 0.005 |
| MgO | 0.547 | Na2O | 0.037 |
| SiO2 | 0.208 | K2O | 0.035 |
| Al2O3 | 0.175 | P2O5 | 0.009 |
| Fe2O3 | 0.084 | SO3 | 0.263 |
| MnO | 0.005 | Total | 100 |
| Composition | Wt% | Composition | Wt% | Composition | Wt% |
|---|---|---|---|---|---|
| CaO | 29.01 | Cr2O3 | 0.18 | P2O5 | 0.12 |
| MgO | 0.37 | V2O5 | 0.15 | SO3 | 1.03 |
| SiO2 | 7.66 | TiO2 | 3.87 | ZrO2 | 0.11 |
| Al2O3 | 23.12 | NiO | 0.06 | SrO | 0.03 |
| Fe2O3 | 30.52 | Na2O | 3.61 | Co3O4 | 0.02 |
| MnO | 0.04 | K2O | 0.10 | Total | 100 |
| No. | Gas Composition (vol%) | Reduction Temperature (°C) |
|---|---|---|
| 1 | 95% H2-5% H2O | 600 |
| 2 | 85% H2-15% H2O | 600 |
| 3 | 75% H2-25% H2O | 600 |
| Component | Mass (gr) | Component | Mass (gr) | Component | Mass (gr) |
|---|---|---|---|---|---|
| CaO | 29.01 | MnO | 0.04 | P2O5 | 0.12 |
| MgO | 0.37 | Cr2O3 | 0.18 | SO3 | 1.03 |
| SiO2 | 7.66 | V2O5 | 0.15 | ZrO2 | 0.11 |
| Al2O3 | 23.12 | TiO2 | 3.87 | SrO | 0.03 |
| Fe2O3 | 25.94 | NiO | 0.06 | Co3O4 | 0.02 |
| Fe3O4 | 1.48 | Na2O | 3.61 | Total | 99.65 |
| FeO | 2.75 | K2O | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hariswijaya, D.; Safarian, J. Studying the Sintering Behavior of H2-Reduced Bauxite Residue Pellets Using High-Temperature Thermal Analysis. Materials 2025, 18, 2378. https://doi.org/10.3390/ma18102378
Hariswijaya D, Safarian J. Studying the Sintering Behavior of H2-Reduced Bauxite Residue Pellets Using High-Temperature Thermal Analysis. Materials. 2025; 18(10):2378. https://doi.org/10.3390/ma18102378
Chicago/Turabian StyleHariswijaya, Dali, and Jafar Safarian. 2025. "Studying the Sintering Behavior of H2-Reduced Bauxite Residue Pellets Using High-Temperature Thermal Analysis" Materials 18, no. 10: 2378. https://doi.org/10.3390/ma18102378
APA StyleHariswijaya, D., & Safarian, J. (2025). Studying the Sintering Behavior of H2-Reduced Bauxite Residue Pellets Using High-Temperature Thermal Analysis. Materials, 18(10), 2378. https://doi.org/10.3390/ma18102378









