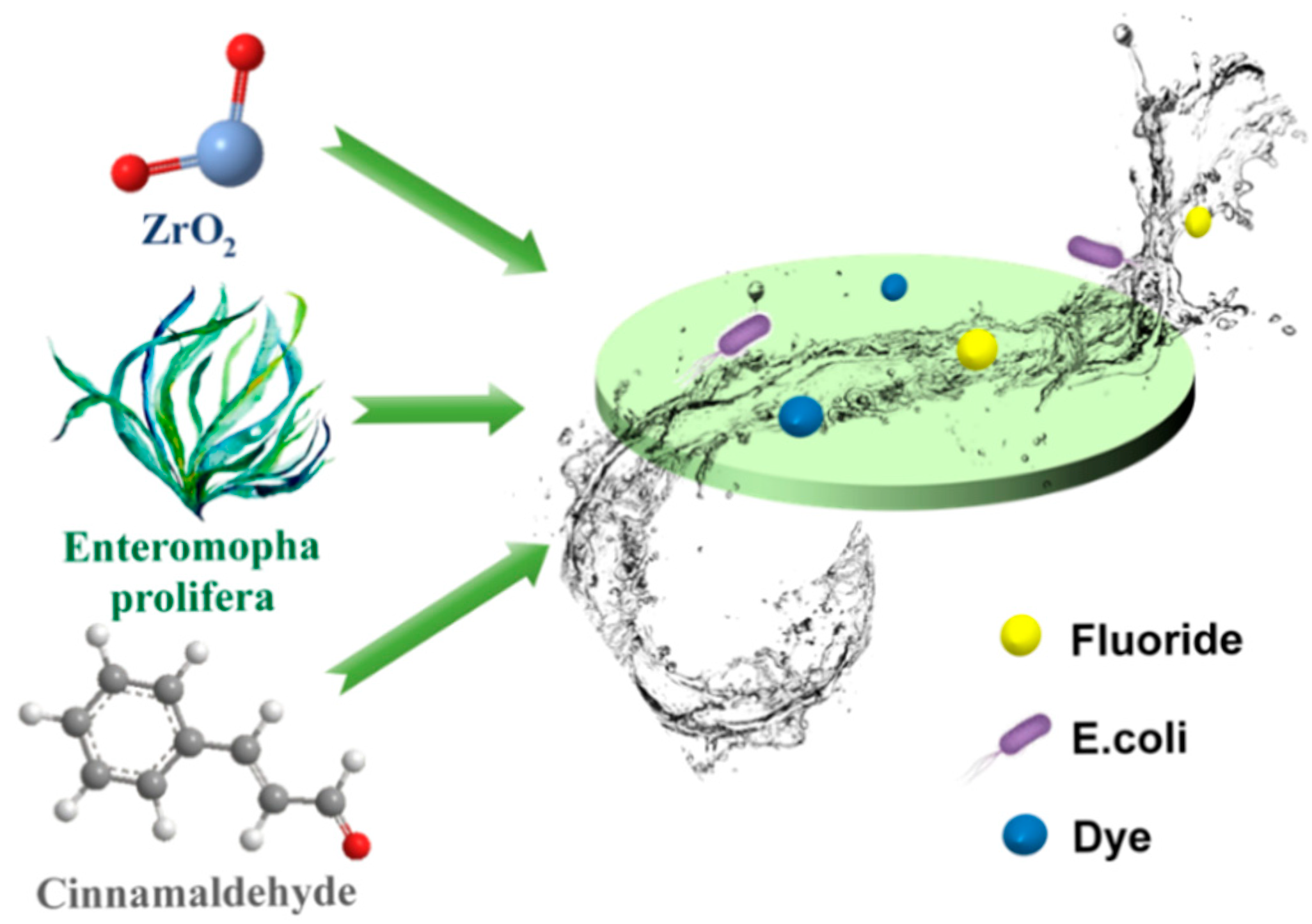
Biomimetic Construction of Enteromorpha prolifera-Based Composite Membranes for Synergistic Purification of Fluoride Ions, Bacteria, and Dye with High Sustainability
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of CIN/EP-ZrO2 Composite Membrane
2.3. Adsorption Experiments of F−
2.4. Antibacterial Testing
2.5. Adsorption Experiment of MB
2.6. Sustainability Analysis
2.7. Characterization Techniques
3. Results and Discussion
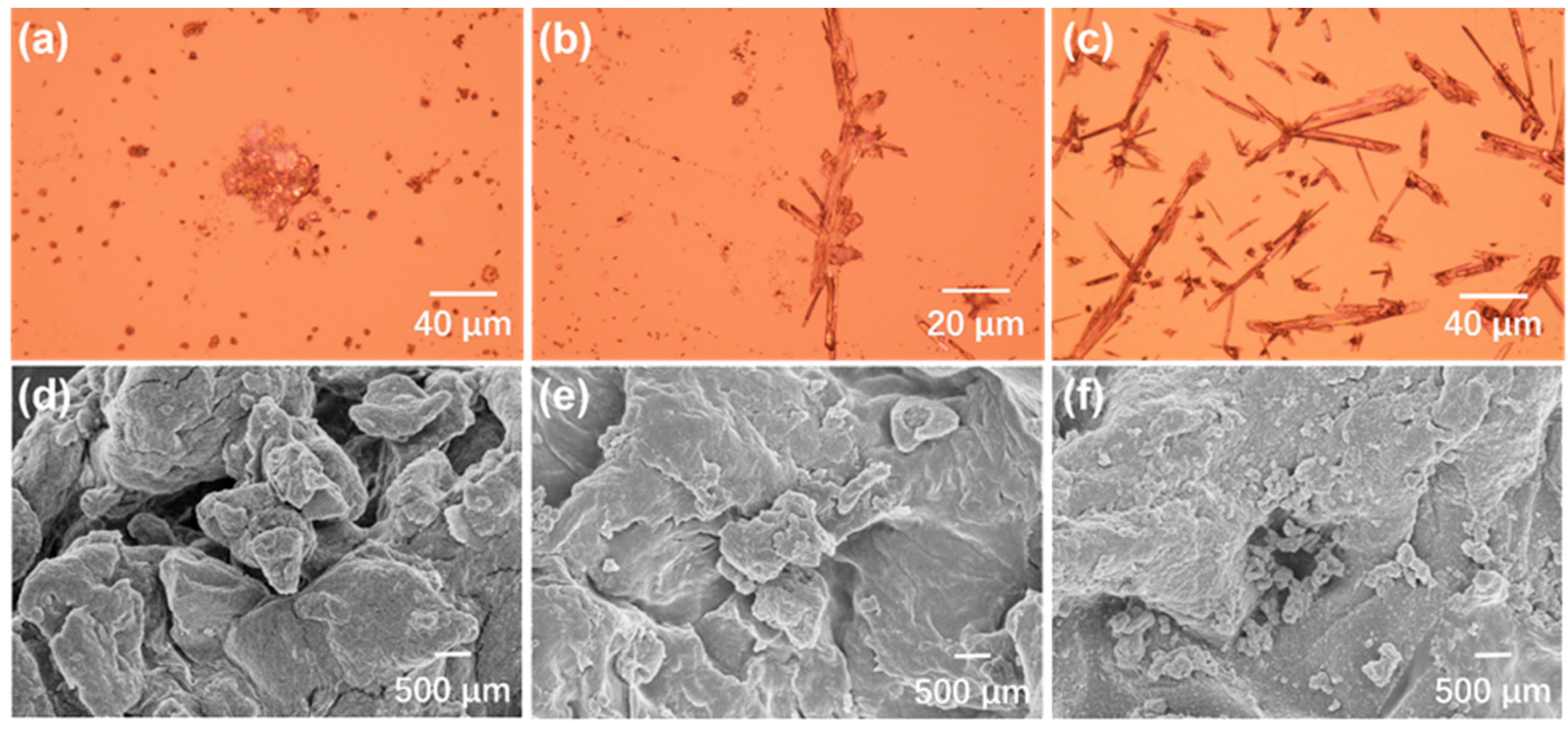
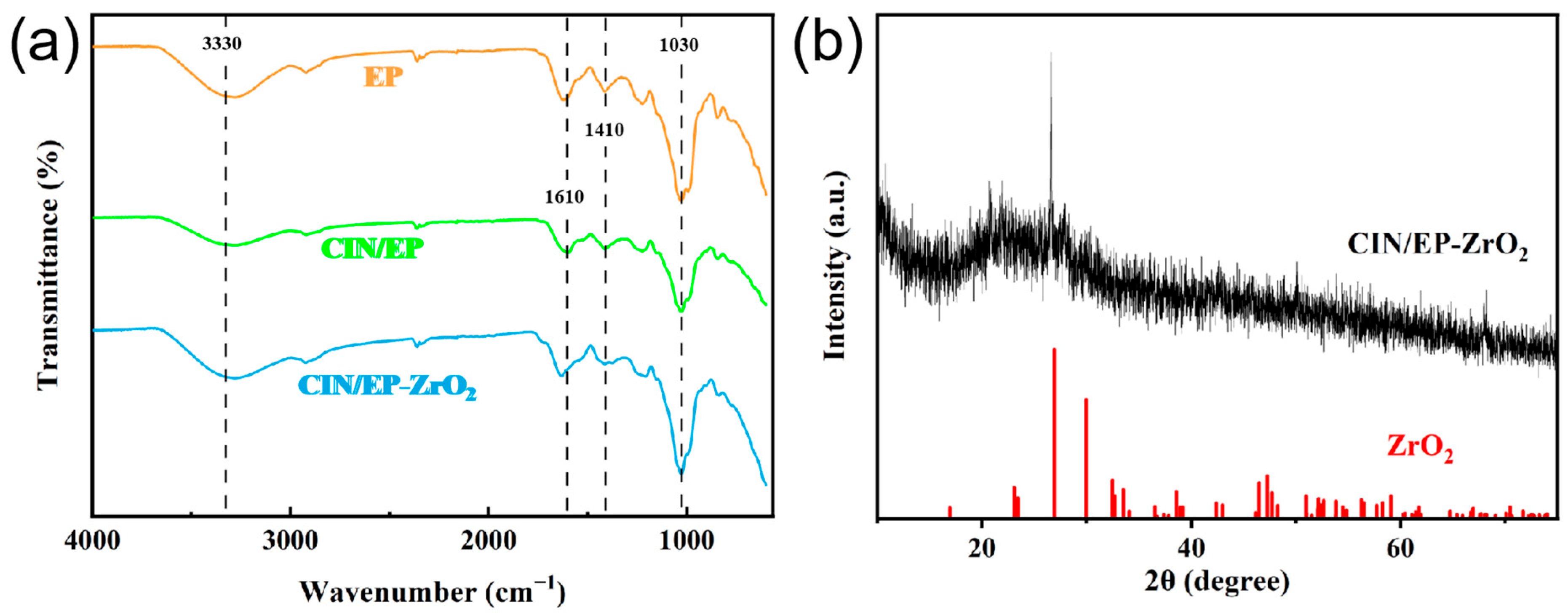
3.1. Preparation and Characterizations of the CIN/EP-ZrO2 Composite Membranes
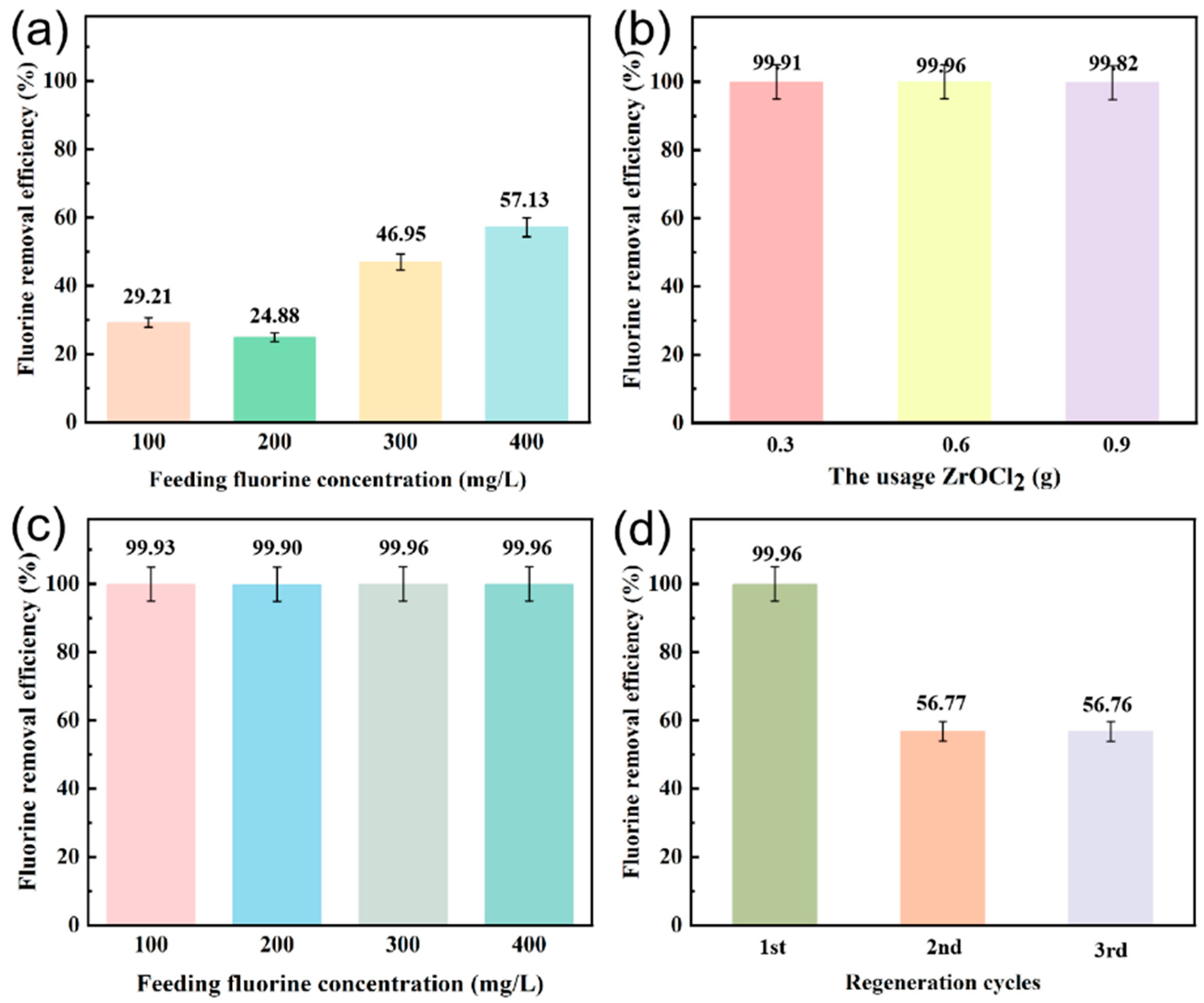
3.2. F− Adsorption of the CIN/EP-ZrO2 Composite Membranes
3.3. Antibacterial Properties of the CIN/EP-ZrO2 Composite Membranes
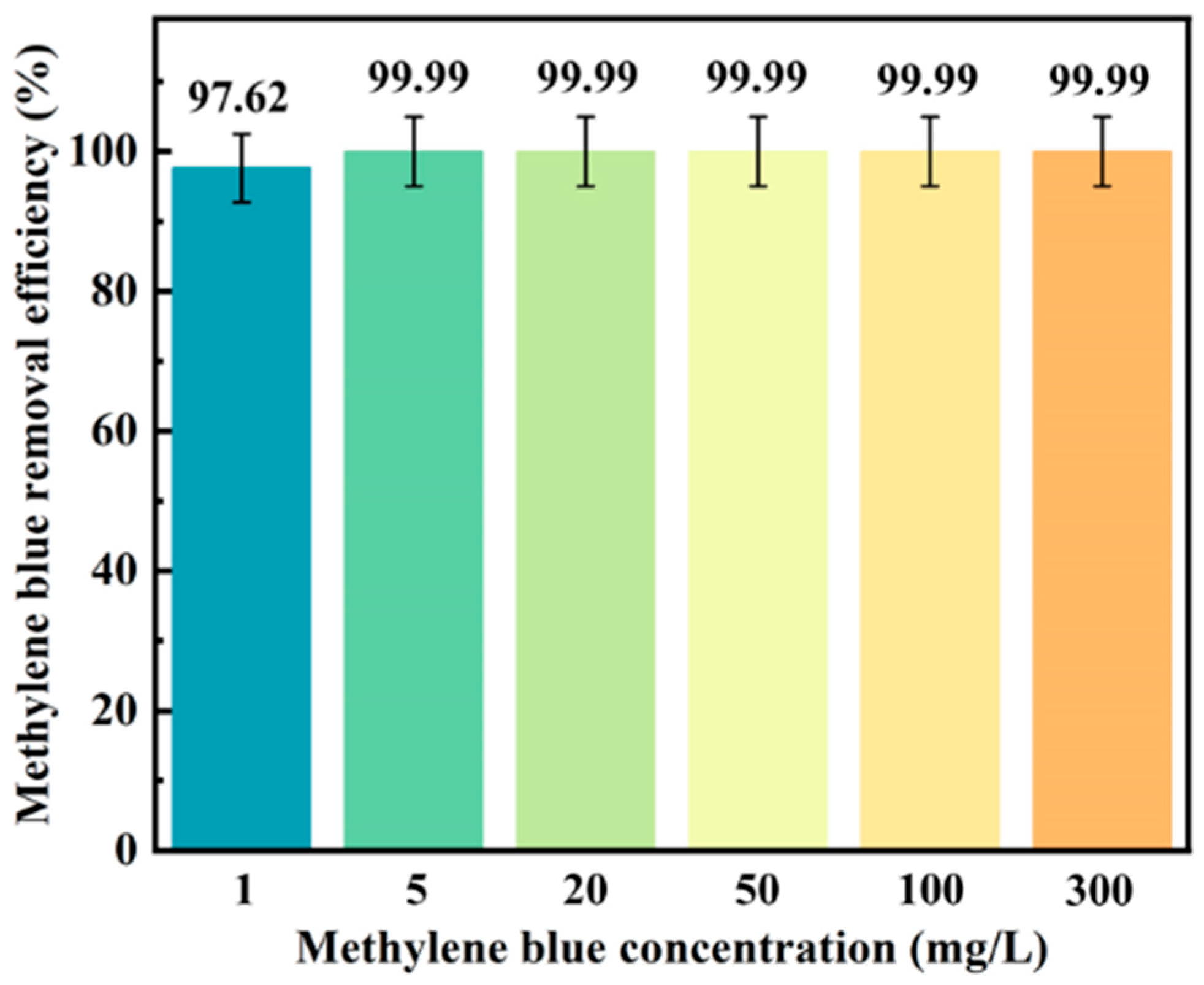
3.4. Dye Adsorption Properties of the CIN/EP-ZrO2 Composite Membranes
3.5. Overall Sustainability Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bera, B.; Saha Chowdhury, S.; Sonawane, V.R.; De, S. High capacity aluminium substituted hydroxyapatite incorporated granular wood charcoal (Al-HApC) for fluoride removal from aqueous medium: Batch and column study. Chem. Eng. J. 2023, 466, 143264. [Google Scholar] [CrossRef]
- Rathnayake, A.; Hettithanthri, O.; Sandanayake, S.; Mahatantila, K.; Rajapaksha, A.U.; Vithanage, M. Essence of hydroxyapatite in defluoridation of drinking water: A review. Environ. Pollut. 2022, 311, 119882. [Google Scholar] [CrossRef]
- Solanki, Y.S.; Agarwal, M.; Gupta, A.B.; Gupta, S.; Shukla, P. Fluoride occurrences, health problems, detection, and remediation methods for drinking water: A comprehensive review. Sci. Total Environ. 2022, 807, 150601. [Google Scholar] [CrossRef]
- Ning, K.; Zheng, S.; He, Y.; Hu, Y.; Hao, S.; Cui, Q.; Chen, H. Removal of fluoride from aqueous solutions through Fe(III) modified water treatment residues. J. Clean. Prod. 2023, 382, 135374. [Google Scholar] [CrossRef]
- Jian, S.; Shi, F.; Zhu, Y.; Jiang, W.; Liu, Y.; Yang, W.; Hu, J.; Jiang, S. Ce-MOFs and Ce/Mn bimetallic MOFs for efficient fluoride removal and their defluoridation mechanism. Colloids Surf. A 2024, 701, 134938. [Google Scholar] [CrossRef]
- Prabhu, S.M.; Chuaicham, C.; Park, C.M.; Jeon, B.-H.; Sasaki, K. Synthesis and characterization of defective UiO-66 for efficient co-immobilization of arsenate and fluoride from single/binary solutions. Environ. Pollut. 2021, 278, 116841. [Google Scholar] [CrossRef]
- Ndiaye, P.I.; Moulin, P.; Dominguez, L.; Millet, J.C.; Charbit, F. Removal of fluoride from electronic industrial effluentby RO membrane separation. Desalination 2005, 173, 25–32. [Google Scholar] [CrossRef]
- Luna, M.D.G.d.; Warmadewanthi; Liu, J.C. Combined treatment of polishing wastewater and fluoride-containing wastewater from a semiconductor manufacturer. Colloids Surf. Physicochem. Eng. Asp. 2009, 347, 64–68. [Google Scholar] [CrossRef]
- Fadaei, A.; Niu, Y. Comparison of Water Defluoridation Using Different Techniques. Int. J. Chem. Eng. 2021, 2021, 2023895. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Q.; Yan, J.; Huang, L.; Arulmani, S.R.B.; Zhang, H.; Xie, S.; Sio, W. The model and mechanism of adsorptive technologies for wastewater containing fluoride: A review. Chemosphere 2023, 340, 139808. [Google Scholar] [CrossRef]
- Huang, L.; Luo, Z.; Huang, X.; Wang, Y.; Yan, J.; Liu, W.; Guo, Y.; Babu Arulmani, S.R.; Shao, M.; Zhang, H. Applications of biomass-based materials to remove fluoride from wastewater: A review. Chemosphere 2022, 301, 134679. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhu, B.-S.; Zhang, K.-S.; Jin, Z.; Sun, B.; Luo, T.; Yu, X.-Y.; Kong, L.-T.; Liu, J.-H. Porous 2-line ferrihydrite/bayerite composites (LFBC): Fluoride removal performance and mechanism. Chem. Eng. J. 2015, 268, 325–336. [Google Scholar] [CrossRef]
- Budyanto, S.; Kuo, Y.-L.; Liu, J.C. Adsorption and precipitation of fluoride on calcite nanoparticles: A spectroscopic study. Sep. Purif. Technol. 2015, 150, 325–331. [Google Scholar] [CrossRef]
- Kabay, N.; Arar, Ö.; Samatya, S.; Yüksel, Ü.; Yüksel, M. Separation of fluoride from aqueous solution by electrodialysis: Effect of process parameters and other ionic species. J. Hazard. Mater. 2008, 153, 107–113. [Google Scholar] [CrossRef]
- He, J.; Cai, X.; Chen, K.; Li, Y.; Zhang, K.; Jin, Z.; Meng, F.; Liu, N.; Wang, X.; Kong, L.; et al. Performance of a novelly-defined zirconium metal-organic frameworks adsorption membrane in fluoride removal. J. Colloid Interface Sci. 2016, 484, 162–172. [Google Scholar] [CrossRef]
- Dhongde, V.; Wasewar, K.L.; De, B.S. Development of nanohybrid adsorbent for defluoridation from aqueous systems. Chemosphere 2017, 188, 354–366. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, W.; Xin, C.; Kong, X.; Hu, X.; Guo, Q. The development of activated carbon from corncob for CO2 capture. RSC Adv. 2022, 12, 33069–33078. [Google Scholar] [CrossRef]
- Swain, S.K.; Mishra, S.; Patnaik, T.; Patel, R.K.; Jha, U.; Dey, R.K. Fluoride removal performance of a new hybrid sorbent of Zr(IV)–ethylenediamine. Chem. Eng. J. 2012, 184, 72–81. [Google Scholar] [CrossRef]
- Mei, L.; Wei, J.; Yang, R.; Ke, F.; Peng, C.; Hou, R.; Liu, J.; Wan, X.; Cai, H. Zirconium/lanthanum-modified chitosan/polyvinyl alcohol composite adsorbent for rapid removal of fluoride. Int. J. Biol. Macromol. 2023, 243, 125155. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, N.; Su, P.Y.; Li, M.; Feng, C.P. Fluoride removal from aqueous solution by Zirconium-Chitosan/Graphene Oxide Membrane. React. Funct. Polym. 2017, 114, 127–135. [Google Scholar] [CrossRef]
- Li, C.; Chen, N.; Zhao, Y.; Li, R.; Feng, C. Polypyrrole-grafted peanut shell biological carbon as a potential sorbent for fluoride removal: Sorption capability and mechanism. Chemosphere 2016, 163, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Li, W.Y.; Han, Y.H.; Wang, L.P.; Wu, H.; He, P.; Wei, G.; Guo, L. Biomimetic ZrO2-modified seaweed residue with excellent fluorine/bacteria removal and uranium extraction properties for wastewater purification. Water Res. 2024, 252, 121219. [Google Scholar] [CrossRef] [PubMed]
- Marzbali, M.H.; Mir, A.A.; Pazoki, M.; Pourjamshidian, R.; Tabeshnia, M. Removal of direct yellow 12 from aqueous solution by adsorption onto spirulina algae as a high-efficiency adsorbent. J. Environ. Chem. Eng. 2017, 5, 1946–1956. [Google Scholar] [CrossRef]
- Andemichael, H.; Lee, J.W. Toxicological study of biofuel ethanol with blue green alga Spirulina platensis. Algal Res. 2016, 18, 110–115. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Lima, E.C.; Royer, B.; Bach, M.V.; Dotto, G.L.; Pinto, L.A.A.; Calvete, T. Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of Reactive Red 120 dye from aqueous effluents. J. Hazard. Mater. 2012, 241–242, 146–153. [Google Scholar] [CrossRef]
- Gomaa, M.; Fawzy, M.A.; Hifney, A.F.; Abdel-Gawad, K.M. Use of the brown seaweed Sargassum latifolium in the design of alginate-fucoidan based films with natural antioxidant properties and kinetic modeling of moisture sorption and polyphenolic release. Food Hydrocoll. 2018, 82, 64–72. [Google Scholar] [CrossRef]
- Liu, Y.M.; Wen, J.; Gao, Y.; Li, T.Y.; Wang, H.F.; Yan, H.; Niu, B.L.; Guo, R.J. Antibacterial graphene oxide coatings on polymer substrate. Appl. Surf. Sci. 2018, 436, 624–630. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Singh, N.; Rao, A.S.; Nandal, A.; Kumar, S.; Yadav, S.S.; Ganaie, S.A.; Narasimhan, B. Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chem. 2020, 338, 127773. [Google Scholar] [CrossRef]
- Chen, X.; Liu, P.; Luo, X.; Huang, A.; Wang, G. Study on the antibacterial activity and mechanism of Cinnamaldehyde against Methicillin-resistant Staphylococcus aureus. Eur. Food Res. Technol. 2024, 250, 1069–1081. [Google Scholar] [CrossRef]
- Pereira, W.A.; Pereira, C.D.S.; Assunção, R.G.; da Silva, I.S.C.; Rego, F.S.; Alves, L.S.R.; Santos, J.S.; Nogueira, F.J.R.; Zagmignan, A.; Thomsen, T.T.; et al. New Insights into the Antimicrobial Action of Cinnamaldehyde towards Escherichia coli and Its Effects on Intestinal Colonization of Mice. Biomolecules 2021, 11, 302. [Google Scholar] [CrossRef]
- Singh, N.; Singh Yadav, S. Antimicrobial and anticancer insights of cinnamaldehyde Schiff bases and metal complexes. Inorg. Chem. Commun. 2024, 167, 112724. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Guo, F.; Meng, Y.; Li, M. Anti-diabetic effects of cinnamaldehyde and berberine and their impacts on retinol-binding protein 4 expression in rats with type 2 diabetes mellitus. Chin. Med. J. 2008, 121, 2124–2128. [Google Scholar] [CrossRef]
- Yuan, X.; Han, L.; Fu, P.; Zeng, H.; Lv, C.; Chang, W.; Runyon, R.S.; Ishii, M.; Han, L.; Liu, K.; et al. Cinnamaldehyde accelerates wound healing by promoting angiogenesis via up-regulation of PI3K and MAPK signaling pathways. Lab. Investig. 2018, 98, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, W.; Lei, Y.; He, P.; Wei, G.; Guo, L. Functionalization of cellulose-based sponges: Design, modification, environmental applications, and sustainability analysis. Carbohydr. Polym. 2024, 348, 122772. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, G.; Liu, B.; Kong, H.; Xiong, Z.; Guo, L.; Wei, G. Biomineralization of ZrO2 nanoparticles on graphene oxide-supported peptide/cellulose binary nanofibrous membranes for high-performance removal of fluoride ions. Chem. Eng. J. 2022, 430, 132721. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, Z.; Wang, Z.; Liu, Y.; He, Z.; Cao, A.; Zhou, L.; Zhu, L.; Zhao, S. Super-adsorptive and photo-regenerable carbon nanotube based membrane for highly efficient water purification. J. Membr. Sci. 2021, 621, 119000. [Google Scholar] [CrossRef]
- Nayanathara, U.; Kottegoda, N.; Perera, I.C.; Mudiyanselage, T.K. Synthesis, photodegradable and antibacterial properties of polystyrene-cinnamaldehyde copolymer film. Polym. Degrad. Stab. 2018, 155, 195–207. [Google Scholar] [CrossRef]
- Ye, Y.; Yan, X.; Li, X.; Huang, S.; Jiang, W.; Liu, D.; Ren, Y.; Cheng, D. Ethanol Treated Mn–Zr Compound for Fluoride Removal and its Adsorption Mechanism. Int. J. Environ. Res. 2024, 18, 54. [Google Scholar] [CrossRef]
- Wang, X.Z.; Pan, S.L.; Zhang, M.; Qi, J.W.; Sun, X.Y.; Gu, C.; Wang, L.J.; Li, J.S. Modified hydrous zirconium oxide/PAN nanofibers for efficient defluoridation from groundwater. Sci. Total Environ. 2019, 685, 401–409. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.; Cao, M.B.; Yang, H.B.; Li, Y.S. Constructing hydrangea-like hierarchical zinc-zirconium oxide microspheres for accelerating fluoride elimination. J. Mol. Liq. 2020, 317, 114133. [Google Scholar] [CrossRef]
- Cho, D.W.; Jeon, B.H.; Jeong, Y.; Nam, I.H.; Choi, U.K.; Kumar, R.; Song, H. Synthesis of hydrous zirconium oxide-impregnated chitosan beads and their application for removal of fluoride and lead. Appl. Surf. Sci. 2016, 372, 13–19. [Google Scholar] [CrossRef]
- Zhou, Q.S.; Lin, X.Y.; Qian, J.; Wang, J.; Luo, X.G. Porous zirconium alginate beads adsorbent for fluoride adsorption from aqueous solutions. RSC Adv. 2015, 5, 2100–2112. [Google Scholar] [CrossRef]
- Zeng, Z.K.; Xu, K.; Zhang, F.X.; Yang, X.; Du, J.F.; Dong, Z.; Bao, Q.; Zhao, L. Facile preparation of zirconium(IV) immobilized carboxymethyl cellulose/multiwalled carbon nanotubes gels by radiation technique and its selective fluoride removal. Int. J. Biol. Macromol. 2024, 282, 137447. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Bhasin, C.P. Synthesis, Characterization and Application of Graphene/Zr Composite Supported on Activated Carbon for Efficient Removal of Fluoride from Drinking Water. J. Water Chem. Technol. 2022, 44, 344–354. [Google Scholar] [CrossRef]
- Riahi, F.; Bagherzadeh, M.; Hadizadeh, Z. Modification of FeO superparamagnetic nanoparticles with zirconium oxide; preparation, characterization and its application toward fluoride removal. RSC Adv. 2015, 5, 72058–72068. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Zhou, D.; Chen, X.F.; Zhao, H.R.; Li, X.; Hughes, S.S.; Zhang, P.C.; He, P.; Zhang, G.R.; et al. Efficient removal of fluoride from neutral wastewater by green synthesized Zr/calcium sulfate whiskers: An experimental and theoretical study. Colloids Surf. Physicochem. Eng. Asp. 2021, 630, 127587. [Google Scholar] [CrossRef]
- Dou, X.M.; Mohan, D.; Pittman, C.U.; Yang, S. Remediating fluoride from water using hydrous zirconium oxide. Chem. Eng. J. 2012, 198–199, 236–245. [Google Scholar] [CrossRef]
- Piascik, J.R.; Wolter, S.D.; Stoner, B.R. Development of a novel surface modification for improved bonding to zirconia. Dent. Mater. 2011, 27, E99–E105. [Google Scholar] [CrossRef]
- Swathi, N.; Kumar, A.G.; Parthasarathy, V.; Sankarganesh, P. Isolation of species and analyzing its crude extract for the determination of in vitro antioxidant and antibacterial activities. Biomass Convers. Biorefinery 2024, 14, 3753–3762. [Google Scholar] [CrossRef]
- Wassie, T.; Niu, K.M.; Xie, C.Y.; Wang, H.H.; Xin, W. Extraction Techniques, Biological Activities and Health Benefits of Marine Algae Polysaccharide. Front. Nutr. 2021, 8, 747928. [Google Scholar] [CrossRef]
- Shen, S.X.; Zhang, T.H.; Yuan, Y.; Lin, S.Y.; Xu, J.Y.; Ye, H.Q. Effects of cinnamaldehyde on and membrane. Food Control 2015, 47, 196–202. [Google Scholar] [CrossRef]
- He, T.F.; Wang, L.H.; Niu, D.B.; Wen, Q.H.; Zeng, X.A. Cinnamaldehyde inhibit Escherichia coli associated with membrane disruption and oxidative damage. Arch. Microbiol. 2019, 201, 451–458. [Google Scholar] [CrossRef]
- Tabassum, N.; Kumar, D.; Verma, D.; Bohara, R.A.; Singh, M.P. Zirconium oxide (ZrO2) nanoparticles from antibacterial activity to cytotoxicity: A next-generation of multifunctional nanoparticles. Mater. Today Commun. 2021, 26, 102156. [Google Scholar] [CrossRef]
- Atia, D.; Zobeidi, A.; Nacer, S.N.; Ghernaout, D.; Elboughdiri, N. Biocomposite of nontronite/sp. for cationic methylene blue dye removal: Optimization, kinetics, and isothermal thermodynamics study. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Yang, R.R.; Bai, F.Q.; Mei, L.P.; Guo, W.; Qiao, H.H.; Chen, G.J.; Liu, J.S.; Ke, F.; Peng, C.Y.; Hou, R.Y.; et al. Zirconium-cerium modified polyvinyl alcohol/NaCMC biocomposite film: Synthesis of films through high-speed shear assisted technique and removal fluoride from water. Carbohydr. Polym. 2024, 339, 122239. [Google Scholar] [CrossRef]
- Yao, G.L.; Zhu, X.T.; Wang, M.Y.; Qiu, Z.W.; Zhang, T.; Qiu, F.X. Controlled Fabrication of the Biomass Cellulose-CeO Nanocomposite Membrane as Efficient and Recyclable Adsorbents for Fluoride Removal. Ind. Eng. Chem. Res. 2021, 60, 5914–5923. [Google Scholar] [CrossRef]
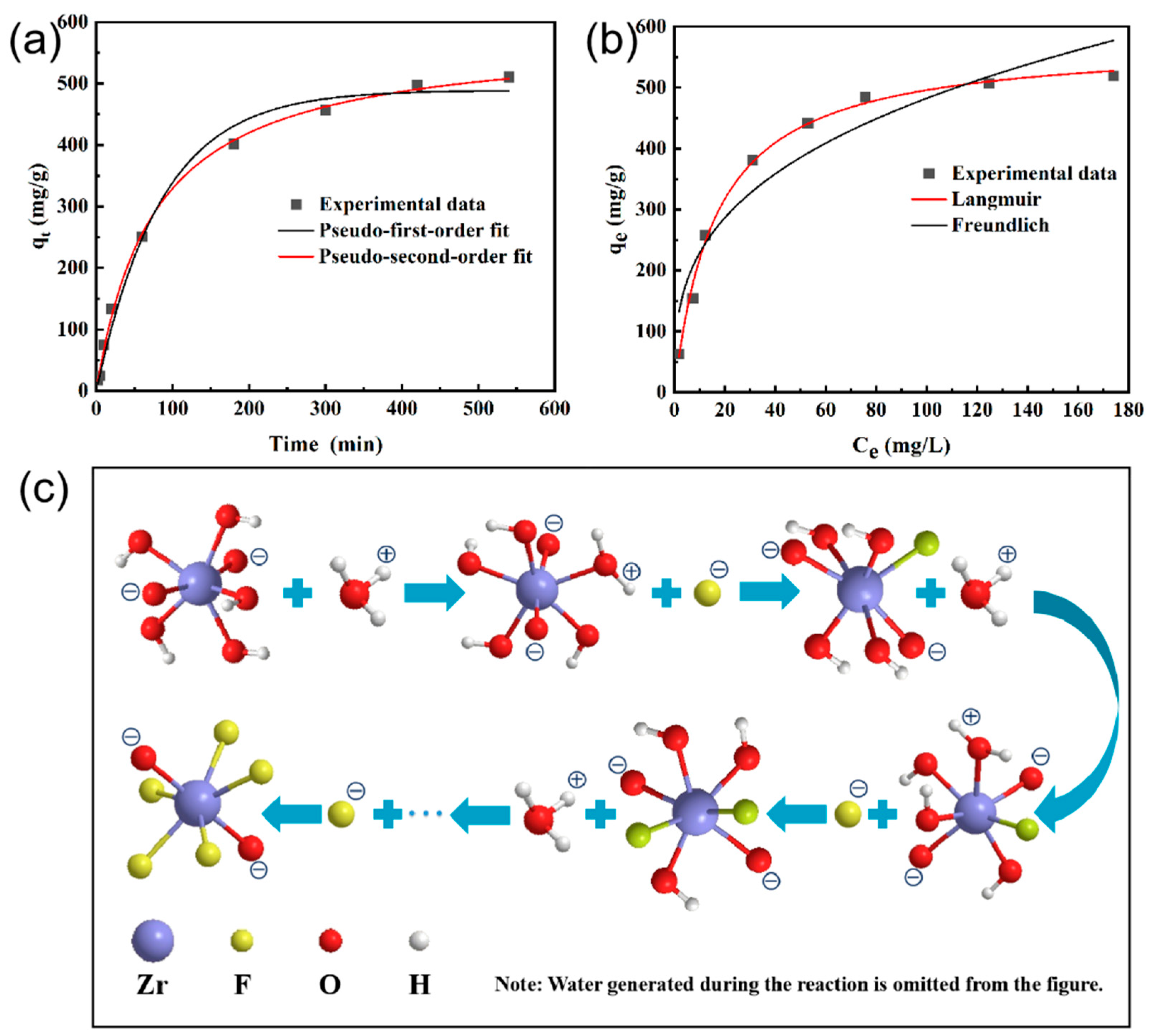


| Adsorbent | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| Qe (mg/g) | k1 | R2 | Qe (mg/g) | k2 | R2 | |
| 489.0815 | 0.01182 | 0.9907 | 579.0373 | 2.2790 × 10−5 | 0.9987 | |
| Isotherm | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF (mg/g) | n | R2 | |
| 577.1421 | 0.06114 | 0.9955 | 108.5427 | 3.0867 | 0.9138 | |
| Adsorption Materials | Adsorption Elements | Amount of Adsorption | Ref. |
|---|---|---|---|
| Mn-Zr-Ethanol composite | F− | 32.87 mg/g | [39] |
| Zr-CS/GO membrane | F− | 29.0588 mg/g | [20] |
| Zirconium oxide/PAN nanofibers | F− | 67.51 mg/g | [40] |
| Hierarchical zinc-zirconium oxide microspheres | F− | 107.41 mg/g | [41] |
| Hydrous zirconium oxide-impregnated CS | F− | 222.2 mg/g | [42] |
| Porous zirconium alginate beads adsorbent | F− | 32.797 mg/g | [43] |
| Zirconium(IV) immobilized carboxymethyl cellulose/multiwalled carbon nanotubes gels | F− | 36.657 mg/g | [44] |
| Graphene/Zr on Activated Carbon | F− | 81.47 mg/g | [45] |
| Fe3O4-ZrO2 NPs | F− | 158.6 mg g | [46] |
| CIN/EP-ZrO2 composite membrane | F− | 577.1421 mg/g | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Lei, Y.; Fan, X.; Wei, G.; Guo, L. Biomimetic Construction of Enteromorpha prolifera-Based Composite Membranes for Synergistic Purification of Fluoride Ions, Bacteria, and Dye with High Sustainability. Materials 2025, 18, 2356. https://doi.org/10.3390/ma18102356
Li W, Lei Y, Fan X, Wei G, Guo L. Biomimetic Construction of Enteromorpha prolifera-Based Composite Membranes for Synergistic Purification of Fluoride Ions, Bacteria, and Dye with High Sustainability. Materials. 2025; 18(10):2356. https://doi.org/10.3390/ma18102356
Chicago/Turabian StyleLi, Wanying, Yu Lei, Xiaoxuan Fan, Gang Wei, and Lei Guo. 2025. "Biomimetic Construction of Enteromorpha prolifera-Based Composite Membranes for Synergistic Purification of Fluoride Ions, Bacteria, and Dye with High Sustainability" Materials 18, no. 10: 2356. https://doi.org/10.3390/ma18102356
APA StyleLi, W., Lei, Y., Fan, X., Wei, G., & Guo, L. (2025). Biomimetic Construction of Enteromorpha prolifera-Based Composite Membranes for Synergistic Purification of Fluoride Ions, Bacteria, and Dye with High Sustainability. Materials, 18(10), 2356. https://doi.org/10.3390/ma18102356







