Ferroelectric Smectic Liquid Crystalline Materials with Different Degree of Chirality
Abstract
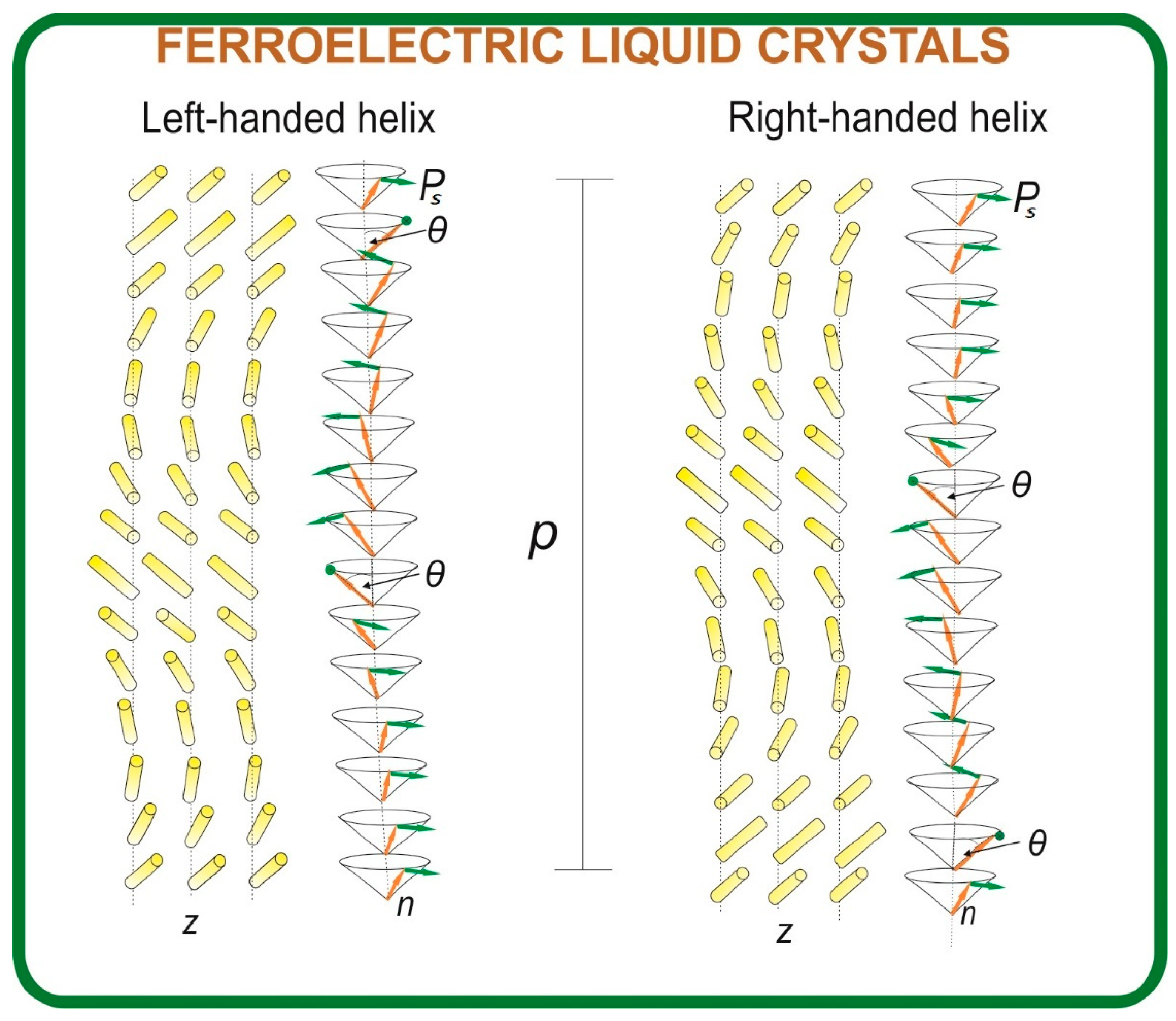
1. Introduction
2. Materials and Methods
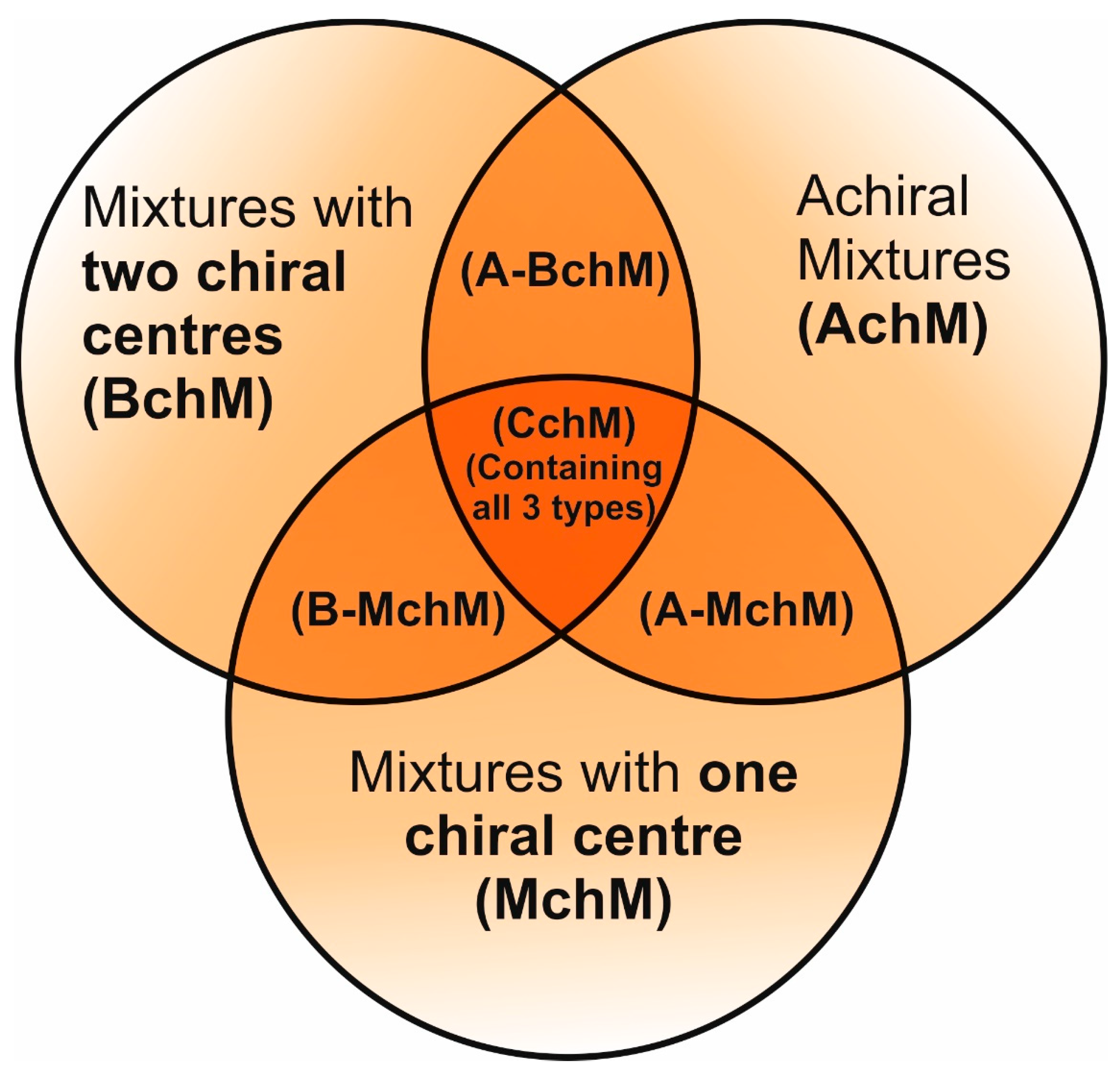
2.1. FLC Mixtures
2.2. Polarizing Optical Microscopy and Differential Scanning Calorimetry
2.3. Helical Pitch Measurement
2.4. Electro-Optical Properties, Spontaneous Polarization, and Tilt Angle
3. Results and Discussion
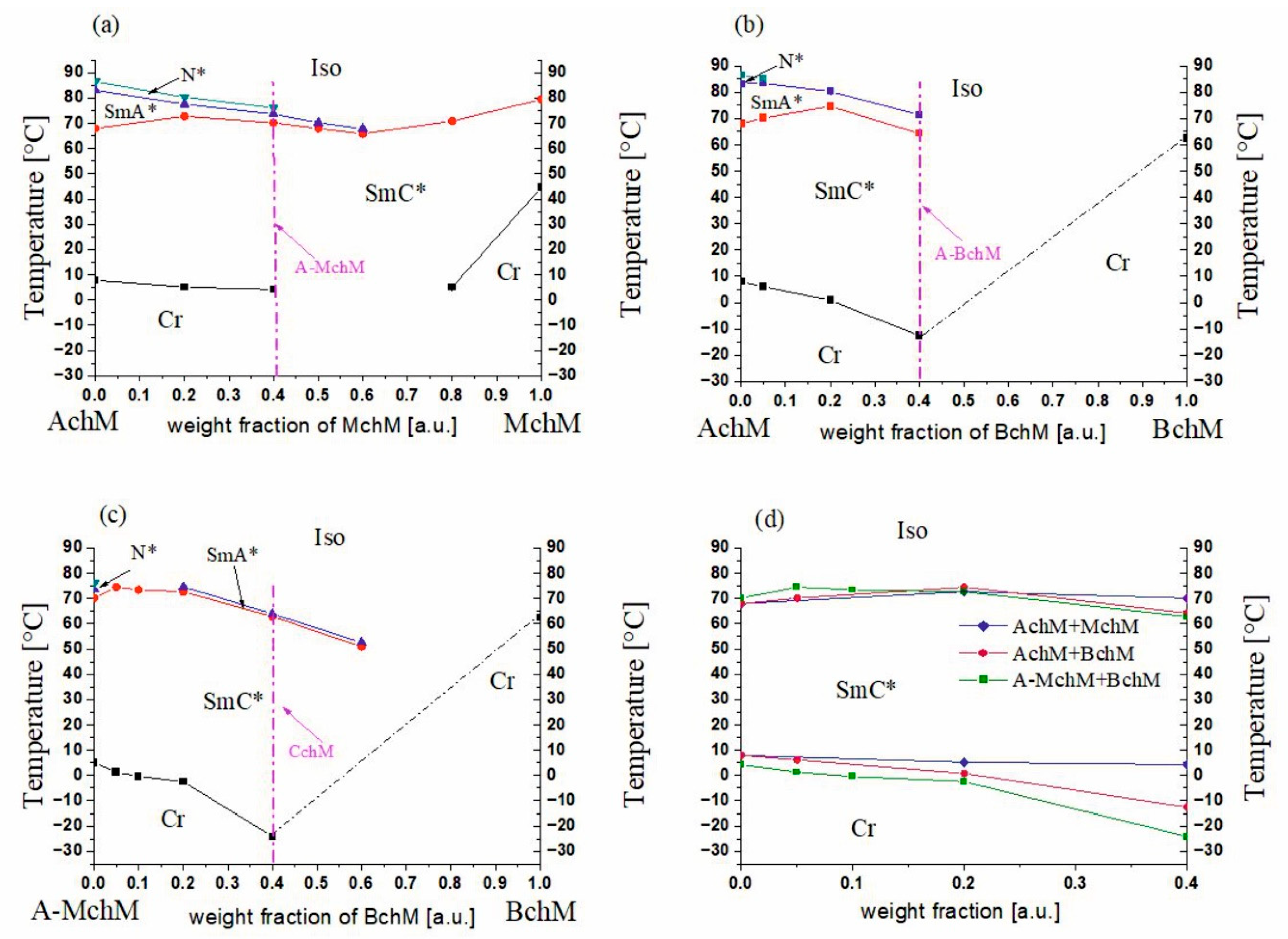
3.1. Mesomorphic Behavior
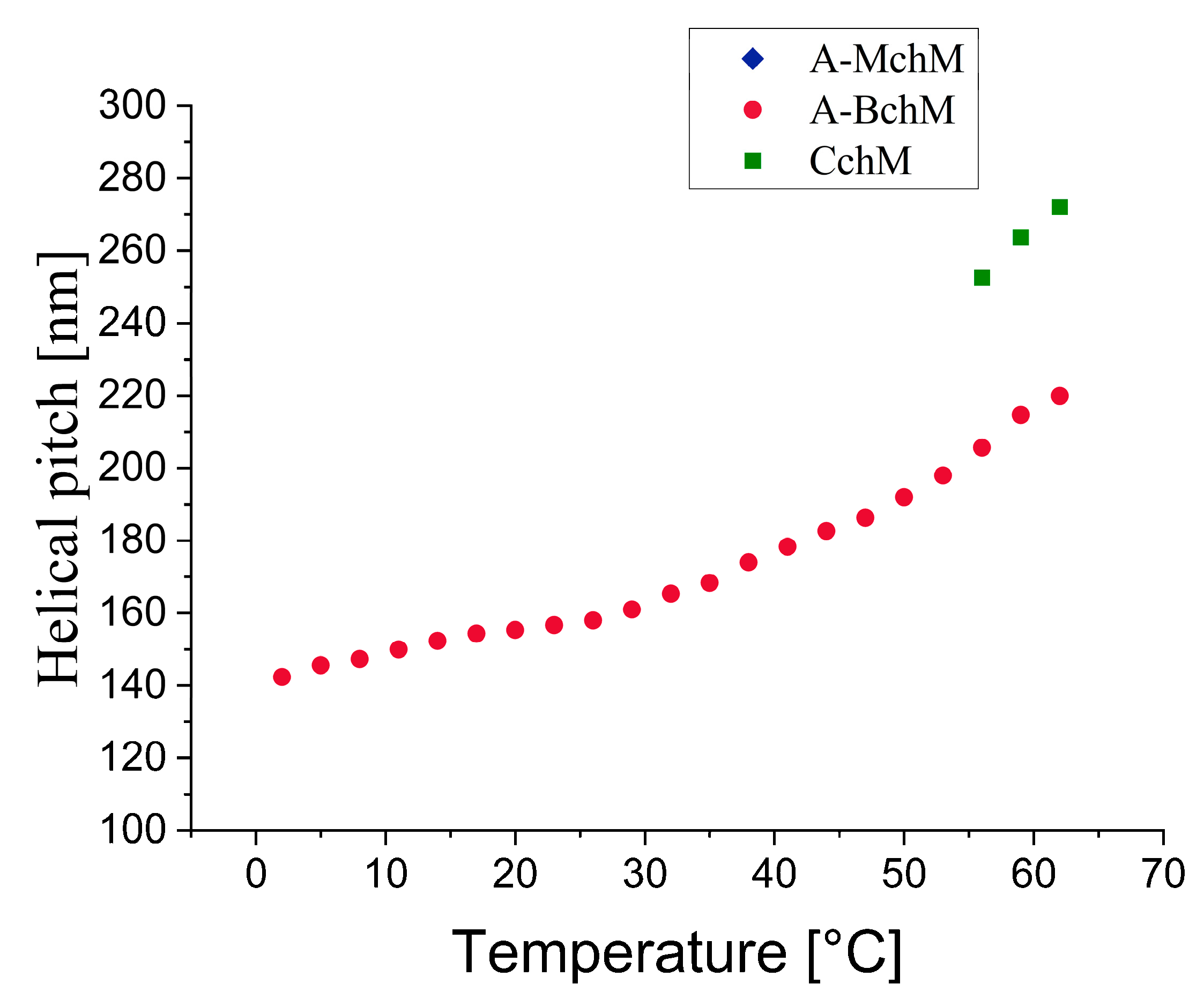
3.2. Helical Pitch
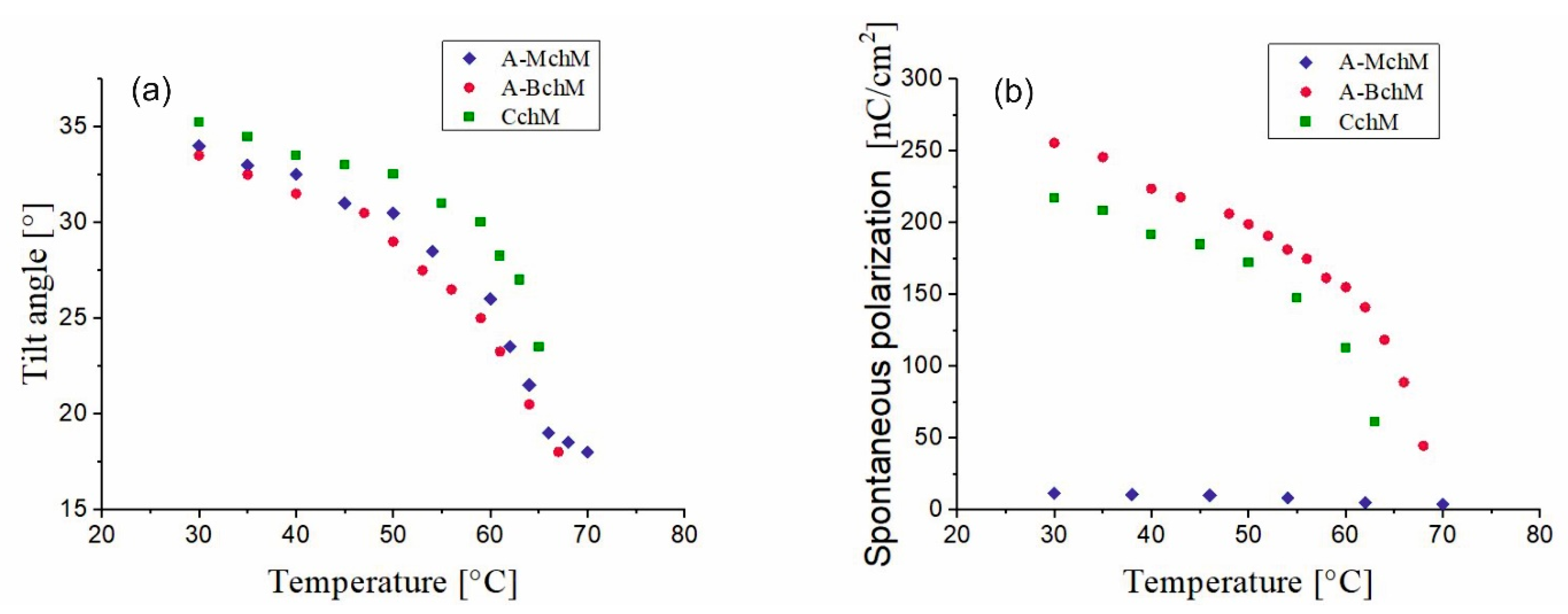
3.3. Tilt Angle and Spontaneous Polarization
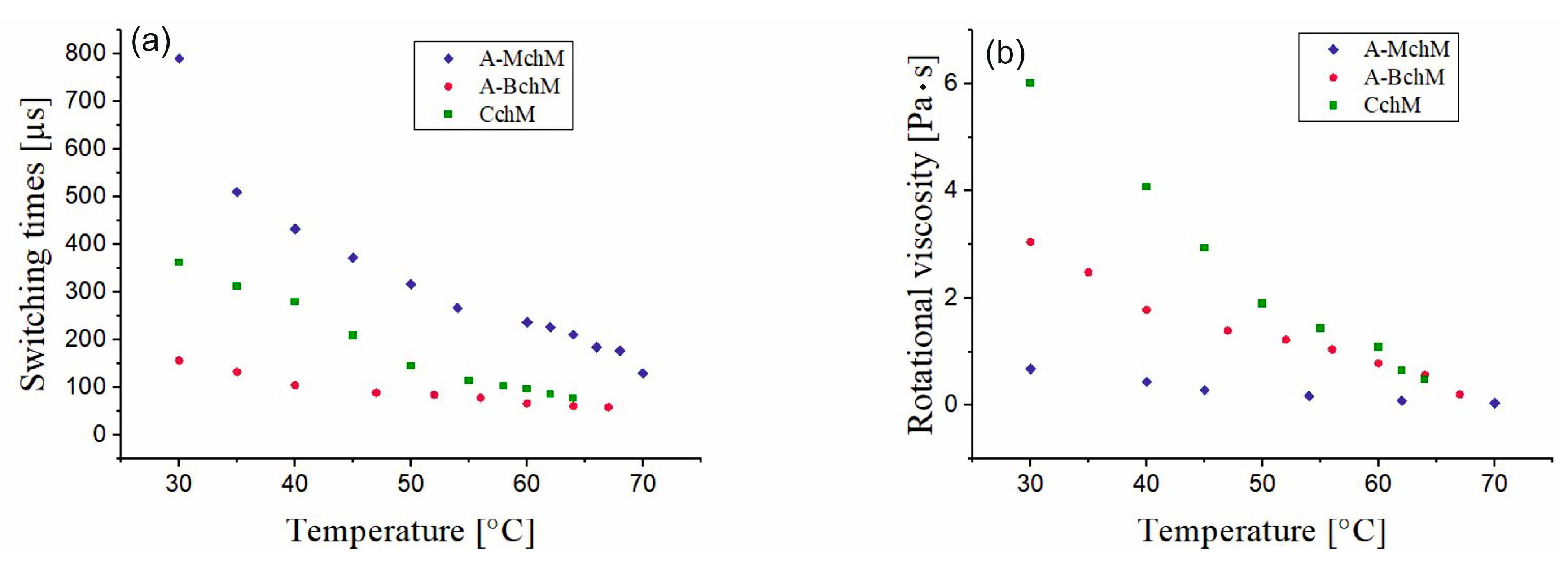
3.4. Switching Time and Rotational Viscosity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lagerwall, J.P.F.; Giesselmann, F. Current Topics in Smectic Liquid Crystal Research. ChemPhysChem 2006, 7, 20–45. [Google Scholar] [CrossRef]
- Coles, H.; Morris, S. Liquid-Crystal Lasers. Nat. Photonics 2010, 4, 676–685. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Scalia, G. A New Era for Liquid Crystal Research: Applications of Liquid Crystals in Soft Matter Nano-, Bio- and Microtechnology. Curr. Appl. Phys. 2012, 12, 1387–1412. [Google Scholar] [CrossRef]
- Swaminathan, V.; Panov, V.P.; Panov, A.; Rodriguez-Lojo, D.; Stevenson, P.J.; Gorecka, E.; Vij, J.K. Design and Electro-Optic Investigations of de Vries Chiral Smectic Liquid Crystals for Exhibiting Broad Temperature Ranges of SmA* and SmC* Phases and Fast Electro-Optic Switching. J. Mater. Chem. C 2020, 8, 4859–4868. [Google Scholar] [CrossRef]
- Brodzeli, Z.; Silvestri, L.; Michie, A.; Guo, Q.; Pozhidaev, E.P.; Chigrinov, V.; Ladouceur, F. Sensors at Your Fibre Tips: A Novel Liquid Crystal-Based Photonic Transducer for Sensing Systems. J. Light. Technol. 2013, 31, 2940–2946. [Google Scholar] [CrossRef]
- Al Abed, A.; Srinivas, H.; Firth, J.; Ladouceur, F.; Lovell, N.H.; Silvestri, L. A Biopotential Optrode Array: Operation Principles and Simulations. Sci. Rep. 2018, 8, 2690. [Google Scholar] [CrossRef] [PubMed]
- Al Abed, A.; Wei, Y.; Almasri, R.M.; Lei, X.; Wang, H.; Firth, J.; Chen, Y.; Gouailhardou, N.; Silvestri, L.; Lehmann, T.; et al. Liquid Crystal Electro-Optical Transducers for Electrophysiology Sensing Applications. J. Neural Eng. 2022, 19, 056031. [Google Scholar] [CrossRef]
- Yoshizawa, A. Ferroelectric Smectic Liquid Crystals. Crystals 2024, 14, 350. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Han, J.; Yang, L.; Li, J.; Song, Z.; Wang, T.; Zhu, J. Advanced Liquid Crystal-Based Switchable Optical Devices for Light Protection Applications: Principles and Strategies. Light Sci. Appl. 2023, 12, 11. [Google Scholar] [CrossRef]
- Clark, N.A.; Lagerwall, S.T. Submicrosecond Bistable Electro-optic Switching in Liquid Crystals. Appl. Phys. Lett. 1980, 36, 899–901. [Google Scholar] [CrossRef]
- Nabadda, E.; Bennis, N.; Czerwinski, M.; Walewska, A.; Jaroszewicz, L.R.; del Mar Sánchez-López, M.; Moreno, I. Ferroelectric Liquid-Crystal Modulator with Large Switching Rotation Angle for Polarization-Independent Binary Phase Modulation. Opt. Lasers Eng. 2022, 159, 107204. [Google Scholar] [CrossRef]
- Hamplová, V.; Bubnov, A.; Kašpar, M.; Novotná, V.; Glogarová, M. New Series of Chiral Ferroelectric Liquid Crystals with the Keto Group Attached to the Molecule Core. Liq. Cryst. 2003, 30, 493–497. [Google Scholar] [CrossRef]
- Bubnov, A.; Novotná, V.; Hamplová, V.; Kašpar, M.; Glogarová, M. Effect of Multilactate Chiral Part of Liquid Crystalline Molecule on Mesomorphic Behaviour. J. Mol. Struct. 2008, 892, 151–157. [Google Scholar] [CrossRef]
- Fitas, J.; Marzec, M.; Kurp, K.; Żurowska, M.; Tykarska, M.; Bubnov, A. Electro-Optic and Dielectric Properties of New Binary Ferroelectric and Antiferroelectric Liquid Crystalline Mixtures. Liq. Cryst. 2017, 44, 1468–1477. [Google Scholar] [CrossRef]
- Bubnov, A.; Podoliak, N.; Hamplová, V.; Tomášková, P.; Havlíček, J.; Kašpar, M. Eutectic Behaviour of Binary Mixtures Composed of Two Isomeric Lactic Acid Derivatives. Ferroelectrics 2016, 495, 105–115. [Google Scholar] [CrossRef]
- Tykarska, M.; Kurp, K.; Zieja, P.; Herman, J.; Stulov, S.; Bubnov, A. New Quaterphenyls Laterally Substituted by Methyl Group and Their Influence on the Self-Assembling Behaviour of Ferroelectric Bicomponent Mixtures. Liq. Cryst. 2022, 49, 821–835. [Google Scholar] [CrossRef]
- Wȩgłowska, D.; Perkowski, P.; Chrunik, M.; Czerwiński, M. The Effect of Dopant Chirality on the Properties of Self-Assembling Materials with a Ferroelectric Order. Phys. Chem. Chem. Phys. 2018, 20, 9211–9220. [Google Scholar] [CrossRef]
- Lalik, S.; Deptuch, A.; Fryń, P.; Jaworska-Gołąb, T.; Węgłowska, D.; Marzec, M. Physical Properties of New Binary Ferroelectric Mixture. J. Mol. Liq. 2019, 274, 540–549. [Google Scholar] [CrossRef]
- Singh, G.; Vijaya Prakash, G.; Kaur, S.; Choudhary, A.; Biradar, A.M. Molecular Relaxation in Homeotropically Aligned Ferroelectric Liquid Crystals. Phys. B Condens. Matter 2008, 403, 3316–3319. [Google Scholar] [CrossRef]
- Kurp, K.; Czerwiński, M.; Tykarska, M.; Salamon, P.; Bubnov, A. Design of Functional Multicomponent Liquid Crystalline Mixtures with Nano-Scale Pitch Fulfilling Deformed Helix Ferroelectric Mode Demands. J. Mol. Liq. 2019, 290, 111329. [Google Scholar] [CrossRef]
- Żurowska, M.; Dziaduszek, J.; Szala, M.; Morawiak, P.; Bubnov, A. Effect of Lateral Fluorine Substitution Far from the Chiral Center on Mesomorphic Behaviour of Highly Titled Antiferroelectric (S) and (R) Enantiomers. J. Mol. Liq. 2018, 267, 504–510. [Google Scholar] [CrossRef]
- Bubnov, A.; Domenici, V.; Hamplová, V.; Kašpar, M.; Veracini, C.A.; Glogarová, M. Orientational and Structural Properties of Ferroelectric Liquid Crystal with a Broad Temperature Range in the SmC* Phase by 13CNMR, X-ray Scattering and Dielectric Spectroscopy. J. Phys. Condens. Matter 2009, 21, 035102. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, M.; Zając, M.; Czerwiński, M.; Morawiak, P.; Bubnov, A.; Deptuch, A. Synthesis and Properties of Highly Tilted Antiferroelectric Liquid Crystalline (R) Enantiomers. Materials 2024, 17, 4967. [Google Scholar] [CrossRef] [PubMed]
- Vimal, T.; Pandey, S.; Gupta, S.K.; Singh, D.P.; Agrahari, K.; Pathak, G.; Kumar, S.; Tripathi, P.K.; Manohar, R. Manifestation of Strong Magneto-Electric Dipolar Coupling in Ferromagnetic Nanoparticles−FLC Composite: Evaluation of Time-Dependent Memory Effect. Liq. Cryst. 2018, 45, 687–697. [Google Scholar] [CrossRef]
- Singh, D.P.; Boussoualem, Y.; Duponchel, B.; Hadj Sahraoui, A.; Kumar, S.; Manohar, R.; Daoudi, A. Pico-Ampere Current Sensitivity and CdSe Quantum Dots Assembly Assisted Charge Transport in Ferroelectric Liquid Crystal. J. Phys. D Appl. Phys. 2017, 50, 325301. [Google Scholar] [CrossRef]
- Agrahari, K.; Vimal, T.; Rastogi, A.; Pandey, K.K.; Gupta, S.; Kurp, K.; Manohar, R. Ferroelectric Liquid Crystal Mixture Dispersed with Tin Oxide Nanoparticles: Study of Morphology, Thermal, Dielectric and Optical Properties. Mater. Chem. Phys. 2019, 237, 121851. [Google Scholar] [CrossRef]
- Misra, A.K.; Roy, A.; Pratap Singh, B.; Pandey, K.K.; Shrivas, R.; Saluja, J.K.; Tripathi, P.K.; Manohar, R. Influence of SiO2 Nanoparticles on the Dielectric Properties and Anchoring Energy Parameters of Pure Ferroelectric Liquid Crystal. J. Dispers. Sci. Technol. 2020, 41, 2136–2142. [Google Scholar] [CrossRef]
- Vimal, T.; Pujar, G.H.; Agrahari, K.; Inamdar, S.R.; Manohar, R. Nanoparticle Surface Energy Transfer (NSET) in Ferroelectric Liquid Crystal-Metallic-Silver Nanoparticle Composites: Effect of Dopant Concentration on NSET Parameters. Phys. Rev. E 2021, 103, 022708. [Google Scholar] [CrossRef]
- Beresnev, L.A.; Chigrinov, V.G.; Dergachevt, D.I.; Poshidaev, E.P.; Fünfschilling, J.; Schadt, M. Deformed Helix Ferroelectric Liquid Crystal Display: A New Electrooptic Mode in Ferroelectric Chiral Smectic C Liquid Crystals. Liq. Cryst. 1989, 5, 1171–1177. [Google Scholar] [CrossRef]
- Pozhidaev, E.; Chigrinov, V.; Murauski, A.; Molkin, V.; Tao, D.; Kwok, H.-S. V-Shaped Electro-Optical Mode Based on Deformed-Helix Ferroelectric Liquid Crystal with Subwavelength Pitch. J. Soc. Inf. Disp. 2012, 20, 273–278. [Google Scholar] [CrossRef]
- Silvestri, L.; Chen, Y.; Brodzeli, Z.; Sirojan, T.; Lu, S.; Liu, Z.; Phung, T.; Ladouceur, F. A Novel Optical Sensing Technology for Monitoring Voltage and Current of Overhead Power Lines. IEEE Sens. J. 2021, 21, 26699–26707. [Google Scholar] [CrossRef]
- Chen, Y.; Silvestri, L.; Lei, X.; Ladouceur, F. Optically Powered Gas Monitoring System Using Single-Mode Fibre for Underground Coal Mines. Int. J. Coal Sci. Technol. 2022, 9, 26. [Google Scholar] [CrossRef]
- Mikhailenko, V.; Krivoshey, A.; Pozhidaev, E.; Popova, E.; Fedoryako, A.; Gamzaeva, S.; Barbashov, V.; Srivastava, A.K.; Kwok, H.S.; Vashchenko, V. The Nano-Scale Pitch Ferroelectric Liquid Crystal Materials for Modern Display and Photonic Application Employing Highly Effective Chiral Components: Trifluoromethylalkyl Diesters of p-Terphenyldicarboxylic Acid. J. Mol. Liq. 2019, 281, 186–195. [Google Scholar] [CrossRef]
- Czerwiński, M.; Gaładyk, K.; Morawiak, P.; Piecek, W.; Chrunik, M.; Kurp, K.; Kula, P.; Jaroszewicz, L.R. Pyrimidine-Based Ferroelectric Mixtures—The Influence of Oligophenyl Based Chiral Doping System. J. Mol. Liq. 2020, 303, 112693. [Google Scholar] [CrossRef]
- Kotova, S.P.; Pozhidaev, E.P.; Samagin, S.A.; Kesaev, V.V.; Barbashov, V.A.; Torgova, S.I. Ferroelectric Liquid Crystal with Sub-Wavelength Helix Pitch as an Electro-Optical Medium for High-Speed Phase Spatial Light Modulators. Opt. Laser Technol. 2021, 135, 106711. [Google Scholar] [CrossRef]
- Fitas, J.; Marzec, M.; Szymkowiak, M.; Jaworska-Gołąb, T.; Deptuch, A.; Tykarska, M.; Kurp, K.; Żurowska, M.; Bubnov, A. Mesomorphic, Electro-Optic and Structural Properties of Binary Liquid Crystalline Mixtures with Ferroelectric and Antiferroelectric Liquid Crystalline Behaviour. Phase Transit. 2018, 91, 1017–1026. [Google Scholar] [CrossRef]
- Padmini, V.; Babu, P.N.; Nair, G.G.; Rao, D.S.S.; Yelamaggad, C.V. Optically Biaxial, Re-Entrant and Frustrated Mesophases in Chiral, Non-Symmetric Liquid Crystal Dimers and Binary Mixtures. Chem. Asian J. 2016, 11, 2897–2910. [Google Scholar] [CrossRef]
- Węgłowska, D.; Chen, Y.; Ladouceur, F.; Silvestri, L.; Węgłowski, R.; Czerwiński, M. Single Ferroelectric Liquid Crystal Compounds Targeted for Optical Voltage Sensing. J. Mol. Liq. 2024, 399, 124454. [Google Scholar] [CrossRef]
- Tykarska, M.; Dąbrowski, R.; Czerwiński, M.; Chełstowska, A.; Piecek, W.; Morawiak, P. The Influence of the Chiral Terphenylate on the Tilt Angle in Pyrimidine Ferroelectric Mixtures. Phase Transit. 2012, 85, 364–370. [Google Scholar] [CrossRef]
- Kowiorski, K.; Kędzierski, J.; Raszewski, Z.; Kojdecki, M.A.; Chojnowska, O.; Garbat, K.; Miszczyk, E.; Piecek, W. Complementary Interference Method for Determining Optical Parameters of Liquid Crystals. Phase Transit. 2016, 89, 403–410. [Google Scholar] [CrossRef]
- Bahr, C.H.; Heppke, G. Optical and Dielectric Investigations on the Electroclinic Effect Exhibited by a Ferroelectric Liquid Crystal with High Spontaneous Polarization. Liq. Cryst. 1987, 2, 825–831. [Google Scholar] [CrossRef]
- Miyasato, K.; Abe, S.; Takezoe, H.; Fukuda, A.; Kuze, E. Direct Method with Triangular Waves for Measuring Spontaneous Polarization in Ferroelectric Liquid Crystals. Jpn. J. Appl. Phys. 1983, 22, 661–663. [Google Scholar] [CrossRef]
- Skarp, K. Rotational Viscosities in Ferroelectric Smectic Liquid Crystals. Ferroelectrics 1988, 84, 119–142. [Google Scholar] [CrossRef]
- Pozhidaev, E.P.; Budynina, E.M.; Kuznetsov, A.V.; Torgova, S.I.; Tkachenko, T.P.; Barbashov, V.A. Short Helix Pitch Ferroelectric Liquid Crystals Induced in Nematic Matrix by Chiral Non-Mesogenic Dopants. J. Mol. Liq. 2023, 391, 123351. [Google Scholar] [CrossRef]
| Numbers of Component | Chemical Formulae | Weight Fraction |
|---|---|---|
| 1 |  | 0.16 |
| 2 | 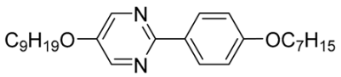 | 0.01 |
| 3 | 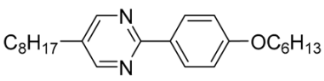 | 0.02 |
| 4 | 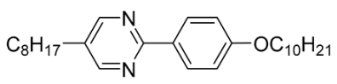 | 0.05 |
| 5 |  | 0.19 |
| 6 | 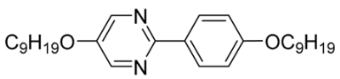 | 0.14 |
| 7 |  | 0.05 |
| 8 |  | 0.13 |
| 9 |  | 0.03 |
| 10 | 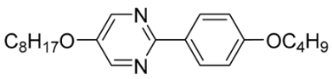 | 0.22 |
| Numbers of Component | Chemical Formulae | Weight Fraction |
|---|---|---|
| 11 |  | 0.52 |
| 12 | 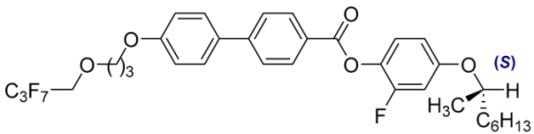 | 0.48 |
| Numbers of Component | Chemical Formulae | Weight Fraction |
|---|---|---|
| 13 |  | 0.44 |
| 14 | 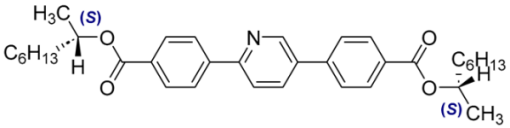 | 0.56 |
| Composition of the Mixture (Weight Fraction) | |||
|---|---|---|---|
| Mixture Acronym | AchM Mixture | MchM Mixture | BchM Mixture |
| 0.2 MchM + 0.8 AchM | 0.80 | 0.20 | - |
| 0.4 MchM + 0.6 AchM (designated as A-MchM) | 0.60 | 0.40 | - |
| 0.5 MchM + 0.5 AchM | 0.50 | 0.50 | - |
| 0.6 MchM + 0.4 AchM | 0.40 | 0.60 | - |
| 0.8 MchM + 0.2 AchM | 0.20 | 0.80 | - |
| 0.05 BchM + 0.95 AchM | 0.95 | - | 0.05 |
| 0.2 BchM + 0.8 AchM | 0.80 | - | 0.20 |
| 0.4BchM + 0.6 AchM (designated as A-BchM) | 0.60 | - | 0.40 |
| 0.4 A-MchM + 0.6 BchM | 0.24 | 0.16 | 0.60 |
| 0.6 A-MchM + 0.4 BchM (designated as CchM) | 0.36 | 0.24 | 0.40 |
| 0.8 A-MchM + 0.2 BchM | 0.48 | 0.32 | 0.20 |
| 0.9 A-MchM + 0.1 BchM | 0.54 | 0.36 | 0.10 |
| 0.95 A-MchM + 0.05 BchM | 0.57 | 0.38 | 0.05 |
| Mixture Acronym | Cr | ▪ | SmC* | ▪ | SmA* | ▪ | N* | ▪ | Iso |
|---|---|---|---|---|---|---|---|---|---|
| A-MchM | ▪ | 4.2 11.74 | ▪ | 70.2 # - | ▪ | 73.6 # - | ▪ | 74.5 5.58 | ▪ |
| A-BchM | ▪ | −12.6 7.28 | ▪ | 64.3 # - | ▪ | 73.5 4.89 | - | - | ▪ |
| CchM | ▪ | −24.1 1.88 | ▪ | 62.9 # - | ▪ | 65.2 3.11 | - | - | ▪ |
| Mixture Acronym | Temperature Range of SmC* [°C] | Properties at 30 °C | ||||
|---|---|---|---|---|---|---|
| p [nm] | θ [°] | PS [nC/cm2] | τ [μs] | γφ [Pa·s] | ||
| A-MchM | 4.2 < SmC* > 74.5 | >2000 | 34.0 | 11 | 790 | 0.67 |
| A-BchM | −12.6 < SmC* > 82.4 | 160 | 34.5 | 255 | 156 | 3.04 |
| CchM | −24.1 < SmC* > 67.2 | <240 | 36.0 | 217 | 362 | 6.00 |
| NFLC-4-eut [44] | 18.0 < SmC* > 60.0 | ~150 | ~30.0 | ~75 | 200 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwiński, M.; Filipow, M.; Łuczak, K.; Węgłowska, D. Ferroelectric Smectic Liquid Crystalline Materials with Different Degree of Chirality. Materials 2025, 18, 2343. https://doi.org/10.3390/ma18102343
Czerwiński M, Filipow M, Łuczak K, Węgłowska D. Ferroelectric Smectic Liquid Crystalline Materials with Different Degree of Chirality. Materials. 2025; 18(10):2343. https://doi.org/10.3390/ma18102343
Chicago/Turabian StyleCzerwiński, Michał, Mateusz Filipow, Klaudia Łuczak, and Dorota Węgłowska. 2025. "Ferroelectric Smectic Liquid Crystalline Materials with Different Degree of Chirality" Materials 18, no. 10: 2343. https://doi.org/10.3390/ma18102343
APA StyleCzerwiński, M., Filipow, M., Łuczak, K., & Węgłowska, D. (2025). Ferroelectric Smectic Liquid Crystalline Materials with Different Degree of Chirality. Materials, 18(10), 2343. https://doi.org/10.3390/ma18102343







