The Effect of Photoreactive Diluents on the Properties of a Styrene-Free Vinyl Ester Resin for Cured-In-Place Pipe (CIPP) Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Sample Preparation
- (a)
- A styrene-free vinyl ester resin (VE) (L050-LCW-03 FC, AOC GmbH, Kaiserslautern, Germany);
- (b)
- Photoreactive mono-, di-, and trifunctional diluents (RDs):
- -
- Acrylic monomers: 1,6-hexanediol diacrylate (HDDA; BASF, Ludwigshafen, Germany), aliphatic urethane acrylates (Laromer UA9028 and Laromer UA19T; BASF, Ludwigshafen, Germany), an aromatic epoxy acrylate (Laromer EA9081, BASF, Ludwigshafen, Germany), trimethylolpropane ethoxylate triacrylate (Photomer 4149; IGM Resins, Waalwijk, The Netherlands), trimethylolpropane triacrylate (TMPTA; Allnex, Drogenbos, Belgium), and pentaerythritol triacrylate (PETIA; Allnex, Drogenbos, Belgium);
- -
- A methacrylic monomer: methyl methacrylate (MMA; Carl Roth, Karlsruhe, Germany);
- -
- A vinyl monomer: vinyl neodecanoate (VeoVa10; Hexion, Columbus, OH, USA).
- (c)
- A radical photoinitiator: A blend of bis(2,4,6-trimethylbenzoyl)-phenylphosphine oxide (~95 wt%) and ethyl(2,4,6-trimethylbenzoyl)-phenyl phosphinate (~5 wt%) (Omnirad 2100; IGM Resins, Waalwijk, The Netherlands).
2.2. Methods
2.2.1. Dynamic Viscosity
2.2.2. Photo-DSC Measurements
2.2.3. DSC Measurements
2.2.4. Gel Content
2.2.5. Mechanical Properties
3. Results and Discussion
3.1. Dynamic Viscosity of the VE-RD Composition
3.2. Photopolymerization Kinetics of the UV-Curable VE-RD Systems
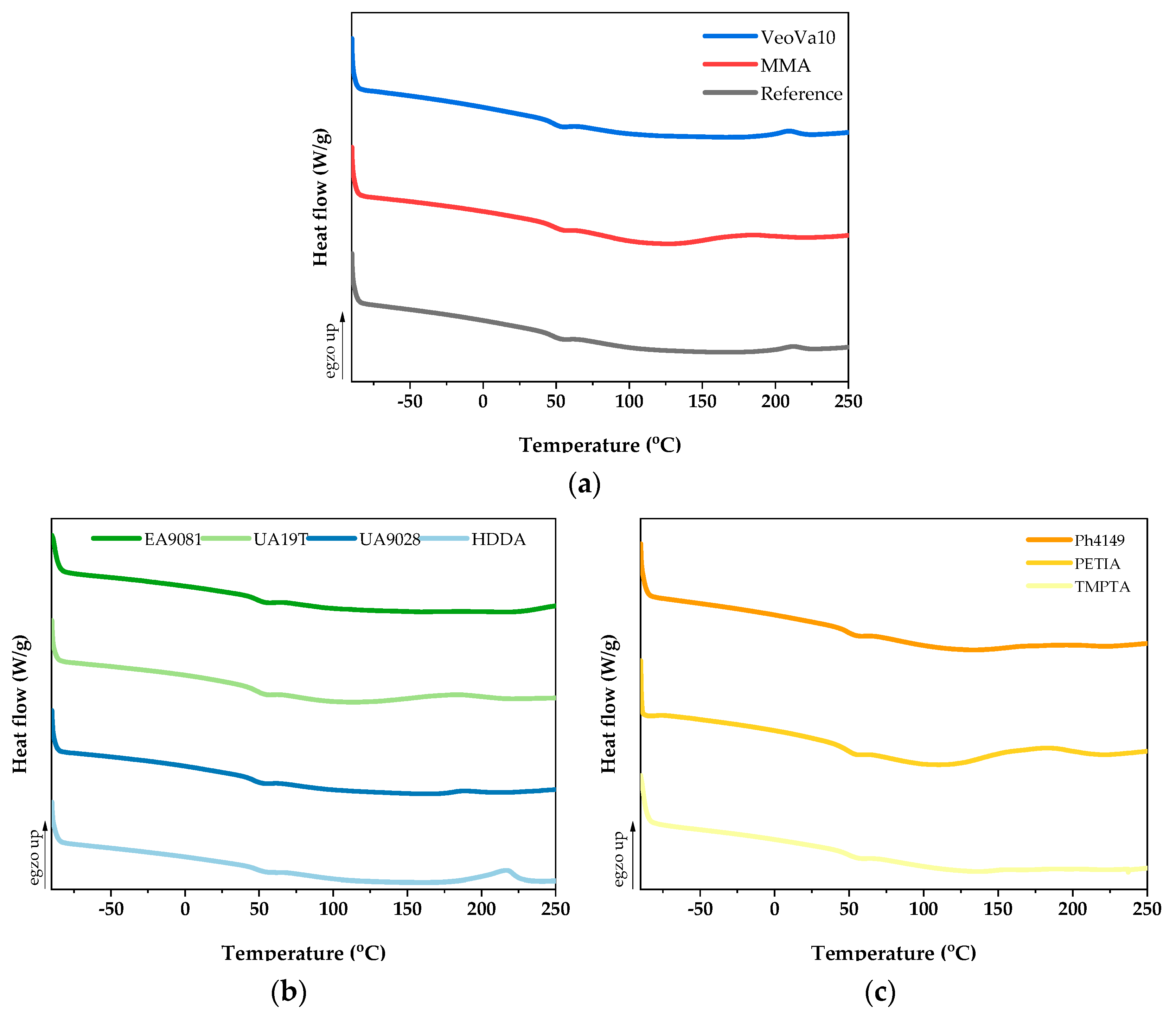
3.3. Thermal Analysis and Gel Content of UV-Cured VE-RD Compositions
3.4. Mechanical Properties of UV-Cured VE-RD Compositions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dvořák, R.; Jakubka, L.; Topolář, L.; Rabenda, M.; Wirowski, A.; Puchýř, J.; Kusák, I.; Pazdera, L. Non-Destructive Characterization of Cured-in-Place Pipe Defects. Materials 2023, 16, 7570. [Google Scholar] [CrossRef] [PubMed]
- Allison, G.R.; Gill, B.L.; Mebarkia, S. UV-Cured Fiber-Reinforced Composites for Trenchless Pipeline Rehabilitation: A Review. Polymers 2021, 13, 2157. [Google Scholar] [CrossRef]
- Kandola, B.K.; Mistik, S.I.; Pornwannachai, W. Thermosetting Resins for Composite Liners: Comparative Study of Vinyl Esters vs. Polyesters. Materials 2020, 13, 112. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Wang, S. Comparative Analysis of Thermal vs. UV-Curing in CIPP Rehabilitation: Energy Efficiency and Mechanical Performance. Materials 2023, 16, 1123. [Google Scholar] [CrossRef]
- Kandelbauer, A.; Tondi, G.; Zaske, O.C.; Goodman, S.H. Unsaturated Polyesters and Vinyl Esters. In Handbook of Thermoset Plastics, 3rd ed.; Dodiuk, H., Goodman, S.H., Eds.; William Andrew Publishing: Norwich, NY, USA, 2014; pp. 111–172. ISBN 978-1-4557-3107-7. [Google Scholar] [CrossRef]
- Yang, Y.; Boogh, L.; Meure, S. Vinyl Ester Resins: Molecular Engineering and Crosslinking Mechanisms. Prog. Polym. Sci. 2022, 134, 101608. [Google Scholar] [CrossRef]
- Decker, C.; Bianchi, C. UV-Curing of Vinyl Ester Resins: Photoinitiator Selection and Depth of Cure. Polym. Int. 2021, 70, 542–551. [Google Scholar] [CrossRef]
- Scott, T.F.; Cook, W.D.; Forsythe, J.S. Photo-DSC Cure Kinetics of Vinyl Ester Resins. I. Influence of Temperature. Polymer 2002, 43, 5839–5845. [Google Scholar] [CrossRef]
- Yang, X.J.; Li, S.H.; Tang, X.D.; Xia, J.L. Research on Preparation, Properties and UV Curing Behaviors of Novel Myrcene-Based Vinyl Ester Resin. Adv. Mater. Res. 2013, 807–809, 2826–2830. [Google Scholar] [CrossRef]
- Tu, R.; Sodano, H.A. Additive Manufacturing of High-Performance Vinyl Ester Resin via Direct Ink Writing with UV-Thermal Dual Curing. Addit. Manuf. 2021, 46, 102180. [Google Scholar] [CrossRef]
- Salman, M.; Markus, W.; Dominguez, J.; Bublitz, D.; Schloegl, S. Prospects in the application of a frontally curable epoxy resin for cured-in-place-pipe rehabilitation. J. Appl. Polym. Sci. 2023, 141, e55024. [Google Scholar] [CrossRef]
- Saenz-Dominguez, I.; Tena, I.; Sarrionandia, M.; Torre, J.; Aurrekoetxea, J. Effect of Ultraviolet Curing Kinetics on the Me-chanical Properties of out of Die Pultruded Vinyl Ester Composites. Compos. Part A Appl. Sci. Manuf. 2018, 109, 280–289. [Google Scholar] [CrossRef]
- Cardona, F.; Rogers, D.; Van Erp, G. Investigation by TGA—FTIR of the Effect of Styrene Content and Thickness on the UV-Curing of Vinylester Resins. J. Thermoplast. Compos. Mater. 2007, 20, 601–615. [Google Scholar] [CrossRef]
- Dominguez-Macaya, A.; Álvarez-Arenas, T.E.G.; Saenz-Dominguez, I.; Tena, I.; Aurrekoetxea, J.; Iturrospe, A. Monitoring the Evolution of Stiffness during Ultraviolet Curing of a Vinyl Ester Resin with Quasi-Normal Air-Coupled Ultrasonic Spec-troscopy. Polym. Test. 2019, 80, 106112. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, Q.; Hu, J.; Xu, Z.; Peng, C. Study on Affecting Factors of Curing Rate in Ultraviolet Curing Adhesives Contain-ing Modified Acrylic Ester Prepolymers. J. Macromol. Sci. Part A 2018, 55, 595–602. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Xia, J.; Song, J.; Huang, K.; Li, M. Renewable myrcene-based UV-curable monomer and its copolymers with acrylated epoxidized soybean oil: Design, preparation, and characterization. BioResources 2015, 10, 2130–2142. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Xia, J.; Song, J.; Huang, K.; Li, M. Novel Renewable Resource-Based UV-Curable Copolymers Derived from Myrcene and Tung Oil: Preparation, Characterization and Properties. Ind. Crops Prod. 2015, 63, 17–25. [Google Scholar] [CrossRef]
- Mao, W.; Li, S.; Li, M.; Yang, X.; Song, J.; Wang, M.; Xia, J.; Huang, K. A Novel Flame Retardant UV-Curable Vinyl Ester Resin Monomer Based on Industrial Dipentene: Preparation, Characterization, and Properties. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Huang, K.; Li, M.; Xia, J. Design, Preparation and Properties of Novel Renewable UV-Curable Copolymers Based on Cardanol and Dimer Fatty Acids. Prog. Org. Coat. 2014, 77, 388–394. [Google Scholar] [CrossRef]
- Bruzzone, A.A.; Lonardo, P.; Diverio, G. University of Genoa, Experimental Characterization of Cured in Place Pipes (CIPP). Available online: https://www.researchgate.net/publication/266329989_Experimental_characterization_of_Cured_In_Place_Pipes_CIPP (accessed on 3 February 2025).
- Ji, H.W.; Yoo, S.S.; Kim, J.; Koo, D.D. The Mechanical Properties of High Strength Reinforced Cured-in-Place Pipe (CIPP) Liner Composites for Urban Water Infrastructure Rehabilitation. Water 2018, 10, 983. [Google Scholar] [CrossRef]
- Xia, Y.; Shi, M.; Zhang, C.; Wang, C.; Sang, X.; Liu, R.; Zhao, P.; An, G.; Fang, H. Analysis of flexural failure mechanism of ultraviolet cured-in-place-pipe materials for buried pipelines rehabilitation based on curing temperature monitoring. Eng. Fail. Anal. 2022, 142, 106763. [Google Scholar] [CrossRef]
- EN ISO 11296-4; Plastics Piping Systems for Renovation of Underground Non-Pressure Drainage and Sewerage Networks—Part 4: Lining with Cured-in-Place Pipes. International Organization for Standardization: Geneva, Switzerland, 2018.
- EN ISO 11297-4; Plastics Piping Systems for Renovation of Underground Drainage and Sewerage Networks Under Pressure—Part 4: Lining with Cured-in-Place Pipes. International Organization for Standardization: Geneva, Switzerland, 2018.
- EN ISO 11298-4; Plastics Piping Systems for Renovation of Underground Water Supply Networks—Part 4: Lining with Cured-in-Place Pipes. International Organization for Standardization: Geneva, Switzerland, 2021.
- Ji Wook, K.; Koo, D.D.; Kang, J.-H. Short- and Long-Term Structural Characterization of Cured-in-Place Pipe Liner with Reinforced Glass Fiber Material. Int. J. Environ. Res. Public Health 2020, 17, 2073. [Google Scholar] [CrossRef]
- Shannon, B.; Fu, G.; Azoor, R.; Deo, R.; Kodikara, J. Long-Term Properties of Cured-in-Place Pipe Liner. Material J. Mater. Civ. Eng. 2022, 34, 04022119. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, C.; Xia, Y.; Wang, C.; Sang, X.; Fang, H.; Wang, N. UV/thermal dual-cured MWCNTs composites for pipeline rehabilitation: Mechanical properties and damage analysis. Constr. Build. Mater. 2024, 450, 138602. [Google Scholar] [CrossRef]
- Abel, T. Laboratory tests and analysis of CIPP epoxy-resin internal liners used in pipelines—Part I: Comparison of tests and engineering calculations. Stud. Geotech. Mech. 2021, 43, 169–180. [Google Scholar] [CrossRef]
- Abel, T. Laboratory tests and analysis of CIPP epoxy resin internal liners used in pipelines—Part II: Comparative analysis with the use of the FEM and engineering algorithms. Stud. Geotech. Mech. 2021, 43, 307–322. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, C.; Wang, C.; Liu, H.; Sang, X.; Liu, R.; Zhao, P.; An, G.; Fang, H.; Shi, M. Prediction of bending strength of glass fiber reinforced methacrylate-based pipeline UV-CIPP rehabilitation materials based on machine learning. Tunn. Undergr. Space Technol. 2023, 140, 105319. [Google Scholar] [CrossRef]
- Matthews, J.C.; Selvakumar, A.; Condit, W. Demonstration and evaluation of an innovative water main rehabilitation technology: Cured-in-Place Pipe (CIPP) lining. Water Pract. Technol. 2012, 7, wpt2012028. [Google Scholar] [CrossRef]
- Pahari, P.; Spears, S.; Liu, J.; Butler, S.; Sonowane, S.; Garcia, A.; Larsen, M.; Proctor, C.R.; Howarter, J.A.; Youngblood, J.P.; et al. Release of Bisphenol A and Other Volatile Chemicals from New Epoxy Drinking Water Pipe Liners: The Role of Manufacturing Conditions. Environ. Sci. Technol. 2025, 59, 1. [Google Scholar] [CrossRef]
- Bavilinezhad, S.; Najafi, M.; Kaushal, V.; Elledge, W.; Kaynak, B. Environmental Impact Assessment of Volatile Organic Compound Emissions during Trenchless Cured-in-Place Pipe Installation. Environments 2024, 11, 169. [Google Scholar] [CrossRef]
- ISO 3219-2:2021; RheologyPart 2: General Principles of Rotational and Oscillatory Rheometry. European Committee for Standardization: Brussels, Belgium, 2021.
- ISO 178:2019; Plastics—Determination of Flexural Properties. International Organization for Standardization: Geneva, Switzerland, 2019.
- Yao, H.; Niu, J.; Zhang, R.; Huang, P. Study of Non-Newtonian Fluids’ Load-Carrying Capacity for Polyoxyethylene Oxide Water-Based Lubricants. Adhesives 2025, 1, 2. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.; Jang, S.; Park, J.; Choi, J.; Lee, S.; Jung, J.; Park, J. Exploring the Effect of the Polyol Structure and the Incorporation of Lignin on the Properties of Bio-Based Polyurethane. Polymers 2025, 17, 604. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization Kinetics of Multifunctional Monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Gziut, K. Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2020, 13, 5661. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Johnson, C.D.; Williams, E.F. Photopolymerization Kinetics of Vinyl Neodecanoate (Veova10) in UV-Curable Coatings: Effects of Photoinitiator Systems and Monomer Functionality. Polymers 2021, 13, 1254. [Google Scholar] [CrossRef]
- Ceylan, G.; Emik, S.; Yalcinyuva, T.; Sunbuloğlu, E.; Bozdag, E.; Unalan, F. The Effects of Cross-Linking Agents on the Mechanical Properties of Poly (Methyl Methacrylate) Resin. Polymers 2023, 15, 2387. [Google Scholar] [CrossRef]
- Gziut, K.; Kowalczyk, A.; Schmidt, B.; Idzik, T.J.; Sośnicki, J.G. Influence of Methacrylate and Vinyl Monomers on Radical Bulk Photopolymerization Process and Properties of Epoxy-Acrylate Structural Adhesives. Polymers 2023, 15, 926. [Google Scholar] [CrossRef]
- Bakhshi, H.; Kuang, G.; Wieland, F.; Meyer, W. Photo-Curing Kinetics of 3D-Printing Photo-Inks Based on Urethane-Acrylates. Polymers 2022, 14, 2974. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Louradour, F.; Lalevée, J.; Fouassier, J.-P. Photopolymerization Reactions: On the Way to a Green and Sustainable Chemistry. Appl. Sci. 2013, 3, 490–514. [Google Scholar] [CrossRef]


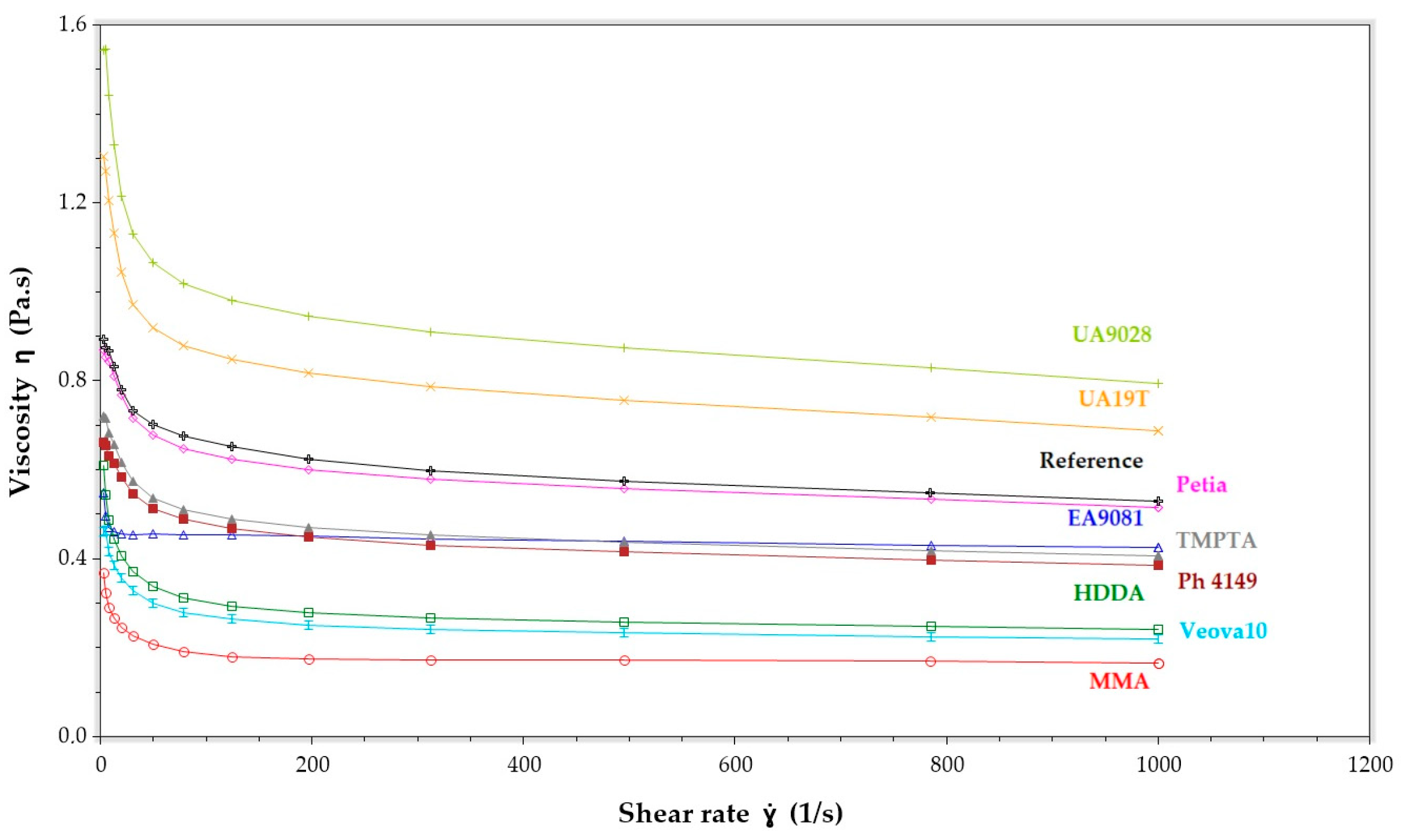
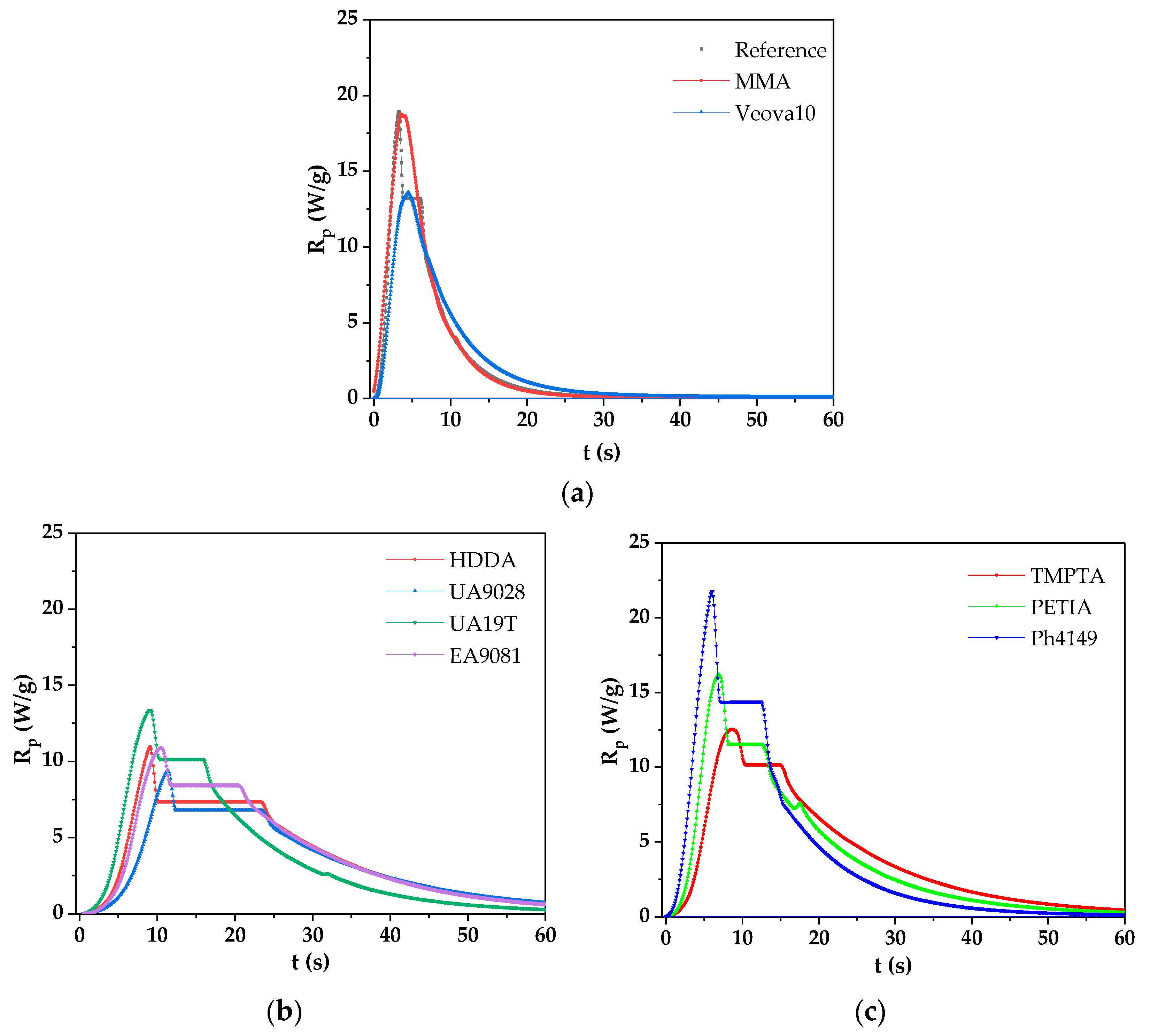

| VE-RD System Symbol | Viscosity (mPa∙s) (25 °C, 50 rpm) | ΔH (J/g) | tmax (s) |
|---|---|---|---|
| VE-0 | 700 | 233 | 6.6 |
| VE-MMA (f = 1) | 210 | 249 (+16) | 7.2 (+0.6) |
| VE-VeoVa10 (f = 1) | 300 | 235 (+2) | 9.0 (+2.4) |
| VE-HDDA (f = 2) | 330 | 242 (+9) | 9.0 (+2.4) |
| VE-UA9028 (f = 2) | 1070 | 219 (−14) | 11.4 (+4.8) |
| VE-UA19T (f = 2) | 920 | 231 (−2) | 9.0 (+2.4) |
| VE-EA9081 (f = 2) | 450 | 250 (+17) | 10.8 (+4.2) |
| VE-TMPTA (f = 3) | 530 | 252 (+19) | 6.0 (−0.6) |
| VE-PETIA (f = 3) | 640 | 240 (+7) | 6.6 (0) |
| VE-Ph4149 (f = 3) | 510 | 253 (+20) | 6.0 (−0.6) |
| Sample | Tg (°C) | Ti (°C) | Tmax (°C) | ΔH (J/g) | GC (wt%) |
|---|---|---|---|---|---|
| VE-0 | 48 | 198 | 211 | 1.2 | 99.99 ± 0.2 |
| VE-MMA | 48 | 140 | 181 | 5.9 | 99.99 ± 0.1 |
| VE-Veova10 | 49 | 194 | 209 | 1.8 | 99.99 ± 0.3 |
| VE-HDDA | 48 | 192 | 216 | 7.9 | 99.88 ± 0.2 |
| VE-UA9028 | 46 | 175 | 181 | 1.0 | 99.97 ± 0.4 |
| VE-UA19T | 50 | 131 | 182 | 7.7 | 99.95 ± 0.4 |
| VE-EA9081 | 49 | 230 | 265 | 4.5 | 99.96 ± 0.2 |
| VE-TMPTA | 49 | 141 | 185 | 3.2 | 99.93 ± 01 |
| VE-PETIA | 49 | 127 | 180 | 13.0 | 99.90 ± 0.2 |
| VE-Ph4149 | 49 | 145 | 187 | 2.9 | 99.95 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasowska, M.; Kowalczyk, A.; Kowalczyk, K.; Oliwa, R.; Oleksy, M. The Effect of Photoreactive Diluents on the Properties of a Styrene-Free Vinyl Ester Resin for Cured-In-Place Pipe (CIPP) Technology. Materials 2025, 18, 2304. https://doi.org/10.3390/ma18102304
Krasowska M, Kowalczyk A, Kowalczyk K, Oliwa R, Oleksy M. The Effect of Photoreactive Diluents on the Properties of a Styrene-Free Vinyl Ester Resin for Cured-In-Place Pipe (CIPP) Technology. Materials. 2025; 18(10):2304. https://doi.org/10.3390/ma18102304
Chicago/Turabian StyleKrasowska, Małgorzata, Agnieszka Kowalczyk, Krzysztof Kowalczyk, Rafał Oliwa, and Mariusz Oleksy. 2025. "The Effect of Photoreactive Diluents on the Properties of a Styrene-Free Vinyl Ester Resin for Cured-In-Place Pipe (CIPP) Technology" Materials 18, no. 10: 2304. https://doi.org/10.3390/ma18102304
APA StyleKrasowska, M., Kowalczyk, A., Kowalczyk, K., Oliwa, R., & Oleksy, M. (2025). The Effect of Photoreactive Diluents on the Properties of a Styrene-Free Vinyl Ester Resin for Cured-In-Place Pipe (CIPP) Technology. Materials, 18(10), 2304. https://doi.org/10.3390/ma18102304








