Effect of Heat Treatment Duration on the Recrystallization and Electrochemical Properties of Cold-Rolled Cantor-Type High-Entropy Alloy
Abstract
1. Introduction
2. Materials and Methods
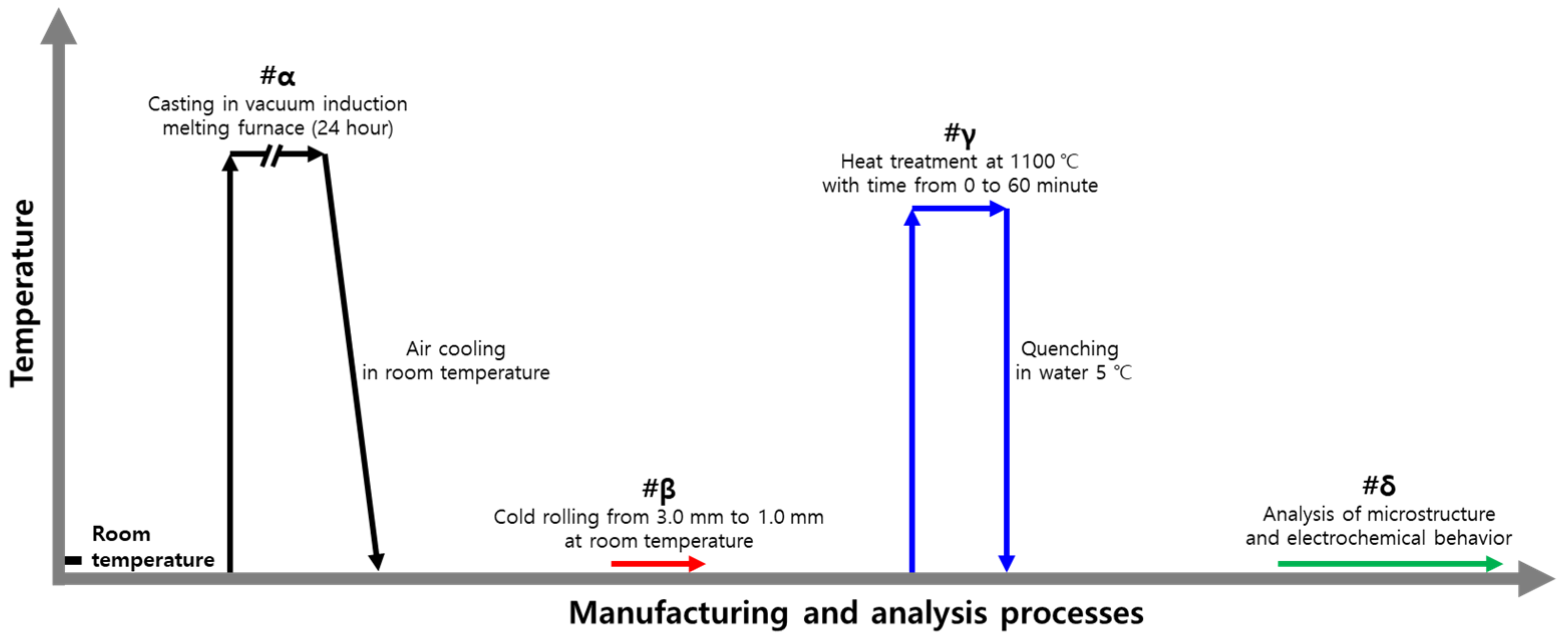
2.1. Manufacturing Process
2.2. Heat Treatment
2.3. Electrochemical Behavior
3. Results and Discussion
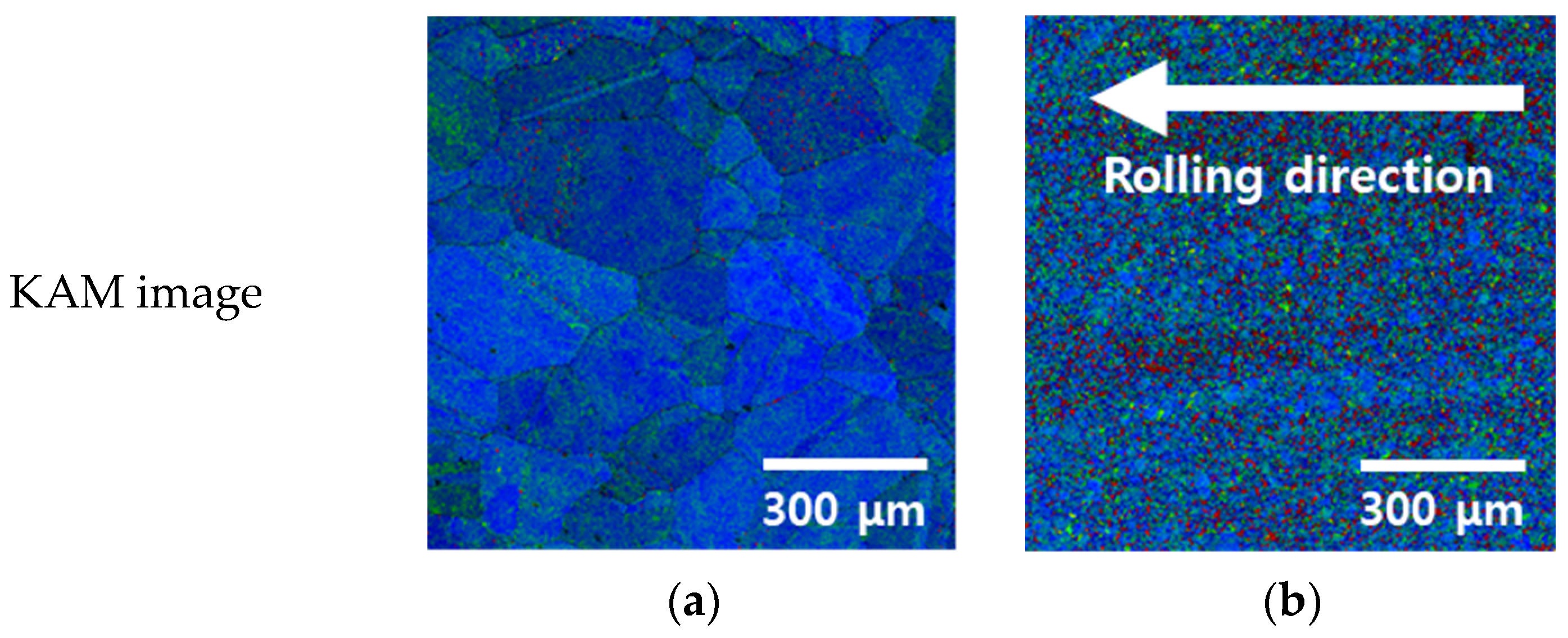
3.1. Microstructure with Manufacturing Process
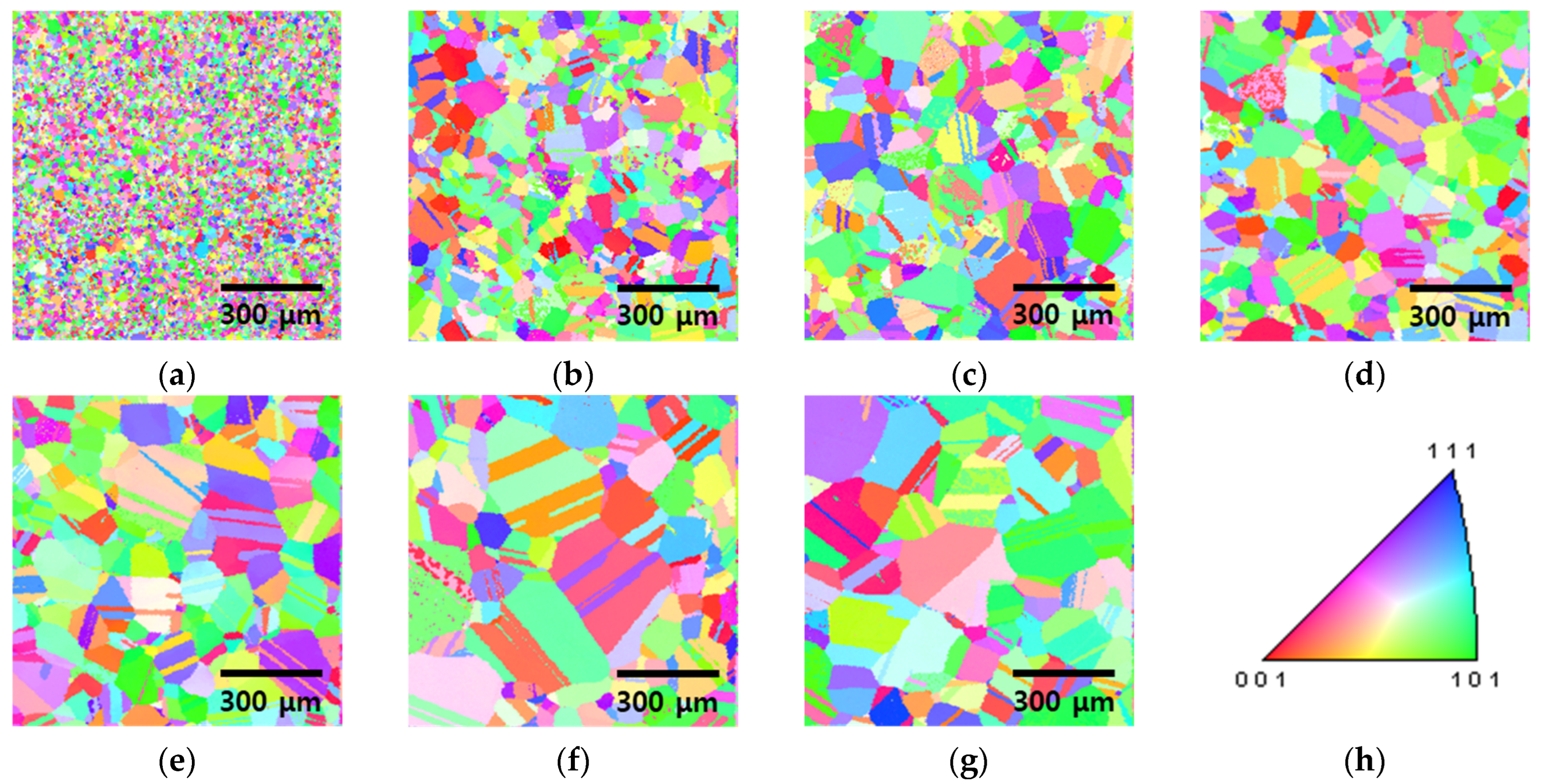
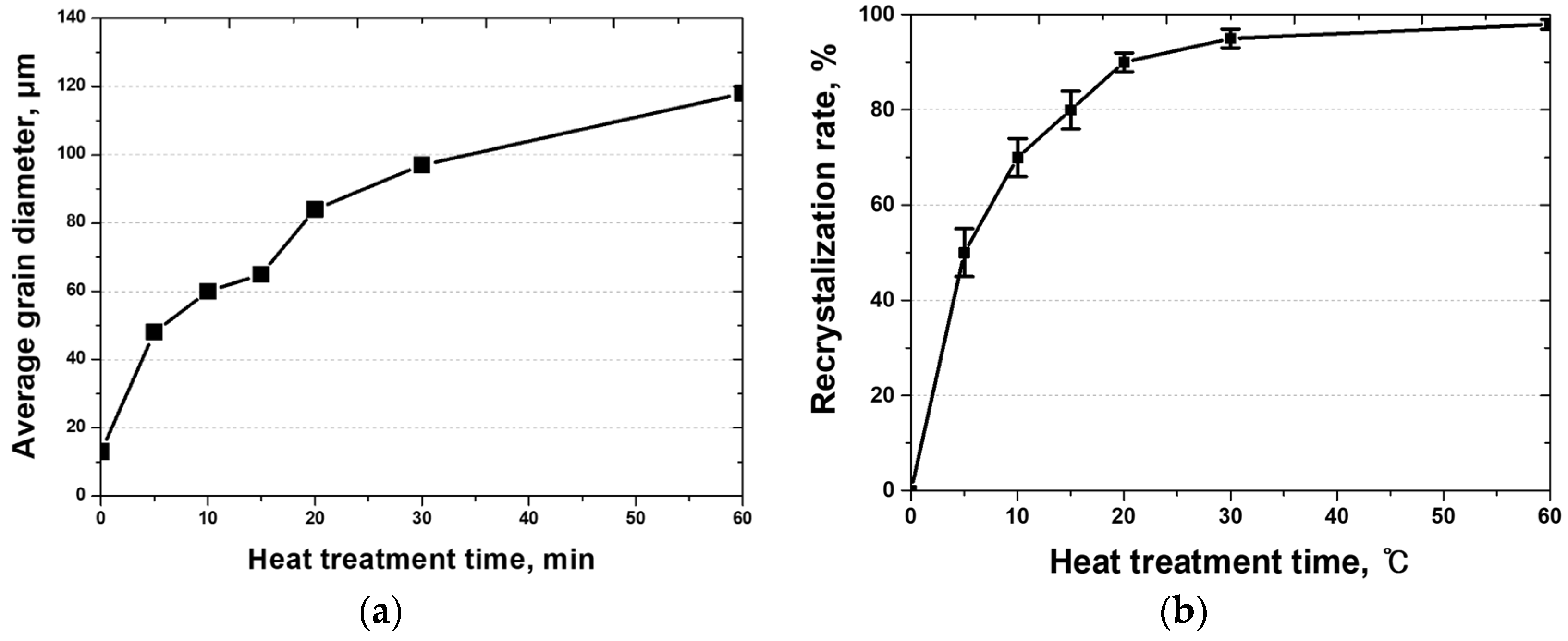
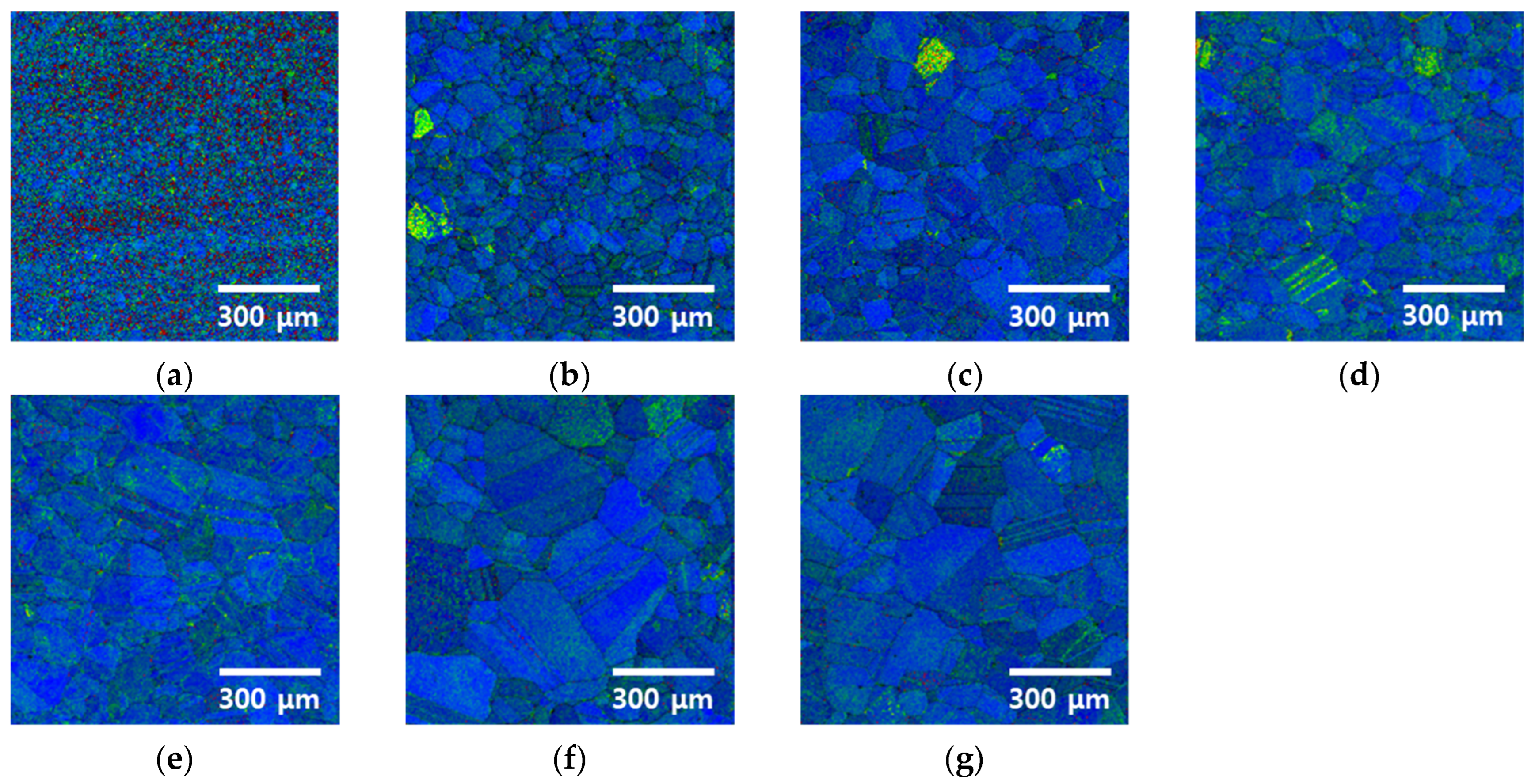
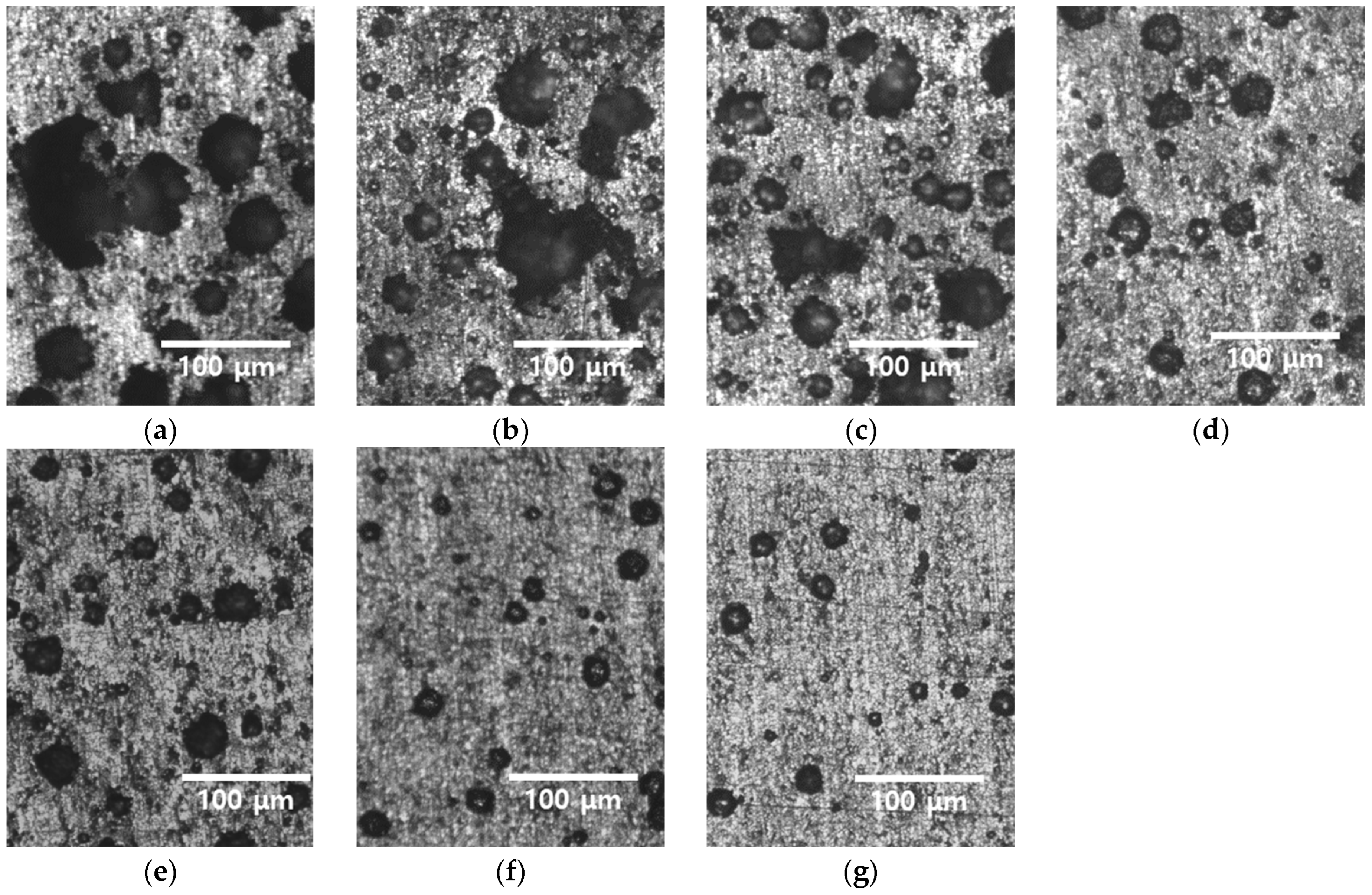
3.2. Recrystallization with Heat Treatment Time
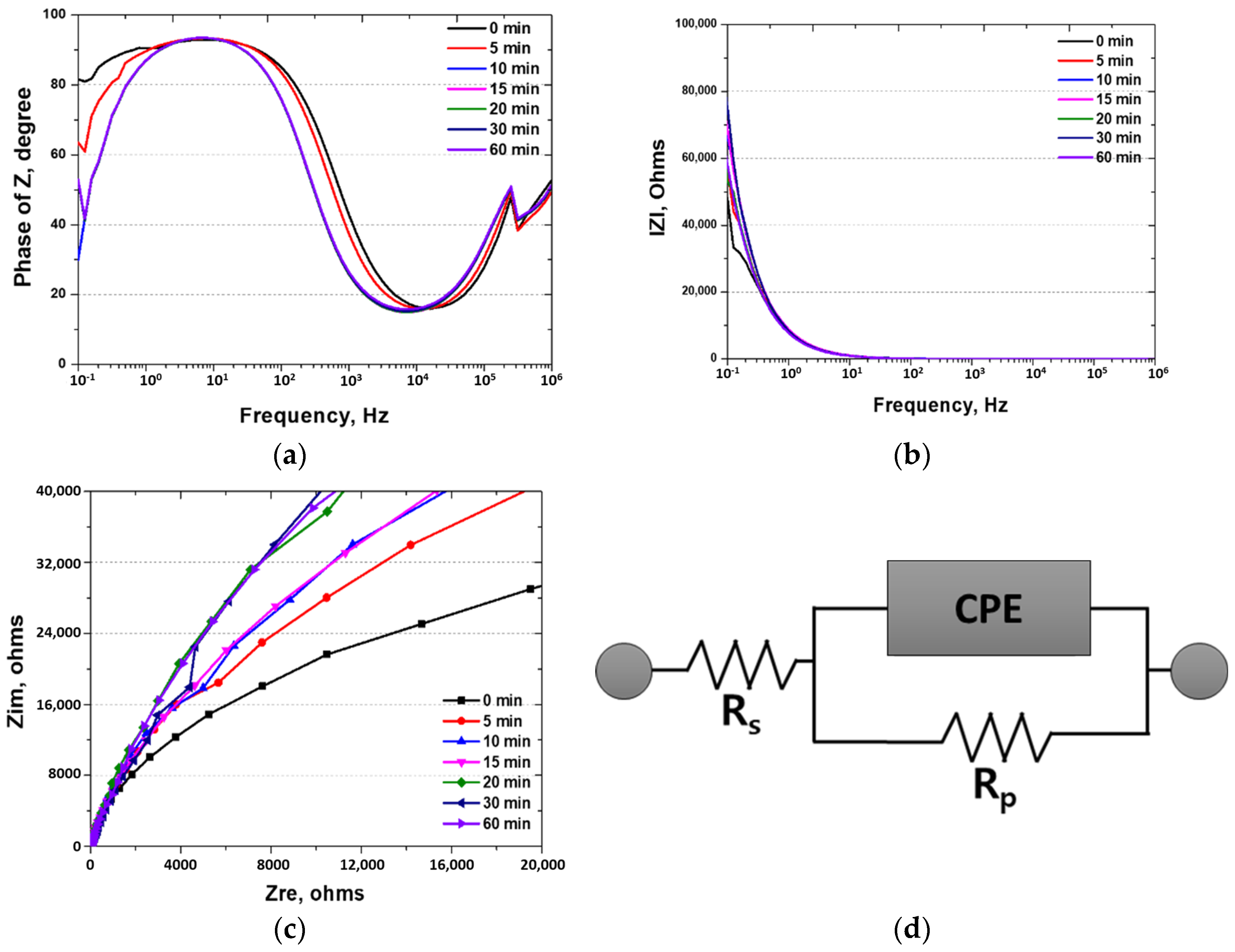
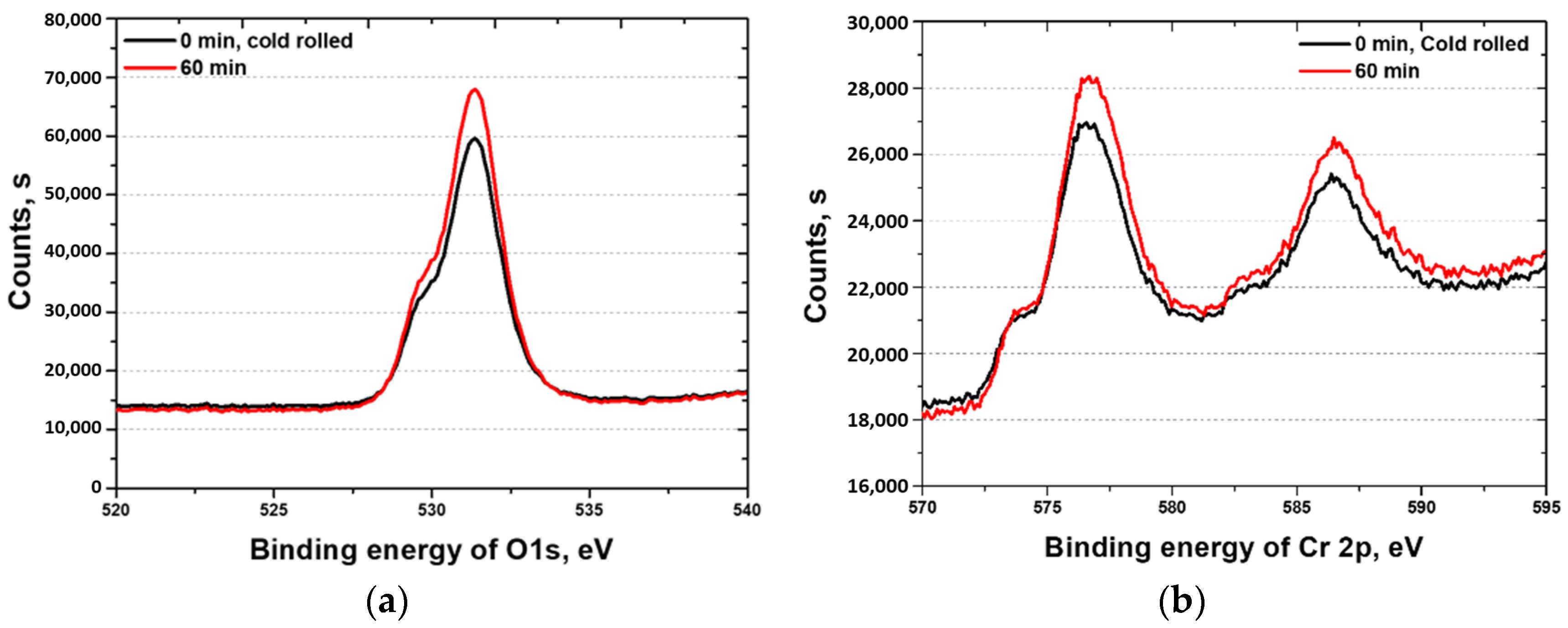
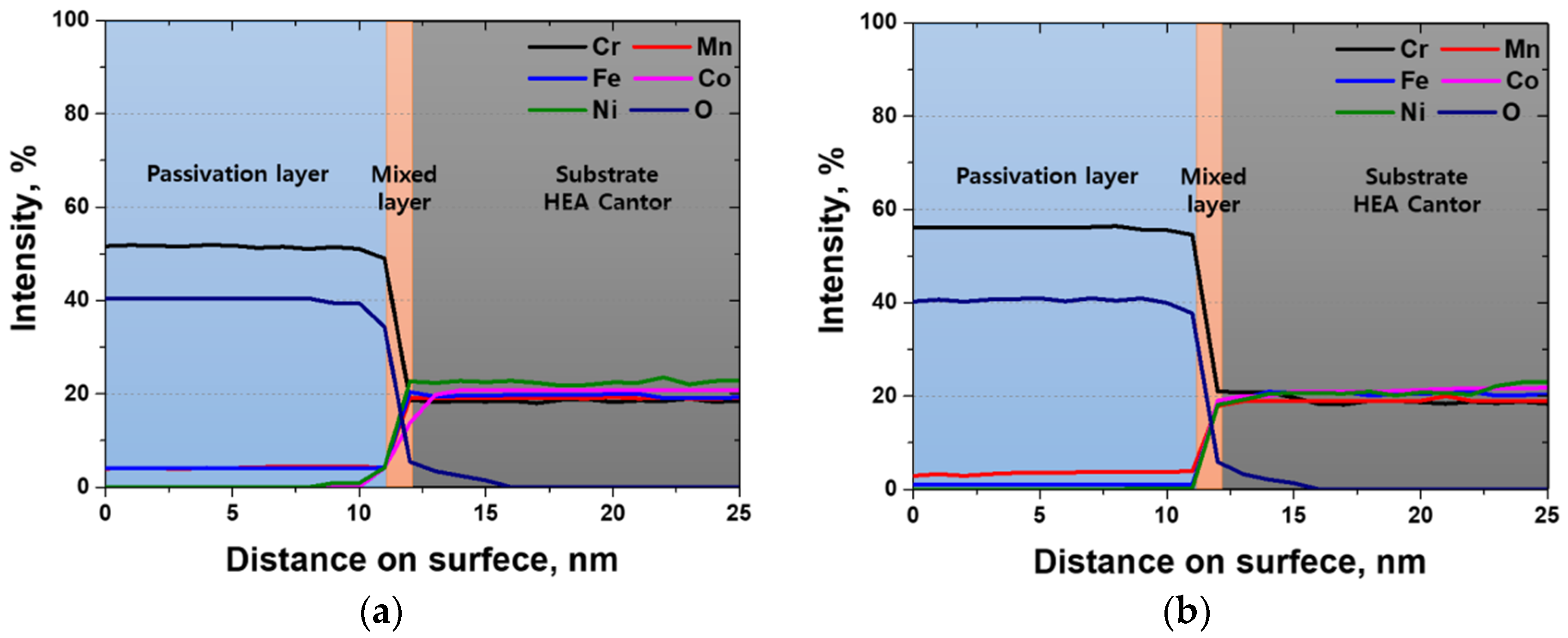
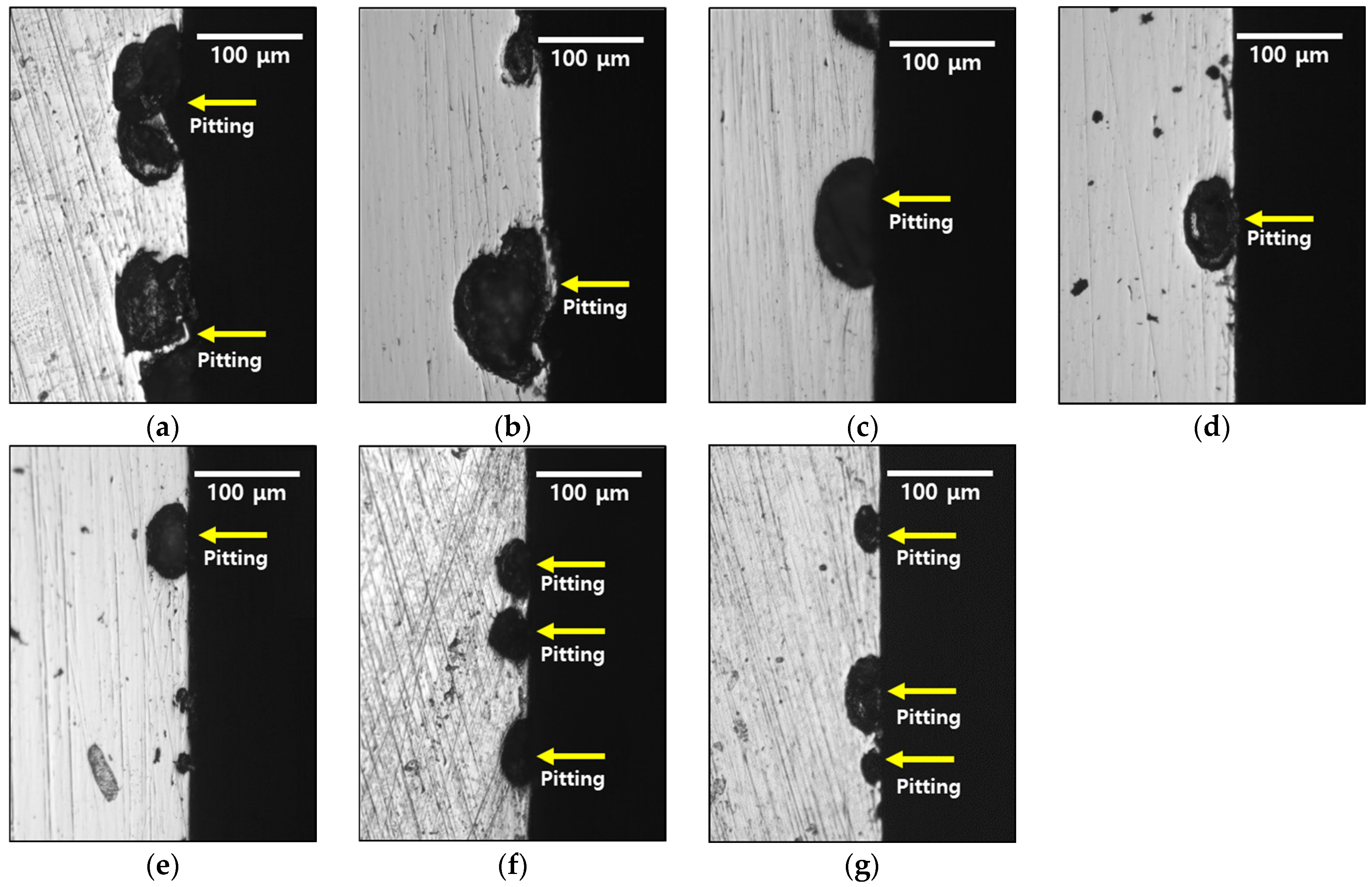
3.3. Electrochemical Behavior with Heat Treatment Time
4. Conclusions
- The as-cast HEA exhibited coarse grains measuring 126 μm and significant grain growth owing to slow cooling in air. Subsequent rolling decreased the grain size to 13 μm and introduced residual stress along the rolling direction. This behavior mirrored those of conventional metals, demonstrating that HEAs exhibit similar microstructural trends upon casting and rolling.
- Heat treatment at 1100 °C induced significant changes in the microstructure of the rolled HEA. The residual stresses decreased prior to grain growth, diminished within 5 min, and vanished within 15 min as the grains continued to grow. The grain size increased from 13 to 48 μm after 5 min and further to 118 μm after 60 min. These results reveal increased grain growth compared with that in conventional alloys, such as stainless steel. Because of the high entropy of the alloy, grain growth was more easily facilitated than in conventional metals, such as stainless steel and pure copper, and this aided in the reduction in residual stresses. Consequently, the high-entropy Cantor alloy required more stringent heat treatment conditions than traditional metals.
- The corrosion resistance of the high-entropy Cantor alloy was influenced by the heat treatment process conducted at 1100 °C for durations ranging from 0 to 60 min. The rolled microstructure exhibited the lowest corrosion resistance, which improved as the grains grew in size. The OCP increased from −0.20 to −0.09 V. Potentiodynamic polarization tests confirmed the enhancement in the corrosion resistance due to heat treatment, with passivation occurring after active polarization. The performance of the passivation layer was further analyzed using the CPT, which increased from 12.6 to 23.1 °C. The state of the passivation layer was verified by performing EIS and XPS, which revealed that the layer primarily consisted of Cr2O3. Cr contributed to the formation of the passivation layer in the high-entropy Cantor alloy, which is important for corrosion resistance.
- The passivation layer in the high-entropy Cantor alloy, which was composed of Cr2O3, played an important role in determining the corrosion resistance. The high-entropy characteristics of the alloy enabled grain growth during the heat treatment. Thus, controlling grain growth through heat treatment is essential because the resultant microstructure significantly impacts the corrosion resistance. As high-entropy Cantor alloys are sensitive to heat treatments, heat treatment processes must be tailored to achieve the desired physical properties, which, in turn, affect corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moniruzzaman, F.N.U.M.; Shakil, S.I.; Shaha, S.K.; Kacher, J.; Nasiri, A.; Haghshenas, M.; Hadadzadeh, A. Study of Direct Aging Heat Treatment of Additively Manufactured PH13–8Mo Stainless Steel: Role of the Manufacturing Process, Phase Transformation Kinetics, and Microstructure Evolution. J. Mater. Res. Technol. 2023, 24, 3772–3787. [Google Scholar] [CrossRef]
- Lervåg, M.; Sørensen, C.; Robertstad, A.; Brønstad, B.M.; Nyhus, B.; Eriksson, M.; Aune, R.; Ren, X.; Akselsen, O.M.; Bunaziv, I. Additive Manufacturing with Superduplex Stainless Steel Wire by Cmt Process. Metals 2020, 10, 272. [Google Scholar] [CrossRef]
- Vukkum, V.B.; Christudasjustus, J.; Darwish, A.A.; Storck, S.M.; Gupta, R.K. Enhanced Corrosion Resistance of Additively Manufactured Stainless Steel by Modification of Feedstock. Npj Mater. Degrad. 2022, 6, 2. [Google Scholar] [CrossRef]
- Rani, K.U.; Kumar, R.; Mahapatra, M.M.; Mulik, R.S.; Świerczyńska, A.; Fydrych, D.; Pandey, C. Wire Arc Additive Manufactured Mild Steel and Austenitic Stainless Steel Components: Microstructure, Mechanical Properties and Residual Stresses. Materials 2022, 15, 7094. [Google Scholar] [CrossRef]
- Skrotzki, W.; Pukenas, A.; Odor, E.; Joni, B.; Ungar, T.; Völker, B.; Hohenwarter, A.; Pippan, R.; George, E.P. Microstructure, Texture, and Strength Development during High-Pressure Torsion of CrMnFeCoNi High-Entropy Alloy. Crystals 2020, 10, 336. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-Entropy Alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Cantor, B. Multicomponent High-Entropy Cantor Alloys. Prog. Mater. Sci. 2021, 120, 100754. [Google Scholar] [CrossRef]
- Koch, C.C. Nanocrystalline High-Entropy Alloys. J. Mater. Res. 2017, 32, 3435–3444. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative Effects of Enthalpy and Entropy on the Phase Stability of Equiatomic High-Entropy Alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Shams, S.A.A.; Jang, G.; Won, J.W.; Bae, J.W.; Jin, H.; Kim, H.S.; Lee, C.S. Low-Cycle Fatigue Properties of CoCrFeMnNi High-Entropy Alloy Compared with Its Conventional Counterparts. Mater. Sci. Eng. A 2020, 792, 139661. [Google Scholar] [CrossRef]
- Han, Z.; Ren, W.; Yang, J.; Du, Y.; Wei, R.; Zhang, C.; Chen, Y.; Zhang, G. The Deformation Behavior and Strain Rate Sensitivity of Ultra-Fine Grained CoNiFeCrMn High-Entropy Alloys at Temperatures Ranging from 77 K to 573 K. J. Alloys Compd. 2019, 791, 962–970. [Google Scholar] [CrossRef]
- George, E.P.; Curtin, W.A.; Tasan, C.C. High Entropy Alloys: A Focused Review of Mechanical Properties and Deformation Mechanisms. Acta Mater. 2020, 188, 435–474. [Google Scholar] [CrossRef]
- Keil, T.; Utt, D.; Bruder, E.; Stukowski, A.; Albe, K.; Durst, K. Solid Solution Hardening in CrMnFeCoNi-Based High Entropy Alloy Systems Studied by a Combinatorial Approach. J. Mater. Res. 2021, 36, 2558–2570. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, W.G.; Kim, Y.H.; Jang, H. Surface Roughness and the Corrosion Resistance of 21Cr Ferritic Stainless Steel. Corros. Sci. 2012, 63, 404–409. [Google Scholar] [CrossRef]
- Large, D.; Scandella, F.; Robineau, A.; Dupoiron, F.; Peultier, J.; Fanica, A.; Petit, B.; Thulin, A.; Pettersson, R.; Weisang-Hoinard, F. Welding of Lean Duplex Stainless Steel Grades: Microstructure, Corrosion Resistance and Mechanical Properties. In NACE CORROSION; NACE: Houston, TX, USA, 2012; p. NACE-2012. [Google Scholar]
- Speidel, M.O. Nitrogen Containing Austenitic Stainless Steels. Mater. Werkst. Entwickl. Fert. Prüfung Eig. Anwendungen Tech. Werkst. 2006, 37, 875–880. [Google Scholar] [CrossRef]
- Fande, A.W.; Taiwade, R.V. Welding of Super Duplex Stainless Steel and Austenitic Stainless Steel:# Xd; Influence and Role of Bicomponent Fluxes. Mater. Manuf. Process. 2023, 38, 434–448. [Google Scholar]
- Jo, H.; Ok, J.-W.; Lee, Y.-S.; Je, Y.; Kim, S.; Kim, S.; Park, J.; Lee, J.; Shin, B.-H.; Yoon, J.-H. Ag-Coated Super Duplex Stainless Steel AISI2507 with or without Crystallization of Secondary Phase as Advanced Li-Ion Battery Case Material. Crystals 2024, 14, 653. [Google Scholar] [CrossRef]
- Nilsson, J.-O. Super Duplex Stainless Steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Franceschi, M.; Bergamo, G.; Bonesso, M.; Dabalà, M. On the Exceptional Stress Corrosion Cracking Susceptibility of Selective Laser Melted 316L Stainless Steel under the Individual Effect of Surface Residual Stresses. Eng. Fail. Anal. 2022, 136, 106192. [Google Scholar] [CrossRef]
- Vignal, V.; Delrue, O.; Heintz, O.; Peultier, J. Influence of the Passive Film Properties and Residual Stresses on the Micro-Electrochemical Behavior of Duplex Stainless Steels. Electrochim. Acta 2010, 55, 7118–7125. [Google Scholar] [CrossRef]
- Li, D.; Liaw, P.K.; Xie, L.; Zhang, Y.; Wang, W. Advanced High-Entropy Alloys Breaking the Property Limits of Current Materials. J. Mater. Sci. Technol. 2024, 186, 219–230. [Google Scholar] [CrossRef]
- Ma, E.; Liu, C. Chemical Inhomogeneities in High-Entropy Alloys Help Mitigate the Strength-Ductility Trade-Off. Prog. Mater. Sci. 2024, 143, 101252. [Google Scholar] [CrossRef]
- Martins, M.; Casteletti, L.C. Sigma Phase Morphologies in Cast and Aged Super Duplex Stainless Steel. Mater. Charact. 2009, 60, 792–795. [Google Scholar] [CrossRef]
- Yoo, Y.-R.; Choi, S.-H.; Kim, Y.-S. Effect of Laser Peening on the Corrosion Properties of 304L Stainless Steel. Materials 2023, 16, 804. [Google Scholar] [CrossRef]
- Balaji, V.; Xavior, A. Development of High Entropy Alloys (HEAs): Current Trends. Heliyon 2024, 10, e26464. [Google Scholar]
- Hoosain, S.E.; Tshabalala, L.C.; Skhosana, S.; Freemantle, C.; Mndebele, N. Investigation of the Properties of Direct Energy Deposition Additive Manufactured 304 Stainless Steel. S. Afr. J. Ind. Eng. 2021, 32, 258–263. [Google Scholar] [CrossRef]
- Saeid, T.; Abdollah-Zadeh, A.; Shibayanagi, T.; Ikeuchi, K.; Assadi, H. EBSD Investigation of Friction Stir Welded Duplex Stainless Steel. World Acad. Sci. Eng. Technol. 2010, 61, 376–379. [Google Scholar]
- Mirzadeh, H.; Cabrera, J.M.; Najafizadeh, A.; Calvillo, P.R. EBSD Study of a Hot Deformed Austenitic Stainless Steel. Mater. Sci. Eng. A 2012, 538, 236–245. [Google Scholar] [CrossRef]
- Fréchard, S.; Martin, F.; Clément, C.; Cousty, J. AFM and EBSD Combined Studies of Plastic Deformation in a Duplex Stainless Steel. Mater. Sci. Eng. A 2006, 418, 312–319. [Google Scholar] [CrossRef]
- Michalska, J.; Chmiela, B. Phase Analysis in Duplex Stainless Steel: Comparison of EBSD and Quantitative Metallography Methods. IOP Conf. Ser. Mater. Sci. Eng. 2014, 55, 012010. [Google Scholar] [CrossRef]
- Rybalka, K.V.; Beketaeva, L.A.; Davydov, A.D. Electrochemical Behavior of Stainless Steel in Aerated NaCl Solutions by Electrochemical Impedance and Rotating Disk Electrode Methods. Russ. J. Electrochem. 2006, 42, 370–374. [Google Scholar] [CrossRef]
- Makhdoom, M.A.; Ahmad, A.; Kamran, M.; Abid, K.; Haider, W. Microstructural and Electrochemical Behavior of 2205 Duplex Stainless Steel Weldments. Surf. Interfaces 2017, 9, 189–195. [Google Scholar] [CrossRef]
- Oh, S.; Kim, D.; Kim, K.C.; Kim, D.-I.; Chung, W.; Shin, B.-H. Electrochemical Properties of Electroless Ni Plated Super Duplex Stainless in 3.5% NaCl Solution. Int. J. Electrochem. Sci. 2023, 18, 100287. [Google Scholar] [CrossRef]
- Sung, C.; Kim, K.; Chung, W.; Shin, B.-H. Electrochemical Properties of UNS S 32750 and UNS S 32760 Annealed Super Duplex Stainless Steels. Int. J. Electrochem. Sci. 2022, 17, 220526. [Google Scholar] [CrossRef]
- Nilsson, J.O.; Wilson, A. Influence of Isothermal Phase Transformations on Toughness and Pitting Corrosion of Super Duplex Stainless Steel SAF 2507. Mater. Sci. Technol. 1993, 9, 545–554. [Google Scholar] [CrossRef]
- Isaacs, H.S.; Ishikawa, Y. Current and Potential Transients during Localized Corrosion of Stainless Steel. J. Electrochem. Soc. 1985, 132, 1288. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, T.-H.; Bae, J.-H.; Chun, D.W. Molybdenum Effects on Pitting Corrosion Resistance of FeCrMnMoNC Austenitic Stainless Steels. Metals 2018, 8, 653. [Google Scholar] [CrossRef]
- Saravanan, P.; Govindaraj, Y.; Khalkho, B.; Srikanth, S.; Kumar, V.; Neelakantan, L. Mechanical Properties and Corrosion Behaviour of Developed High Nitrogen High Manganese Stainless Steels. Materwiss Werksttech 2023, 54, 615–626. [Google Scholar] [CrossRef]
- Seo, M.; Hultquist, G.; Leygraf, C.; Sato, N. The Influence of Minor Alloying Elements (Nb, Ti and Cu) on the Corrosion Resistivity of Ferritic Stainless Steel in Sulfuric Acid Solution. Corros. Sci. 1986, 26, 949–960. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Babić, R.; Grubač, Z.; Petrović, Ž.; Lajçi, N. High Corrosion Resistance of Austenitic Stainless Steel Alloyed with Nitrogen in an Acid Solution. Corros. Sci. 2011, 53, 2176–2183. [Google Scholar] [CrossRef]
- Guerrini, E.; Cristiani, P.; Grattieri, M.; Santoro, C.; Li, B.; Trasatti, S. Electrochemical Behavior of Stainless Steel Anodes in Membraneless Microbial Fuel Cells. J. Electrochem. Soc. 2013, 161, H62. [Google Scholar] [CrossRef]
- Paulraj, P.; Garg, R. Effect of Intermetallic Phases on Corrosion Behavior and Mechanical Properties of Duplex Stainless Steel and Super-Duplex Stainless Steel. Adv. Sci. Technol. Res. J. 2015, 9, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, W.; Edström, K. XPS Study of Duplex Stainless Steel as a Possible Current Collector in a Li-Ion Battery. Electrochim. Acta 2012, 79, 82–94. [Google Scholar] [CrossRef]
- Lim, J.; Shin, B.-H.; Kim, D.-I.; Bae, J.-S.; Ok, J.-W.; Kim, S.; Park, J.; Lee, J.I.; Yoon, J.-H. Effect of Annealing after Casting and Cold Rolling on Microstructure and Electrochemical Behavior of High-Entropy Alloy, Cantor. Metalss 2024, 14, 846. [Google Scholar] [CrossRef]
- Mayyas, A.; Steward, D.; Mann, M. The Case for Recycling: Overview and Challenges in the Material Supply Chain for Automotive Li-Ion Batteries. Sustain. Mater. Technol. 2019, 19, e00087. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Park, J.; Ok, J.-W.; Kim, S.; Shin, B.-H.; Yoon, J.-H. Study of Effects of Post-Weld Heat Treatment Time on Corrosion Behavior and Manufacturing Processes of Super Duplex Stainless SAF 2507 for Advanced Li-Ion Battery Cases. Materials 2024, 17, 4107. [Google Scholar] [CrossRef]
- Barbosa, C.A.; Sokolowski, A. Development of UNS S 32760 Super-Duplex Stainless Steel Produced in Large Diameter Rolled Bars. Rem Rev. Esc. Minas 2013, 66, 201–208. [Google Scholar] [CrossRef]
- Gundgire, T.; Jokiaho, T.; Santa-aho, S.; Rautio, T.; Järvenpää, A.; Vippola, M. Comparative Study of Additively Manufactured and Reference 316 L Stainless Steel Samples–Effect of Severe Shot Peening on Microstructure and Residual Stresses. Mater. Charact. 2022, 191, 112162. [Google Scholar] [CrossRef]
- Soria, L.; Herrera, E.J. A Reliable Technique to Determine Pitting Potentials of Austenitic Stainless Steels by Potentiodynamic Methods. Weld. Int. 1992, 6, 959–964. [Google Scholar] [CrossRef]
- Masarapu, C.; Subramanian, V.; Zhu, H.; Wei, B. Long—Cycle Electrochemical Behavior of Multiwall Carbon Nanotubes Synthesized on Stainless Steel in Li Ion Batteries. Adv. Funct. Mater. 2009, 19, 1008–1014. [Google Scholar] [CrossRef]
- Faraji, H.; Yıldız, Ç.; Arshad, A.; Arıcı, M.; Choukairy, K.; El Alami, M. Passive Thermal Management Strategy for Cooling Multiple Portable Electronic Components: Hybrid Nanoparticles Enhanced Phase Change Materials as an Innovative Solution. J. Energy Storage 2023, 70, 108087. [Google Scholar] [CrossRef]
- Sung, C.; Shin, B.-H.; Chung, W. Effect of Solution Annealing on Austenite Morphology and Pitting Corrosion of Super Duplex Stainless Steel UNS S 32750. Int. J. Electrochem. Sci. 2021, 16, 210813. [Google Scholar] [CrossRef]




| Cr | Mn | Fe | Co | Ni | C | O | N | P | |
|---|---|---|---|---|---|---|---|---|---|
| at.% | 20.0 ± 0.02 | 20.0 ± 0.02 | 20.0 ± 0.01 | 20.0 ± 0.01 | 20.0 ± 0.01 | 0.05 | 0.0004 | 0.0004 | 0.0002 |
| wt.% | 18.5 ± 0.01 | 19.6 ± 0.01 | 19.9 ± 0.01 | 21.1 ± 0.01 | 20.9 ± 0.01 | 0.01 | 0.0001 | 0.0001 | 0.0001 |
| Ecorr | Icorr | Epit | |
|---|---|---|---|
| 0 min | −0.29 V | 30 × 10−8 A/cm2 | −0.06 V |
| 30 min | 0.21 V | 10 × 10−8 A/cm2 | 0.19 V |
| 60 min | −0.15 V | 5 × 10−8 A/cm2 | 0.28 V |
| Condition | Rs | CPE | Rp | |
|---|---|---|---|---|
| n | P | |||
| 0 min | 7.1 ohms | 0.68 | 5.7 × 104 | 39 kohms |
| 5 min | 7.2 ohms | 0.71 | 6.9 × 104 | 49 kohms |
| 10 min | 7.1 ohms | 0.71 | 7.1 × 104 | 51 kohms |
| 15 min | 7.2 ohms | 0.72 | 7.2 × 104 | 52 kohms |
| 20 min | 7.2 ohms | 0.72 | 7.3 × 104 | 53 kohms |
| 30 min | 7.2 ohms | 0.72 | 7.5 × 104 | 54 kohms |
| 60 min | 7.2 ohms | 0.72 | 7.8 × 104 | 56 kohms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, B.-H.; Lim, J.; Kim, D.-I.; Ok, J.-W.; Kim, S.; Park, J.; Hong, J.; Lee, T.; Yoon, J.-H.; Lee, J.I. Effect of Heat Treatment Duration on the Recrystallization and Electrochemical Properties of Cold-Rolled Cantor-Type High-Entropy Alloy. Materials 2025, 18, 2298. https://doi.org/10.3390/ma18102298
Shin B-H, Lim J, Kim D-I, Ok J-W, Kim S, Park J, Hong J, Lee T, Yoon J-H, Lee JI. Effect of Heat Treatment Duration on the Recrystallization and Electrochemical Properties of Cold-Rolled Cantor-Type High-Entropy Alloy. Materials. 2025; 18(10):2298. https://doi.org/10.3390/ma18102298
Chicago/Turabian StyleShin, Byung-Hyun, Jinsurang Lim, Doo-In Kim, Jung-Woo Ok, Seongjun Kim, Jinyong Park, Jonggi Hong, Taekyu Lee, Jang-Hee Yoon, and Je In Lee. 2025. "Effect of Heat Treatment Duration on the Recrystallization and Electrochemical Properties of Cold-Rolled Cantor-Type High-Entropy Alloy" Materials 18, no. 10: 2298. https://doi.org/10.3390/ma18102298
APA StyleShin, B.-H., Lim, J., Kim, D.-I., Ok, J.-W., Kim, S., Park, J., Hong, J., Lee, T., Yoon, J.-H., & Lee, J. I. (2025). Effect of Heat Treatment Duration on the Recrystallization and Electrochemical Properties of Cold-Rolled Cantor-Type High-Entropy Alloy. Materials, 18(10), 2298. https://doi.org/10.3390/ma18102298






