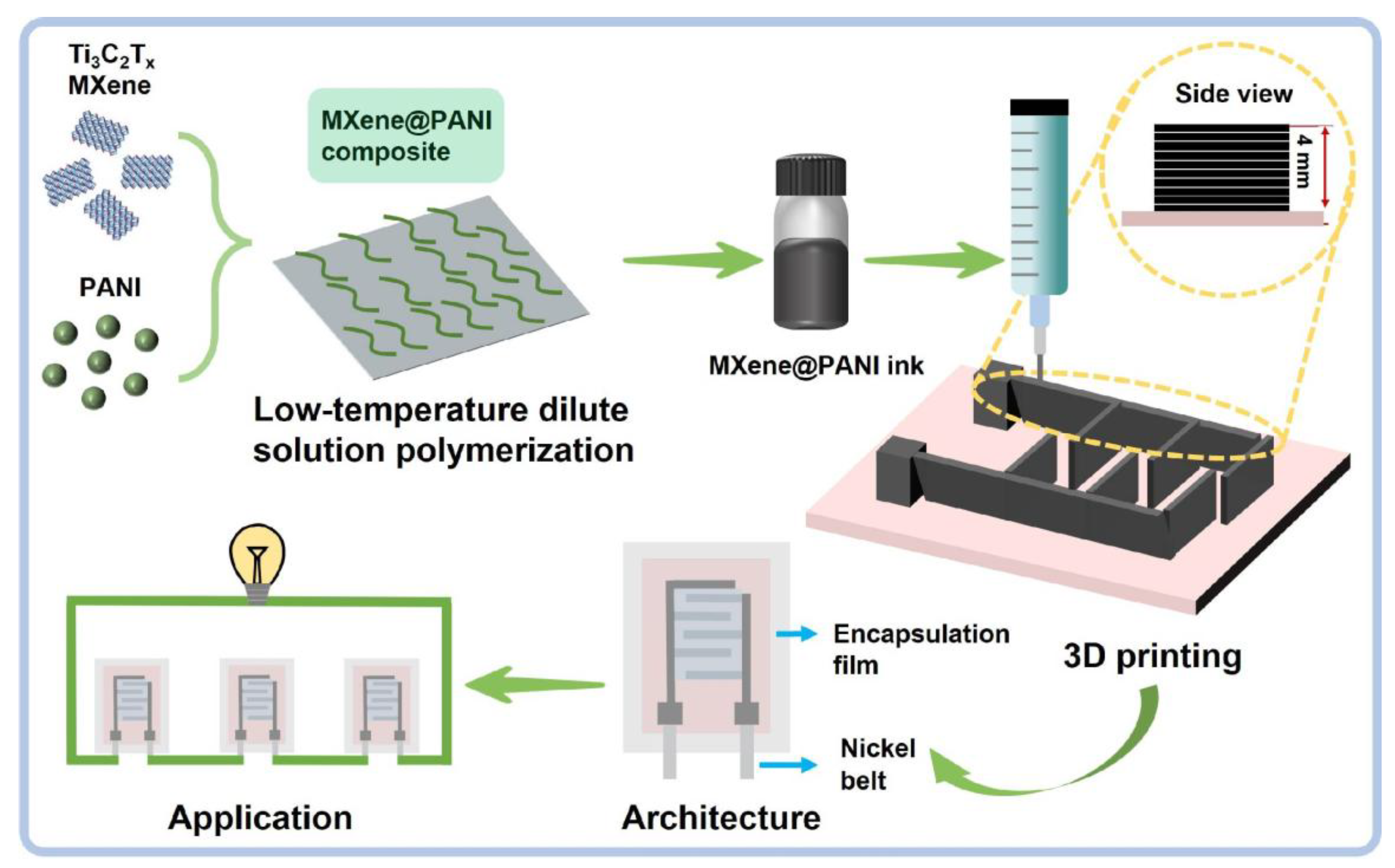
Three-Dimensional Printed MXene@PANI Hierarchical Architecture for High-Performance Micro-Supercapacitors
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
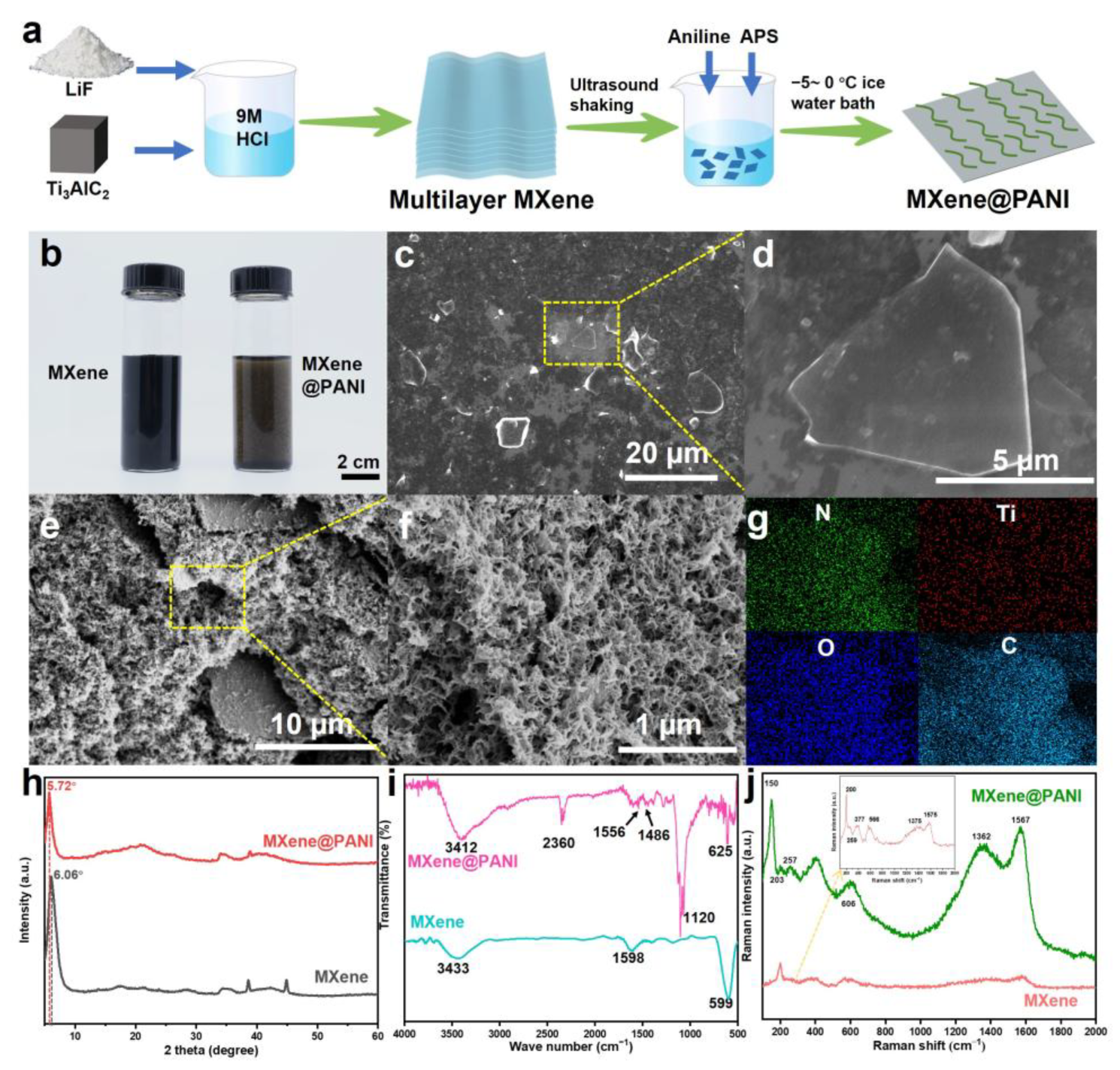
2.2. Synthesis of Single-Layer Ti3C2Tx Nanosheets
2.3. Synthesis of MXene@PANI Composites
2.4. Preparation of MXene Ink and MXene@PANI Ink
2.5. Fabrication of PVA/Na2SO4 Gel Electrolyte
2.6. Fabrication of MSCs
2.7. Material Characterization
2.8. Electrochemical Measurements
3. Results and Discussion
3.1. Fabrication and Characterization of MXene@PANI Composites
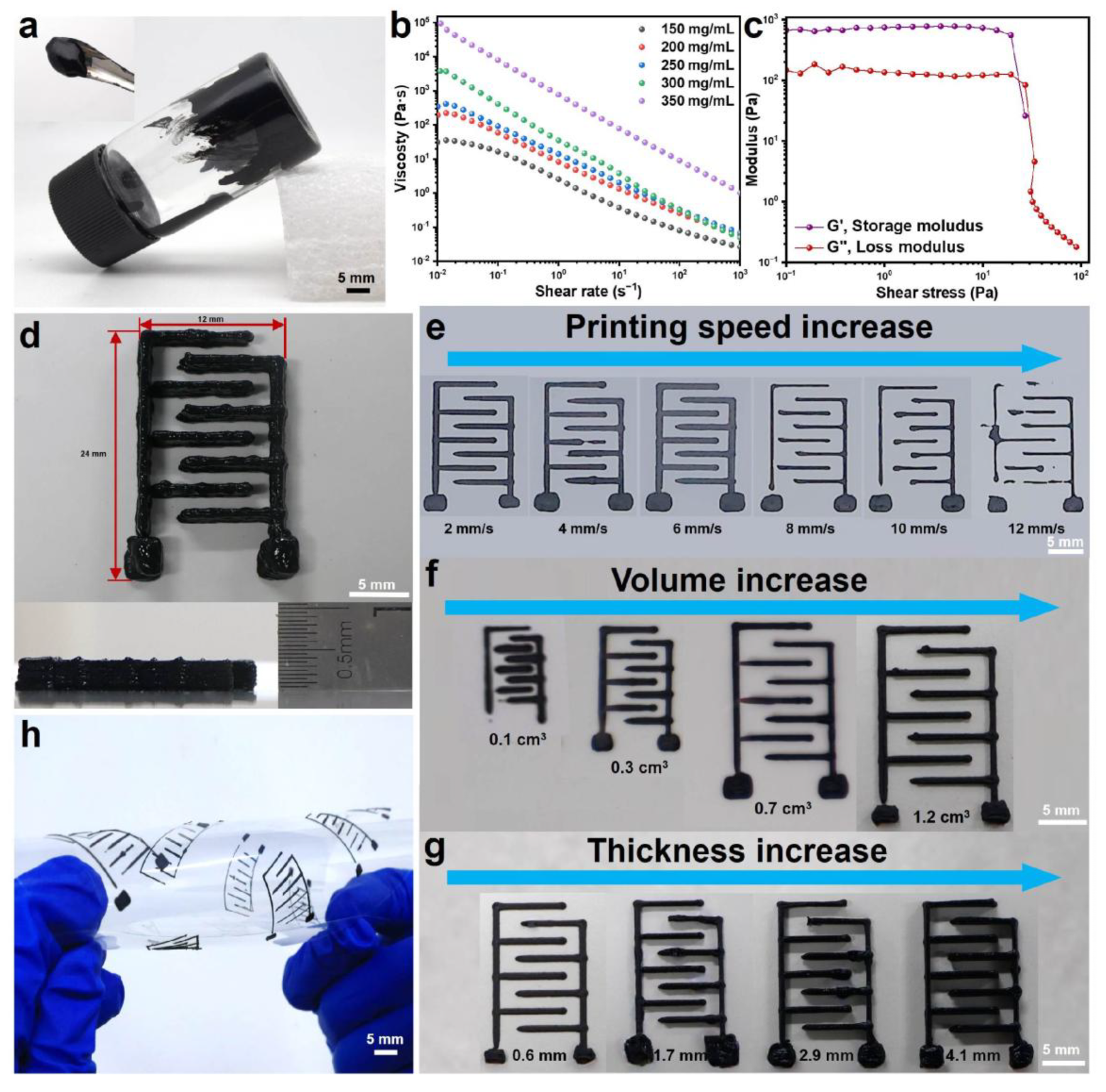
3.2. Preparation and Regulation of MXene@PANI Composite Ink
3.3. Regulation of Printing Parameters
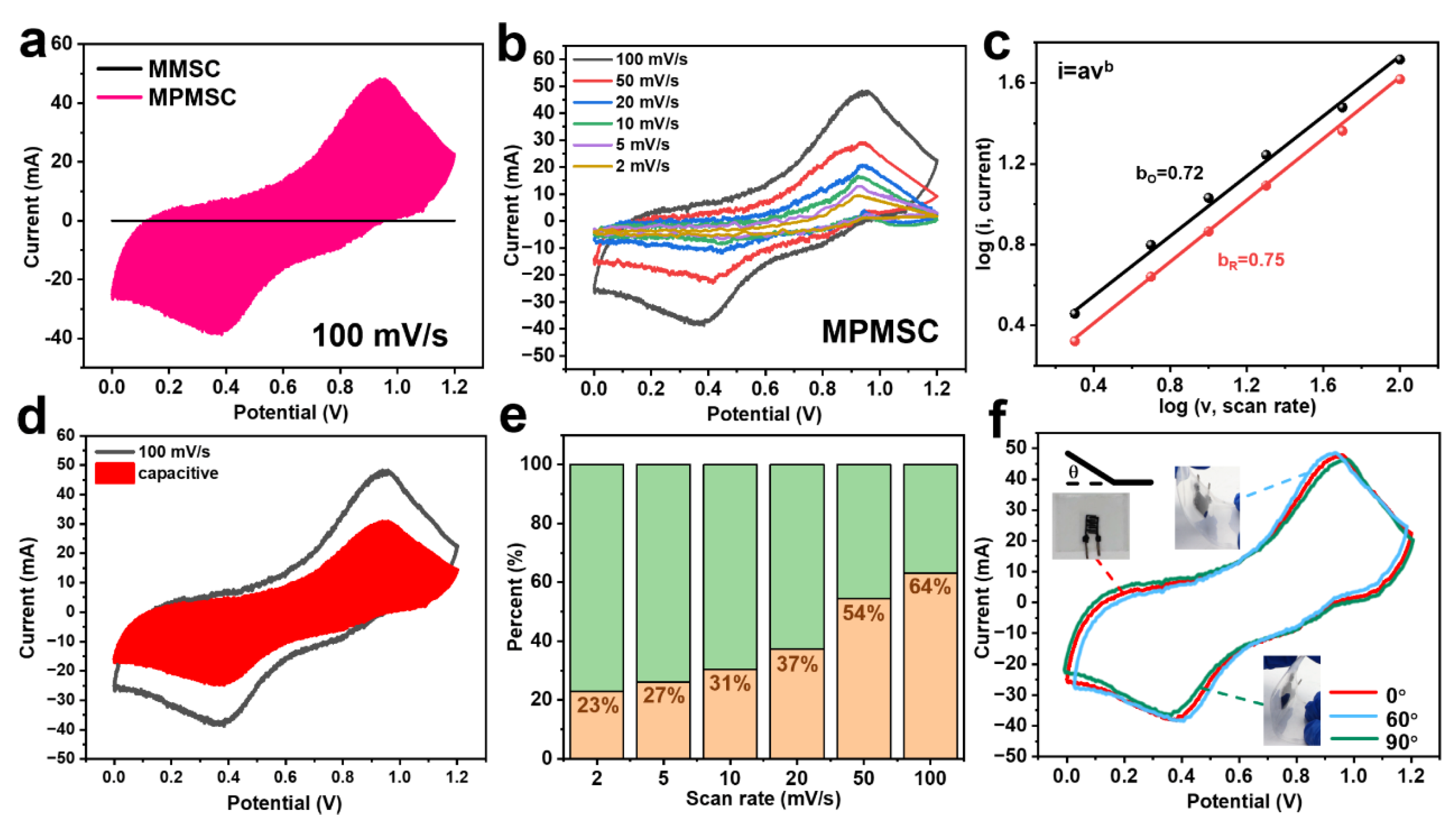
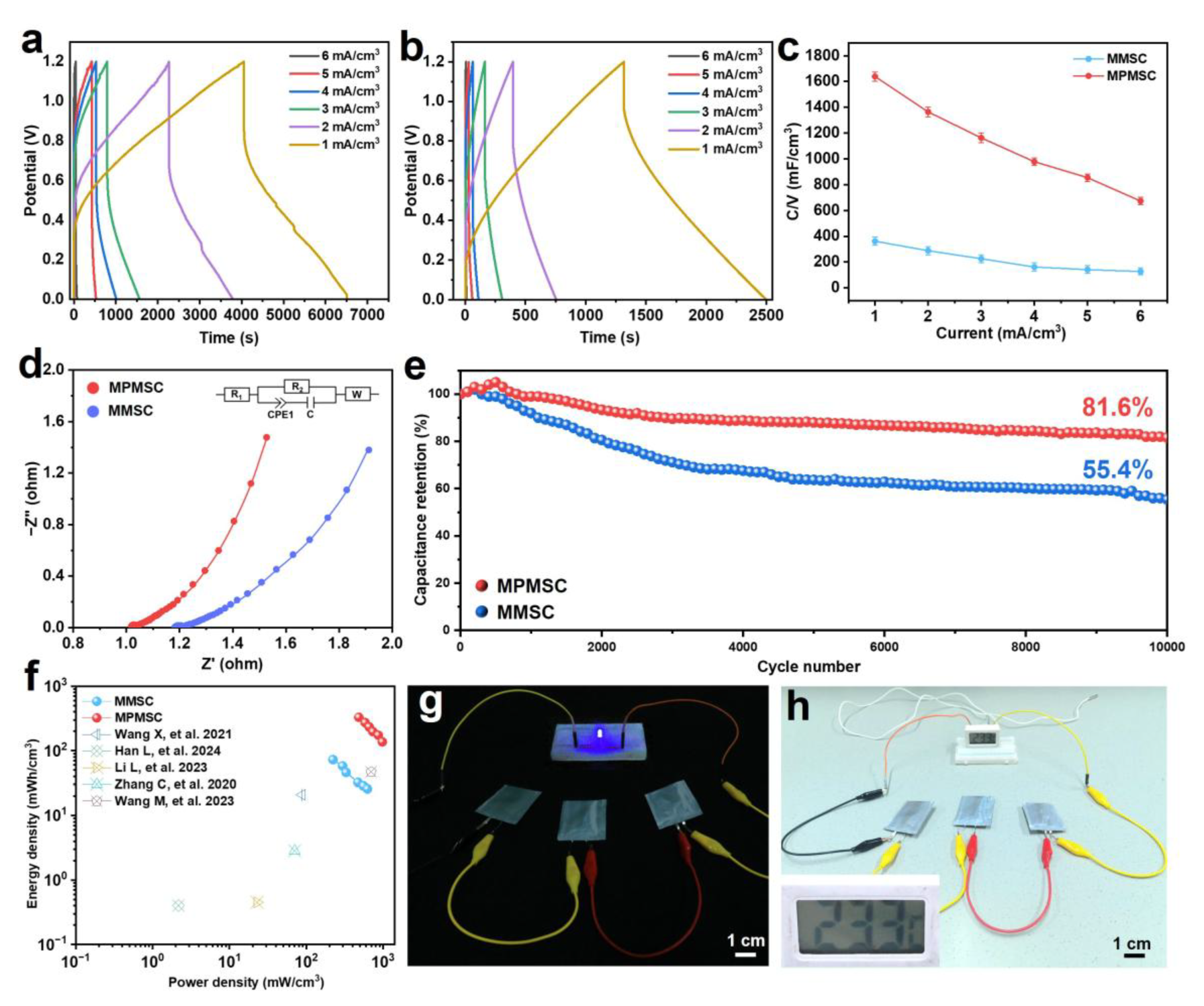
3.4. Assembly and Electrochemical Performance Testing of MMSCs and MPMSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1D | One-dimensional |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| MSCs | Micro-supercapacitors |
| PANI | Polyaniline |
| MBs | Micro-batteries |
| APS | Ammonium persulfate |
| m-Ti3C2 | Multilayer Ti3C2 |
| s-Ti3C2 | Single-layer Ti3C2 |
| PET | Polyethylene terephthalate |
| MILD | Minimally intensive layer delamination |
| EDLC | Electrical double-layer capacitance |
| SEM | Scanning electron microscopy |
| AFM | Atomic force microscopy |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared |
| XPS | X-ray photoelectron spectroscopy |
| BET | Brunauer–Emmett–Teller |
| CV | Cyclic voltammetry |
| GCD | Galvanostatic charge/discharge |
| EIS | Electrochemical impedance spectroscopy |
| CPE | Constant phase element |
| MMSCs | MXene MSCs |
| MPMSCs | MXene@PANI MSCs |
References
- Wang, S.; Zheng, S.H.; Shi, X.Y.; Das, P.; Li, L.M.; Zhu, Y.Y.; Lu, Y.; Feng, X.L.; Wu, Z.S. Monolithically integrated micro-supercapacitors with high areal number density produced by surface adhesive-directed electrolyte assembly. Nat. Commun. 2024, 15, 2850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.F.; McKeon, L.; Kremer, M.P.; Park, S.H.; Ronan, O.; Ascaso, A.S.; Barwich, S.; Coileáin, C.; McEvoy, N.; Nerl, H.C.; et al. Additive-free MXene inks and direct printing of micro-supercapacitors. Nat. Commun. 2019, 10, 1795. [Google Scholar] [CrossRef]
- Kyeremateng, N.A.; Brousse, T.; Pech, D. Microsupercapacitors as miniaturized energy-storage components for on-chip electronics. Nat. Nanotechnol. 2017, 12, 7–15. [Google Scholar] [CrossRef]
- Gao, C.; Huang, J.C.; Xiao, Y.K.; Zhang, G.Q.; Dai, C.L.; Li, Z.L.; Zhao, Y.; Jiang, L.; Qu, L.T. A seamlessly integrated device of micro-supercapacitor and wireless charging with ultrahigh energy density and capacitance. Nat. Commun. 2021, 12, 2647. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, P.; Soomro, R.A.; Zhu, Q.Z.; Xu, B. Advances in the synthesis of 2D MXenes. Adv. Mater. 2021, 33, 2103148. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.T.; Zhang, H.T.; Huang, H.C.; Wang, Z.X.; Xu, Z.; Zhao, H.B.; Wang, Y.C.; Chen, N.J.; Yang, W.Q. High-voltage asymmetric MXene-based on-chip micro-supercapacitors. Nano Energy 2020, 74, 104928. [Google Scholar] [CrossRef]
- Xia, Y.; Mathis, T.S.; Zhao, M.Q.; Anasori, B.; Dang, A.; Zhou, Z.H.; Cho, H.; Gogotsi, Y.; Yang, S. Thickness-independent capacitance of vertically aligned liquid-crystalline MXenes. Nature 2018, 557, 409–412. [Google Scholar] [CrossRef]
- Kim, E.; Lee, B.J.; Maleski, K.; Chae, Y.; Leea, Y.; Gogotsi, Y.; Ahn, C.W. Microsupercapacitor with a 500 nm gap between MXene/CNT electrodes. Nano Energy 2021, 81, 105616. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhang, P.; Sun, N.; Anasori, B.; Zhu, Q.Z.; Liu, H.; Gogotsi, Y.; Xu, B. Self-Assembly of Transition Metal Oxide Nanostructures on MXene Nanosheets for Fast and Stable Lithium Storage. Adv. Mater. 2018, 30, 1707334. [Google Scholar] [CrossRef]
- Zhu, M.S.; Huang, Y.; Deng, Q.H.; Zhou, J.; Pei, Z.X.; Xue, Q.; Huang, Y.; Wang, Z.F.; Li, H.F.; Huang, Q.; et al. Highly Flexible, Freestanding Supercapacitor Electrode with Enhanced Performance Obtained by Hybridizing Polypyrrole Chains with MXene. Adv. Energy Mater. 2016, 6, 1600969. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, N.S.; Ma, Y.N.; Wang, S.L.; Liu, W.J.; Luo, C.; Zhang, H.; Cheng, F.; Rao, J.Y.; Hu, X.K.; et al. Highly Self-Healable 3D Microsupercapacitor with MXene-Graphene Composite Aerogel. ACS Nano 2018, 12, 4224–4232. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/Graphene Films for Ultrafast Supercapacitors with Outstanding Volumetric Capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Ma, L.; Hamidinejad, M.; Liang, C.Y.; Zhao, B.; Habibpour, S.; Yu, A.P.; Filleter, T.; Park, C.B. Enhanced electromagnetic wave absorption performance of polymer/SiC-nanowire/MXene (Ti3C2Tx) composites. Carbon 2021, 179, 408–416. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Q.; Chang, J.; Chen, L.D. Enhanced thermoelectric properties of CNT/PANI composite nanofibers by highly orienting the arrangement of polymer chains. J. Mater. Chem. 2012, 22, 17612–17618. [Google Scholar] [CrossRef]
- Feng, X.M.; Li, R.M.; Ma, Y.W.; Chen, R.F.; Shi, N.E.; Fan, Q.L.; Huang, W. One-Step Electrochemical Synthesis of Graphene/Polyaniline Composite Film and Its Applications. Adv. Funct. Mater. 2011, 21, 2989–2996. [Google Scholar] [CrossRef]
- Rajkumar, S.; Ezhilarasi, J.C.; Saranya, P.; Merlin, J.P. Fabrication of CoWO4/PANI composite as electrode material for energy storage applications. J. Power Sources 2022, 162, 110500. [Google Scholar] [CrossRef]
- Xu, H.Z.; Zheng, D.H.; Liu, F.Q.; Li, W.; Lin, J.J. Synthesis of an MXene/polyaniline composite with excellent electrochemical properties. J. Mater. Chem. A 2020, 8, 5853–5858. [Google Scholar] [CrossRef]
- Chen, J.K.; Han, D.; Deng, J.H.; Li, B.B.; Wang, T.Y.; Cao, L.G.; Zhang, L.L.; Lai, L.F. Unlocking Maximum Synergy: Screen-Printing Fabrication of Heterostructured Microsupercapacitor Stacks. Small Methods 2024, 8, 2301506. [Google Scholar] [CrossRef]
- Li, C.L.; Wang, S.; Wang, X.R.; Bai, W.Y.; Sun, H.; Pan, F.; Chi, Y.; Wang, Z. High-Temperature Supercapacitors Based on MXene with Ultrahigh Volumetric Capacitance. ACS Mater. Lett. 2023, 5, 2084–2095. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.M.; Liu, D.D.; Li, X.L.; Xiao, H.H.; Ma, Y.; Xu, M.; Yuan, G.H.; Chen, G.R. Opening MXene Ion Transport Channels by Intercalating PANI Nanoparticles from the Self-Assembly Approach for High Volumetric and Areal Energy Density Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 30633–30642. [Google Scholar] [CrossRef]
- Han, L.; Li, Y.Q.; Chen, C.; Liu, L.K.; Lu, Z.C. Multifunctional enhanced energy density of flexible wide-temperature supercapacitors based on MXene/PANI conductive hydrogel. Chem. Eng. J. 2024, 485, 149951. [Google Scholar] [CrossRef]
- Zhou, J.H.; Kang, Q.; Xu, S.C.; Li, X.G.; Liu, C.; Ni, L.; Chen, N.N.; Lu, C.L.; Wang, X.Z.; Peng, L.M.; et al. Ultrahigh rate capability of 1D/2D polyaniline/titanium carbide (MXene) nanohybrid for advanced asymmetric supercapacitors. Nano Res. 2022, 15, 285–295. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhang, D.Z.; Zhang, H.B.; Gong, L.K.; Yang, Y.; Zhao, W.H.; Yu, S.J.; Yin, Y.D.; Sun, D.F. In situ polymerized polyaniline/MXene (V2C) as building blocks of supercapacitor and ammonia sensor self-powered by electromagnetic-triboelectric hybrid generator. Nano Energy 2021, 88, 106242. [Google Scholar] [CrossRef]
- Zhang, C.; McKeon, L.; Kremer, M.P.; Park, S.H.; Ronan, O.; Seral-Ascaso, A.; Barwich, S.; Coileáin, C.Ó.; McEvoy, N.; Nerl, H.C.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Xiong, Z.; Jiang, C.; Zhang, Y.; Fu, P.; Du, F. Electrochemical polymerization of polyaniline/single-walled carbon nanotube bilayer films with enhanced thermoelectric properties. Prog. Org. Coat. 2024, 194, 108612. [Google Scholar] [CrossRef]
- Létiche, M.; Brousse, K.; Demortière, A.; Huang, P.H.; Daffos, B.; Pinaud, S.; Respaud, M.; Chaudret, B.; Roussel, P.; Buchaillot, L.; et al. Sputtered Titanium Carbide Thick Film for High Areal Energy on Chip Carbon-Based Micro-Supercapacitors. Adv. Funct. Mater. 2017, 27, 1606813. [Google Scholar] [CrossRef]
- Zhang, C.; Kremer, M.P.; Seral-Ascaso, A.; Park, S.-H.; McEvoy, N.; Anasori, B.; Gogotsi, Y.; Nicolosi, V. Stamping of Flexible, Coplanar Micro-Supercapacitors Using MXene Inks. Adv. Funct. Mater. 2018, 28, 1705506. [Google Scholar] [CrossRef]
- Lewis, J.A. Direct Ink Writing of 3D Functional Materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Fu, K.; Yao, Y.; Dai, J.; Hu, L. Progress in 3D Printing of Carbon Materials for Energy-Related Applications. Adv. Mater. 2017, 29, 1603486. [Google Scholar] [CrossRef]
- Shen, K.; Ding, J.W.; Yang, S.B. 3D Printing Quasi-Solid-State Asymmetric Micro-Supercapacitors with Ultrahigh Areal Energy Density. Adv. Energy Mater. 2018, 8, 1800408. [Google Scholar] [CrossRef]
- Li, L.; Meng, J.; Bao, X.R.; Huang, Y.P.; Yan, X.P.; Qian, H.L.; Zhang, C.; Liu, T.X. Direct-Ink-Write 3D Printing of Programmable Micro-Supercapacitors from MXene-Regulating Conducting Polymer Inks. Adv. Energy Mater. 2023, 13, 2203683. [Google Scholar] [CrossRef]
- Yu, L.; Li, W.; Wei, C. 3D Printing of NiCoP/Ti3C2 MXene Architectures for Energy Storage Devices with High Areal and Volumetric Energy Density. Nano-micro lett. 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Chiou, N.-R.; Lu, C.M.; Guan, J.J.; Lee, L.J.; Epstein, A.J. Growth and alignment of polyaniline nanofibres with superhydrophobic, superhydrophilic and other properties. Nat. Nanotechnol. 2007, 2, 354–357. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zu, S.; Han, B.; Wei, Z. Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Y.; Geng, H.; Yang, W.; Chen, X. Boosting Ion Diffusion Kinetics of MXene Inks with Water-in-Salt Electrolyte for Screen-Printed Micro-Supercapacitors. Adv. Funct. Mater. 2024, 34, 2400887. [Google Scholar] [CrossRef]
- Ding, R.J.; Xiong, J.H.; Yan, Q.; Chen, Z.; Peng, Q.Y.; He, X.D. Achieving fast interfacial solar vapor generation and aqueous acid purification using Ti3C2Tx MXene/PANI non-woven fabrics. Mater. Horiz. 2023, 6, d3mh00060e. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.; Jang, J. Fabrication of Highly Flexible, Scalable, and High-Performance Supercapacitors Using Polyaniline/Reduced Graphene Oxide Film with Enhanced Electrical Conductivity and Crystallinity. Adv. Funct. Mater. 2024, 24, 2489–2499. [Google Scholar] [CrossRef]
- Zhao, L.J.; Wang, K.; Wei, W.; Wang, L.L.; Han, W. High-performance flexible sensing devices based on polyaniline/MXene nanocomposites. InfoMat. 2019, 1, 407–416. [Google Scholar] [CrossRef]
- Liu, P.; Pan, R.Y.; Li, B.Q.; Su, Z.; Lin, B.C.; Tong, M.M. Mild and Efficient Method for the In Situ Preparation of High-Quality MXene Materials Enabled by Hexafluoro Complex Anion Contained Salts Etching. Adv. Funct. Mater. 2024, 34, 2308532. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Y.W.; Wen, X.Y.; Xiong, J.L.; Song, H.R.; Li, Z.; Zu, D.Y.; Shen, Y.M.; Li, C.P. A high-performance flexible gas sensor based on self-assembled PANI-CeO2 nanocomposite thin film for trace-level NH3 detection at room temperature. Anal. Chim. Acta. 2022, 1225, 340256. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Chen, N.J.; Zhou, B.; Zhou, X.F.; Pu, B.; Bai, J.; Tang, Q.; Liu, Y.; Yang, W.Q. NH3-Induced In Situ Etching Strategy Derived 3D-Interconnected Porous MXene/Carbon Dots Films for High Performance Flexible Supercapacitors. Nano-Micro Lett. 2023, 15, 231. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Romate, X.F.; Bosque, A.D.; Artigas-Arnaudas, J.; Muñoz, B.K.; Sánchez, M.; Ureña, A. A proof of concept of a structural supercapacitor made of graphene coated woven carbon fibers: EIS study and mechanical performance. Electrochim. Acta 2021, 370, 137746. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, S.K.; Cai, D.; Cao, J.M.; Wang, L.L.; Han, W. Planar supercapacitor with high areal capacitance based on Ti3C2/Polypyrrole composite film. Electrochim. Acta. 2020, 330, 135277. [Google Scholar] [CrossRef]
- Wang, M.X.; Chen, Z.W.; Dong, L.; Wu, J.J.; Li, C.; Gao, Q.; Shi, J.; Zhu, C.H.; Morikawa, H. Conductance-stable and integrated helical fiber electrodes toward stretchy energy storage and self-powered sensing utilization. Chem. Eng. J. 2023, 457, 141164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Wang, Y.; Yu, H.; Zhang, Y. Three-Dimensional Printed MXene@PANI Hierarchical Architecture for High-Performance Micro-Supercapacitors. Materials 2025, 18, 2277. https://doi.org/10.3390/ma18102277
Zhang A, Wang Y, Yu H, Zhang Y. Three-Dimensional Printed MXene@PANI Hierarchical Architecture for High-Performance Micro-Supercapacitors. Materials. 2025; 18(10):2277. https://doi.org/10.3390/ma18102277
Chicago/Turabian StyleZhang, Anyi, Yiming Wang, Haidong Yu, and Yabin Zhang. 2025. "Three-Dimensional Printed MXene@PANI Hierarchical Architecture for High-Performance Micro-Supercapacitors" Materials 18, no. 10: 2277. https://doi.org/10.3390/ma18102277
APA StyleZhang, A., Wang, Y., Yu, H., & Zhang, Y. (2025). Three-Dimensional Printed MXene@PANI Hierarchical Architecture for High-Performance Micro-Supercapacitors. Materials, 18(10), 2277. https://doi.org/10.3390/ma18102277




