Investigation of the Corrosion Behavior of L245 Steel in 3.5 wt.% NaCl Solution with Varying Concentrations of Na2S2O3
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Weight-Loss Experiment
2.3. Corrosion Product Analysis
2.4. Electrochemical Testing
3. Results
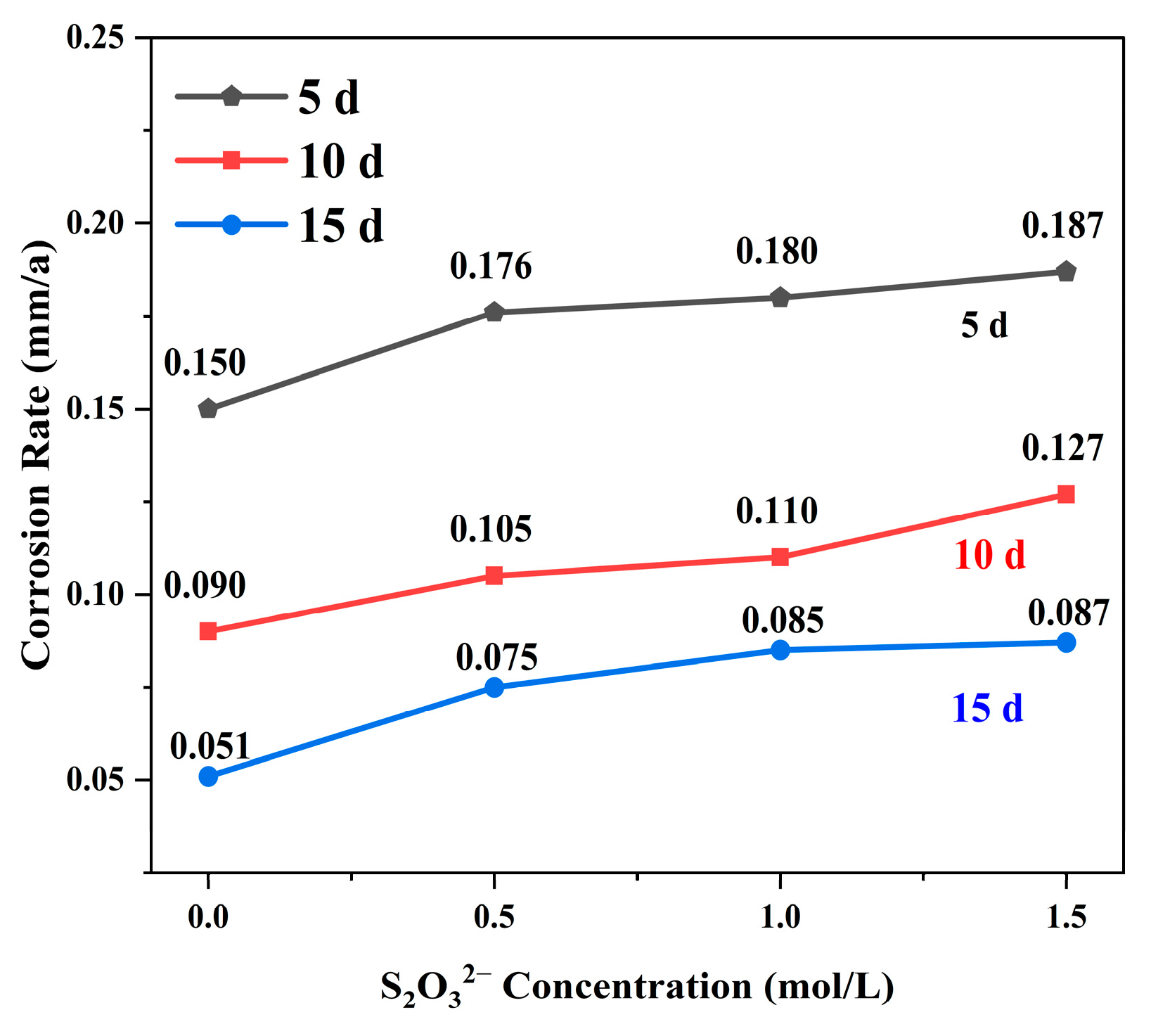
3.1. Corrosion Weight Loss Test
3.2. Analysis of the Corrosion Product
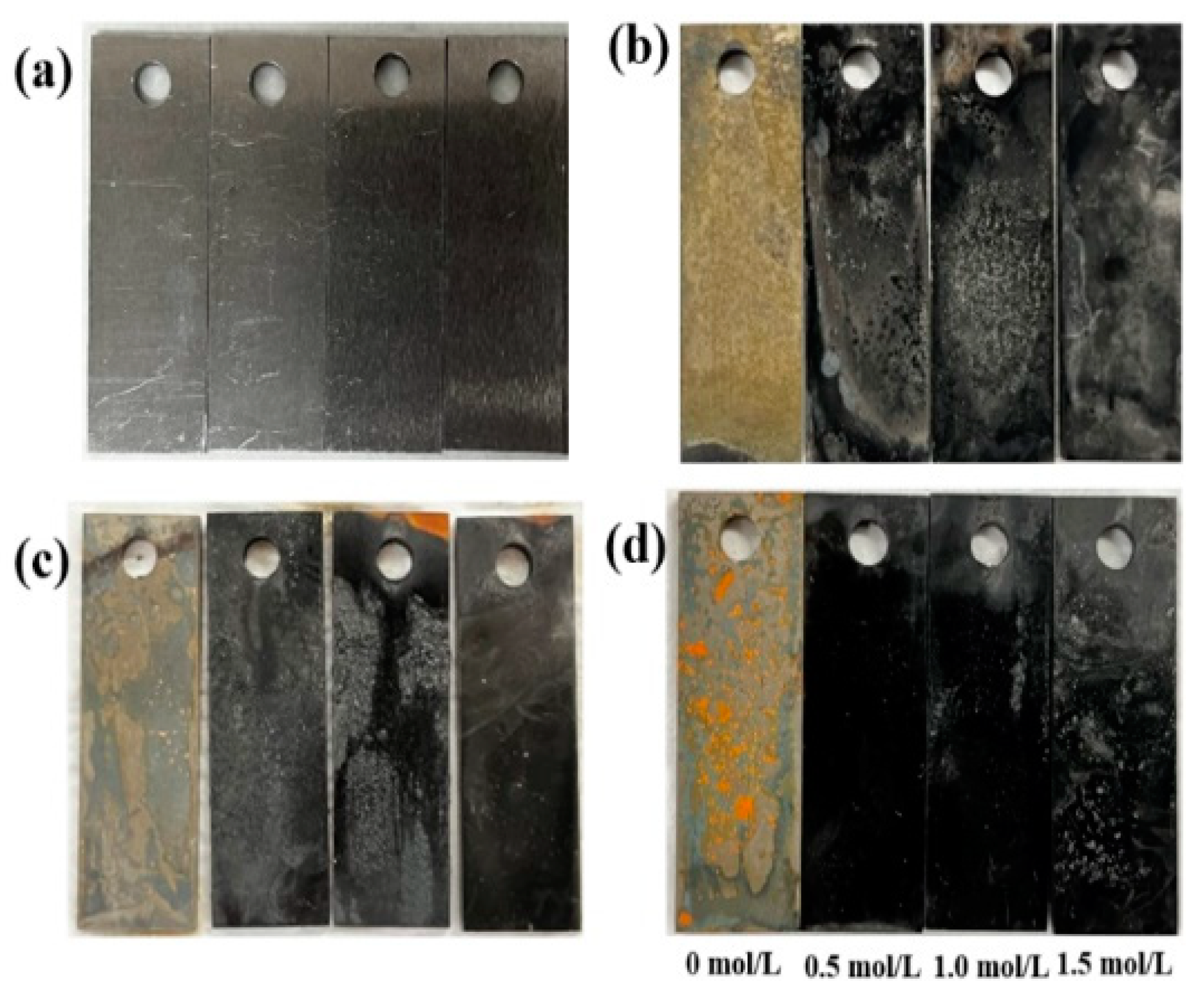
3.2.1. Macroscopic Corrosion Morphology
3.2.2. Phase Analysis by XRD
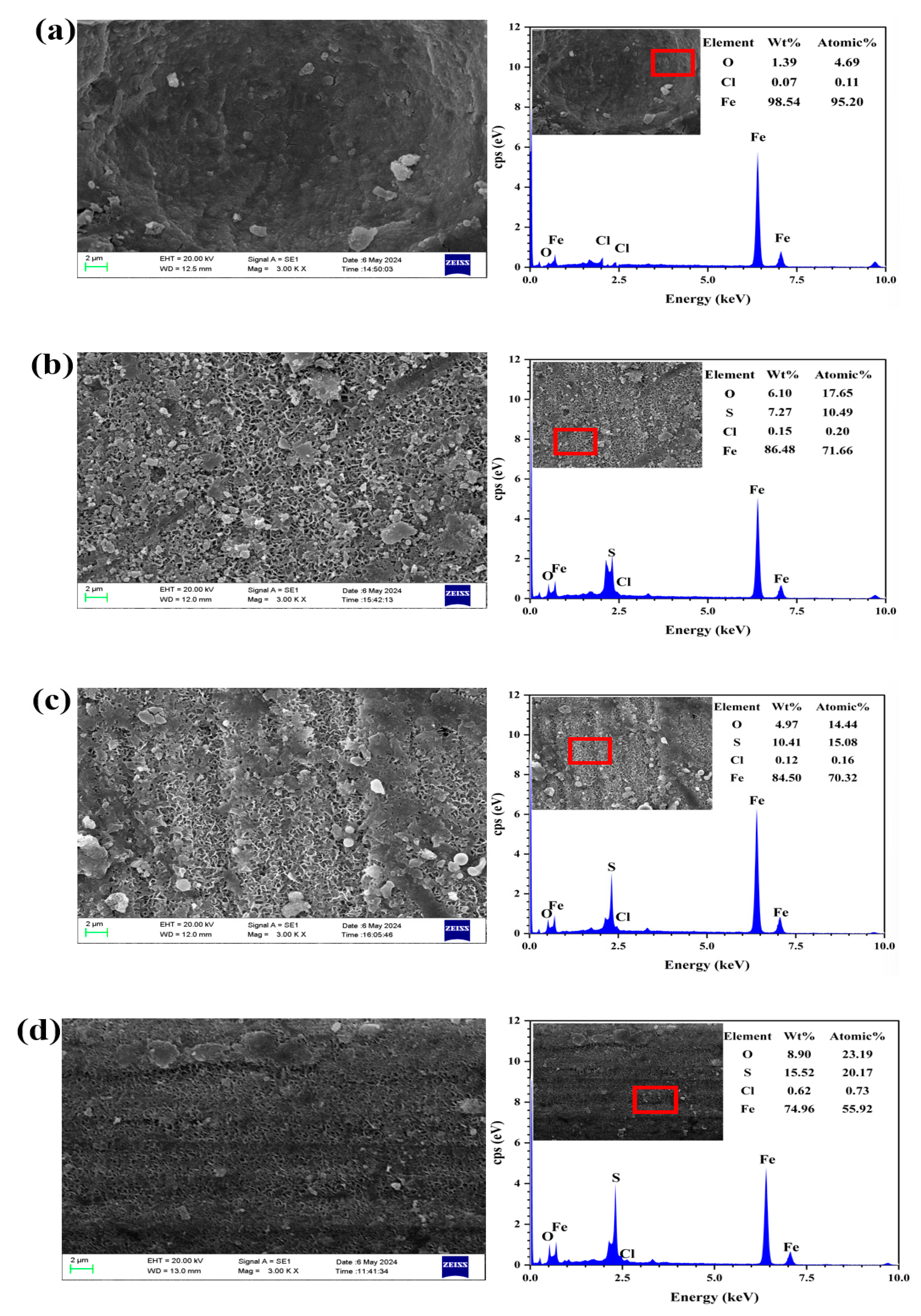
3.2.3. Corrosion Product and Elemental Composition Analysis by SEM-EDS
3.3. Corrosion Electrochemical Testing
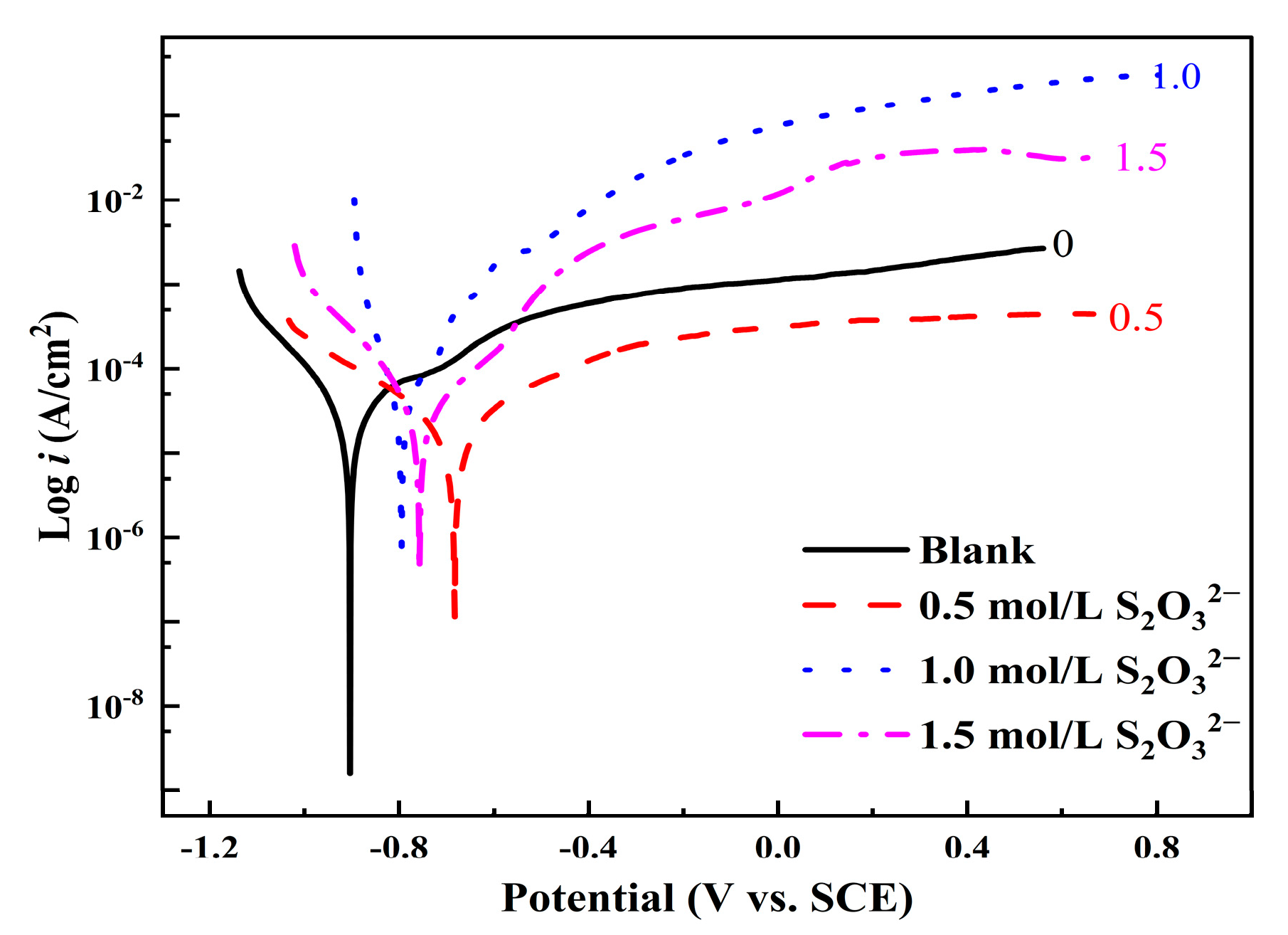
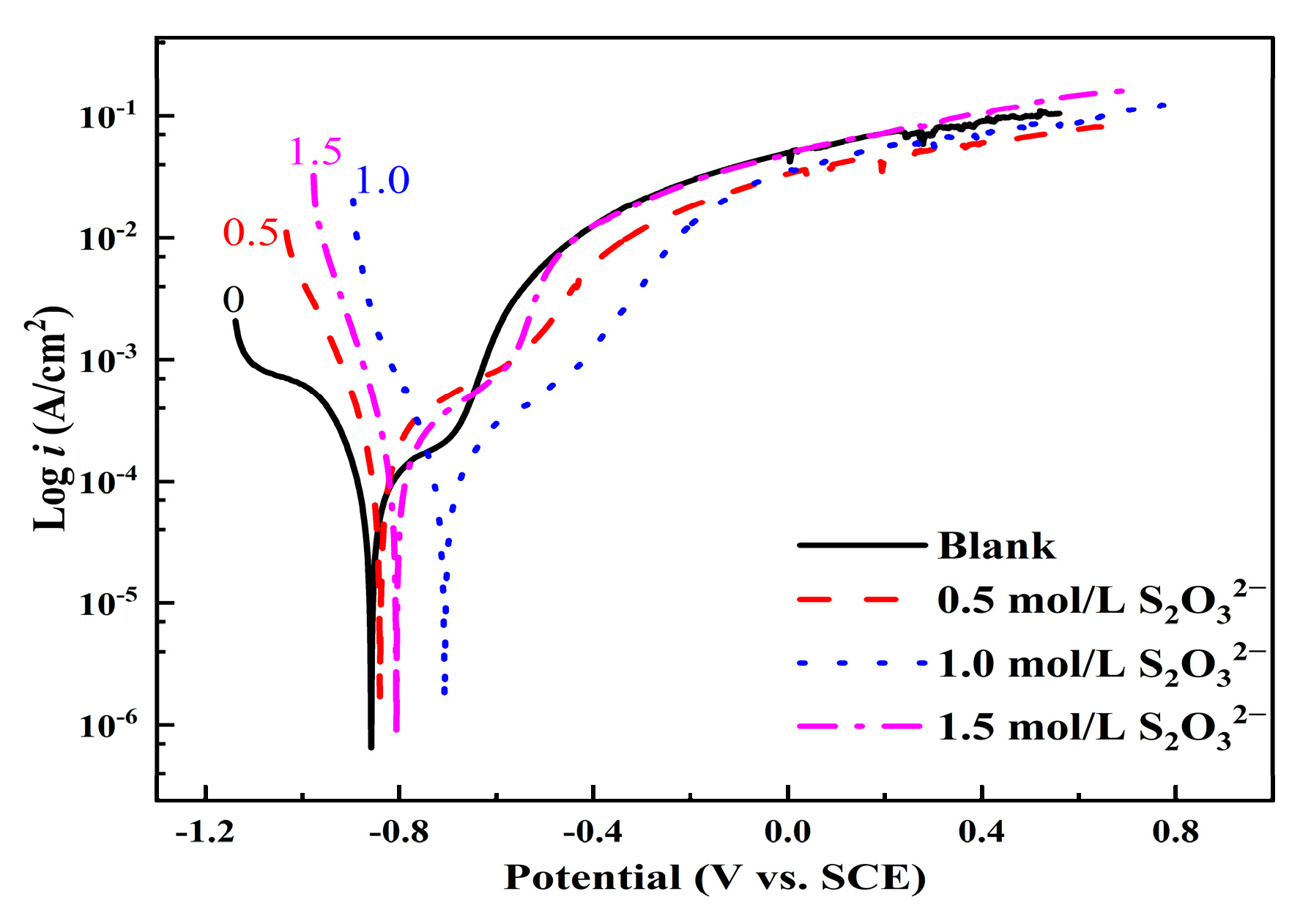
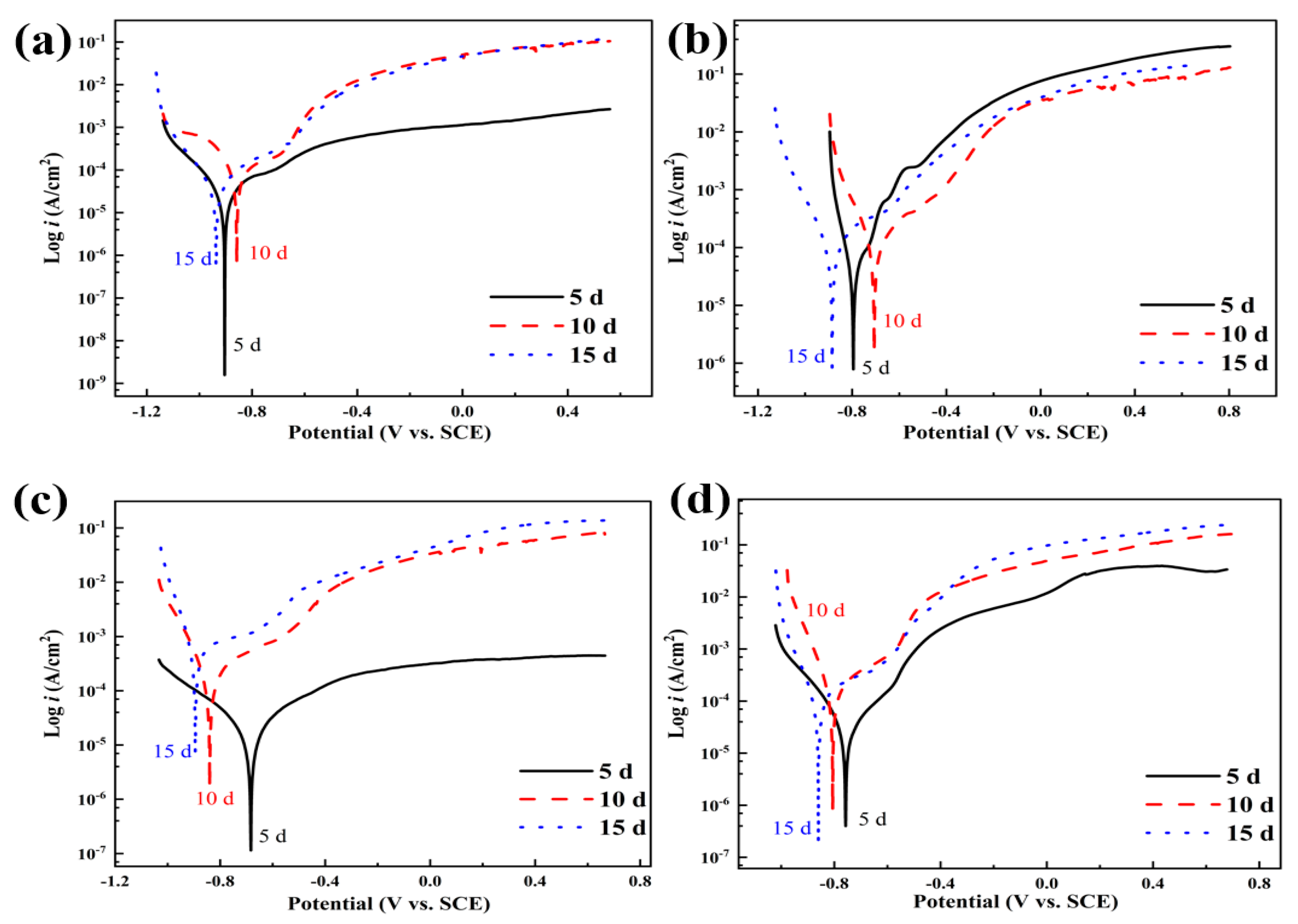
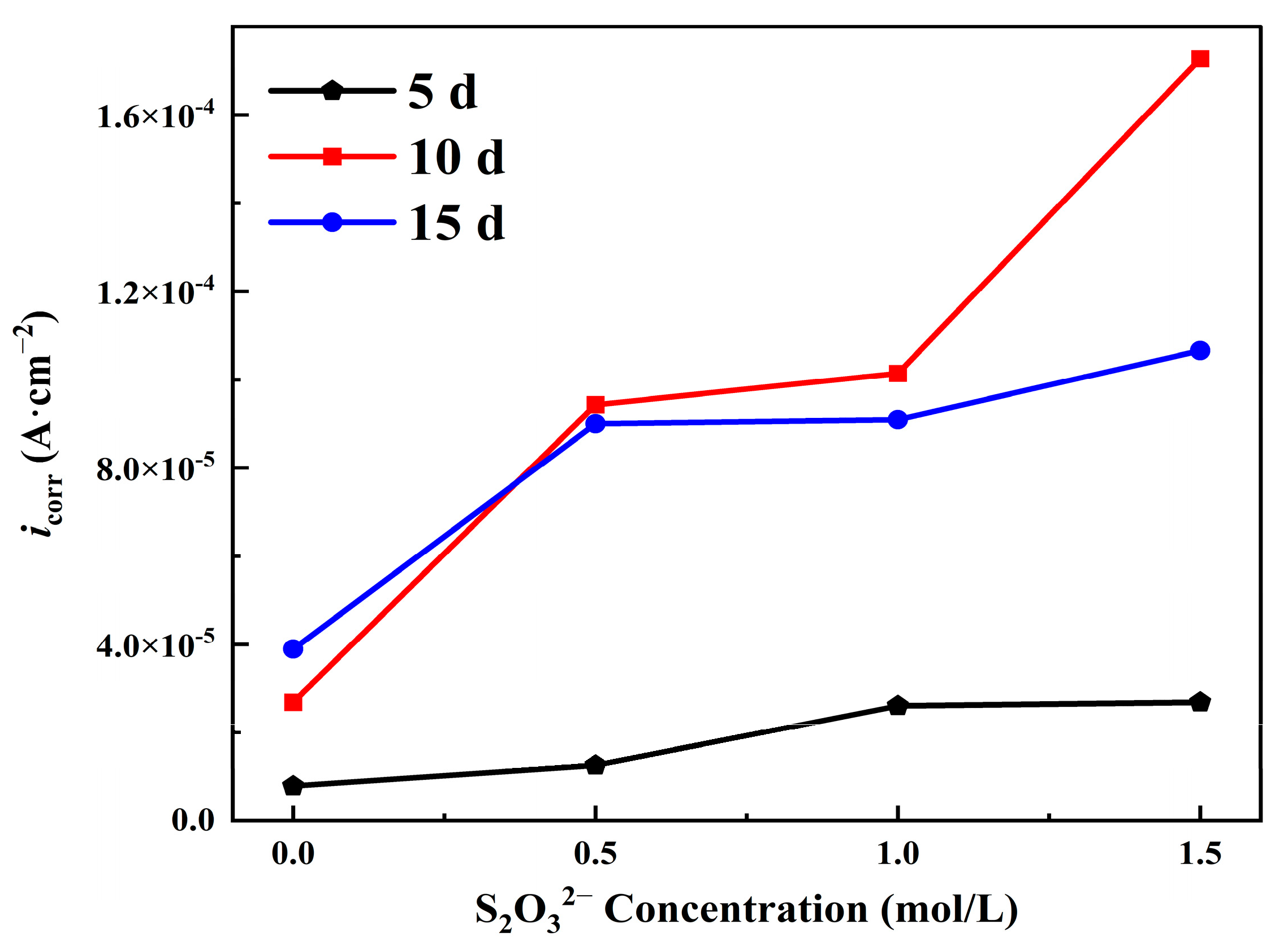
3.3.1. Potentiodynamic Polarization Curve
3.3.2. Electrochemical Impedance Spectroscopy Test
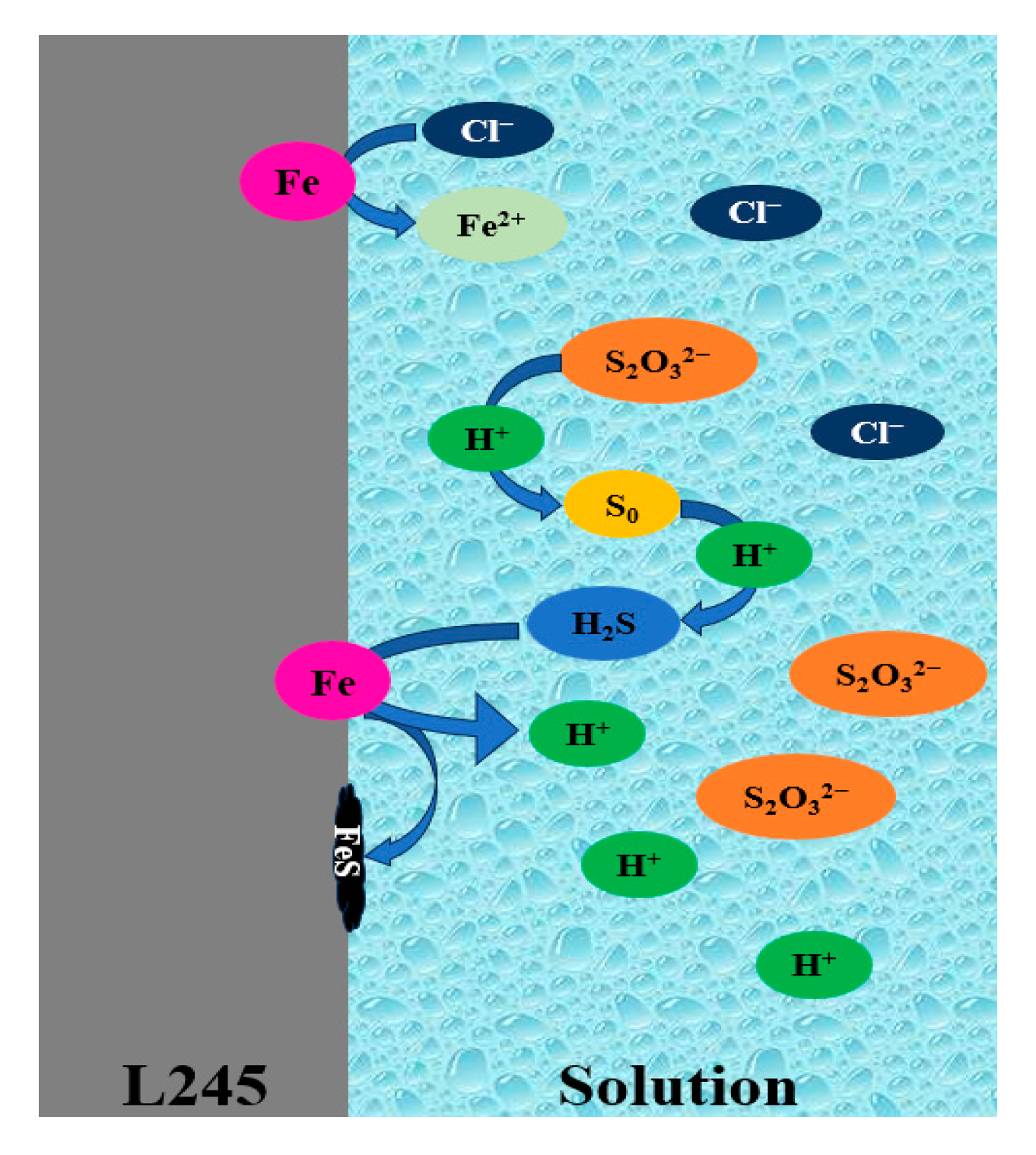
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Zhang, Q.; Qu, D.; Xu, K.; Song, X. Corrosion behavior of L360N and L415N mild steel in a shale gas gathering environment—Laboratory and on-site studies. J. Nat. Gas Sci. Eng. 2020, 82, 103492. [Google Scholar] [CrossRef]
- Liao, K.; Qin, M.; He, G.; Yang, N.; Zhang, S. Study on corrosion mechanism and the risk of the shale gas gathering pipelines. Eng. Fail. Anal. 2021, 128, 105622. [Google Scholar] [CrossRef]
- Baranwal, P.K.; Rajaraman, P.V. Electrochemical investigation on effect of sodium thiosulfate (Na2S2O3) and ammonium chloride (NH4Cl) on carbon steel corrosion. J. Mater. Res. Technol. JMRT 2019, 8, 1366–1378. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, Y.; Matsuda, K.; Zou, Z. Characterization of the effect of hydrogen sulfide on the corrosion of X80 pipeline steel in saline solution. Corros. Sci. 2016, 102, 455–468. [Google Scholar] [CrossRef]
- Ezuber, H.M. Influence of temperature and thiosulfate on the corrosion behavior of steel in chloride solutions saturated in CO2. Mater. Des. 2009, 30, 3420–3427. [Google Scholar] [CrossRef]
- Tsujikawa, S.; Miyasaka, A.; Ueda, M.; Ando, S.; Shibata, T.; Haruna, T.; Katahira, M.; Yamane, Y.; Aoki, T.; Yamada, T. Alternative for evaluating sour gas resistance of low-alloy steels and corrosion-resistant alloys. Corrosion 1993, 49, 409–419. [Google Scholar] [CrossRef]
- Kappes, M.; Frankel, G.S.; Sridhar, N.; Carranza, R.M. Corrosion behavior of carbon steel in acidified, thiosulfate-containing Brines. Corrosion 2012, 68, 872–884. [Google Scholar] [CrossRef]
- Dai, J.; Feng, H.; Li, H.B.; Jiang, Z.H.; Li, H.; Zhang, S.C.; Zhou, P.; Zhang, T. Nitrogen significantly enhances corrosion resistance of 316L stainless steel in thiosulfate-chloride solution. Corros. Sci. 2020, 174, 108792. [Google Scholar] [CrossRef]
- Al-Mamun, N.S.; Haider, W.; Shabib, I. Corrosion resistance of additively manufactured 316L stainless steel in chloride-thiosulfate environment. Electrochim. Acta 2020, 362, 137039. [Google Scholar] [CrossRef]
- Ezuber, H.M. Effect of temperature and thiosulphate on the corrosion behavior of 90-10 copper-nickel alloys in seawater. Anti-Corros. Methods Mater. 2009, 56, 168–172. [Google Scholar] [CrossRef]
- Tromans, D.; Frederick, L. Effect of thiosulfate on crevice corrosion of stainless steels. Corrosion 1984, 40, 633–639. [Google Scholar] [CrossRef]
- Xia, D.H.; Behnamian, Y.; Luo, J.L. Review-Factors influencing sulfur induced corrosion on the secondary side in pressurized water reactors. J. Electrochem. Soc. 2019, 166, C49–C64. [Google Scholar] [CrossRef]
- Newman, R.C.; Isaacs, H.S.; Alman, B. Effects of sulfur compounds on the pitting behavior of type 304 stainless steel in near-neutral chloride solutions. Corrosion 1982, 38, 261–265. [Google Scholar] [CrossRef]
- Duret-Thual, C.; Costa, D.; Yang, W.P.; Marcus, P. The role of thiosulfates in the pitting corrosion of Fe-17Cr alloys in neutral chloride solutions: Electrochemical and XPS study. Corros. Sci. 1997, 39, 913–933. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Nakhaie, D.; Zakeri, M.; Moayedz, M.H. Effect of thiosulfate on pitting corrosion of 316SS I. Critical pitting temperature and pit chemistry. J. Electrochem. Soc. 2015, 162, 71–77. [Google Scholar] [CrossRef]
- Nakhaie, D.; Zakeri, M.; Naghizadeh, M.; Moayedz, M.H. Effect of thiosulfate on pitting corrosion of 316SS II. Metastable pitting and transition to stability. J. Electrochem. Soc. 2015, 162, 121–127. [Google Scholar] [CrossRef]
- Xia, D.H.; Song, S.Z.; Zhu, R.K.; Behnamian, Y.; Chen, C.; Wang, J.H.; Luo, J.L.; Lu, Y.C.; Kilmas, S. A mechanistic study on thiosulfate-enhanced passivity degradation of Alloy 800 in chloride solutions. Electrochim. Acta 2013, 111, 510–525. [Google Scholar] [CrossRef]
- Fu, C.; Wang, J.; Wang, H.; Wang, K. Effect of thiosulfate ions on the electrochemical behavior of alloy 800. Int. J. Electrochem. Sci. 2013, 8, 10335–10349. [Google Scholar] [CrossRef]
- Zakeri, M.; Naghizadeh, M.; Nakhaie, D.; Moayed, M.H. Pit transition potential and repassivation potential of stainless steel in thiosulfate solution. J. Electrochem. Soc. 2016, 163, C275–C281. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, S.; Wang, L.; Man, C.; Liu, Z.; Wu, J.; Wang, X.; Chen, S.; Li, X. Passivation behavior and surface chemistry of 2507 super duplex stainless steel in acidified artificial seawater containing thiosulfate. J. Electrochem. Soc. 2017, 164, C856–C868. [Google Scholar] [CrossRef]
- Choudhary, L.; Macdonald, D.D.; Alfantazi, A. Role of thiosulfate in the corrosion of steels: A review. Corrosion 2015, 75, 1147–1168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, X.; Lin, P.; Chou, K. Electrochemical behavior of 2205 Duplex stainless steel in a chloride-thiosulfate environment. Int. J. Electrochem. Sci. 2019, 14, 4144–4160. [Google Scholar] [CrossRef]
- Ning, F.; Tan, J.; Zhang, Z.; Wang, X.; Wu, X.; Han, E.H.; Ke, W. Crevice corrosion behaviors of Alloy 690 and 405 stainless steel in chloride solution with different concentrations of thiosulfate. J. Nucl. Mater. 2023, 575, 154226. [Google Scholar] [CrossRef]
- ISO 8407:2021; Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens. International Standard: Geneva, Switzerland, 2021.
- Guo, L.; Chen, P.; Huang, C.; Zhang, J.; Xue, Y.; Zhang, X.; Tang, J.; Wang, S.; Wang, H. Study on under-deposit corrosion behavior of X65 steel in simulated CO2 saturated produced water. Int. J. Electrochem. Sci. 2024, 19, 100731. [Google Scholar] [CrossRef]
- Carvalho, M.B.L.; Bott, I.S.; Forero, A.B.; Ponciano, J.A.C. The corrosion process of an API 5L X80 Welded joint in a system with different pH and H2S concentration. Mater. Res. 2022, 25, 20210443. [Google Scholar] [CrossRef]
- Heuer, J.K.; Stubbins, J.F. An XPS characterization of FeCO3 films from CO2 corrosion. Corros. Sci. 1999, 41, 1231–1243. [Google Scholar] [CrossRef]
- Yan, Q.; Yin, Q.; Cui, J.; Wang, X.; Qiao, Y.; Zhou, H. Effect of temperature on corrosion behavior of E690 steel in 3.5 wt.% NaCl solution. Mater. Res. Express 2021, 8, 016528. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, B.; Cao, J. Corrosion mechanism of Q235A under 3.5% NaCl salt spray. Mater. Trans. 2020, 61, 2342–2347. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, M.; Yuan, Y.; Guo, S. Effects of Na2S2O3 concentrations and solutions temperature on anti-corrosion property of 6061 aluminum alloy in marine environment. J. Mater. Eng. Perform. 2024. [Google Scholar] [CrossRef]
- Wu, S.; Wang, J.; Song, S.; Xia, D.; Zhang, Z.; Gao, Z.; Wang, J.; Jin, W.; Hu, W. Factors influencing passivity breakdown on UNS N08800 in nuetral chloride and thiosulfate solutions. J. Electrochem. Soc. 2017, 164, C94–C103. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corrosion Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Behnamian, Y.; Gao, Z.; Wang, J.; Xia, D. Pitting growth rate on Alloy 800 in chloride solutions containing thiosulfate: Image analysis assessment. Corros. Eng. Sci. Technol. 2018, 53, 206–213. [Google Scholar] [CrossRef]
- Nishimura, T.; Katayama, H.; Noda, K.; Kodama, T. Electrochemical behavior of rust formed on carbon steel in wet/dry environment containing chloride ions. Corrosion 2000, 56, 935–941. [Google Scholar] [CrossRef]
- Li, G.; Huang, B.; Zhang, S.; Wen, X.; Li, T. Investigation of corrosion product films on steel surfaces of drilling materials in a wet hydrogen sulfide environment. Int. J. Electrochem. Sci. 2020, 15, 121–136. [Google Scholar] [CrossRef]
- Tykodi, R.J. In praise of thiosulfate. J. Chem. Educ. 1990, 67, 146. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.; He, L.; Singh, P.M. Effect of thiosulfate on metastable pitting of 304L and S32101 in chloride- and thiosulfate-containing environment. Corrosion 2016, 72, 628–635. [Google Scholar] [CrossRef]
- Gao, M.; Pang, X.; Gao, K. The growth mechanism of CO2 corrosion product films. Corros. Sci. 2010, 53, 557–568. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Z.; Liu, Y.; Lu, Y.; Wang, C.; Hu, W. Effect of temperature on corrosion behavior of X65 steel in simulated deep sea environment. Int. J. Electrochem. Sci. 2019, 14, 161–172. [Google Scholar] [CrossRef]


| Element | C | Si | Mn | P | S | Cr | Ni | Mo | V | Nb | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L245 | 0.202 | 0.254 | 0.396 | 0.0189 | 0.0139 | 0.023 | 0.020 | 0.018 | 0.0014 | 0.003 | 0.0025 | Bal. |
| API 5L | ≤0.26 | - | ≤1.20 | ≤0.030 | ≤0.030 | - | - | - | Nb + V ≤ 0.06; Nb + V+Ti ≤ 0.15 | |||
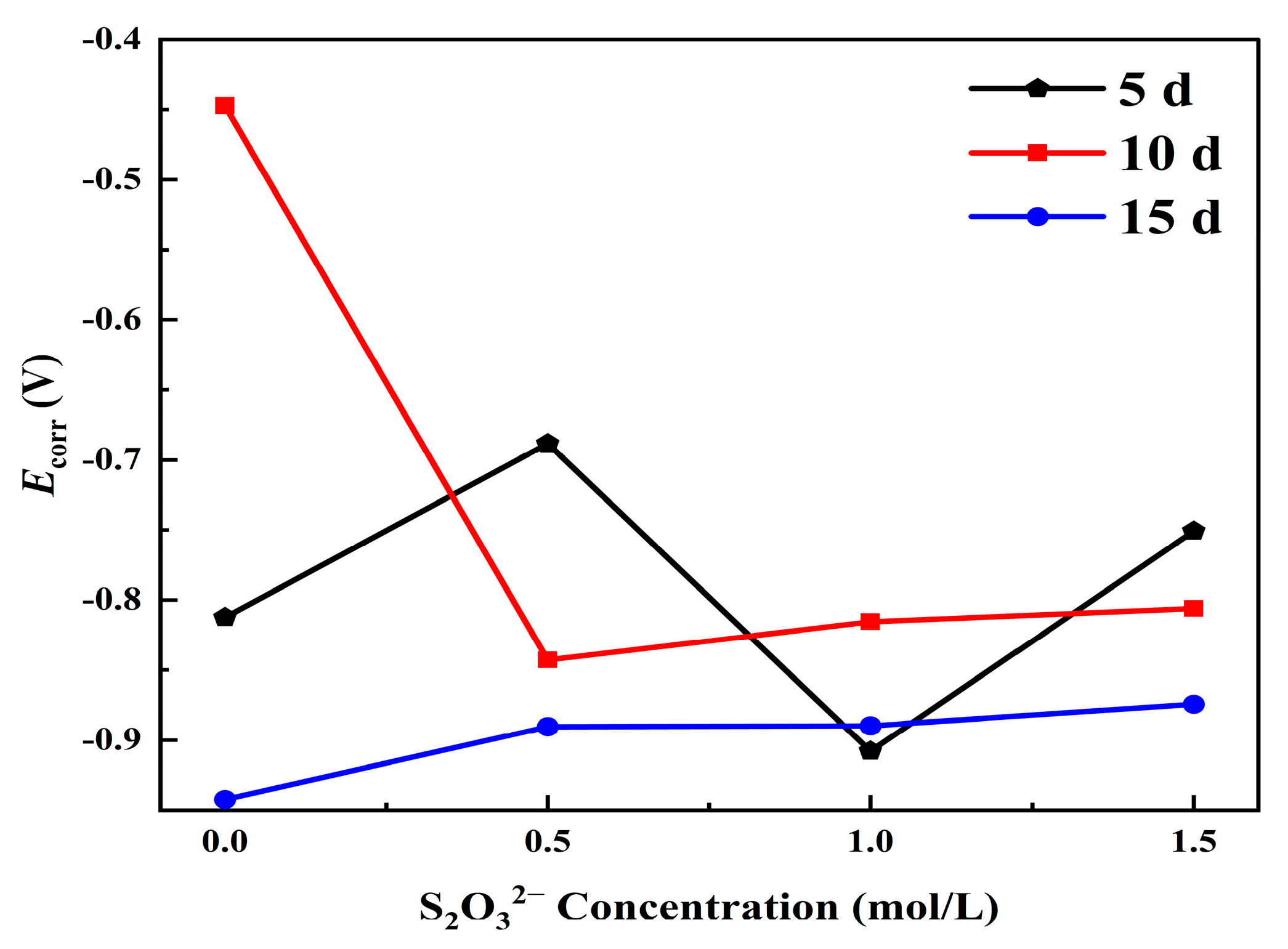
| Days | Corrosion Medium Concentration | Βa (mV/dec) | −βc (mV/dec) | Ecorr (V) | Icorr (A/cm2) |
|---|---|---|---|---|---|
| 5 d | 3.5 wt.%NaCl | 257 | 146 | −0.81 | 7.8 × 10−6 |
| 3.5 wt.% NaCl + 0.5 mol/L S2O32− | 213 | 186 | −0.69 | 1.3 × 10−5 | |
| 3.5 wt.% NaCl + 1.0 mol/L S2O32− | 73 | 29 | −0.91 | 2.6 × 10−5 | |
| 3.5 wt.% NaCl + 1.5 mol/L S2O32− | 192 | 139 | −0.75 | 2.7 × 10−5 | |
| 10 d | 3.5 wt.% NaCl | 359 | 170 | −0.45 | 2.7 × 10−5 |
| 3.5 wt.% NaCl + 0.5 mol/L S2O32− | 291 | 104 | −0.84 | 9.4 × 10−5 | |
| 3.5 wt.% NaCl + 1 mol/L S2O32− | 205 | 126 | −0.82 | 1.0 × 10−4 | |
| 3.5 wt.% NaCl + 1.5 mol/L S2O32− | 196 | 30 | −0.81 | 1.7 × 10−4 | |
| 15 d | 3.5 wt.% NaCl | 213 | 127 | −0.94 | 3.9 × 10−5 |
| 3.5 wt.% NaCl + 0.5 mol/L S2O32− | 250 | 120 | −0.89 | 9.0 × 10−5 | |
| 3.5 wt.% NaCl + 1 mol/L S2O32− | 250 | 69 | −0.89 | 9.1 × 10−5 | |
| 3.5 wt.% NaCl + 1.5 mol/L S2O32− | 335 | 81 | −0.87 | 1.1 × 10−4 |
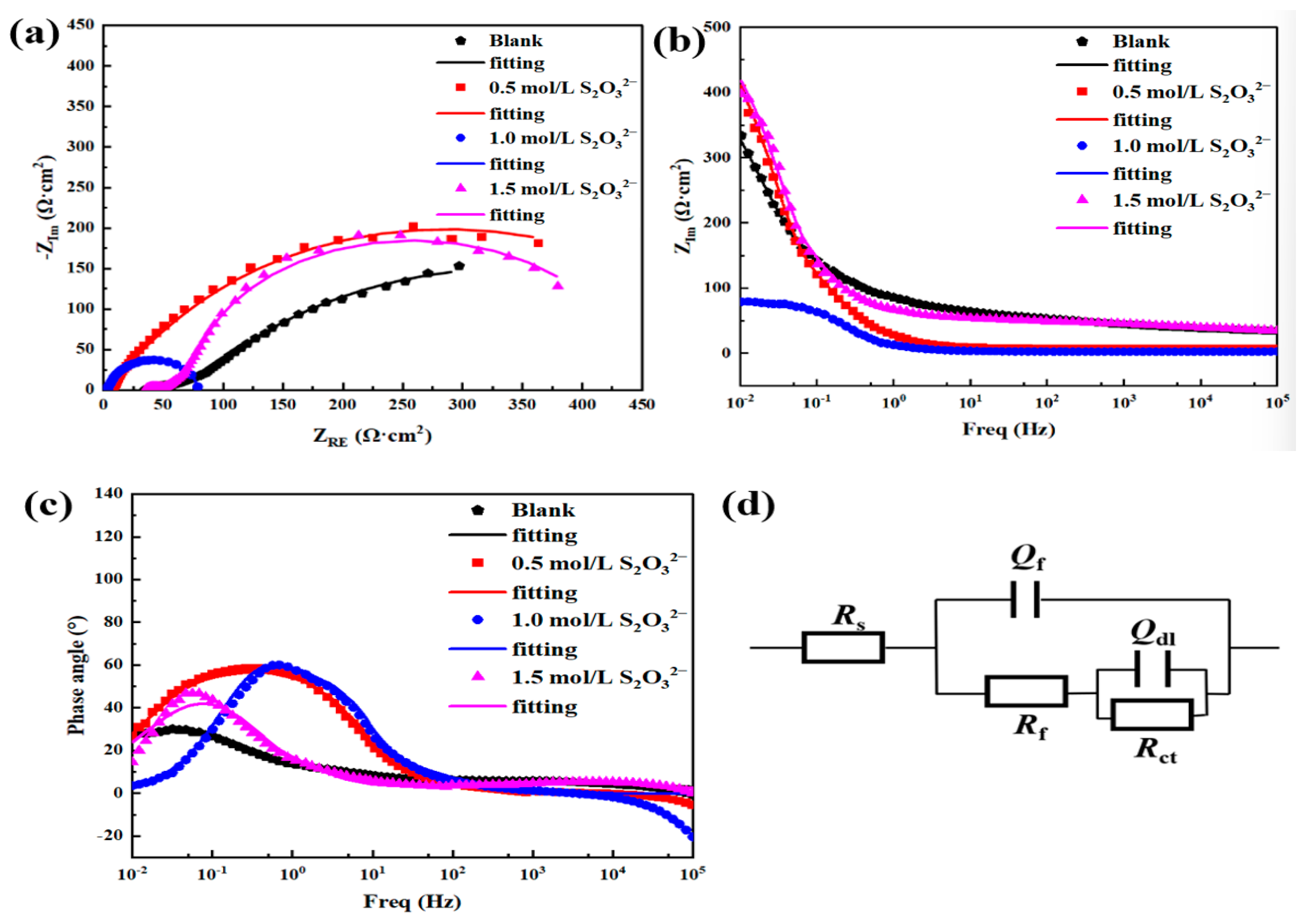
| EEC Parameters | 3.5 wt.% NaCl | 3.5 wt.% NaCl + 0.5 mol/L S2O32− | 3.5 wt.% NaCl + 1 mol/L S2O32− | 3.5 wt.% NaCl + 1.5 mol/L S2O32− |
|---|---|---|---|---|
| Rs (Ωcm−2) | 33.00 | 7.15 | 2.76 | 33.26 |
| Qf (Ω−1sncm−2) | 3.141 × 10−3 | 9.683 × 10−3 | 7.092 × 10−3 | 1.021 × 10−3 |
| nf | 0.3400 | 0.8046 | 0.7649 | 0.4319 |
| Rf (Ωcm−2) | 48.87 | 29.26 | 27.33 | 24.04 |
| Qdl (Ω−1sncm−2) | 9.350 × 10−3 | 8.866 × 10−3 | 8.088 × 10−3 | 1.095 × 10−2 |
| ndl | 0.6047 | 0.8841 | 0.8574 | 0.8392 |
| Rct (Ωcm−2) | 1046 | 527 | 438 | 283 |
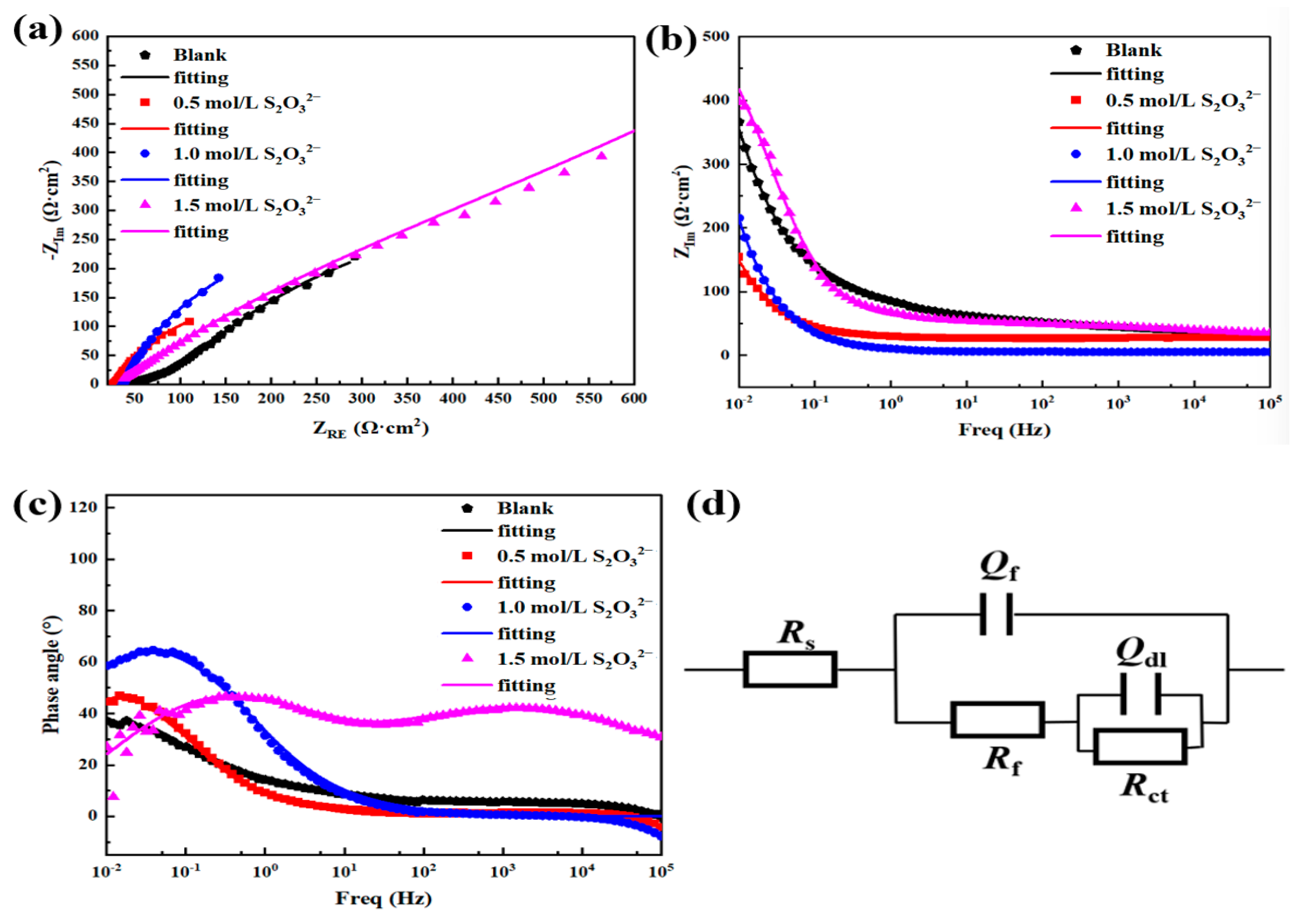
| EEC Parameters | 3.5 wt.% NaCl | 3.5 wt.% NaCl + 0.5 mol/L S2O32− | 3.5 wt.% NaCl + 1 mol/L S2O32− | 3.5 wt.% NaCl + 1.5 mol/L S2O32− |
|---|---|---|---|---|
| RS (Ωcm−2) | 31.77 | 27.21 | 25.64 | 24.31 |
| Qf (Ω−1sncm−2) | 2.060 × 10−3 | 1.847 × 10−2 | 2.587 × 10−2 | 1.180 × 10−4 |
| nf | 0.3691 | 0.8341 | 0.8190 | 0.4635 |
| Rf (Ωcm−2) | 37.37 | 5.15 | 3.48 | 1.71 |
| Qdl (Ω−1sncm−2) | 1.085 × 10−2 | 4.269 × 10−2 | 1.802 × 10−2 | 4.651 × 10−2 |
| ndl | 0.5156 | 0.7140 | 0.8545 | 0.8000 |
| Rct (Ωcm−2) | 217 | 539 | 472 | 457 |
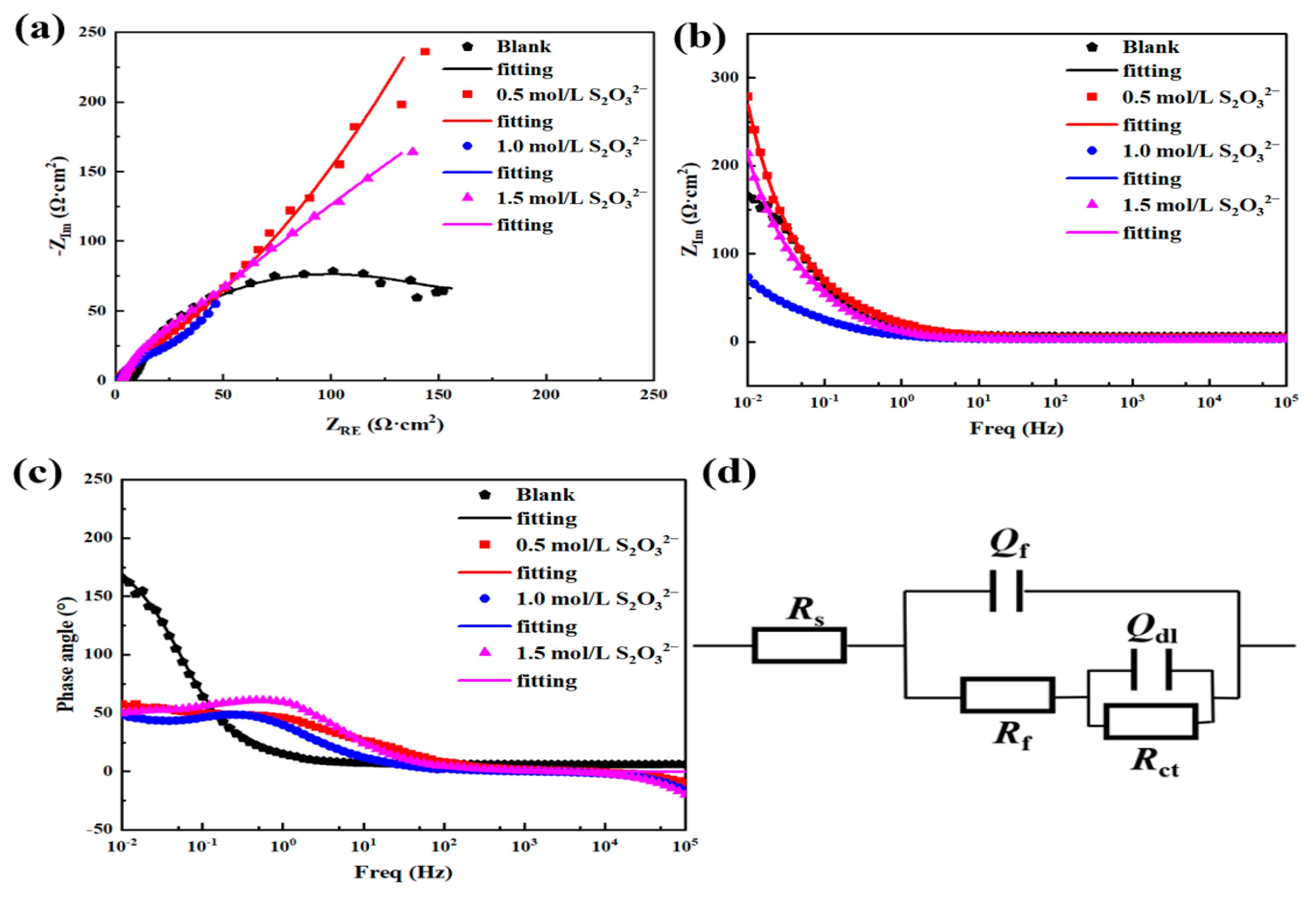
| EEC Parameters | 3.5 wt.% NaCl | 3.5 wt.% NaCl + 0.5 mol/L S2O32− | 3.5 wt.% NaCl + 1.0 mol/L S2O32− | 3.5 wt.% NaCl + 1.5 mol/L S2O32− |
|---|---|---|---|---|
| RS (Ωcm−2) | 6.240 | 0.690 | 0.549 | 2.599 |
| Qf (Ω−1sncm−2) | 1.420 × 10−3 | 1.966 × 10−3 | 7.457 × 10−3 | 2.022 × 10−2 |
| nf | 0.8490 | 0.8311 | 0.7952 | 0.7638 |
| Rf (Ωcm−2) | 41 | 110 | 61 | 22 |
| Qdl (Ω−1sncm−2) | 1.156 × 10−2 | 1.807 × 10−2 | 1.378 × 10−2 | 2.416 × 10−2 |
| ndl | 0.8289 | 0.5769 | 0.5503 | 0.7082 |
| Rct (Ωcm−2) | 201.4 | 627 | 525 | 487 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, M.; He, Y.; Li, J.; Zhang, L.; Liu, C.; Wang, L.; Wen, Z.; Zhang, X.; Wang, S. Investigation of the Corrosion Behavior of L245 Steel in 3.5 wt.% NaCl Solution with Varying Concentrations of Na2S2O3. Materials 2025, 18, 2270. https://doi.org/10.3390/ma18102270
Bao M, He Y, Li J, Zhang L, Liu C, Wang L, Wen Z, Zhang X, Wang S. Investigation of the Corrosion Behavior of L245 Steel in 3.5 wt.% NaCl Solution with Varying Concentrations of Na2S2O3. Materials. 2025; 18(10):2270. https://doi.org/10.3390/ma18102270
Chicago/Turabian StyleBao, Mingyu, Yan He, Jing Li, Lingfan Zhang, Chang Liu, Lei Wang, Zidan Wen, Xiaoyan Zhang, and Shuliang Wang. 2025. "Investigation of the Corrosion Behavior of L245 Steel in 3.5 wt.% NaCl Solution with Varying Concentrations of Na2S2O3" Materials 18, no. 10: 2270. https://doi.org/10.3390/ma18102270
APA StyleBao, M., He, Y., Li, J., Zhang, L., Liu, C., Wang, L., Wen, Z., Zhang, X., & Wang, S. (2025). Investigation of the Corrosion Behavior of L245 Steel in 3.5 wt.% NaCl Solution with Varying Concentrations of Na2S2O3. Materials, 18(10), 2270. https://doi.org/10.3390/ma18102270







