Gum Rosin in Medical and Pharmaceutical Applications: From Conventional Uses to Modern Advancements
Abstract
1. Introduction
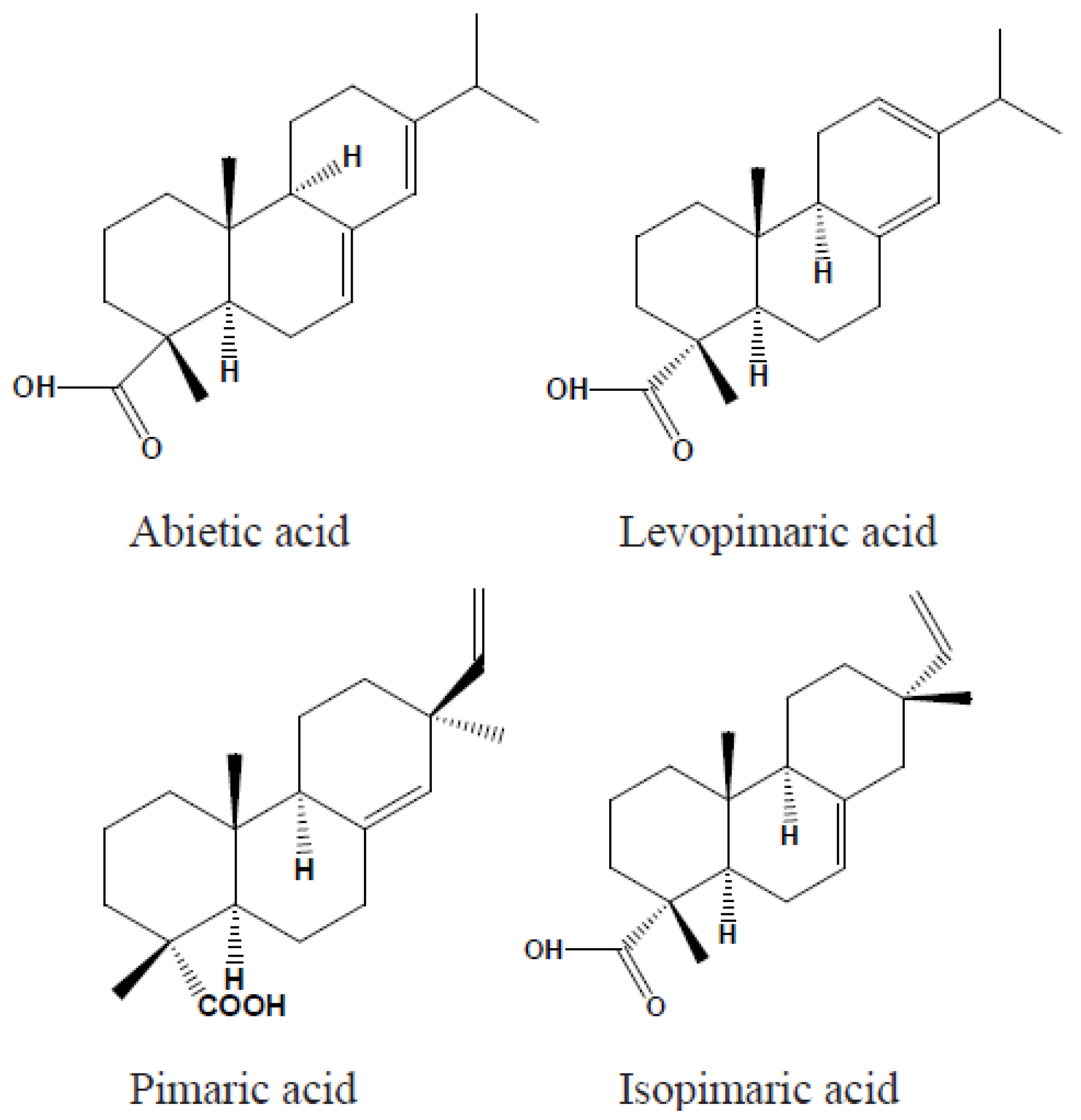
2. Chemical and Physical Properties
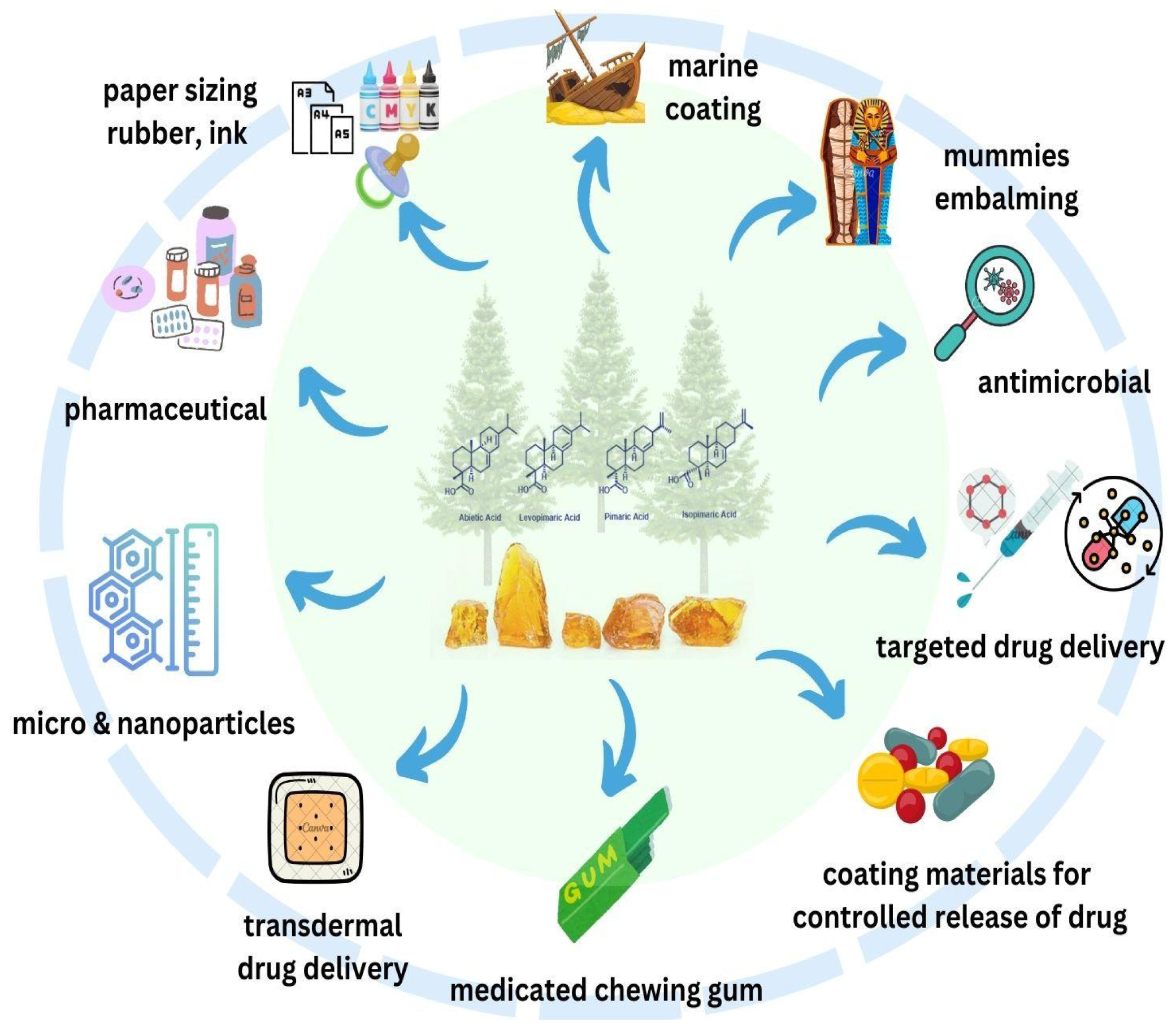
3. General Uses
4. Medical and Pharmaceutical Applications
4.1. Antimicrobial Agents
4.2. Anticancer Agents
4.3. Corrosion Retardant for Biodegradable Implants
4.4. Remineralization-Promoting Tooth Varnish
4.5. Pharmaceutical Dosage Forms
5. Drug Delivery System
5.1. Taste-Masking Agent
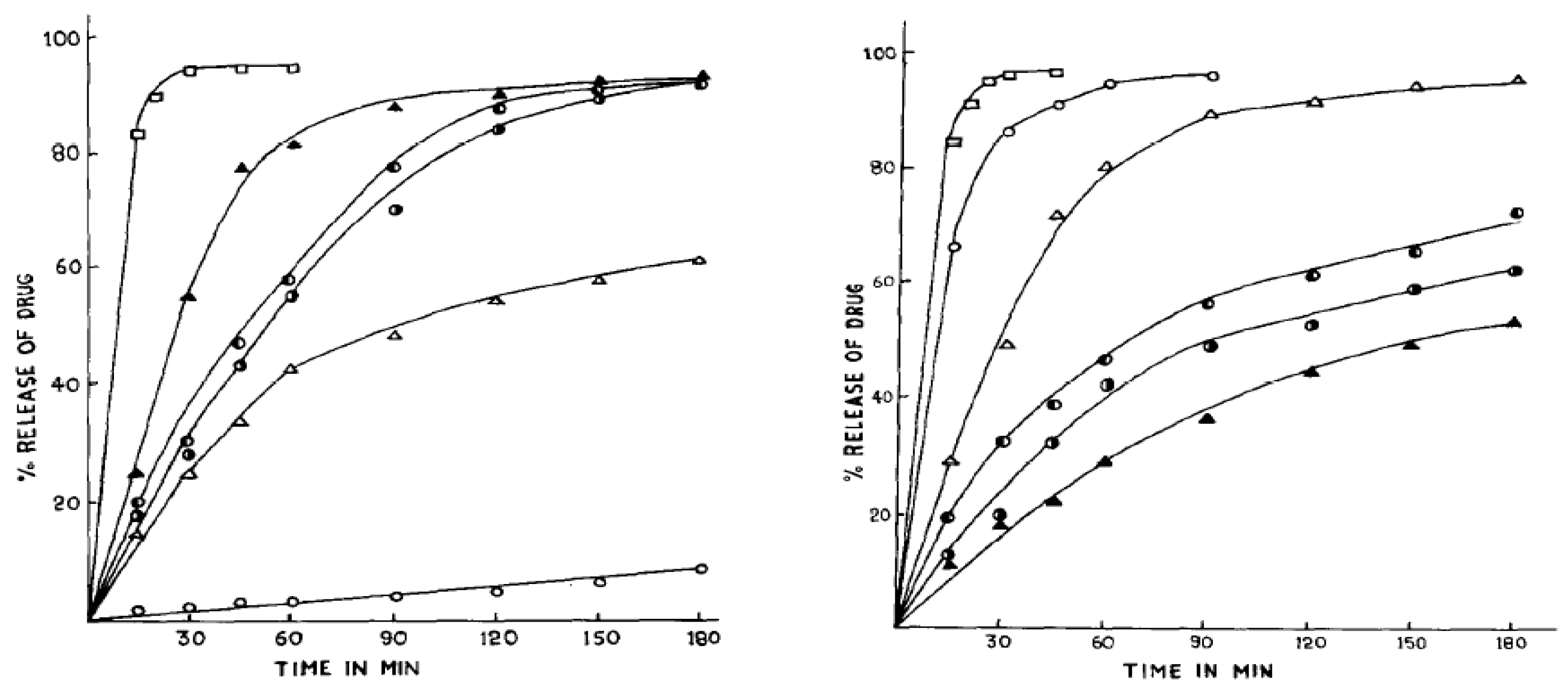
5.2. Coating Materials for Controlled-Release Dosage Forms
5.3. Medicated Chewing Gum
5.4. Transdermal Drug Delivery System
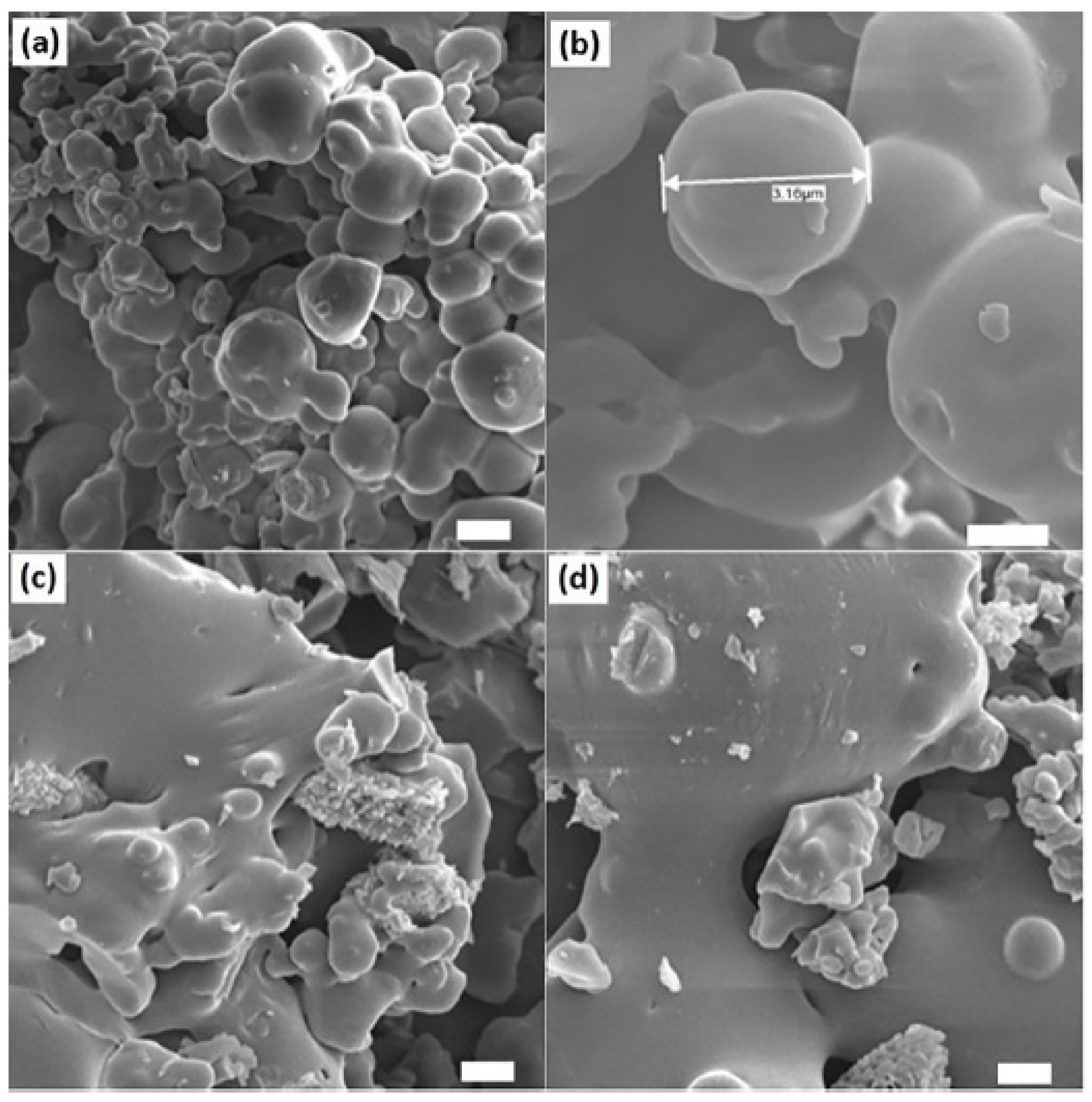
5.5. Micro- and Nanoparticles
5.6. Targeted Drug Delivery Systems
5.6.1. Targeted Delivery for Anticancer Drugs
5.6.2. Targeted Delivery of 5-ASA, an Anti-Inflammatory Drug
5.6.3. Targeted Delivery of Periodontitis Medication
6. Challenges of Gum Rosin as Pharmaceutical Excipient for Frug Delivery System
6.1. Allergenic Activity and Occupational Exposure
6.2. Brittleness and Mechanical Weakness
6.3. Hydrophobicity and Drug Release Modulation
6.4. Other Challenging Characteristics
7. Strategies to Optimize the Application of Gum Rosin
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, B.K.; Gidwani, B.; Vyas, A. Rosin: Recent Advances and Potential Applications in Novel Drug Delivery System. J. Bioact. Compat. Polym. 2016, 31, 111–126. [Google Scholar] [CrossRef]
- Park, S.A.; Son, J.; Kim, A.J.; Oh, S.; Bae, J.M. Effect of Adhesive Components in Experimental Fluoride Varnish on Fluoride Release Within 30 Days In Vitro Study. Dent. Mater. J. 2024, 43, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.R.; Vidhura, M.; Dora, S. Biopolymers Based on Rosin. Curr. Res. Biopolym. 2018, 18, 1–6. [Google Scholar]
- Mitchell, G.R.; Biscaia, S.; Mahendra, V.S.; Mateus, A. High Value Materials from the Forests. Adv. Mater. Phys. Chem. 2016, 6, 54–60. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Y.; Ni, C.; Yu, L. Preparation and Evaluation of Natural Rosin-Based Zinc Resins for Marine Antifouling. Prog. Org. Coat. 2021, 157, 106270. [Google Scholar] [CrossRef]
- Cai, W.; Tai, H.-C. String Theories: Chemical Secrets of Italian Violins and Chinese Guqins. AsiaChem Mag. 2020, 1, 10–17. [Google Scholar] [CrossRef]
- Hamnerius, N.; Dahlin, J.; Bruze, M.; Nilsson, K.; Sukakul, T.; Svedman, C. Colophonium-Related Allergic Contact Dermatitis Caused by Medical Adhesive Tape Used to Prevent Skin Lesions in Soldiers. Acta Derm. Venereol. 2023, 103, 1–6. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, D.; Wei, X.; Liang, J.; Chen, X.; Wang, L. Green Catalytic Conversion of Hydrogenated Rosin to Glycerol Esters Using Subcritical CO2 in Water and the Associated Kinetics. J. Supercrit. Fluids 2017, 125, 12–21. [Google Scholar] [CrossRef]
- Satturwar, P.M.; Fulzele, S.V.; Dorle, A.K. Evaluation of Polymerized Rosin for the Formulation and Development of Transdermal Drug Delivery System: A Technical Note. AAPS PharmSciTech 2005, 6, E649–E654. [Google Scholar] [CrossRef]
- Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K.; Wierzbicka, E. Advances in Rosin-Based Chemicals: The Latest Recipes, Applications and Future Trends. Molecules 2019, 24, 1651. [Google Scholar] [CrossRef]
- Morkhade, D.M.; Nande, V.S.; Barabde, U.V.; Joshi, S.B. Study of Biodegradation and Biocompatibility of PEGylated Rosin Derivatives. J. Bioact. Compat. Polym. 2017, 32, 628–640. [Google Scholar] [CrossRef]
- Mardiah, M.; Samadhi, T.W.; Wulandari, W.; Aqsha, A.; Situmorang, Y.A.; Indarto, A. Recent Progress on Catalytic of Rosin Esterification Using Different Agents of Reactant. AgriEngineering 2023, 5, 2155–2169. [Google Scholar] [CrossRef]
- Kanlaya, P.; Sumrit, W.; Amorn, P. Synthesis and Characterization of Water Soluble Rosin-Polyethylene Glycol 1500 Derivative. Int. J. Chem. Eng. Appl. 2016, 7, 277–281. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, J.K.; Chandel, A.K.; Sharma, L.; Mallick, N.; Singh, S.P. Biomedical Applications of Microbially Engineered Polyhydroxyalkanoates: An Insight into Recent Advances, Bottlenecks, and Solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. [Google Scholar] [CrossRef]
- Joshi, S.; Singh, V. Gelatin–Rosin Gum Complex Nanoparticles: Preparation, Characterization and Colon Targeted Delivery of 5-Fluorouracil. Chem. Pap. 2020, 74, 4241–4252. [Google Scholar] [CrossRef]
- Mahendra, V. Rosin Product Review. Appl. Mech. Mater. 2019, 890, 77–91. [Google Scholar] [CrossRef]
- Li, X.Q.; Chen, Y. Rosin: A Comprehensive Review on Traditional Uses, Phytochemistry, and Pharmacology. Fitoterapia 2024, 177, 106068. [Google Scholar] [CrossRef]
- Qiu, H.; Chen, X.; Wei, X.; Liang, J.; Zhou, D.; Wang, L. The Emulsifying Properties of Hydrogenated Rosin Xylitol Ester as a Biomass Surfactant for Food: Effect of PH and Salts. Molecules 2020, 25, 302. [Google Scholar] [CrossRef]
- Pratapwar, A.S.; Sakarkar, D.M. Applications of Rosin Derivatives in the Development of Novel Drug Delivery System (NDDS): A Contemporary View. J. Qual. Assur. Pharma Anal. 2015, 1, 100–109. [Google Scholar]
- Pics, P.I.; Rodenburg, L.; Chemicals, E. Skin Sensitisation of Rosin and Its Derivatives in Relation to Their Chemistry; Pine Chemicals Association International: Fernandina Beach, FL, USA, 2016; pp. 1–79. Available online: https://assets.noviams.com/novi-file-uploads/pcai/PDFs/eXoGZKnpM6xzTaRY7aBtegnX-0aab39e7.pdf (accessed on 12 March 2025).
- Yamaguchi, T.; Nasu, D.; Masani, K. Effect of Grip-Enhancing Agents on Sliding Friction between a Fingertip and a Baseball. Commun. Mater. 2022, 3, 92. [Google Scholar] [CrossRef]
- Xie, W.; Yan, Q.; Fu, H. Study on Novel Rosin-based Polyurethane Reactive Hot Melt Adhesive. Polym. Adv. Technol. 2021, 32, 4415–4423. [Google Scholar] [CrossRef]
- Biswas, M.; Kundu, S.; Debnath, S. Tall Oil Rosin: A Substitute for Gum Rosin in Development of Offset Printing Ink. NIP Digit. Fabr. Conf. 2018, 34, 44–48. [Google Scholar] [CrossRef]
- Bezzekhami, M.A.; Belalia, M.; Hamed, D.; Bououdina, M.; Berfai, B.B.; Harrane, A. Nanoarchitectonics of Starch Nanoparticles Rosin Catalyzed by Algerian Natural Montmorillonite (Maghnite-H+) for Enhanced Antimicrobial Activity. J. Inorg. Organomet. Polym. Mater. 2023, 33, 193–206. [Google Scholar] [CrossRef]
- Leite, L.S.F.; Bilatto, S.; Paschoalin, R.T.; Soares, A.C.; Moreira, F.K.V.; Oliveira, O.N.; Mattoso, L.H.C.; Bras, J. Eco-Friendly Gelatin Films with Rosin-Grafted Cellulose Nanocrystals for Antimicrobial Packaging. Int. J. Biol. Macromol. 2020, 165, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Abdalkarim, S.Y.H.; Yu, H.-Y.; Zhu, J.; Zhou, Y.; Guan, Y. Bifunctional Reinforcement of Green Biopolymer Packaging Nanocomposites with Natural Cellulose Nanocrystal–Rosin Hybrids. ACS Appl. Bio Mater. 2020, 3, 1944–1954. [Google Scholar] [CrossRef]
- Moustafa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT Bionanocomposites with Antimicrobial Natural Rosin for Green Packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; An, X.; Liu, H.; Nie, S.; Cao, H.; Xu, Q.; Lu, B. Improving Sizing Performance of Middle Layer of Liquid Packaging Board Containing High-Yield Pulp. Cellulose 2020, 27, 4707–4719. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Liu, H.; Shang, S.; Song, Z.; Song, J. Preparation and Properties of Room Temperature Vulcanized Silicone Rubber Based on Rosin-Grafted Polydimethylsiloxane. RSC Adv. 2018, 8, 14684–14693. [Google Scholar] [CrossRef]
- Rosu, L.; Mustata, F.; Rosu, D.; Varganici, C.-D.; Rosca, I.; Rusu, T. Bio-Based Coatings from Epoxy Resins Crosslinked with a Rosin Acid Derivative for Wood Thermal and Anti–Fungal Protection. Prog. Org. Coat. 2021, 151, 106008. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Degen, G.; Engel, K.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; Manco, M.; et al. Follow-up of the Re-evaluation of Glycerol Esters of Wood Rosins (E 445) as a Food Additive. EFSA J. 2022, 20, 7353. [Google Scholar] [CrossRef]
- Sapbamrer, R.; Naksata, M.; Hongsibsong, S.; Chittrakul, J.; Chaiut, W. Efficiency of Gum Rosin-Coated Personal Protective Clothing to Protect against Chlorpyrifos Exposure in Applicators. Int. J. Environ. Res. Public Health 2022, 19, 2594. [Google Scholar] [CrossRef] [PubMed]
- Naksata, M.; Watcharapasorn, A.; Hongsibsong, S.; Sapbamrer, R. Development of Personal Protective Clothing for Reducing Exposure to Insecticides in Pesticide Applicators. Int. J. Environ. Res. Public Health 2020, 17, 3303. [Google Scholar] [CrossRef]
- Phun, L.; Snead, D.; Hurd, P.; Jing, F. Industrial Applications of Pine-Chemical-Based Materials. In Sustainable Polymers from Biomass; Wiley: Hoboken, NJ, USA, 2017; pp. 151–179. [Google Scholar] [CrossRef]
- Gu, S.; Liu, M.; Xu, R.; Han, X.; Lou, Y.; Kong, Y.; Gao, Y.; Shang, S.; Song, Z.; Song, J.; et al. Ecofriendly Controlled-Release Insecticide Carrier: PH-/Temperature-Responsive Rosin-Derived Hydrogels for Avermectin Delivery Against Mythimna separata (Walker). Langmuir 2024, 40, 10992–11010. [Google Scholar] [CrossRef] [PubMed]
- Vainio-Kaila, T.; Hänninen, T.; Kyyhkynen, A.; Ohlmeyer, M.; Siitonen, A.; Rautkari, L. Effect of Volatile Organic Compounds from Pinus Sylvestris and Picea Abies on Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae and Salmonella enterica Serovar Typhimurium. Holzforschung 2017, 71, 905–912. [Google Scholar] [CrossRef]
- Badr, M.M.; Awadallah-F, A.; Azzam, A.M.; Mady, A.H. Influence of Gamma Irradiation on Rosin Properties and Its Antimicrobial Activity. Sci. Rep. 2023, 13, 4500. [Google Scholar] [CrossRef]
- Sipponen, A.; Peltola, R.; Jokinen, J.J.; Laitinen, K.; Lohi, J.; Rautio, M.; Männistö, M.; Sipponen, P.; Lounatmaa, K. Effects of Norway Spruce (Picea abies) Resin on Cell Wall and Cell Membrane of Staphylococcus aureus. Ultrastruct. Pathol. 2009, 33, 128–135. [Google Scholar] [CrossRef]
- Majeed, Z.; Mushtaq, M.; Ajab, Z.; Guan, Q.; Mahnashi, M.H.; Alqahtani, Y.S.; Ahmad, B. Rosin Maleic Anhydride Adduct Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus. Polímeros 2020, 30, 1–7. [Google Scholar] [CrossRef]
- Chang, R.; Lata, R.; Rohindra, D. Study of Mechanical, Enzymatic Degradation and Antimicrobial Properties of Poly(Butylene Succinate)/Pine-Resin Blends. Polym. Bull. 2020, 77, 3621–3635. [Google Scholar] [CrossRef]
- Kanerva, M.; Matrenichev, V.; Layek, R.; Takala, T.; Laurikainen, P.; Sarlin, E.; Elert, A.M.; Yudin, V.; Seitsonen, J.; Ruokolainen, J.; et al. Comparison of Rosin and Propolis Antimicrobials in Cellulose Acetate Fibers Against Staphylococcus aureus. BioResources 2020, 15, 3756–3773. [Google Scholar] [CrossRef]
- Nirmala, R.; Woo-il, B.; Navamathavan, R.; Kalpana, D.; Lee, Y.S.; Kim, H.Y. Influence of Antimicrobial Additives on the Formation of Rosin Nanofibers via Electrospinning. Colloids Surfaces B Biointerfaces 2013, 104, 262–267. [Google Scholar] [CrossRef]
- Kanerva, M.; Puolakka, A.; Takala, T.M.; Elert, A.M.; Mylläri, V.; Jönkkäri, I.; Sarlin, E.; Seitsonen, J.; Ruokolainen, J.; Saris, P.; et al. Antibacterial Polymer Fibres by Rosin Compounding and Melt-Spinning. Mater. Today Commun. 2019, 20, 100527. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, J.; Yang, X.; Liu, H.; Xu, X.; Ma, L.; Shang, S.; Song, Z. Construction of Antimicrobial and Biocompatible Cotton Textile Based on Quaternary Ammonium Salt from Rosin Acid. Int. J. Biol. Macromol. 2020, 150, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.P.; Yao, K.; Wilbon, P.A.; Zhang, W.; Ren, L.; Zhou, J.; Nagarkatti, M.; Wang, C.; Chu, F.; et al. Robust Antimicrobial Compounds and Polymers Derived from Natural Resin Acids. Chem. Commun. 2012, 48, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Jindal, R.; Sharma, R.; Maiti, M.; Kaur, A.; Sharma, P.; Mishra, V.; Jana, A.K. Synthesis and Characterization of Novel Reduced Gum Rosin-Acrylamide Copolymer-Based Nanogel and Their Investigation for Antibacterial Activity. Polym. Bull. 2017, 74, 2995–3014. [Google Scholar] [CrossRef]
- Jokinen, J.J.; Sipponen, A. Refined Spruce Resin to Treat Chronic Wounds: Rebirth of an Old Folkloristic Therapy. Adv. Wound Care 2016, 5, 198–207. [Google Scholar] [CrossRef]
- Sipponen, A.; Kuokkanen, O.; Tiihonen, R.; Kauppinen, H.; Jokinen, J.J. Natural Coniferous Resin Salve Used to Treat Complicated Surgical Wounds: Pilot Clinical Trial on Healing and Costs. Int. J. Dermatol. 2012, 51, 726–732. [Google Scholar] [CrossRef]
- Fei, B.-L.; Tu, S.; Wei, Z.; Wang, P.; Qiao, C.; Chen, Z.-F. Optically Pure Chiral Copper(II) Complexes of Rosin Derivative as Attractive Anticancer Agents with Potential Anti-Metastatic and Anti-Angiogenic Activities. Eur. J. Med. Chem. 2019, 176, 175–186. [Google Scholar] [CrossRef]
- El-Hallouty, S.M.; Soliman, A.A.F.; Nassrallah, A.; Salamatullah, A.; Alkaltham, M.S.; Kamal, K.Y.; Hanafy, E.A.; Gaballa, H.S.; Aboul-Soud, M.A.M. Crude Methanol Extract of Rosin Gum Exhibits Specific Cytotoxicity against Human Breast Cancer Cells via Apoptosis Induction. Anticancer Agents Med. Chem. 2020, 20, 1028–1036. [Google Scholar] [CrossRef]
- Gumelar, M.D.; Putri, N.A.; Anggaravidya, M.; Anawati, A. Corrosion Behavior of Biodegradable Material AZ31 Coated with Beeswax-Colophony Resin. AIP Conf. Proc. 2018, 1964, 020035. [Google Scholar] [CrossRef]
- Satturwar, P.M.; Fulzele, S.V.; Dorle, A.K. Biodegradation and in Vivo Biocompatibility of Rosin: A Natural Film-Forming Polymer. AAPS PharmSciTech 2003, 4, 1–6. [Google Scholar] [CrossRef]
- Abou Neel, E.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.; Bozec, L.; Mudera, V. Demineralization-Remineralization Dynamics in Teeth and Bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- Tulumbaci, F.; Gungormus, M. In Vitro Remineralization of Primary Teeth with a Mineralization-Promoting Peptide Containing Dental Varnish. J. Appl. Oral. Sci. 2020, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Durmuş, E.; Kölüş, T.; Çoban, E.; Yalçınkaya, H.; Ülker, H.E.; Çelik, İ. In Vitro Determination of the Remineralizing Potential and Cytotoxicity of Non-Fluoride Dental Varnish Containing Bioactive Glass, Eggshell, and Eggshell Membrane. Eur. Arch. Paediatr. Dent. 2023, 24, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Baik, A.; Alamoudi, N.; El-Housseiny, A.; Altuwirqi, A. Fluoride Varnishes for Preventing Occlusal Dental Caries: A Review. Dent. J. 2021, 9, 64. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The Evolution of Commercial Drug Delivery Technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Otte, A.; Park, K. Evolution of Drug Delivery Systems: From 1950 to 2020 and Beyond. J. Control. Release 2022, 342, 53–65. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Montazam, S.H. Comparison of Microencapsulation by Emulsion-Solvent Extraction/Evaporation Technique Using Derivatives Cellulose and Acrylate-Methacrylate Copolymer as Carriers. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 144–152. [Google Scholar]
- Geraili, A.; Xing, M.; Mequanint, K. Design and Fabrication of Drug-delivery Systems toward Adjustable Release Profiles for Personalized Treatment. VIEW 2021, 2, 1–24. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, K.; Lee, J.; Chandler, D.; Wang, J.; Wang, C.; Chu, F.; Tang, C. Well-Defined Renewable Polymers Derived from Gum Rosin. Macromolecules 2010, 43, 5922–5924. [Google Scholar] [CrossRef]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Abdela Siraj, E. Targeted Drug Delivery—From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef]
- de la Rosa-Ramírez, H.; Dominici, F.; Ferri, J.M.; Luzi, F.; Puglia, D.; Torre, L.; López-Martínez, J.; Samper, M.D. Pentaerythritol and Glycerol Esters Derived from Gum Rosin as Bio-Based Additives for the Improvement of Processability and Thermal Stability of Polylactic Acid. J. Polym. Environ. 2023, 31, 5446–5461. [Google Scholar] [CrossRef]
- Jacob, S. Rosin Microspheres as Taste Masking Agent in Oral Drug Delivery System. Int. J. Pharm. Sci. Res. 2012, 3, 3116–3124. [Google Scholar]
- Ratnaparkhi, M.P.; Karnawat, G.R.; Andhale, R.S. Natural Polymers in Fast Dissolving Tablets. Res. J. Pharm. Technol. 2021, 14, 2859–2866. [Google Scholar] [CrossRef]
- Chen, C.; Li, Z.; Hu, Y.; Huang, Q.; Li, X.; Qing, Y.; Wu, Y. Rosin Acid and SiO2 Modified Cotton Fabric to Prepare Fluorine-Free Durable Superhydrophobic Coating for Oil-Water Separation. J. Hazard. Mater. 2022, 440, 129797. [Google Scholar] [CrossRef]
- Zaoui, A.; Mahendra, V.; Mitchell, G.; Cherifi, Z.; Harrane, A.; Belbachir, M. Design, Synthesis and Thermo-Chemical Properties of Rosin Vinyl Imidazolium Based Compounds as Potential Advanced Biocompatible Materials. Waste Biomass Valorization 2020, 11, 3723–3730. [Google Scholar] [CrossRef]
- Pathak, Y.V.; Nikore, R.L.; Dorle, A.K. Study of Rosin and Rosin Esters as Coating Materials. Int. J. Pharm. 1985, 24, 351–354. [Google Scholar] [CrossRef]
- Pathak, Y.V.; Dorle, A.K. Study of Rosin and Rosin Derivatives as Coating Materials for Controlled Release of Drug. J. Control. Release 1987, 5, 63–68. [Google Scholar] [CrossRef]
- Burakale, P.; Sudke, S.; Bhise, M.; Tare, H.; Kachave, R. Exploring Film Forming Ability of Newly Synthesized Rosin Esters. Int. J. Drug Deliv. Technol. 2023, 13, 908–912. [Google Scholar] [CrossRef]
- Wang, M.; Guo, X.; Yang, X.; Zhang, J.; Yang, M.; Song, J.; Han, C.; Liu, L. Preparation and Application of Rosin@apatite Hybrid Material with New Natural Surfactants Based on In-Situ Reaction by Sol-Gel Method. Mater. Res. Express 2024, 11, 075003. [Google Scholar] [CrossRef]
- Thivya, P.; Durgadevi, M.; Sinija, V.R.N. Biodegradable Medicated Chewing Gum: A Modernized System for Delivering Bioactive Compounds. Futur. Foods 2021, 4, 100054. [Google Scholar] [CrossRef]
- Pandit, A.; Joshi, S.B. Formulation Development of Chewing Gum as a Novel Drug Delivery System for Diltiazem Hydrochloride. Indian. Drugs 2006, 43, 724–728. [Google Scholar]
- Gudigennavar, A.S.; Chandragirvar, P.C.; Gudigennavar, A.S. Role of Fluvastatin Sodium Loaded Polymeric Nanoparticles in the Treatment of Hyperlipidemia: Fabrication and Characterization. Ger. J. Pharm. Biomater. 2023, 1, 14–26. [Google Scholar] [CrossRef]
- Danışman-Kalındemirtaş, F.; Birman, H.; Karakuş, S.; Kilislioğlu, A.; Erdem-Kuruca, S. Preparation and Biological Evaluation of Novel 5-Fluorouracil and Carmofur Loaded Polyethylene Glycol/Rosin Ester Nanocarriers as Potential Anticancer Agents and Ceramidase Inhibitors. J. Drug Deliv. Sci. Technol. 2022, 73, 103456. [Google Scholar] [CrossRef]
- Rathore, C.; Rathbone, M.J.; Chellappan, D.K.; Tambuwala, M.M.; Pinto, T.D.J.A.; Dureja, H.; Hemrajani, C.; Gupta, G.; Dua, K.; Negi, P. Nanocarriers: More than Tour de Force for Thymoquinone. Expert. Opin. Drug Deliv. 2020, 17, 479–494. [Google Scholar] [CrossRef]
- Singh, V.; Joshi, S.; Malviya, T. Carboxymethyl Cellulose-Rosin Gum Hybrid Nanoparticles: An Efficient Drug Carrier. Int. J. Biol. Macromol. 2018, 112, 390–398. [Google Scholar] [CrossRef]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s Magic Bullet Concept: 100 Years of Progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef]
- Madhavi, C.; Kumara Babu, P.; Sreekanth Reddy, O.; Ujwala, G.; Subha, M.C.S. Formulation and Evaluation of 6-Thioguanine-Loaded Gum Rosin/Poly(Ethylene Oxide) Blend Microspheres for Controlled Release Applications. Mater. Today Proc. 2023, 92, 892–898. [Google Scholar] [CrossRef]
- Khaing, E.M.; Intaraphairot, T.; Chuenbarn, T.; Chantadee, T.; Phaechamud, T. Natural Resin-Based Solvent Exchange Induced in-Situ Forming Gel for Vancomycin HCl Delivery to Periodontal Pocket. Mater. Today Proc. 2021, 47, 3585–3593. [Google Scholar] [CrossRef]
- Chuenbarn, T.; Sirirak, J.; Tuntarawongsa, S.; Okonogi, S.; Phaechamud, T. Design and Comparative Evaluation of Vancomycin HCl-Loaded Rosin-Based In Situ Forming Gel and Microparticles. Gels 2022, 8, 231. [Google Scholar] [CrossRef]
- Khaing, E.M.; Mahadlek, J.; Okonogi, S.; Phaechamud, T. Lime Peel Oil–Incorporated Rosin-Based Antimicrobial In Situ Forming Gel. Gels 2022, 8, 169. [Google Scholar] [CrossRef]
- Karlberg, A.-T.; Bohlinder, K.; Boman, A.; Hacksell, U.; Hermansson, J.; Jacobsson, S.; Nilsson, J.L.G. Identification of 15-Hydroperoxyabietic Acid as a Contact Allergen in Portuguese Colophony. J. Pharm. Pharmacol. 1988, 40, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Gafvert, E.; Nilsson, U.; Karlberg, A.-T.; Magnusson, K.; Nilsson, J.L.G. Rosin Allergy: Identification of a Dehydroabietic Acid Peroxide with Allergenic Properties. Arch. Dermatol. Res. 1992, 284, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Gafvert, E.; Shao, L.P.; Karlberg, A.-T.; Nilsson, U.; Nilsson, J.L.G. Contact Allergy to Resin Acid Hydroperoxides. Hapten Binding via Free Radicals and Epoxides. Chem. Res. Toxicol. 1994, 7, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Hausen, B.M.; Hessling, C. Contact Allergy Due to Colophony (VI). The Sensitizing Capacity of Minor Resin Acids and 7 Commercial Modified-colophony Products. Contact Dermat. 1990, 23, 90–95. [Google Scholar] [CrossRef]
- SADHRA, S.; FOULDS, I.S.; GRAY, C.N. Identification of Contact Allergens in Unmodified Rosin Using a Combination of Patch Testing and Analytical Chemistry Techniques. Br. J. Dermatol. 1996, 134, 662–668. [Google Scholar] [CrossRef]
- Shao, L.P.; Gäfvert, E.; Nilsson, U.; Karlberg, A.-T.; Nilsson, J.L.G. 15-Hydroperoxydehydroabietic Acid—A Contact Allergen in Colophony from Pinus Species. Phytochemistry 1995, 38, 853–857. [Google Scholar] [CrossRef]
- Khan, L.; Saeed, M.A. 13β, 14β-Dihydroxy-13α-isopropylabietic Acid, an Elicitor of Contact Allergy. J. Pharm. Sci. 1994, 83, 909–910. [Google Scholar] [CrossRef]
- Gäfvert, E.; Bordalo, O.; Karlberg, A. Patch Testing with Allergens from Modified Rosin (Colophony) Discloses Additional Cases of Contact Allergy. Contact Dermatitis 1996, 35, 290–298. [Google Scholar] [CrossRef]
- Illing, H.P.A.; Malmfors, T.; Rodenburg, L. Skin Sensitization and Possible Groupings for ‘Read across’ for Rosin Based Substances. Regul. Toxicol. Pharmacol. 2009, 54, 234–241. [Google Scholar] [CrossRef]
- Karlberg, A.; Lidén, C. Comparison of Colophony Patch Test Preparations. Contact Dermat. 1988, 18, 158–165. [Google Scholar] [CrossRef]
- Lyon, C.C.; Tucker, S.C.; Gäfvert, E.; Karlberg, A.-T.; Beck, M.H. Contact Dermatitis from Modified Rosin in Footwear. Contact Dermat. 1999, 41, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Schnuch, A.; Uter, W.; Geier, J.; Gefeller, O. Epidemiology of Contact Allergy: An Estimation of Morbidity Employing the Clinical Epidemiology and Drug-utilization Research (CE-DUR) Approach. Contact Dermat. 2002, 47, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.P.; Linneberg, A.; Menné, T.; Johansen, J.D. The Epidemiology of Contact Allergy in the General Population—Prevalence and Main Findings. Contact Dermat. 2007, 57, 287–299. [Google Scholar] [CrossRef]
- Diepgen, T.L.; Ofenloch, R.F.; Bruze, M.; Bertuccio, P.; Cazzaniga, S.; Coenraads, P.-J.; Elsner, P.; Goncalo, M.; Svensson, Å.; Naldi, L. Prevalence of Contact Allergy in the General Population in Different European Regions. Br. J. Dermatol. 2016, 174, 319–329. [Google Scholar] [CrossRef]
- Lagrelius, M.; Wahlgren, C.; Matura, M.; Kull, I.; Lidén, C. High Prevalence of Contact Allergy in Adolescence: Results from the Population-based BAMSE Birth Cohort. Contact Dermat. 2016, 74, 44–51. [Google Scholar] [CrossRef]
- Mortz, C.G.; Bindslev-Jensen, C.; Andersen, K.E. Prevalence, Incidence Rates and Persistence of Contact Allergy and Allergic Contact Dermatitis in The Odense Adolescence Cohort Study: A 15-Year Follow-Up. Br. J. Dermatol. 2013, 168, 318–325. [Google Scholar] [CrossRef]
- Barbaud, A.; Collet, E.; Le Coz, C.J.; Meaume, S.; Gillois, P. Contact Allergy in Chronic Leg Ulcers: Results of a Multicentre Study Carried out in 423 Patients and Proposal for an Updated Series of Patch Tests. Contact Dermat. 2009, 60, 279–287. [Google Scholar] [CrossRef]
- Christoffers, W.A.; Coenraads, P.; Schuttelaar, M. Bullous Allergic Reaction Caused by Colophonium in Medical Adhesives. Contact Dermat. 2014, 70, 256–257. [Google Scholar] [CrossRef]
- Goossens, A.; Armingaud, P.; Avenel-Audran, M.; Begon-Bagdassarian, I.; Constandt, L.; Giordano-Labadie, F.; Girardin, P.; Coz, C.J.L.E.; Milpied-Homsi, B.; Nootens, C.; et al. An Epidemic of Allergic Contact Dermatitis Due to Epilating Products. Contact Dermat. 2002, 47, 67–70. [Google Scholar] [CrossRef]
- Koh, D.; Leow, Y.H.; Goh, C.L. Occupational Allergic Contact Dermatitis in Singapore. Sci. Total Environ. 2001, 270, 97–101. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Gao, B.-A.; Cheng, H.-Y.; Li, L.-F. Survey of Occupational Allergic Contact Dermatitis and Patch Test among Clothing Employees in Beijing. BioMed Res. Int. 2017, 2017, 3102358. [Google Scholar] [CrossRef]
- Suuronen, K.; Aalto-Korte, K.; Piipari, R.; Tuomi, T.; Jolanki, R. Occupational Dermatitis and Allergic Respiratory Diseases in Finnish Metalworking Machinists. Occup. Med. 2007, 57, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, A.-T.; Gäfvert, E.; Lidén, C. Environmentally Friendly Paper May Increase Risk of Hand Eczema in Rosin-Sensitive Persons. J. Am. Acad. Dermatol. 1995, 33, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Raison-Peyron, N.; Nilsson, U.; Du-Thanh, A.; Karlberg, A.-T. Contact Dermatitis From Unexpected Exposure to Rosin From a Toilet Seat. Dermatitis 2013, 24, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Wujanto, L.; Wakelin, S. Allergic Contact Dermatitis to Colophonium in a Sanitary Pad–an Overlooked Allergen? Contact Dermat. 2012, 66, 161–162. [Google Scholar] [CrossRef]
- Elms, J.; Fishwick, D.; Robinson, E.; Burge, S.; Huggins, V.; Barber, C.; Williams, N.; Curran, A. Specific IgE to Colophony? Occup. Med. 2005, 55, 234–237. [Google Scholar] [CrossRef][Green Version]
- Downs, A.M.R.; Sansom, J.E. Colophony Allergy: A Review. Contact Dermat. 1999, 41, 305–310. [Google Scholar] [CrossRef]
- Karlberg, A.-T.; Boman, A.; Nilsson, J.L.G. Hydrogenation Reduces the Allergenicity of Colophony (Rosin). Contact Dermat. 1988, 19, 22–29. [Google Scholar] [CrossRef]
- Karlberg, A.-T.; Hagvall, L. Colophony: Rosin in Unmodified and Modified Form. In Kanerva’s Occupational Dermatology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 607–624. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Liu, Y.; Wang, C.; Chu, F. Preparation and Characterization of Natural Rosin Stabilized Nanoparticles via Miniemulsion Polymerization and Their Pressure-Sensitive Adhesive Applications. Ind. Crops Prod. 2018, 124, 244–253. [Google Scholar] [CrossRef]




| Application Area | Function/Role | Key Benefits/Properties | References |
|---|---|---|---|
| Adhesives and Tackifiers | Acts as a tackifier in hot-melt and pressure-sensitive adhesives; also used as a grip enhancer (e.g., in sports and dance floors). | Improves initial stickiness by enhancing wetting and bonding; cost effective (accounts for 30–50% of formulation); enhances mechanical performance and adhesion. | [21,22] |
| Printing Inks | Binder/Film-Former in Offset, Heatset, and Package Printing. | Enhances pigment dispersion, adhesion, water resistance, gloss, quick-drying properties, and emulsification stability. | [23] |
| Paper and Packaging | Enhances wet strength in paper and is used in biodegradable packaging films and nanocomposites. | Increases paper hydrophobicity; packaging films exhibit improved tensile, viscoelastic, and antimicrobial properties along with enhanced barrier performance. | [24,25,26,27,28] |
| Rubber and Elastomers | Functions as an extender in synthetic rubbers and as a crosslinking agent in silicone rubber. | Improves processability, tensile strength, filler dispersion, and overall quality of vulcanizates; enhances thermal and mechanical properties in silicone rubbers. | [29] |
| Coatings | Utilized as a film-forming component in protective wood coatings. | Enhances adhesion and hardness; provides resistance to moisture and fungal decay through an effective barrier formation. | [30] |
| Food and Beverage | As a food additive primarily used as a stabilizer and emulsifier to maintain uniformity and prevent separation of ingredients. | Enhances the stability and consistency of emulsions in flavored drinks, improves the shelf life and appearance of food products, and complies with updated safety standards. | [31] |
| Textile Coatings | Applied as a protective coating on fabrics, such as personal protective clothing against pesticides. | Provides a hydrophobic barrier while maintaining acceptable breathability and comfort; performance comparable to commercial-grade PPC. | [32,33] |
| Industrial Applications | Enhances adhesion, flexibility, durability, and resistance properties across various industrial applications, such as adhesives, paints, inks, epoxy resins, plastics, paper sizing, surfactants, tires, insulation. | Improves adhesion, water resistance, printability, thermal stability, impact resistance, and chemical resistance. | [34] |
| Insecticidal Application | Used as an effective insecticide. | Have strong insecticidal efficacy against oriental armyworms, minimal toxicity toward aquatic organisms, and an ecofriendly, sustainable design. | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siboro, S.A.P.; Salma, S.A.; Syuhada; Putri, K.S.S.; Yuliati, F.; Lee, W.-K.; Lim, K.-T. Gum Rosin in Medical and Pharmaceutical Applications: From Conventional Uses to Modern Advancements. Materials 2025, 18, 2266. https://doi.org/10.3390/ma18102266
Siboro SAP, Salma SA, Syuhada, Putri KSS, Yuliati F, Lee W-K, Lim K-T. Gum Rosin in Medical and Pharmaceutical Applications: From Conventional Uses to Modern Advancements. Materials. 2025; 18(10):2266. https://doi.org/10.3390/ma18102266
Chicago/Turabian StyleSiboro, Sonita Afrita Purba, Sabrina Aufar Salma, Syuhada, Kurnia Sari Setio Putri, Frita Yuliati, Won-Ki Lee, and Kwon-Taek Lim. 2025. "Gum Rosin in Medical and Pharmaceutical Applications: From Conventional Uses to Modern Advancements" Materials 18, no. 10: 2266. https://doi.org/10.3390/ma18102266
APA StyleSiboro, S. A. P., Salma, S. A., Syuhada, Putri, K. S. S., Yuliati, F., Lee, W.-K., & Lim, K.-T. (2025). Gum Rosin in Medical and Pharmaceutical Applications: From Conventional Uses to Modern Advancements. Materials, 18(10), 2266. https://doi.org/10.3390/ma18102266







