From Sodium Storage Mechanism to Design of High-Capacity Carbon-Based Anode: A Review
Abstract
1. Introduction
- (1)
- Elucidate sodium storage mechanisms in carbon matrices, with emphasis on how microstructural and compositional characteristics (e.g., closed pores, graphitic domains, mesopores/micropores, heteroatom doping, and sodiophilic interfaces) govern storage behavior (Figure 1);
- (2)
- Establish structure–performance relationships between carbon microarchitecture and sodium storage capacity;
- (3)
- Analyze recent advances in synthesis methodologies for precise microstructure control and strategies for capacity enhancement;
- (4)
- Discuss appropriate characterization techniques for different carbon architectures while addressing common analytical pitfalls;
- (5)
- Provide perspectives on future development directions for high-capacity carbon anodes, proposing actionable pathways for advancing SIB technology.
2. Mechanism for Sodium-Ion Storage
2.1. Graphite
2.2. Pseudo-Graphite
2.3. Closed Pores
2.4. Micro- and Mesoporous Structures
3. Synthesis and Characterization
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Zhang, Z.; Zheng, Y.; Luo, Y.; Jiang, X.; Wang, Y.; Wang, Z.; Wu, Y.; Zhang, Y.; Liu, X.; et al. Sodium-Ion Battery at Low Temperature: Challenges and Strategies. Nanomaterials 2024, 14, 1604. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kang, S.-H.; Slater, M.; Rood, S.; Vaughey, J.T.; Karan, N.; Balasubramanian, M.; Johnson, C.S. Enabling Sodium Batteries Using Lithium-Substituted Sodium Layered Transition Metal Oxide Cathodes. Adv. Energy Mater. 2011, 1, 333–336. [Google Scholar] [CrossRef]
- Ellis, B.L.; Nazar, L.F. Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid State Mater. Sci. 2012, 16, 168–177. [Google Scholar] [CrossRef]
- Zhao, J.; He, X.-X.; Lai, W.-H.; Yang, Z.; Liu, X.-H.; Li, L.; Qiao, Y.; Xiao, Y.; Li, L.; Wu, X.; et al. Catalytic Defect-Repairing Using Manganese Ions for Hard Carbon Anode with High-Capacity and High-Initial-Coulombic-Efficiency in Sodium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300444. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Choi, A.; Choi, N.-S.; Kim, J.; Lee, J.; Ryu, J.H.; Oh, S.M.; Lee, K.T. An Amorphous Red Phosphorus/Carbon Composite as a Promising Anode Material for Sodium Ion Batteries. Adv. Mater. 2013, 25, 3045–3049. [Google Scholar] [CrossRef]
- Li, W.; Hu, S.; Luo, X.; Li, Z.; Sun, X.; Li, M.; Liu, F.; Yu, Y. Confined Amorphous Red Phosphorus in MOF-Derived N-Doped Microporous Carbon as a Superior Anode for Sodium-Ion Battery. Adv. Mater. 2017, 29, 1605820. [Google Scholar] [CrossRef]
- Shi, S.; Sun, C.; Yin, X.; Shen, L.; Shi, Q.; Zhao, K.; Zhao, Y.; Zhang, J. FeP Quantum Dots Confined in Carbon-Nanotube-Grafted P-Doped Carbon Octahedra for High-Rate Sodium Storage and Full-Cell Applications. Adv. Funct. Mater. 2020, 30, 1909283. [Google Scholar] [CrossRef]
- Liu, J.; Kopold, P.; Wu, C.; van Aken, P.A.; Maier, J.; Yu, Y. Uniform Yolk–shell Sn4P3@C Nanospheres as High-capacity and Cycle-stable Anode Materials for Sodium-ion Batteries. Energy Environ. Sci. 2015, 8, 3531–3538. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.; Zheng, J.; Wang, G.; Fu, W.; Hao, Y.; Zhao, Y.; Cao, X.; Lin, Z.; Liu, J.; et al. FeSe2 Graphite Intercalation Compound as Anode Materials for Sodium Ion Batteries. ACS Appl. Electron. Mater. 2023, 5, 6964–6973. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Hu, Y.-S.; Li, H.; Zhou, Z.; Armand, M.; Chen, L. Disodium Terephthalate (Na2C8H4O4) as High Performance Anode Material for Low-Cost Room-Temperature Sodium-Ion Battery. Adv. Energy Mater. 2012, 2, 962–965. [Google Scholar] [CrossRef]
- Zhang, N.; Han, X.; Liu, Y.; Hu, X.; Zhao, Q.; Chen, J. 3D Porous γ-Fe2O3@C Nanocomposite as High-Performance Anode Material of Na-Ion Batteries. Adv. Energy Mater. 2015, 5, 1401123. [Google Scholar] [CrossRef]
- Yin, X.; Sarkar, S.; Shi, S.; Huang, Q.-A.; Zhao, H.; Yan, L.; Zhao, Y.; Zhang, J. Recent Progress in Advanced Organic Electrode Materials for Sodium-Ion Batteries: Synthesis, Mechanisms, Challenges and Perspectives. Adv. Funct. Mater. 2020, 30, 1908445. [Google Scholar] [CrossRef]
- Xu, Z.; Ye, J.; Pan, Y.; Liu, K.; Liu, X.; Shui, J. Necklace-Like Sn@C Fiber Self-Supporting Electrode for High-Performance Sodium-Ion Battery. Energy Technol. 2022, 10, 2101024. [Google Scholar] [CrossRef]
- Choi, I.; Ha, S.; Kim, K.-H. Review and Recent Advances in Metal Compounds as Potential High-Performance Anodes for Sodium Ion Batteries. Energies 2024, 17, 2646. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Ming, F.; Alshareef, H.N. Sodium-ion Battery Anodes: Status and Future Trends. EnergyChem 2019, 1, 100012. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.P.; Sougrati, M.T.; Feng, Z.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. A Review on Design Strategies for Carbon Based Metal Oxides and Sulfides Nanocomposites for High Performance Li and Na Ion Battery Anodes. Adv. Energy Mater. 2017, 7, 1601424. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, S.; Shu, C.; Tang, Z.; Wang, X.; Wu, Y.; Tang, W. Advances and Perspectives of Hard Carbon Anode Modulated by Defect/hetero Elemental Engineering for Sodium Ion Batteries. Mater. Today 2025, 85, 231–252. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Chen, Y.; Li, S.; Tang, P.; Xie, Y.; Xie, S.; Miao, Z.; Zhu, J.; Yan, X. Graphitization Induction Effect of Hard Carbon for Sodium-Ion Storage. Adv. Funct. Mater. 2025, 35, 2424629. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Zhang, Y.; Zhang, N. Hard Carbon for Sodium Storage: Mechanism and Performance Optimization. Nano Res 2024, 17, 6038–6057. [Google Scholar] [CrossRef]
- Guo, Y.; Ji, S.; Liu, F.; Zhu, Z.; Xiao, J.; Liu, K.; Zhang, Y.; Liao, S.; Zeng, X. A Review of the Preparation and Characterization Techniques for Closed Pores in Hard Carbon and Their Functions in Sodium-ion Batteries. Energy Mater. 2025, 5, 500030. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, H.; Gao, F.; Zhang, D.; Zhang, Z.; Liu, Y.; Shang, Z.; Gao, C.; Luo, L.; Terrones, M.; et al. Overview of Hard Carbon Anode for Sodium-ion Batteries: Influencing Factors and Strategies to Extend Slope and Plateau regions. Carbon 2024, 228, 119354. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, C.; He, X.-X.; Zhao, J.; Yang, Z.; Li, L.; Wu, X.; Li, L.; Chou, S.-L. Boosting the Development of Hard Carbon for Sodium-Ion Batteries: Strategies to Optimize the Initial Coulombic Efficiency. Adv. Funct. Mater. 2024, 34, 2302277. [Google Scholar] [CrossRef]
- Togonon, J.J.H.; Chiang, P.-C.; Lin, H.-J.; Tsai, W.-C.; Yen, H.-J. Pure Carbon-based Electrodes for Metal-ion Batteries. Carbon Trends 2021, 3, 100035. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, R.; Wang, H.; Zhou, S.; Pan, Z.; Huang, Y.; Sun, D.; Tang, Y.; Ji, X.; Amine, K.; et al. Revealing the Closed Pore Formation of Waste Wood-derived Hard Carbon for Advanced Sodium-ion Battery. Nat. Commun. 2023, 14, 6024. [Google Scholar] [CrossRef]
- He, Q.; Chen, H.; Chen, X.; Zheng, J.; Que, L.; Yu, F.; Zhao, J.; Xie, Y.; Huang, M.; Lu, C.; et al. Tea-Derived Sustainable Materials. Adv. Funct. Mater. 2024, 34, 2310226. [Google Scholar] [CrossRef]
- You, S.; Zhang, Q.; Liu, J.; Deng, Q.; Sun, Z.; Cao, D.; Liu, T.; Amine, K.; Yang, C. Hard Carbon with an Opened Pore Structure for Enhanced Sodium Storage Performance. Energy Environ. Sci. 2024, 17, 8189–8197. [Google Scholar] [CrossRef]
- Izanzar, I.; Dahbi, M.; Kiso, M.; Doubaji, S.; Komaba, S.; Saadoune, I. Hard Carbons Issued from Date Palm as Efficient Anode Materials for Sodium-ion Batteries. Carbon 2018, 137, 165–173. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Zhu, Y.; Ji, K.; Wang, C.; Ruan, D.; Xia, Y. Engineering Hard Carbon with High Initial Coulomb Efficiency for Practical Sodium-ion Batteries. J. Power Sources 2021, 492, 229656. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Z.; Zhang, R.; Fan, C.; Liu, J.; Liu, J. Anode of Anthracite Hard Carbon Hybridized by Phenolic Epoxy Resin toward Enhanced Performance for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2024, 7, 6704–6716. [Google Scholar] [CrossRef]
- Mou, D.; Lin, Y.; Zhu, X. Sucrose-anthracite Composite Supplemented by KOH&HCl Washing Strategy for High-Performance Carbon Anode Material of Sodium-ion Batteries. J. Power Sources 2025, 628, 235924. [Google Scholar]
- Zhao, Y.; Hu, Z.; Zhou, W.; Gao, P.; Liu, Z.; Liu, J.; Fan, C.; Liu, J. Advanced Structural Engineering Design for Tailored Microporous Structure via Adjustable Graphite Sheet Angle to Enhance Sodium-Ion Storage in Anthracite-Based Carbon Anode. Adv. Funct. Mater. 2024, 34, 2405174. [Google Scholar] [CrossRef]
- Wang, K.; Sun, F.; Wang, H.; Wu, D.; Chao, Y.; Gao, J.; Zhao, G. Altering Thermal Transformation Pathway to Create Closed Pores in Coal-Derived Hard Carbon and Boosting of Na+ Plateau Storage for High-Performance Sodium-Ion Battery and Sodium-Ion Capacitor. Adv. Funct. Mater. 2022, 32, 2203725. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.-S.; Qi, X.; Rong, X.; Li, H.; Huang, X.; Chen, L. Advanced Sodium-ion Batteries Using Superior Low Cost Pyrolyzed Anthracite Anode: Towards Practical Applications. Energy Storage Mater. 2016, 5, 191–197. [Google Scholar] [CrossRef]
- Zhou, L.; Cui, Y.; Niu, P.; Ge, L.; Zheng, R.; Liang, S.; Xing, W. Biomass-derived Hard Carbon Material for High-capacity Sodium-ion Battery Anode through Structure Regulation. Carbon 2025, 231, 119733. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Wang, Y.; Tian, X.; Qiao, Y.; Han, P. Regulation Plateau Capacity and Initial Coulombic Efficiency of Furfural Residues-derived Hard Carbon via Components Engineering. J. Power Sources 2025, 625, 235664. [Google Scholar] [CrossRef]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. Low-Defect and Low-Porosity Hard Carbon with High Coulombic Efficiency and High Capacity for Practical Sodium Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, Z.; Pan, Z.; Huang, Y.; Zhao, L.; Zhang, X.; Sun, D.; Tang, Y.; Dhmees, A.S.; Wang, H. Regulating Closed Pore Structure Enables Significantly Improved Sodium Storage for Hard Carbon Pyrolyzing at Relatively Low Temperature. SusMat 2022, 2, 357–367. [Google Scholar] [CrossRef]
- Song, M.; Yi, Z.; Xu, R.; Chen, J.; Cheng, J.; Wang, Z.; Liu, Q.; Guo, Q.; Xie, L.; Chen, C. Towards Enhanced Sodium Storage of Hard Carbon Anodes: Regulating the Oxygen Content in Precursor by Low-temperature Hydrogen Reduction. Energy Storage Mater. 2022, 51, 620–629. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, R.; Pu, X.; Zhao, A.; Chen, W.; Song, C.; Fang, Y.; Cao, Y. Access to Advanced Sodium-ion Batteries by Presodiation: Principles and Applications. J. Energy Chem. 2024, 92, 162–175. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, R.; He, B.; Jin, J.; Gong, Y.; Wang, H. Recent Advances on Pre-sodiation in Sodium-ion Capacitors: A Mini Review. Electrochem. Commun. 2021, 129, 107090. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Shu, C.; Hua, W.; Wang, X.; Zhao, Y.; Luo, J.; Tang, Z.; Wu, Y.; Tang, W. Research Progress and Perspectives on Pre-Sodiation Strategies for Sodium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2409628. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Dai, W.; Cheng, X.; Zuo, J.; Lei, H.; Liu, W.; Fu, Z. On the Practicability of the Solid-State Electrochemical Pre-Sodiation Technique on Hard Carbon Anodes for Sodium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2403841. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, K.; Zheng, J.; Zhao, Y.; Cai, X.; Liu, C.; Zhang, M.; Shen, Z. Closed-Pore Hard Carbon Nanospheres via Aldol Condensation for Sodium Storage. ACS Appl. Nano Mater. 2025, 8, 2785–2796. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Zheng, J.; Lin, Y.; Liu, C.; Zheng, J.; Zhang, C.; Zhang, K.; Shen, Z. Designing Spherical Sucrose-Derived Hard Carbon Materials with Abundant Closed Pores via Amino-Aldehyde Condensation. Energy Fuels 2025, 39, 5974–5985. [Google Scholar] [CrossRef]
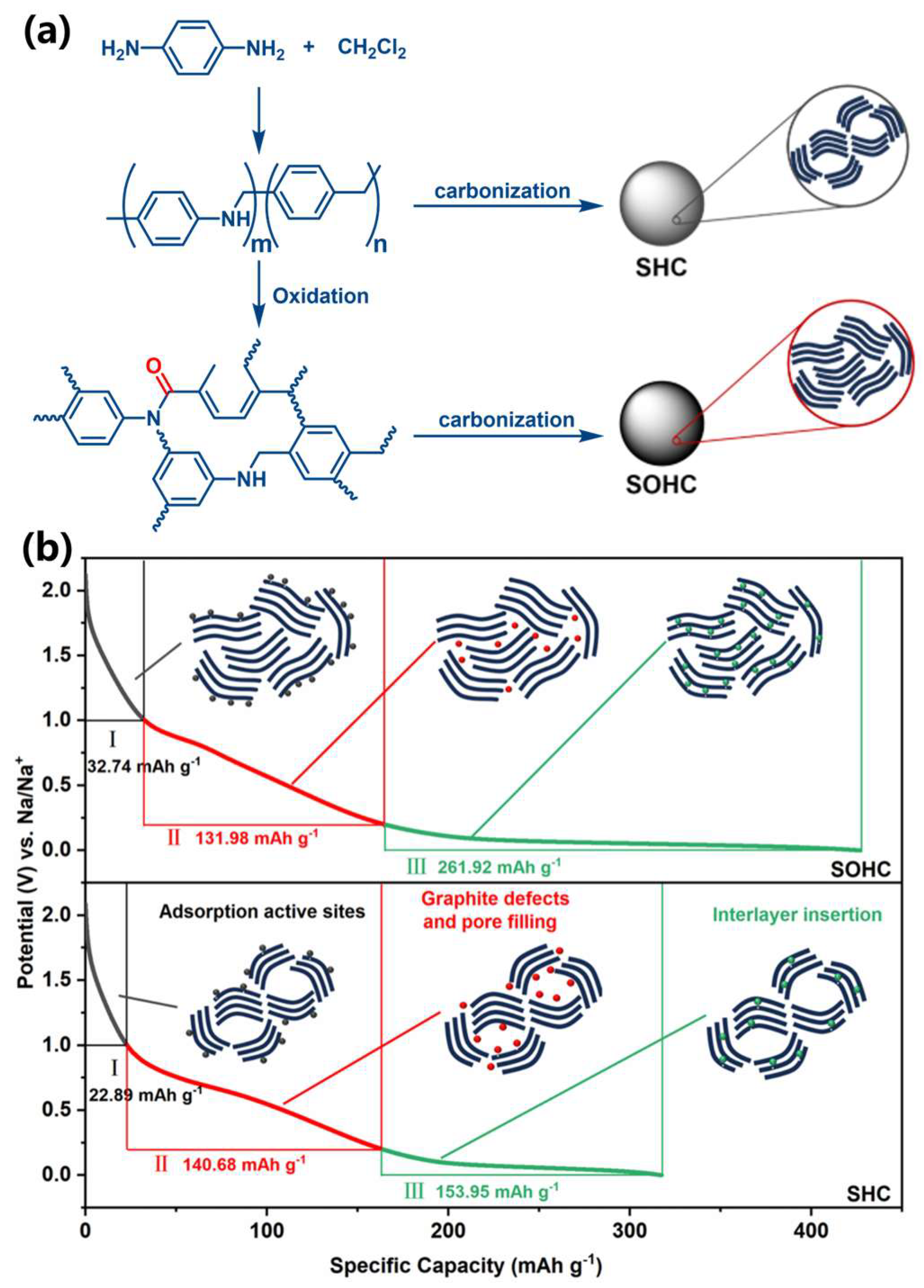
- Shao, W.; Cao, Q.; Liu, S.; Zhang, T.; Song, Z.; Song, C.; Weng, Z.; Jian, X.; Hu, F. Replacing “Alkyl” with “Aryl” for Inducing Accessible Channels to Closed Pores as Plateau-dominated Sodium-ion Battery Anode. SusMat 2022, 2, 319–334. [Google Scholar] [CrossRef]
- Song, M.; Xie, L.; Su, F.; Yi, Z.; Guo, Q.; Chen, C.-M. New Insights into the Effect of Hard Carbons Microstructure on the Diffusion of Sodium Ions into Closed Pores. Chin. Chem. Lett. 2024, 35, 109266. [Google Scholar] [CrossRef]
- Rathnayake, R.M.N.M.; Duignan, T.T.; Searles, D.J.; Zhao, X.S. Exploring the Effect of Interlayer Distance of Expanded Graphite for Sodium Ion Storage Using First Principles Calculations. Phys. Chem. Chem. Phys. 2021, 23, 3063–3070. [Google Scholar] [CrossRef]
- Nobuhara, K.; Nakayama, H.; Nose, M.; Nakanishi, S.; Iba, H. First-principles Study of Alkali Metal-graphite Intercalation Compounds. J. Power Sources 2013, 243, 585–587. [Google Scholar] [CrossRef]
- Fan, L.; Ma, R.; Zhang, Q.; Jia, X.; Lu, B. Graphite Anode for a Potassium-Ion Battery with Unprecedented Performance. Angew. Chem. Int. Ed. 2019, 58, 10500–10505. [Google Scholar] [CrossRef]
- Chen, J.; Fan, X.; Ji, X.; Gao, T.; Hou, S.; Zhou, X.; Wang, L.; Wang, F.; Yang, C.; Chen, L.; et al. Intercalation of Bi nanoparticles into Graphite Results in an Ultra-fast and Ultra-stable Anode Material for Sodium-ion Batteries. Energy Environ. Sci. 2018, 11, 1218–1225. [Google Scholar] [CrossRef]
- Kim, H.; Lim, K.; Yoon, G.; Park, J.-H.; Ku, K.; Lim, H.-D.; Sung, Y.-E.; Kang, K. Exploiting Lithium–Ether Co-Intercalation in Graphite for High-Power Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1700418. [Google Scholar] [CrossRef]
- Komaba, S.; Hasegawa, T.; Dahbi, M.; Kubota, K. Potassium Intercalation into Graphite to Realize High-voltage/High-power potassium-ion Batteries and Potassium-ion Capacitors. Electrochem. Commun. 2015, 60, 172–175. [Google Scholar] [CrossRef]
- Jache, B.; Adelhelm, P. Use of Graphite as a Highly Reversible Electrode with Superior Cycle Life for Sodium-Ion Batteries by Making Use of Co-Intercalation Phenomena. Angew. Chem. Int. Ed. 2014, 53, 10169–10173. [Google Scholar] [CrossRef]
- Kachmar, A.; Goddard, W.A. Free Energy Landscape of Sodium Solvation into Graphite. J. Phys. Chem. C 2018, 122, 20064–20072. [Google Scholar] [CrossRef]
- Moriwake, H.; Kuwabara, A.; Fisher, C.A.J.; Ikuhara, Y. Why is Sodium-intercalated Graphite Unstable? RSC Adv. 2017, 7, 36550–36554. [Google Scholar] [CrossRef]
- Lenchuk, O.; Adelhelm, P.; Mollenhauer, D. New Insights into the Origin of Unstable Sodium Graphite Intercalation Compounds. Phys. Chem. Chem. Phys. 2019, 21, 19378–19390. [Google Scholar] [CrossRef]
- Karunarathna, R.; Ranasinghe Arachchige, H.; Karunarathne, S.; Wijesinghe, W.P.S.L.; Sandaruwan, C.; Mantilaka, M.P.G.; Kannangara, Y.Y.; Abdelkader, A.M. Intercalating Graphite-Based Na-Ion Battery Anodes with Integrated Magnetite. Small Sci. 2025, 5, 2400405. [Google Scholar] [CrossRef] [PubMed]
- Cohn, A.P.; Share, K.; Carter, R.; Oakes, L.; Pint, C.L. Ultrafast Solvent-Assisted Sodium Ion Intercalation into Highly Crystalline Few-Layered Graphene. Nano Lett. 2016, 16, 543–548. [Google Scholar] [CrossRef]
- Kamiyama, A.; Kubota, K.; Igarashi, D.; Youn, Y.; Tateyama, Y.; Ando, H.; Gotoh, K.; Komaba, S. MgO-Template Synthesis of Extremely High Capacity Hard Carbon for Na-Ion Battery. Angew. Chem. Int. Ed. 2021, 60, 5114–5120. [Google Scholar] [CrossRef]
- Chen, X.; Tian, J.; Li, P.; Fang, Y.; Fang, Y.; Liang, X.; Feng, J.; Dong, J.; Ai, X.; Yang, H.; et al. An Overall Understanding of Sodium Storage Behaviors in Hard Carbons by an “Adsorption-Intercalation/Filling” Hybrid Mechanism. Adv. Energy Mater. 2022, 12, 2200886. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Z.; Liu, Y.; Cao, Y.; Zhu, Q.; Liu, H.; Wang, Z.; Zhang, P.; Xu, B. Extended “Adsorption–Insertion” Model: A New Insight into the Sodium Storage Mechanism of Hard Carbons. Adv. Energy Mater. 2019, 9, 1901351. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Q.; Sun, B.; Gu, J.; Yu, B.; Zhang, W.; Zhang, D. N-doped Catalytic Graphitized Hard Carbon for Jigh-performance Lithium/Sodium-ion Batteries. Sci. Rep. 2018, 8, 9934. [Google Scholar]
- Qiu, R.; Zhao, S.; Ju, Z.; Huang, Y.; Zheng, L.; Lian, R.; Tao, X.; Hong, Z. Sodiophilic Skeleton Based on the Packing of Hard Carbon Microspheres for Stable Sodium Metal Anode without Dead Sodium. J. Energy Chem. 2022, 73, 400–406. [Google Scholar] [CrossRef]
- Sun, Z.; Jin, H.; Ye, Y.; Xie, H.; Jia, W.; Jin, S.; Ji, H. Guiding Sodium Deposition through a Sodiophobic−Sodiophilic Gradient Interfacial Layer for Highly Stable Sodium Metal Anodes. ACS Appl. Energy Mater. 2021, 4, 2724–2731. [Google Scholar] [CrossRef]
- Zhang, K.-Y.; Liu, H.-H.; Cao, J.-M.; Yang, J.-L.; Su, M.-Y.; Wang, X.-Y.; Gu, Z.-Y.; Wang, J.; Li, B.; Wang, Y.; et al. Microstructure Reconstruction via Confined Carbonization Achieves Highly Available Sodium Ion Diffusion Channels in Hard Carbon. Energy Storage Mater. 2024, 73, 103839. [Google Scholar] [CrossRef]
- Feng, X.; Li, Y.; Li, Y.; Liu, M.; Zheng, L.; Gong, Y.; Zhang, R.; Wu, F.; Wu, C.; Bai, Y. Unlocking the Local Structure of Hard Carbon to Grasp Sodium-ion Diffusion Behavior for Advanced Sodium-ion Batteries. Energy Environ. Sci. 2024, 17, 1387–1396. [Google Scholar] [CrossRef]
- Han, J.; Johnson, I.; Lu, Z.; Kudo, A.; Chen, M. Effect of Local Atomic Structure on Sodium Ion Storage in Hard Amorphous Carbon. Nano Lett. 2021, 21, 6504–6510. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, J.; Zhao, Y.; Zhang, K.; Fu, W.; Wang, G.; Wang, H.; Hao, Y.; Lin, Z.; Cao, X.; et al. Designing Hard Carbon Microsphere Structure via Halogenation Amination and Oxidative Polymerization Reactions for Sodium ion Insertion Mechanism Investigation. J. Colloid Interface Sci. 2024, 668, 202–212. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, D.; Fu, Z.; Zhou, J.; Liu, R.; Zhang, R.; Sun, D.; Dhmees, A.S.; Tang, Y.; Wang, H. Regulating Pseudo-Graphitic Domain and Closed Pores to Facilitate Plateau Sodium Storage Capacity and Kinetics for Hard Carbon. Small Methods 2024, 8, 2400509. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Wei, J.; Diao, C.; Li, F.; Du, C.; Bai, Z.; Zhang, Y.; Malyi, O.I.; Chen, X.; et al. Pushing Slope-to Plateau-type Behavior in Hard Carbon for Sodium-ion Batteries via Local Structure Rearrangement. Energy Environ. Sci. 2025. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, G.; Xu, C.; Li, J.; Liu, Y.; Li, B.; Wang, A.; Sun, K. Tailored Regulation of Graphite Microcrystals via Tandem Catalytic Carbonization for Enhanced Electrochemical Performance of Hard Carbon in the Low-Voltage Plateau. Adv. Funct. Mater. 2025, 35, 2416061. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, F.; Cheng, Y.; Chen, M.; Hou, J.; Yang, D.; Zhang, Y.; Yang, W.; Liu, G.; Zhang, Y.; et al. Identifying the Importance of Functionalization Evolution During Pre-oxidation Treatment in Producing Economical Asphalt-derived Hard Carbon for Na-ion Batteries. Energy Storage Mater. 2024, 73, 103808. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Z.; Lin, P.; Zhong, Y.; Wang, X.; Yuan, Y.; Tu, J. Oxygen-Crosslinker Effect on the Electrochemical Characteristics of Asphalt-Based Hard Carbon Anodes for Sodium-Ion Batteries. Adv. Energy Mater. 2025, 15, 2403084. [Google Scholar] [CrossRef]
- Iglesias, L.K.; Antonio, E.N.; Martinez, T.D.; Zhang, L.; Zhuo, Z.; Weigand, S.J.; Guo, J.; Toney, M.F. Revealing the Sodium Storage Mechanisms in Hard Carbon Pores. Adv. Energy Mater. 2023, 13, 2302171. [Google Scholar] [CrossRef]
- Stratford, J.M.; Kleppe, A.K.; Keeble, D.S.; Chater, P.A.; Meysami, S.S.; Wright, C.J.; Barker, J.; Titirici, M.-M.; Allan, P.K.; Grey, C.P. Correlating Local Structure and Sodium Storage in Hard Carbon Anodes: Insights from Pair Distribution Function Analysis and Solid-State NMR. J. Am. Chem. Soc. 2021, 143, 14274–14286. [Google Scholar] [CrossRef]
- Qiu, C.; Li, A.; Qiu, D.; Wu, Y.; Jiang, Z.; Zhang, J.; Xiao, J.; Yuan, R.; Jiang, Z.; Liu, X.; et al. One-Step Construction of Closed Pores Enabling High Plateau Capacity Hard Carbon Anodes for Sodium-Ion Batteries: Closed-Pore Formation and Energy Storage Mechanisms. ACS Nano 2024, 18, 11941–11954. [Google Scholar] [CrossRef]
- Li, Y.; Vasileiadis, A.; Zhou, Q.; Lu, Y.; Meng, Q.; Li, Y.; Ombrini, P.; Zhao, J.; Chen, Z.; Niu, Y.; et al. Origin of Fast Charging in Hard Carbon Anodes. Nat. Energy 2024, 9, 134–142. [Google Scholar] [CrossRef]
- Sun, D.; Zhao, L.; Sun, P.; Zhao, K.; Sun, Y.; Zhang, Q.; Li, Z.; Ma, Z.; Zheng, F.; Yang, Y.; et al. Rationally Regulating Closed Pore Structures by Pitch Coating to Boost Sodium Storage Performance of Hard Carbon in Low-voltage Platforms. Adv. Funct. Mater. 2024, 34, 2403642. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, Z.; Xie, L.; Mao, Y.; Ji, W.; Liu, Z.; Wei, X.; Su, F.; Chen, C.-M. Releasing Free Radicals in Precursor Triggers the Formation of Closed Pores in Hard Carbon for Sodium-Ion Batteries. Adv. Mater. 2024, 36, 2401249. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, P.; Wang, H.; Meng, Q.; Qi, X.; Ai, G.; Xie, F.; Rong, X.; Xiong, Y.; Lu, Y.; et al. Unlocking Plateau Capacity with Versatile Precursor Crosslinking for Carbon Anodes in Na-ion Batteries. Energy Storage Mater. 2024, 70, 103543. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. An In Situ Small-Angle X-Ray Scattering Study of Sodium Insertion into a Nanoporous Carbon Anode Material within an Operating Electrochemical Cell. J. Electrochem. Soc. 2000, 147, 4428. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hu, M.; Zhang, H.; Yang, W.; Lv, R. Pore Structure Regulation of Hard Carbon: Towards Fast and High-capacity Sodium-ion Storage. J. Colloid Interface Sci. 2020, 566, 257–264. [Google Scholar] [CrossRef]
- Chen, X.; Sawut, N.; Chen, K.; Li, H.; Zhang, J.; Wang, Z.; Yang, M.; Tang, G.; Ai, X.; Yang, H.; et al. Filling Carbon: A Microstructure-engineered Hard Carbon for Efficient Alkali Metal Ion Storage. Energy Environ. Sci. 2023, 16, 4041–4053. [Google Scholar] [CrossRef]
- Tian, Y.-R.; Yi, Z.-L.; Su, F.-Y.; Xie, L.-J.; Zhang, X.-F.; Li, X.-F.; Cheng, J.-Y.; Chen, J.-P.; Chen, C.-M. Regulating the Pore Structure of Activated Carbon by Pitch for High-Performance Sodium-Ion Storage. ACS Appl. Mater. Interfaces 2024, 16, 17553–17562. [Google Scholar] [CrossRef]
- Kumar, H.; Detsi, E.; Abraham, D.P.; Shenoy, V.B. Fundamental Mechanisms of Solvent Decomposition Involved in Solid-Electrolyte Interphase Formation in Sodium Ion Batteries. Chem. Mater. 2016, 28, 8930–8941. [Google Scholar] [CrossRef]
- Golozar, M.; Hovington, P.; Paolella, A.; Bessette, S.; Lagacé, M.; Bouchard, P.; Demers, H.; Gauvin, R.; Zaghib, K. In Situ Scanning Electron Microscopy Detection of Carbide Nature of Dendrites in Li–Polymer Batteries. Nano Lett. 2018, 18, 7583–7589. [Google Scholar] [CrossRef]
- Aslam, M.K.; Niu, Y.; Hussain, T.; Tabassum, H.; Tang, W.; Xu, M.; Ahuja, R. How to Avoid Dendrite Formation in Metal Batteries: Innovative Strategies for Dendrite Suppression. Nano Energy 2021, 86, 106142. [Google Scholar] [CrossRef]
- Wu, S.; Peng, H.; Xu, J.; Huang, L.; Liu, Y.; Xu, X.; Wu, Y.; Sun, Z. Nitrogen/phosphorus Co-doped Ultramicropores Hard Carbon Spheres for Rapid Sodium Storage. Carbon 2024, 218, 118756. [Google Scholar] [CrossRef]
- Lan, N.; Li, J.; Zeng, L.; Luo, D.; Du, D.; Li, X.; He, H.; Zhang, C. Hyper-Crosslinking to Customize Ultrathin-Wall Closed Pores in Pitch-Derived Carbon for Sodium-Ion Batteries. Adv. Mater. 2025. [Google Scholar] [CrossRef] [PubMed]
- Weaving, J.S.; Lim, A.; Millichamp, J.; Neville, T.P.; Ledwoch, D.; Kendrick, E.; McMillan, P.F.; Shearing, P.R.; Howard, C.A.; Brett, D.J.L. Elucidating the Sodiation Mechanism in Hard Carbon by Operando Raman Spectroscopy. ACS Appl. Energy Mater. 2020, 3, 7474–7484. [Google Scholar] [CrossRef]
- Song, Z.; Di, M.; Zhang, X.; Wang, Z.; Chen, S.; Zhang, Q.; Bai, Y. Nanoconfined Strategy Optimizing Hard Carbon for Robust Sodium Storage. Adv. Energy Mater. 2024, 14, 2401763. [Google Scholar] [CrossRef]
- He, Y.; Liu, D.; Jiao, J.; Liu, Y.; He, S.; Zhang, Y.; Cheng, Q.; Fang, Y.; Mo, X.; Pan, H.; et al. Pyridinic N-Dominated Hard Carbon with Accessible Carbonyl Groups Enabling 98% Initial Coulombic Efficiency for Sodium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2403144. [Google Scholar] [CrossRef]
- Qiu, D.; Zhao, W.; Zhang, B.; Ahsan, M.T.; Wang, Y.; Zhang, L.; Yang, X.; Hou, Y. Ni-Single Atoms Modification Enabled Kinetics Enhanced and Ultra-Stable Hard Carbon Anode for Sodium-Ion Batteries. Adv. Energy Mater. 2024, 14, 2400002. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.; Ye, W.; Xiao, B.; Sun, Z.; Cheng, Y.; Wang, M.-S. Regulating the Wettability of Hard Carbon through Open Mesochannels for Enhanced K+ Storage. Small 2023, 19, 2300605. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, M.; Liu, W.; Zhu, H.; Cheng, Y.; Wang, M.-S. Pore-size tuning of hard carbon to optimize its wettability for efficient Na+ storage. J. Mater. Chem. A 2024, 12, 13703–13712. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Xiang, Y.; Li, C.; Zhu, H.; Li, C.; Zou, G.; Hou, H.; Ji, X. Bridging Structure and Performance: Decoding Sodium Storage in Hard Carbon Anodes. ACS Nano 2025, 19, 14627–14651. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Zhang, W.; Quadrelli, A.; Jarvis, S.; Chen, J.; Lu, H.; Mangayarkarasi, N.; Niu, Y.; Tao, J.; et al. Operando Nano-mapping of Sodium-diglyme Co-intercalation and SEI Formation in Sodium Ion Batteries’ Graphene Anodes. Appl. Phys. Rev. 2024, 11, 021422. [Google Scholar] [CrossRef]
- Brennhagen, A.; Cavallo, C.; Wragg, D.S.; Sottmann, J.; Koposov, A.Y.; Fjellvåg, H. Understanding the (De) Sodiation Mechanisms in Na-Based Batteries through Operando X-Ray Methods. Batter. Supercaps 2021, 4, 1039–1063. [Google Scholar] [CrossRef]
- Gotoh, K.; Yamakami, T.; Nishimura, I.; Kometani, H.; Ando, H.; Hashi, K.; Shimizu, T.; Ishida, H. Mechanisms for Overcharging of Carbon Electrodes in Lithium-ion/Sodium-ion Batteries Analysed by Operando Solid-state NMR. J. Mater. Chem. A 2020, 8, 14472–14481. [Google Scholar] [CrossRef]
- Deringer, V.L.; Merlet, C.; Hu, Y.; Lee, T.H.; Kattirtzi, J.A.; Pecher, O.; Csanyi, G.; Elliott, S.R.; Grey, C.P. Towards An Atomistic Understanding of Disordered Carbon Electrode Materials. Chem. Commun. 2018, 54, 5988–5991. [Google Scholar] [CrossRef] [PubMed]
- Deringer, V.L.; Csanyi, G. Machine Learning Based Interatomic Potential for Amorphous Carbon. Phys. Rev. B 2017, 95, 94203. [Google Scholar] [CrossRef]
- Bartok, A.P.; Payne, M.C.; Kondor, R.; Csanyi, G. Gaussian Approximation Potentials: The Accuracy of Quantum Mechanics, without the Electrons. Phys. Rev. Lett. 2010, 104, 136403. [Google Scholar] [CrossRef]
- Liu, J.; You, Y.; Huang, L.; Zheng, Q.; Sun, Z.; Fang, K.; Sha, L.; Liu, M.; Zhan, X.; Zhao, J.; et al. Precisely Tunable Instantaneous Carbon Rearrangement Enables Low-Working-Potential Hard Carbon Toward Sodium-Ion Batteries with Enhanced Energy Density. Adv. Mater. 2024, 36, 2407369. [Google Scholar] [CrossRef]
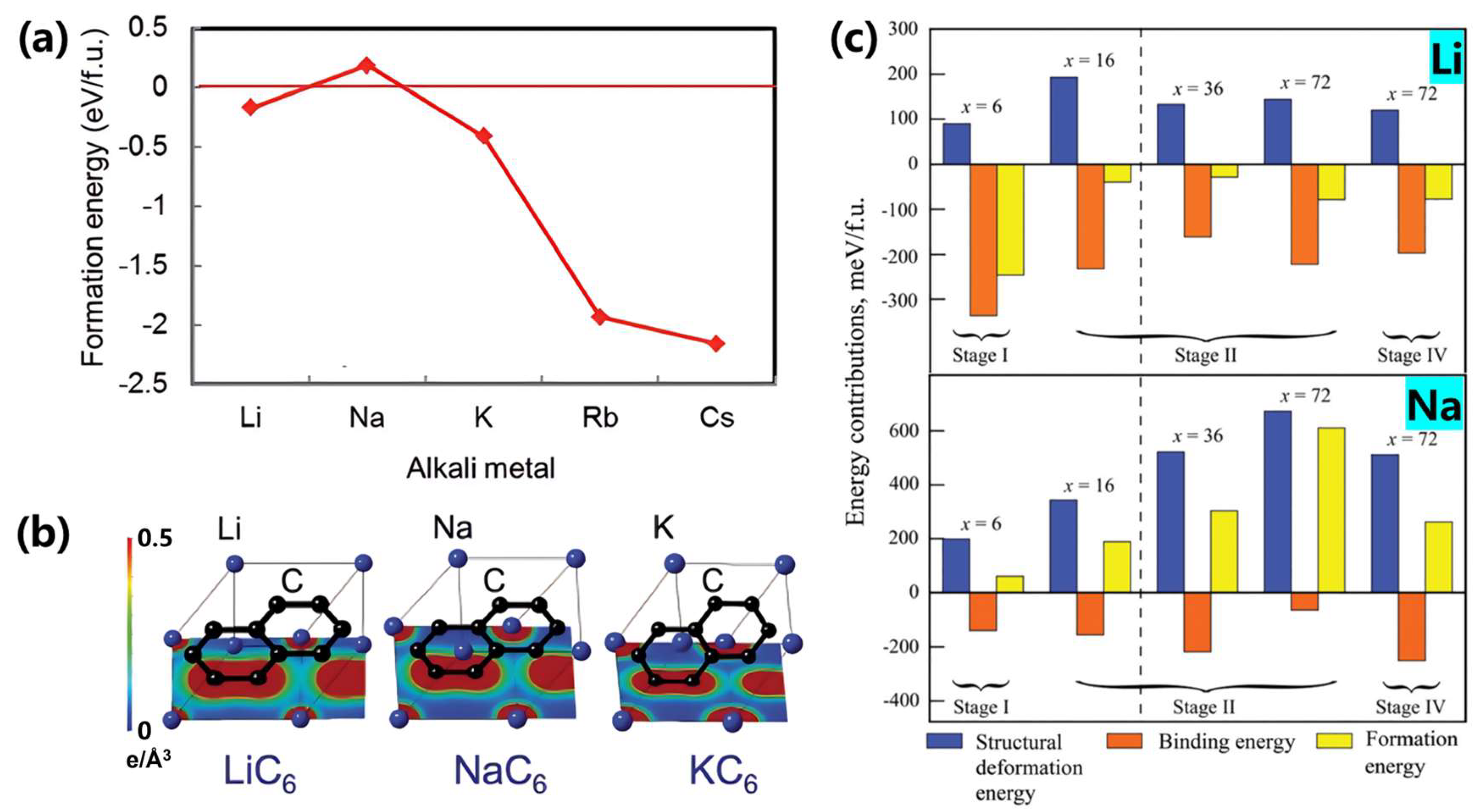
- Hérold, A.; Marêché, J.-F.; Lelaurain, M. Intercalation of sodium with its halides into graphite. Carbon 2000, 38, 1955–1963. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. A Comprehensive Review of Lithium Salts and Beyond for Rechargeable Batteries: Progress and Perspectives. Mater. Sci. Eng. R Rep. 2018, 134, 1–21. [Google Scholar] [CrossRef]
- Bordet, F.; Ahlbrecht, K.; Tübke, J.; Ufheil, J.; Hoes, T.; Oetken, M.; Holzapfel, M. Anion Intercalation into Graphite from a Sodium-containing Electrolyte. Electrochim. Acta 2015, 174, 1317–1323. [Google Scholar] [CrossRef]




| Method | Analysis Focus | Physical Significance |
|---|---|---|
| X-ray Diffraction (XRD) | Crystalline Structure | Analyzes long-range ordering, interlayer spacing (d002), crystallite size, and graphitization degree. |
| Raman Spectroscopy | Disorders/Defects | Intensity ratio of D-band (defects) to G-band (graphitic, ID/IG) reflects disorder and defect density. |
| Scanning Electron Microscopy (SEM) | Morphology | Observes surface morphology, particle size distribution, and mesoporous structures. |
| Transmission Electron Microscopy (TEM) | Microstructure | High-resolution imaging of nanopores, carbon layer stacking, and local defects. |
| BET Surface Area and Pore Analysis | Pore Structure | Measures specific surface area, pore size distribution, and pore volume; correlates with ion transport and storage sites. |
| X-ray Photoelectron Spectroscopy (XPS) | Surface Chemistry | Identifies surface elemental composition, functional groups (e.g., C-O, C=O), and SEI components. |
| Fourier Transform Infrared Spectroscopy (FTIR) | Chemical Bonds and Functional Groups | Detects functional groups (e.g., oxygen-containing groups) and chemical bonding types. |
| Selected Area Electron Diffraction (SAED) | Local Crystalline Structure | Determines local crystallinity (graphitic microdomains) or amorphous regions. |
| Thermogravimetric Analysis (TGA) | Thermal Stability | Evaluates the precursor carbonization process. |
| True Density Measurement | Bulk Density and Closed Porosity | Measures skeletal density excluding pores; evaluates closed-pore content for Na/Li storage. |
| Cyclic Voltammetry (CV) | Reaction Kinetics | Redox peaks reveal Na storage mechanisms; peak separation indicates reversibility. |
| Galvanostatic Charge–Discharge | Capacity and Cycling Performance | Discharge curve slope correlates with storage mechanisms; cycling stability reflects structural robustness. |
| Electrochemical Impedance Spectroscopy (EIS) | Interface Resistance | High-frequency semicircle (charge transfer resistance) and low-frequency slope (ion diffusion) reveal kinetic limitations. |
| Nuclear Magnetic Resonance (NMR) | Ion Storage Mechanism | The 13C/23Na NMR probes ion environments and diffusion in pores/defects. |
| Small-Angle X-ray Scattering (SAXS) | Nanoscale Porosity | Quantifies pore size/shape (1–100 nm), linking closed pores to Na storage capacity. |
| X-ray Absorption Near Edge Structure (XANES) | Electronic Structure and Local Coordination | Probes electronic states, oxidation states, and local atomic environments. |
| Research Direction | Key Parameters | Performance and Advantages |
|---|---|---|
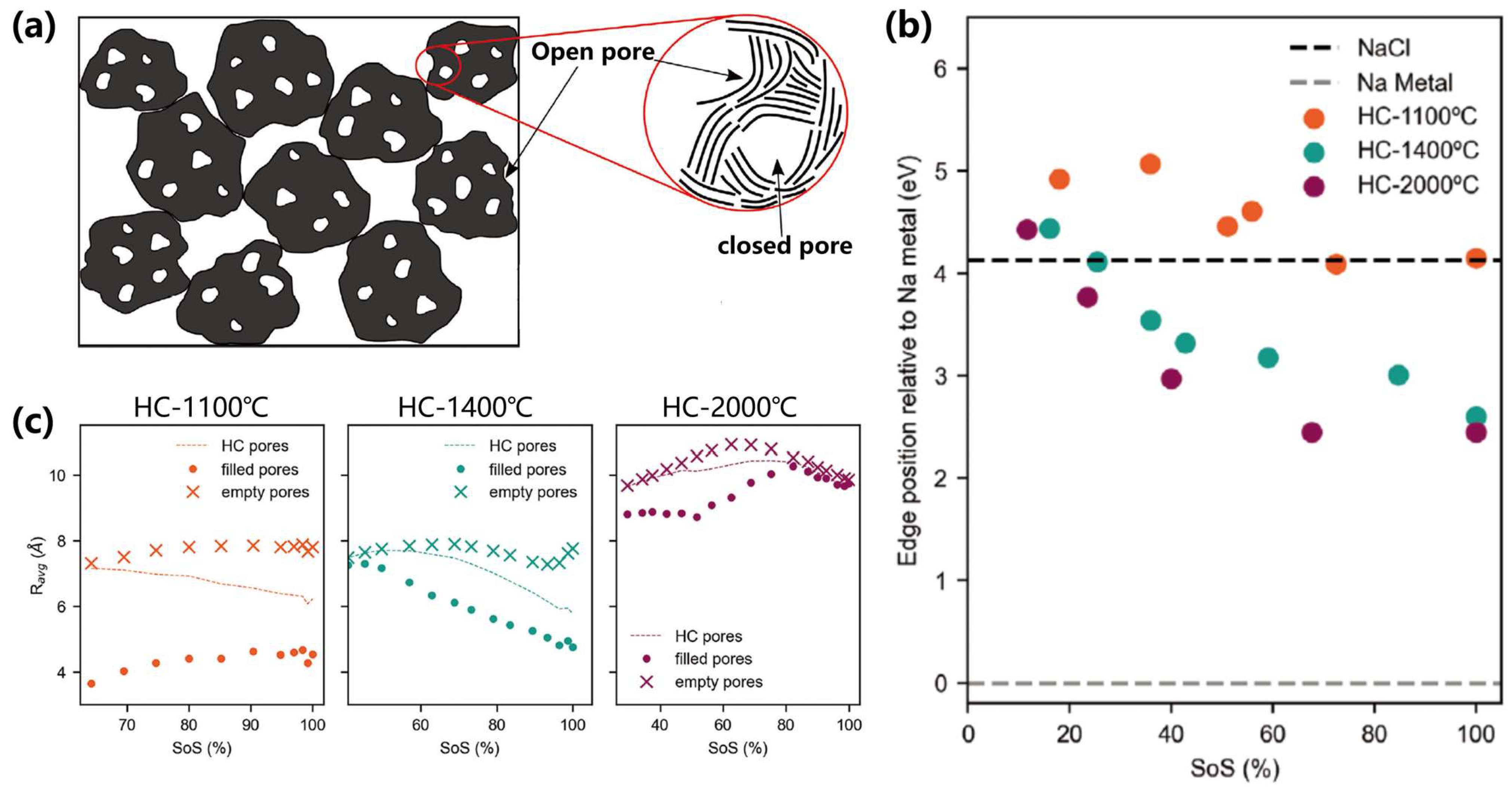
| Closed-Pore Design | Pore size: <1 nm; Carbonization: 1100–2000 °C | Achieves 481.5 mAh/g capacity with 81% plateau contribution via optimized closed pores; 20% higher capacity than traditional hard carbon [77]. |
| Pseudo-graphitic Domain Regulation | Interlayer spacing: 0.36–0.40 nm; Doping: 1–5 wt%; | Delivers 339 mAh/g total capacity (262 mAh/g from pseudo-graphitic domains) through expanded interlayer spacing, overcoming graphite limitations (<35 mAh/g) [69]. |
| Defect Engineering | Pre-oxidation treatment; Low surface area | Enhances sloping capacity (>150 mAh/g) and ICE (>85%) via controlled defect generation; cost-effective for scalable production [70]. |
| Sodiophilic Interface Modification | Ag loading: 1–10% | Reduces overpotential to 8 mV (vs. 23.6 mV for Cu and 12.5 mV for unmodified HC) and maintains stable cycling for 500 cycles with 493 mAh g−1 capacity retention; significantly outperforms unmodified HC (fails after 120 cycles) and Cu substrates (unstable deposition) [64]. |
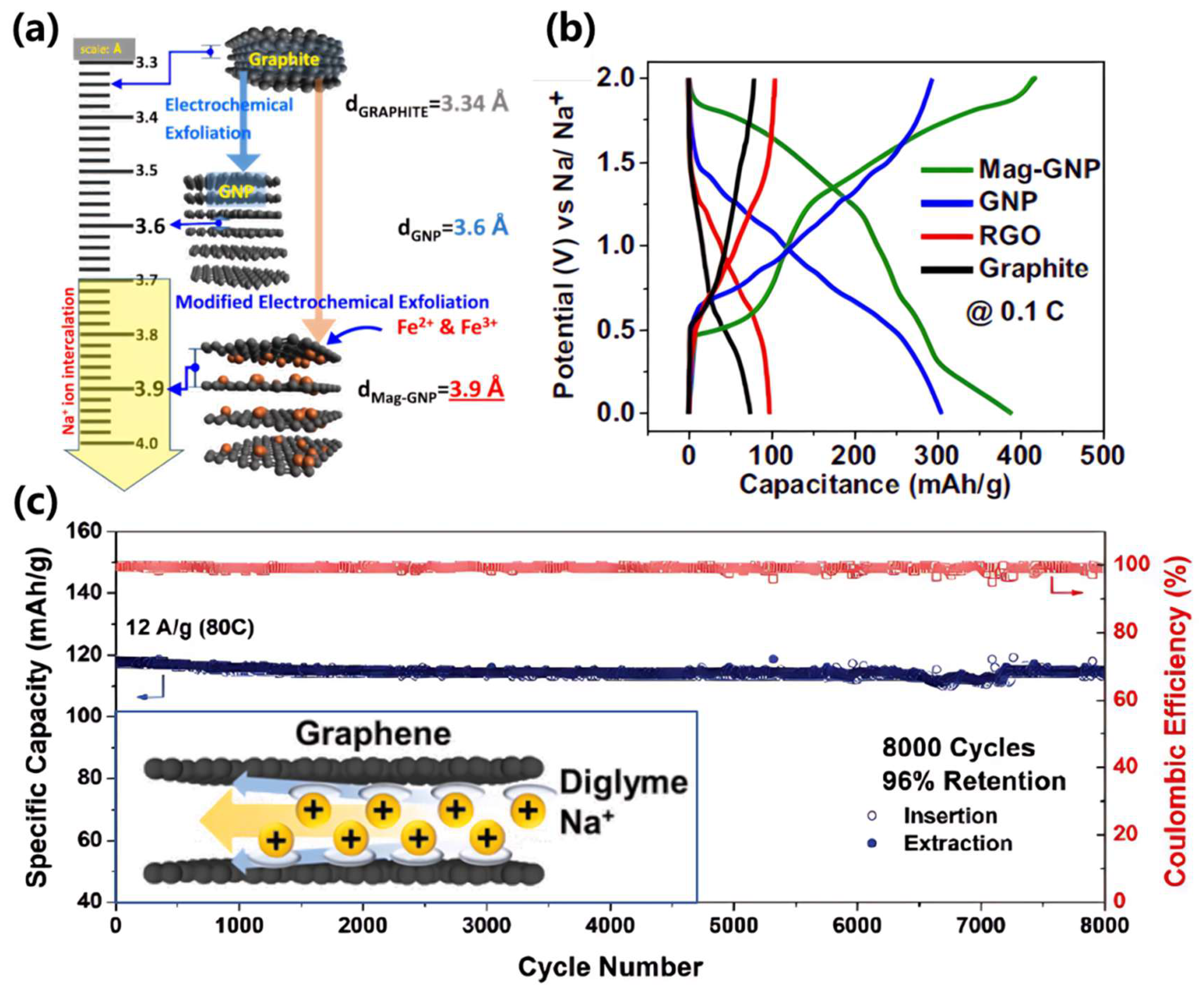
| Interlayer Spacing Control | Interlayer spacing: >0.4 nm; Solvent: Diglyme | Enables high-rate performance (~100 mAh/g@30 A/g) and 98% capacity retention over 8000 cycles; breaks graphite’s limitations [59]. |
| Heteroatom Doping | Dopants (N/S/O/P); Carbonization: 1300–1600 °C | Boosts capacity by 15–20% and rate capability (>2C) through enhanced conductivity; Fe-catalyzed graphitization improves efficiency [90]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Shen, Z. From Sodium Storage Mechanism to Design of High-Capacity Carbon-Based Anode: A Review. Materials 2025, 18, 2248. https://doi.org/10.3390/ma18102248
Zhou Y, Shen Z. From Sodium Storage Mechanism to Design of High-Capacity Carbon-Based Anode: A Review. Materials. 2025; 18(10):2248. https://doi.org/10.3390/ma18102248
Chicago/Turabian StyleZhou, Yujun, and Zhongrong Shen. 2025. "From Sodium Storage Mechanism to Design of High-Capacity Carbon-Based Anode: A Review" Materials 18, no. 10: 2248. https://doi.org/10.3390/ma18102248
APA StyleZhou, Y., & Shen, Z. (2025). From Sodium Storage Mechanism to Design of High-Capacity Carbon-Based Anode: A Review. Materials, 18(10), 2248. https://doi.org/10.3390/ma18102248






