Comparative Evaluation of Corrosion Resistance of AISI 316L and Ti6Al4V Dental Materials Under Simulated Inflammatory Conditions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Surface Analysis
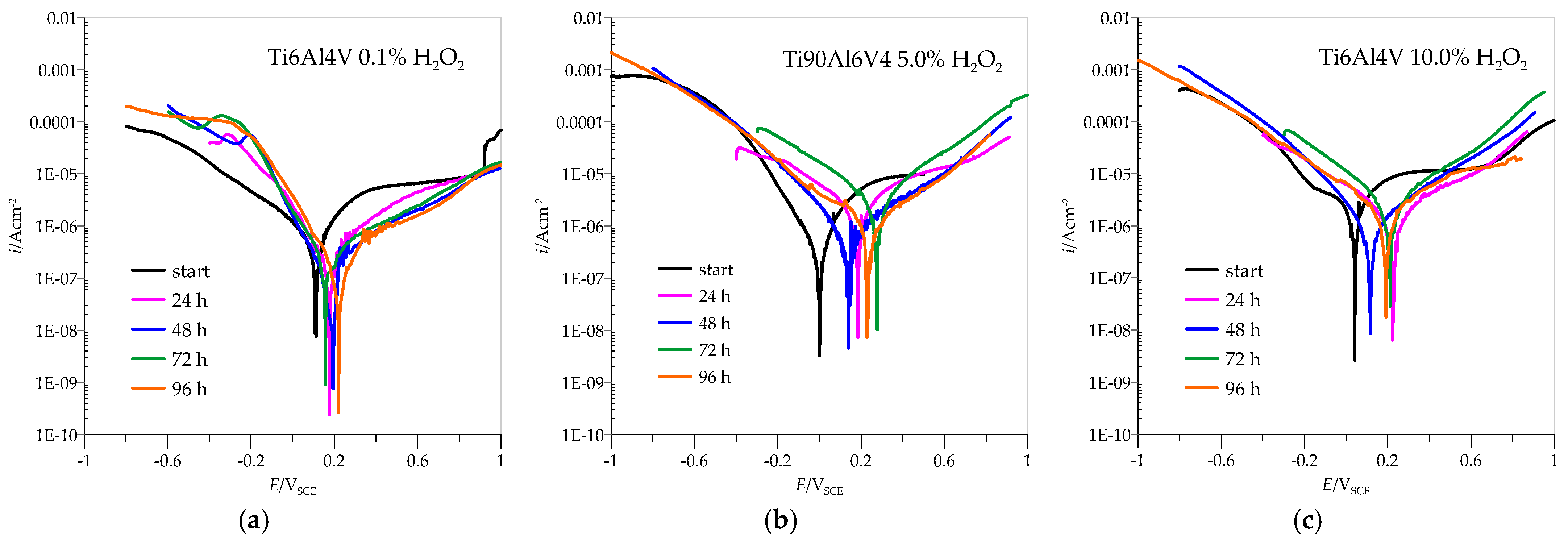
3.2. Potentiodynamic Measurements
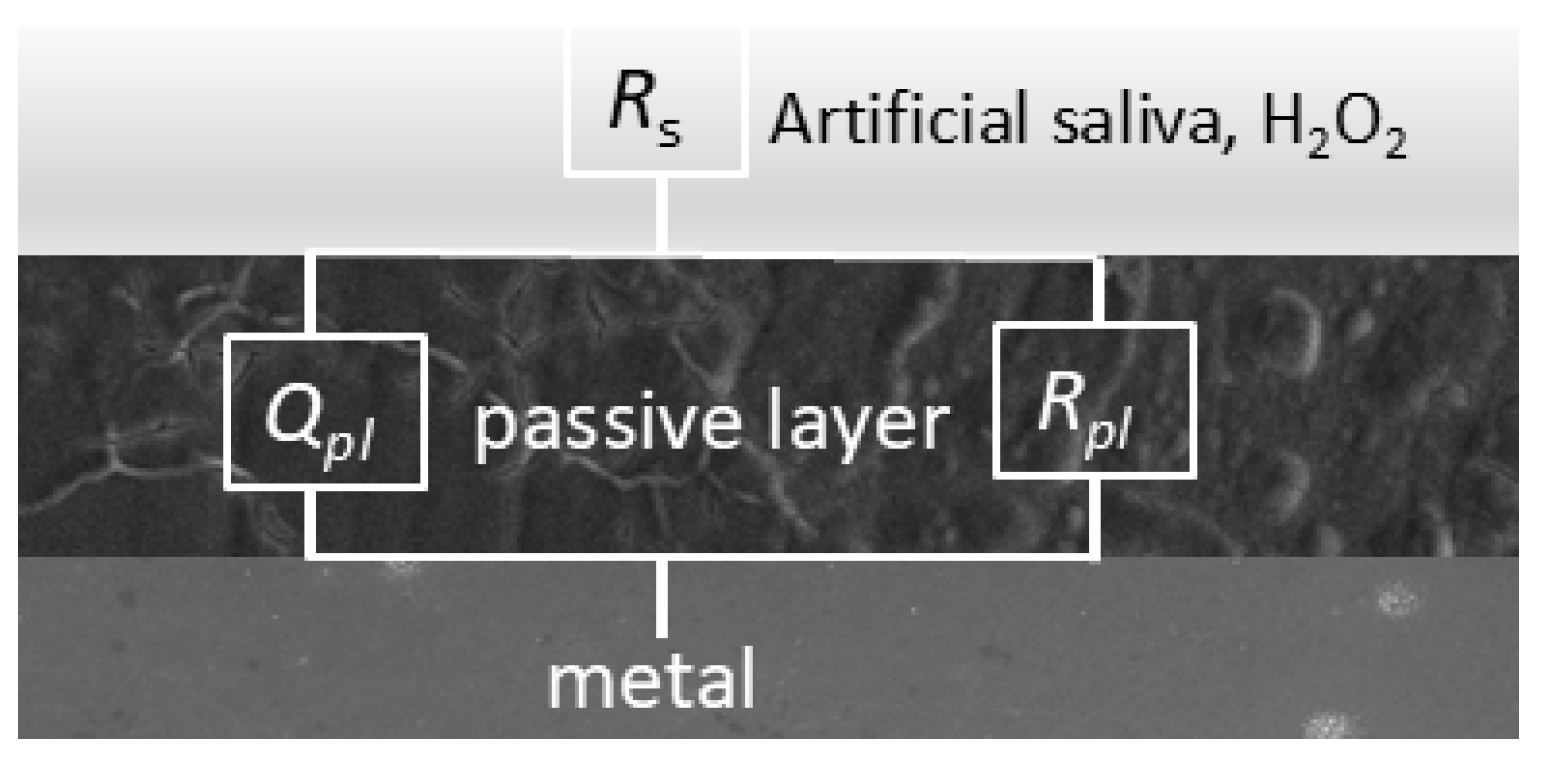
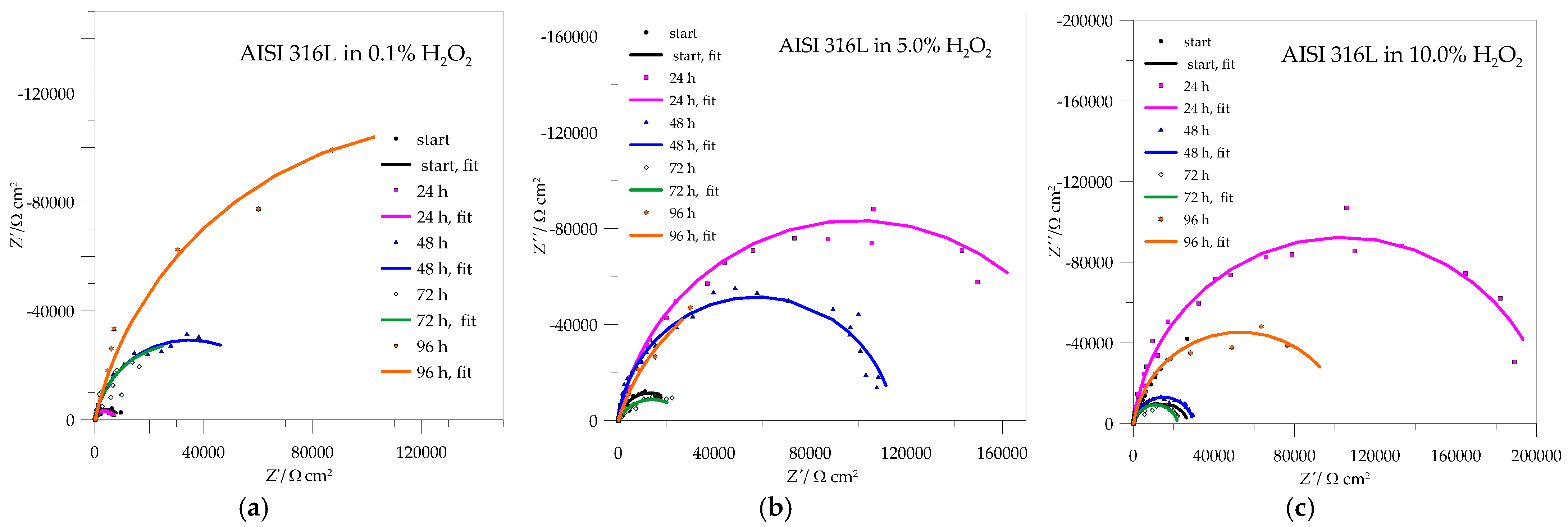
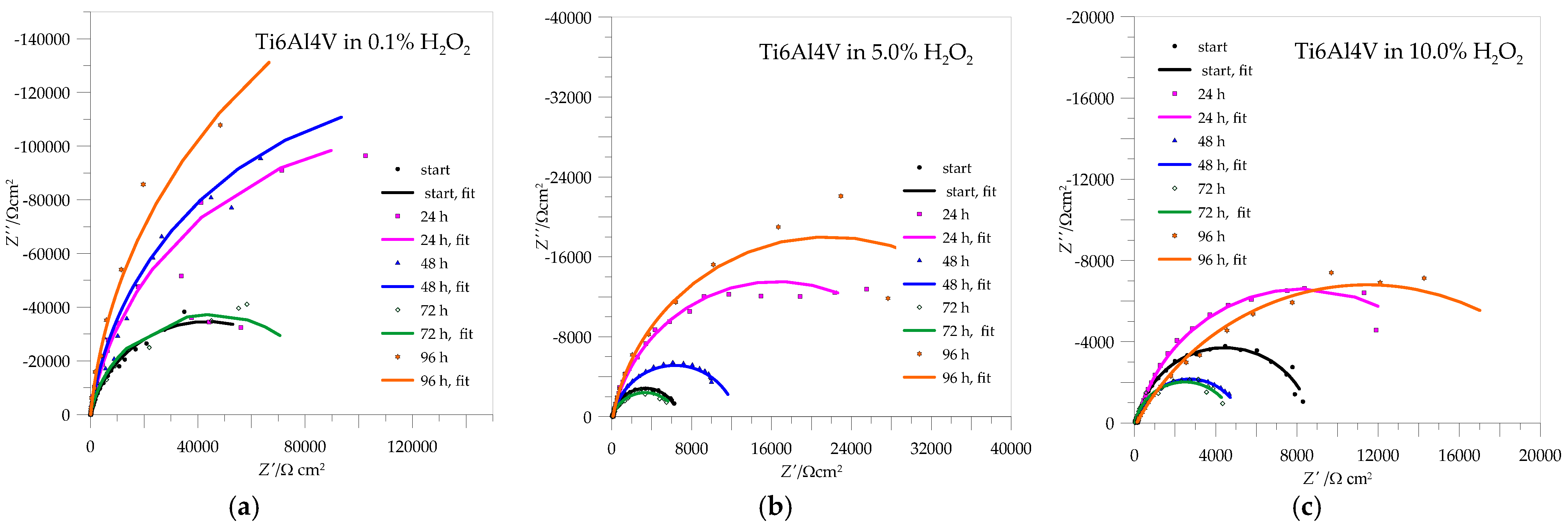
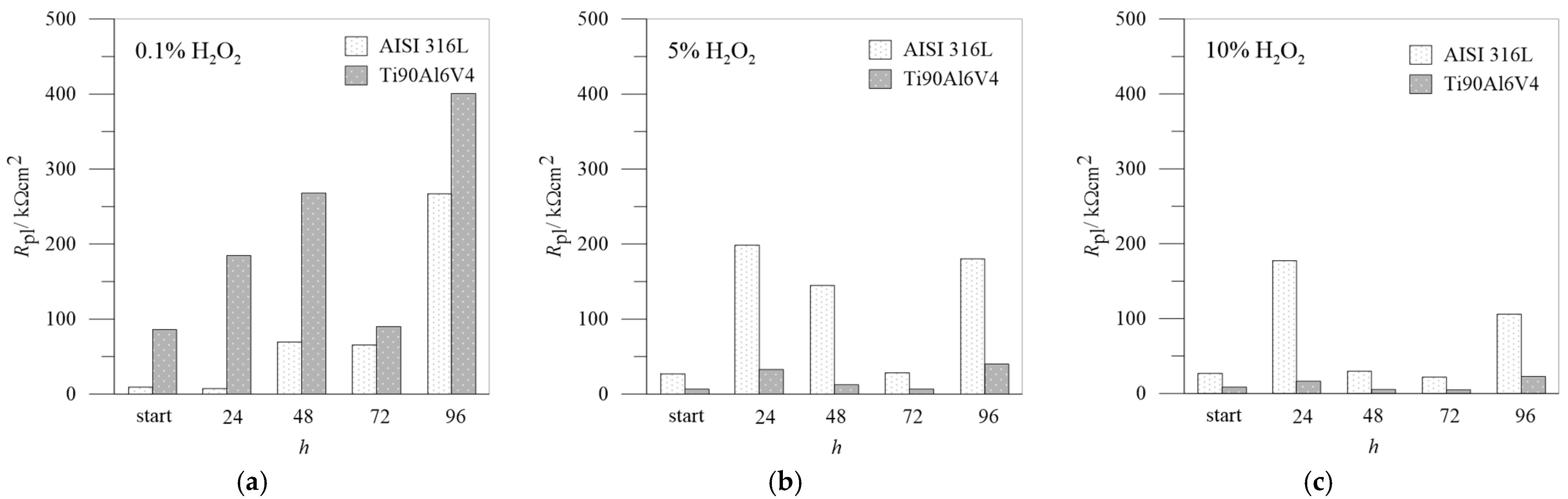
3.3. EIS Measurements
3.4. Corrosion Rate
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, R.; Bhattacharjee, C. 16-Titanium-based nanocomposite materials for dental implant systems. In Applications of Nanocomposite Materials in Dentistry; Asiri, A.M., Inamuddin, Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 271–284. [Google Scholar]
- Alhamad, M.; Barão, V.A.R.; Sukotjo, C.; Cooper, L.F.; Mathew, M.T. Ti-Ions and/or Particles in Saliva Potentially Aggravate Dental Implant Corrosion. Materials 2021, 14, 5733. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.; Romanos, G. Potential Causes of Titanium Particle and Ion Release in Implant Dentistry: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef] [PubMed]
- Leban, M.B.; Kosec, T.; Finšgar, M. Corrosion characterization and ion release in SLM-manufactured and wrought Ti6Al4V alloy in an oral environment. Corros. Sci. 2022, 209, 110716. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Gatto, R.; Monaco, A. Periodontitis, implant loss and peri-implantitis. A meta-analysis. Clin. Oral Implant. Res. 2015, 26, e8–e16. [Google Scholar] [CrossRef]
- Li, Y.; Wong, C.; Xiong, J.; Hodgson, P.; Wen, C. Cytotoxicity of Titanium and Titanium Alloying Elements. J. Dent. Res. 2010, 89, 493–497. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, K.; Zhang, B. In vitro study of platelet adhesion on medical nickel-free stainless steel surface. Mater. Lett. 2005, 59, 1785–1789. [Google Scholar] [CrossRef]
- Hamza, H.M.; Deen, K.M.; Haider, W. Microstructural examination and corrosion behavior of selective laser melted and conventionally manufactured Ti6Al4V for dental applications. Mater. Sci. Eng. C 2020, 113, 110980. [Google Scholar] [CrossRef]
- Nguyen, A.-N.; Kung, K.-C.; Chen, K.-C.; Hsu, C.-W.; Huang, C.-L.; Lee, T.-M. Characteristics and biological responses of selective laser melted Ti6Al4V modified by micro-arc oxidation. J. Dent. Sci. 2024, 19, 1426–1433. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Méndez-Ramírez, C.T.; Baltazar-Zamora, M.Á.; Estupinán-López, F.; Bautista-Margulis, R.G.; Cuevas-Rodríguez, J.; De Flores los Rios, J.P.; Almeraya-Calderón, F. Corrosion of Titanium Alloys Anodized Using Electrochemical Techniques. Metals 2023, 13, 476. [Google Scholar] [CrossRef]
- Alam, M.J.; Cameron, D.C. Preparation and Characterization of TiO2 Thin Films by Sol-Gel Method. J. Sol-Gel Sci. Technol. 2002, 25, 137–145. [Google Scholar] [CrossRef]
- Löbl, P.; Huppertz, M.; Mergel, D. Nucleation and growth in TiO2 films prepared by sputtering and evaporation. Thin Solid Film. 1994, 251, 72–79. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Codeluppi, S.; Cordioli, A.; Pedeferri, M.P. Effect of thermal oxidation on titanium oxides’ characteristics. J. Exp. Nanosci. 2009, 4, 365–372. [Google Scholar] [CrossRef]
- Garcia-Cabezón, C.; Rodriguez-Mendez, M.L.; Amigo Borrás, V.; Raquel, B.; Rodriguez Cabello, J.C.; Ibañez Fonseca, A.; Martin-Pedrosa, F. Application of Plasma Electrolytic Oxidation Coating on Powder Metallurgy Ti-6Al-4V for Dental Implants. Metals 2020, 10, 1167. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Huang, H.-H.; Tsai, Y.-H.; Kuo, Y.-L.; Lee, J.-W.; Lee, Y.-J.; Linn, T.Y.; Chen, P. Creating an extracellular matrix-like three-dimension structure to enhance the corrosion resistance and biological responses of titanium implants. J. Dent. Sci. 2024, 19, S70–S80. [Google Scholar] [CrossRef] [PubMed]
- Porcayo-Calderon, J.; Casales-Diaz, M.; Salinas-Bravo, V.M.; Martinez-Gomez, L. Corrosion Performance of Fe-Cr-Ni Alloys in Artificial Saliva and Mouthwash Solution. Bioinorg. Chem. Appl. 2015, 2015, 930802. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Zhao, X.; Sheng, Y.; Li, W.; Chang, C.-L.; Zhang, Q.; Yu, Z.; Wang, X. Microstructure, mechanical properties and corrosion behaviors of biomedical Ti-Zr-Mo-xMn alloys for dental application. Corros. Sci. 2019, 161, 108195. [Google Scholar] [CrossRef]
- Fojt, J.; Joska, L.; Malek, J.; Sefl, V. Corrosion behavior of Ti–39Nb alloy for dentistry. Mater. Sci. Eng. C 2015, 56, 532–537. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Beline, T.; Ribeiro, A.L.R.; Rangel, E.C.; da Cruz, N.C.; Landers, R.; Faverani, L.P.; Vaz, L.G.; Fais, L.M.G.; Vicente, F.B.; et al. Development of binary and ternary titanium alloys for dental implants. Dent. Mater. 2017, 33, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Wang, B.L.; Li, L.; Zheng, Y.F. Corrosion behavior of Ti–5Ag alloy with and without thermal oxidation in artificial saliva solution. Dent. Mater. 2011, 27, 214–220. [Google Scholar] [CrossRef]
- Prestat, M.; Vucko, F.; Holzer, L.; Thierry, D. Microstructural aspects of Ti6Al4V degradation in H2O2-containing phosphate buffered saline. Corros. Sci. 2021, 190, 109640. [Google Scholar] [CrossRef]
- Torres Pérez, A.I.; Fernández Fairén, M.; Torres Pérez, Á.A.; Gil Mur, J. Use of Porous Titanium Trabecular as a Bone Defect Regenerator: In Vivo Study. Metals 2022, 12, 327. [Google Scholar] [CrossRef]
- Eid, Y. The myths of trabecular metal: ‘the next best thing to bone’. Egypt. Orthop. J. 2013, 48, 327–329. [Google Scholar] [CrossRef]
- Balla, V.K.; Bodhak, S.; Bose, S.; Bandyopadhyay, A. Porous tantalum structures for bone implants: Fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010, 6, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Milošev, I.; Strehblow, H.-H. The behavior of stainless steels in physiological solution containing complexing agent studied by X-ray photoelectron spectroscopy. J. Biomed. Mater. Res. 2000, 52, 404–412. [Google Scholar] [CrossRef]
- Kocijan, A.; Merl, D.K.; Jenko, M. The corrosion behaviour of austenitic and duplex stainless steels in artificial saliva with the addition of fluoride. Corros. Sci. 2011, 53, 776–783. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Y.; Liu, X.; Liu, P.; Li, X.; Zhang, M.; Zhou, E.; Zhao, Z.; Wang, X.; Zhang, Y.; et al. Accelerated corrosion of 316L stainless steel in a simulated oral environment via extracellular electron transfer and acid metabolites of subgingival microbiota. Bioact. Mater. 2024, 35, 56–66. [Google Scholar] [CrossRef]
- Eduok, U. Microbiologically induced intergranular corrosion of 316L stainless steel dental material in saliva. Mater. Chem. Phys. 2024, 313, 128799. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C. Nucleation of corrosion pits in Ringer’s solution containing bovine serum. Corros. Sci. 2007, 49, 4296–4306. [Google Scholar] [CrossRef]
- Lima, A.R.; Pinto, A.M.P.; Toptan, F.; Alves, A.C. Impact of simulated inflammation and food breakdown on the synergistic interaction between corrosion and wear on titanium. Corros. Sci. 2024, 228, 111839. [Google Scholar] [CrossRef]
- Sheit, H.M.K.; Mohan, K.S.; Srinivasan, P.; Muthu, S.E.; Dinesh, A.; Rajeswari, B.; Priya, L.S.; Gnanasekaran, L.; Iqbal, M. Anti-corrosive efficiency of salvadora persica plant stick powder on SS 316L orthodontic wire in artificial saliva. Results Chem. 2024, 12, 101894. [Google Scholar] [CrossRef]
- Tian, K.V.; Passaretti, F.; Nespoli, A.; Placidi, E.; Condò, R.; Andreani, C.; Licoccia, S.; Chass, G.A.; Senesi, R.; Cozza, P. Composition—Nanostructure Steered Performance Predictions in Steel Wires. Nanomaterials 2019, 9, 1119. [Google Scholar] [CrossRef]
- Seo, B.; Kanematsu, H.; Nakamoto, M.; Miyabayashi, Y.; Suzuki, M.; Tanaka, T. Corrosion and antibacterial performance of 316L stainless steel with copper patterns by super-spread wetting of liquid copper. Surf. Coat. Technol. 2023, 462, 129496. [Google Scholar] [CrossRef]
- Echeverrigaray, F.G.; Echeverrigaray, S.; Delamare, A.P.L.; Wanke, C.H.; Figueroa, C.A.; Baumvol, I.J.R.; Aguzzoli, C. Antibacterial properties obtained by low-energy silver implantation in stainless steel surfaces. Surf. Coat. Technol. 2016, 307, 345–351. [Google Scholar] [CrossRef]
- Wang, X.; Ye, X.; Zhang, L.; Shao, Y.; Zhou, X.; Lu, M.; Chu, C.; Xue, F.; Bai, J. Corrosion and antimicrobial behavior of stainless steel prepared by one-step electrodeposition of silver at the grain boundaries. Surf. Coat. Technol. 2022, 439, 128428. [Google Scholar] [CrossRef]
- Xi, T.; Shahzad, M.B.; Xu, D.; Sun, Z.; Zhao, J.; Yang, C.; Qi, M.; Yang, K. Effect of copper addition on mechanical properties, corrosion resistance and antibacterial property of 316L stainless steel. Mater. Sci. Eng. C 2017, 71, 1079–1085. [Google Scholar] [CrossRef]
- Lv, J.; Liang, T. Improved corrosion resistance of 316L stainless steel by nanocrystalline and electrochemical nitridation in artificial saliva solution. Appl. Surf. Sci. 2015, 359, 158–165. [Google Scholar] [CrossRef]
- Nakagawa, M.; Matsuya, S.; Shiraishi, T.; Ohta, M. Effect of Fluoride Concentration and pH on Corrosion Behavior of Titanium for Dental Use. J. Dent. Res. 1999, 78, 1568–1572. [Google Scholar] [CrossRef]
- Zhang, H.; Man, C.; Dong, C.; Wang, L.; Li, W.; Kong, D.; Wang, L.; Wang, X. The corrosion behavior of Ti6Al4V fabricated by selective laser melting in the artificial saliva with different fluoride concentrations and pH values. Corros. Sci. 2021, 179, 109097. [Google Scholar] [CrossRef]
- Milošev, I.; Kapun, B.; Šelih, V.S. The effect of fluoride ions on the corrosion behaviour of Ti metal, and Ti6-Al-7Nb and Ti-6Al-4V alloys in artificial saliva. Acta Chim. Slov. 2013, 60, 543. [Google Scholar]
- Souza, J.C.M.; Barbosa, S.L.; Ariza, E.; Celis, J.P.; Rocha, L.A. Simultaneous degradation by corrosion and wear of titanium in artificial saliva containing fluorides. Wear 2012, 292–293, 82–88. [Google Scholar] [CrossRef]
- Turkina, A.Y.; Makeeva, I.M.; Dubinin, O.N.; Bondareva, J.V.; Chernodoubov, D.A.; Shibalova, A.A.; Arzukanyan, A.V.; Antoshin, A.A.; Timashev, P.S.; Evlashin, S.A. The Impact of Commercially Available Dry Mouth Products on the Corrosion Resistance of Common Dental Alloys. Materials 2023, 16, 4195. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-García, A.; Pérez-Alvarez, J.; Barrera, C.C.; Medina, J.C.; Almaguer-Flores, A.; Sánchez, R.B.; Rodil, S.E. The effect of simulated inflammatory conditions on the surface properties of titanium and stainless steel and their importance as biomaterials. Mater. Sci. Eng. C 2016, 66, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Mabilleau, G.; Bourdon, S.; Joly-Guillou, M.L.; Filmon, R.; Baslé, M.F.; Chappard, D. Influence of fluoride, hydrogen peroxide and lactic acid on the corrosion resistance of commercially pure titanium. Acta Biomater. 2006, 2, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Handzlik, P.; Fitzner, K. Corrosion resistance of Ti and Ti–Pd alloy in phosphate buffered saline solutions with and without H2O2 addition. Trans. Nonferrous Met. Soc. China 2013, 23, 866–875. [Google Scholar] [CrossRef]
- Freeman, B.A.; Crapo, J.D. Biology of disease: Free radicals and tissue injury. Lab. Invest 1982, 47, 412–426. [Google Scholar]
- ZView, ZPlot, CorrView, CorrWare, version 2.8; Scribner Associates, Inc.: Southern Pines, NC, USA, 1990–1999.
- Motyka, M. Martensite Formation and Decomposition during Traditional and AM Processing of Two-Phase Titanium Alloys—An Overview. Metals 2021, 11, 481. [Google Scholar] [CrossRef]
- Boukamp, B.A. Equivalent Circuit Users Manual; Report CT88/265/128; University of Twente, Department of Chemical Technology: Enschede, The Netherlands, 1989. [Google Scholar]
- Macdonald, J.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; Macdonald, J.R., Ed.; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Liao, H.; Watson, W.; Dizon, A.; Tribollet, B.; Vivier, V.; Orazem, M.E. Physical properties obtained from measurement model analysis of impedance measurements. Electrochim. Acta 2020, 354, 136747. [Google Scholar] [CrossRef]
- Shreir, L.L.; Jarman, R.A.; Burstein, G.T. (Eds.) Corrosion, 3rd ed.; Butterworth-Heinemann: London, UK, 1994; Volume 1, pp. 1-88–1-90. [Google Scholar]
- ASTM G102; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM: West Conshohocken, PA, USA, 2023.
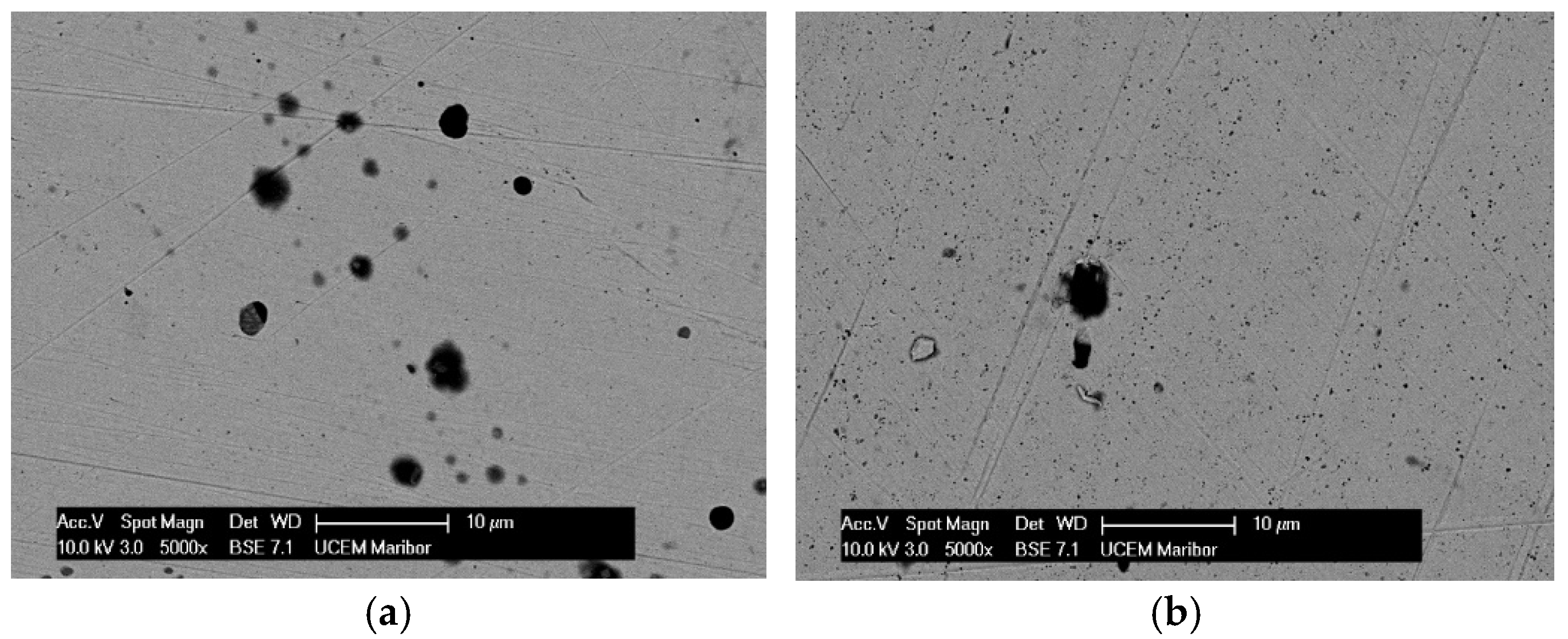
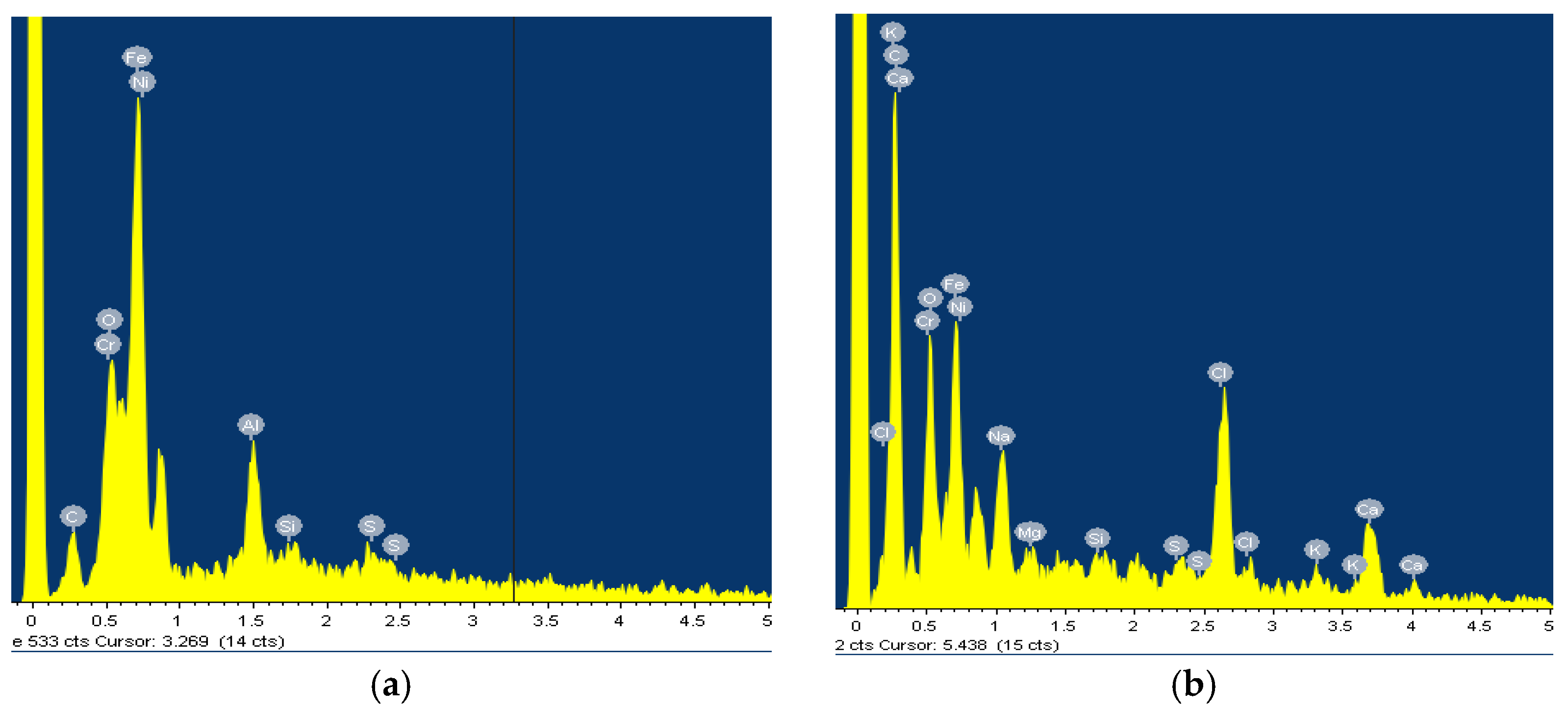

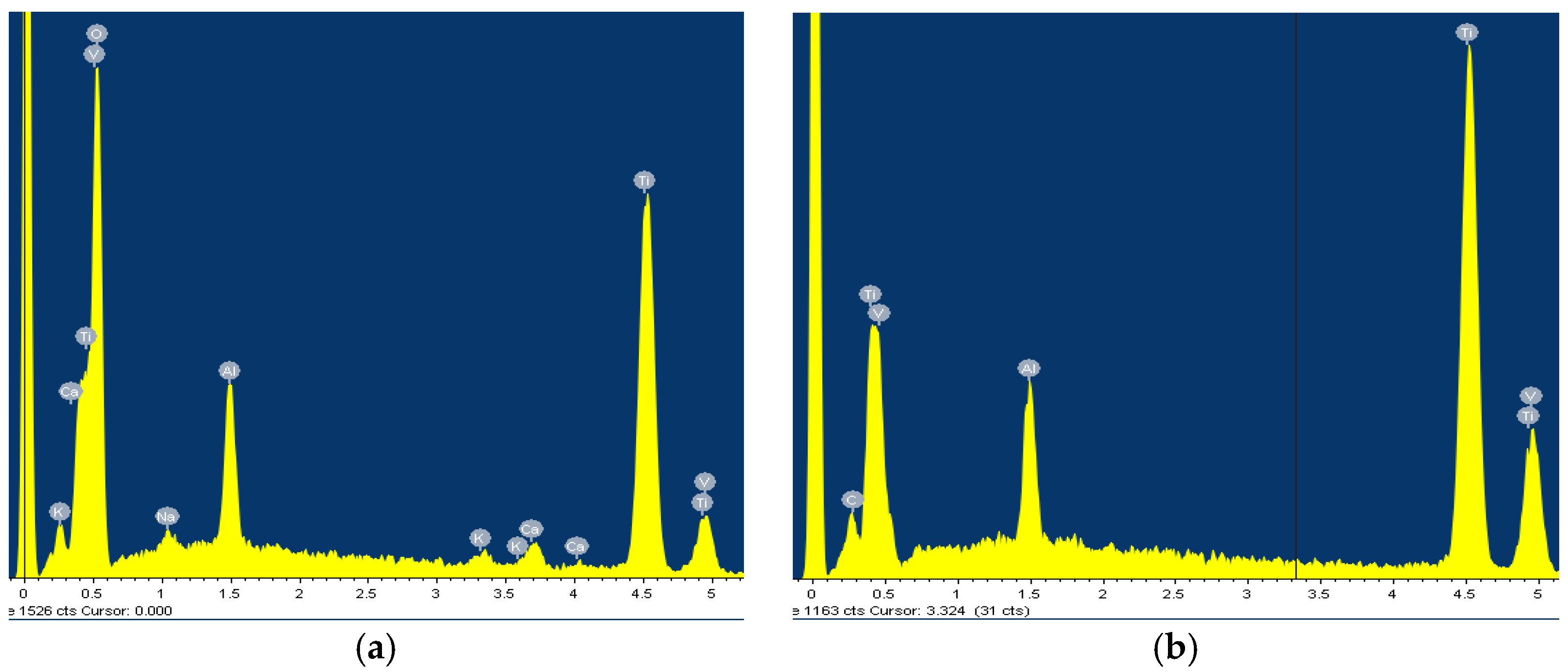
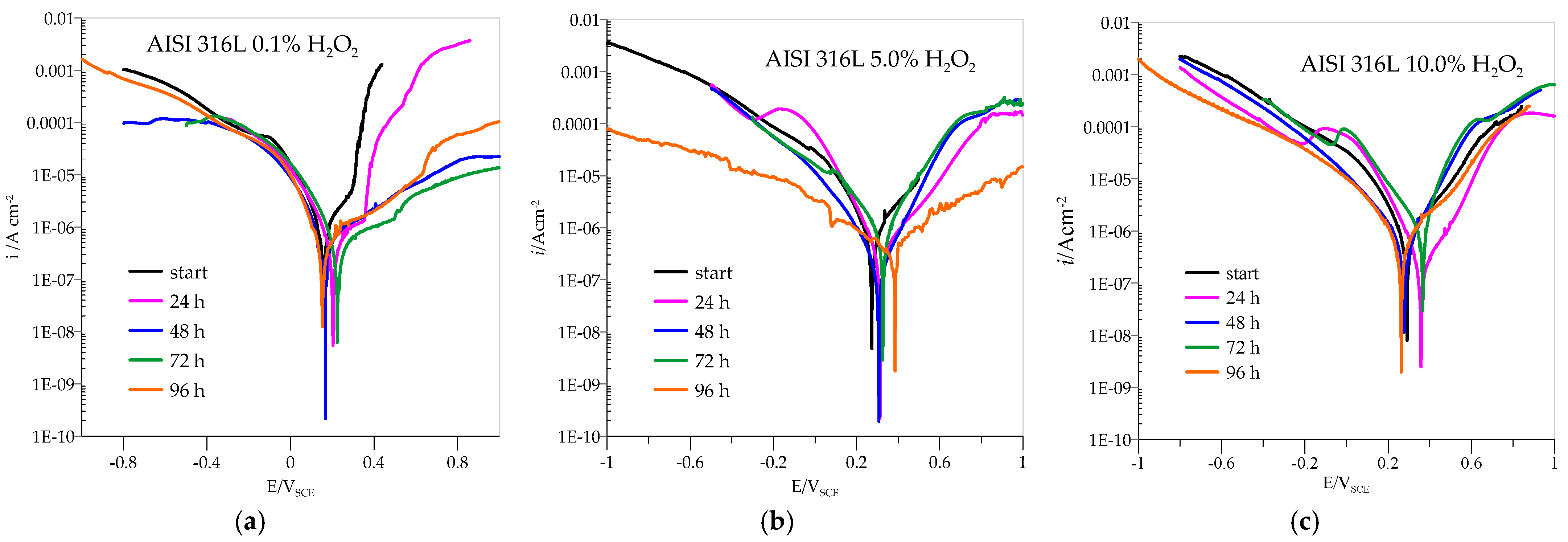

| 0.1% H2O2 | 5.0% H2O2 | 10.0% H2O2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| h | icorr (A/cm2) | Ecorr (VSCE) | ipas (A/cm2) | Epas (VSCE) | icorr (A/cm2) | Ecorr (VSCE) | ipas (A/cm2) | Epas (VSCE) | icorr (A/cm2) | Ecorr (VSCE) | ipas (A/cm2) | Epas (VSCE) |
| 0 | 6.93·10−7 | 0.1573 | 4.69·10−8 | 0.159 | 4.54·10−7 | 0.2738 | 7.52·10−7 | 0.309 | 4.62·10−7 | 0.2908 | 2.81·10−8 | 0.291 |
| 24 | 4.12·10−7 | 0.2032 | 5.05·10−8 | 0.205 | 1.48·10−7 | 0.3120 | 7.8·10−9 | 0.314 | 1.93·10−7 | 0.3580 | 9.05·10−9 | 0.359 |
| 48 | 2.92·10−7 | 0.1659 | 0.159·10−7 | 0.168 | 1.69·10−7 | 0.3074 | 1.23·10−8 | 0.308 | 5.78·10−7 | 0.2770 | 5.55·10−8 | 0.280 |
| 72 | 2.47·10−7 | 0.2252 | 1.097·10−7 | 0.230 | 4.50·10−7 | 0.3262 | 4.78·10−9 | 0.320 | 8.62·10−7 | 0.3671 | 8.98·10−8 | 0.367 |
| 96 | 2.08·10−7 | 0.1510 | 3.59·10−8 | 0.153 | 1.51·10−7 | 0.3840 | 5.44·10−8 | 0.386 | 4.61·10−7 | 0.2626 | 2.19·10−8 | 0.263 |
| 0.1% H2O2 | 5.0% H2O2 | 10.0% H2O2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| h | icorr (A/cm2) | Ecorr (VSCE) | ipas (A/cm2) | Epas (VSCE) | icorr (A/cm2) | Ecorr (VSCE) | ipas (A/cm2) | Epas (VSCE) | icorr (A/cm2) | Ecorr (VSCE) | ipas (A/cm2) | Epas (VSCE) |
| 0 | 3.18·10−7 | 0.1110 | 7.2·10−8 | 0.113 | 7.20·10−7 | 0.0016 | 6.52·10−8 | 0.0051 | 12.5·10−7 | 0.0432 | 1.14·10−5 | 0.401 |
| 24 | 1.41·10−7 | 0.1752 | 3.56·10−8 | 0.180 | 3.57·10−7 | 0.1845 | 2.64·10−7 | 0.191 | 4.17·10−7 | 0.2271 | 7.8·10−9 | 0.314 |
| 48 | 0.73·10−7 | 0.1556 | 1.48·10−8 | 0.199 | 4.98·10−7 | 0.1377 | 1.08·10−7 | 0.148 | 17.28·10−7 | 0.1169 | 1.23·10−8 | 0.308 |
| 72 | 3.22·10−7 | 0.1257 | 4.83·10−7 | 0.161 | 6.24·10−7 | 0.2762 | 2.51·10−7 | 0.208 | 19.42·10−7 | 0.2120 | 6.62·10−6 | 0.400 |
| 96 | 0.56·10−7 | 0.2223 | 2.72·10−7 | 0.240 | 3.36·10−7 | 0.2250 | 1.01·10−7 | 0.234 | 4.78·10−7 | 0.1914 | 4.75·10−6 | 0.364 |
| Rpl (kΩ cm2) | Qpl·10−5 (sn Ω−1 cm−2) | n | Rpl (kΩ cm2) | Qpl·10−5(sn Ω−1 cm−2) | n | Rpl (kΩ cm2) | Qpl·10−5 (sn Ω−1 cm−2) | n | |
|---|---|---|---|---|---|---|---|---|---|
| h | 0.1% H2O2 | 5.0% H2O2 | 10% H2O2 | ||||||
| 0 | 9.22 | 2.57 | 0.85 | 26.99 | 1.78 | 0.89 | 26.91 | 2.15 | 0.85 |
| 24 | 7.31 | 4.27 | 0.90 | 198.63 | 1.19 | 0.88 | 170.33 | 1.39 | 0.92 |
| 48 | 69.51 | 3.22 | 0.89 | 114.96 | 1.53 | 0.92 | 29.78 | 2.04 | 0.91 |
| 72 | 65.50 | 1.97 | 0.90 | 28.36 | 7.37 | 0.70 | 21.95 | 2.76 | 0.89 |
| 96 | 267.08 | 2.36 | 0.86 | 180.28 | 1.19 | 0.81 | 105.91 | 1.91 | 0.90 |
| Rpl (kΩ cm2) | Qpl·10−5 (sn Ω−1 cm−2) | n | Rpl (kΩ cm2) | Qpl·10−5 (sn Ω−1 cm−2) | n | Rpl (kΩ cm2) | Qpl·10−5 (sn Ω−1 cm−2) | n | |
|---|---|---|---|---|---|---|---|---|---|
| h | 0.1% H2O2 | 5.0% H2O2 | 10.0% H2O2 | ||||||
| 0 | 86.00 | 3.40 | 0.85 | 6.52 | 3.25 | 0.91 | 8.60 | 3.02 | 0.90 |
| 24 | 184.81 | 3.19 | 0.94 | 32.8 | 4.17 | 0.88 | 16.34 | 24.1 | 0.86 |
| 48 | 268.00 | 3.43 | 0.91 | 12.39 | 18.33 | 0.88 | 5.19 | 22.75 | 0.88 |
| 72 | 89.91 | 4.64 | 0.88 | 6.62 | 36.05 | 0.80 | 4.90 | 47.54 | 0.87 |
| 96 | 400.58 | 4.13 | 0.93 | 40.00 | 4.13 | 0.94 | 22.70 | 3.65 | 0.68 |
| r (mm/year) | ||||||
|---|---|---|---|---|---|---|
| 0.1% H2O2 | 5.0% H2O2 | 10.0% H2O2 | ||||
| h | Ti6Al4V | AISI 316L | Ti6Al4V | AISI 316L | Ti6Al4V | AISI 316L |
| 0 | 2.82·10−3 | 7.23·10−3 | 6.23·10−3 | 4.74·10−3 | 11.10·10−3 | 4.82·10−3 |
| 24 | 1.25·10−3 | 4.30·10−3 | 3.12·10−3 | 1.54·10−3 | 3.69·10−3 | 2.02·10−3 |
| 48 | 0.65·10−3 | 3.04·10−3 | 4.32·10−3 | 1.76·10−3 | 11.12·10−3 | 2.14·10−3 |
| 72 | 0.78·10−3 | 2.57·10−3 | 5.41·10−3 | 4.69·10−3 | 12.05·10−3 | 9.01·10−3 |
| 96 | 0.49·10−3 | 2.17·10−3 | 2.91·10−3 | 1.57·10−3 | 3.08·10−3 | 2.52·10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slemnik, M. Comparative Evaluation of Corrosion Resistance of AISI 316L and Ti6Al4V Dental Materials Under Simulated Inflammatory Conditions. Materials 2025, 18, 2243. https://doi.org/10.3390/ma18102243
Slemnik M. Comparative Evaluation of Corrosion Resistance of AISI 316L and Ti6Al4V Dental Materials Under Simulated Inflammatory Conditions. Materials. 2025; 18(10):2243. https://doi.org/10.3390/ma18102243
Chicago/Turabian StyleSlemnik, Mojca. 2025. "Comparative Evaluation of Corrosion Resistance of AISI 316L and Ti6Al4V Dental Materials Under Simulated Inflammatory Conditions" Materials 18, no. 10: 2243. https://doi.org/10.3390/ma18102243
APA StyleSlemnik, M. (2025). Comparative Evaluation of Corrosion Resistance of AISI 316L and Ti6Al4V Dental Materials Under Simulated Inflammatory Conditions. Materials, 18(10), 2243. https://doi.org/10.3390/ma18102243




