Improvement of Surface Properties of Carbon Steel Through Electrospark Coatings from Multicomponent Hard Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Coating Material
2.2. Materials and Equipment for ESD and Deposition Conditions
- -
- Substrate
- -
- Equipment for ESD
- -
- Short-circuit current—0.1–2 A;
- -
- Voltage—U = 80 V;
- -
- Capacitance—C = 5–100 µF;
- -
- Energy of a single pulse—E = CU2/2; E = 0.02–0.3 J;
- -
- Vibration frequency—100 Hz;
- -
- Pulse duration ≈ 100 µs.
2.3. Types of Research, Methodology of Measurements and Research Equipment
3. Results and Discussion
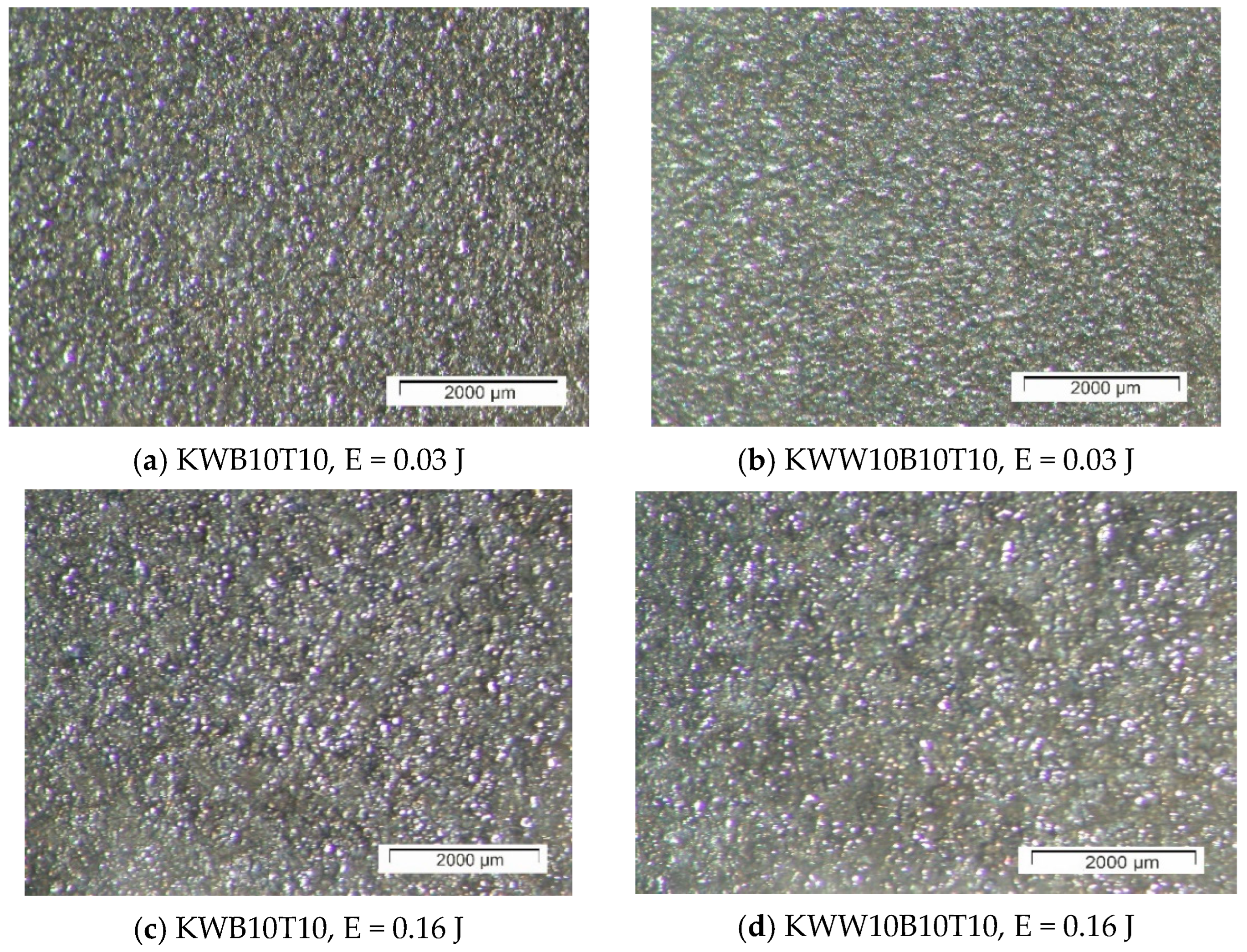
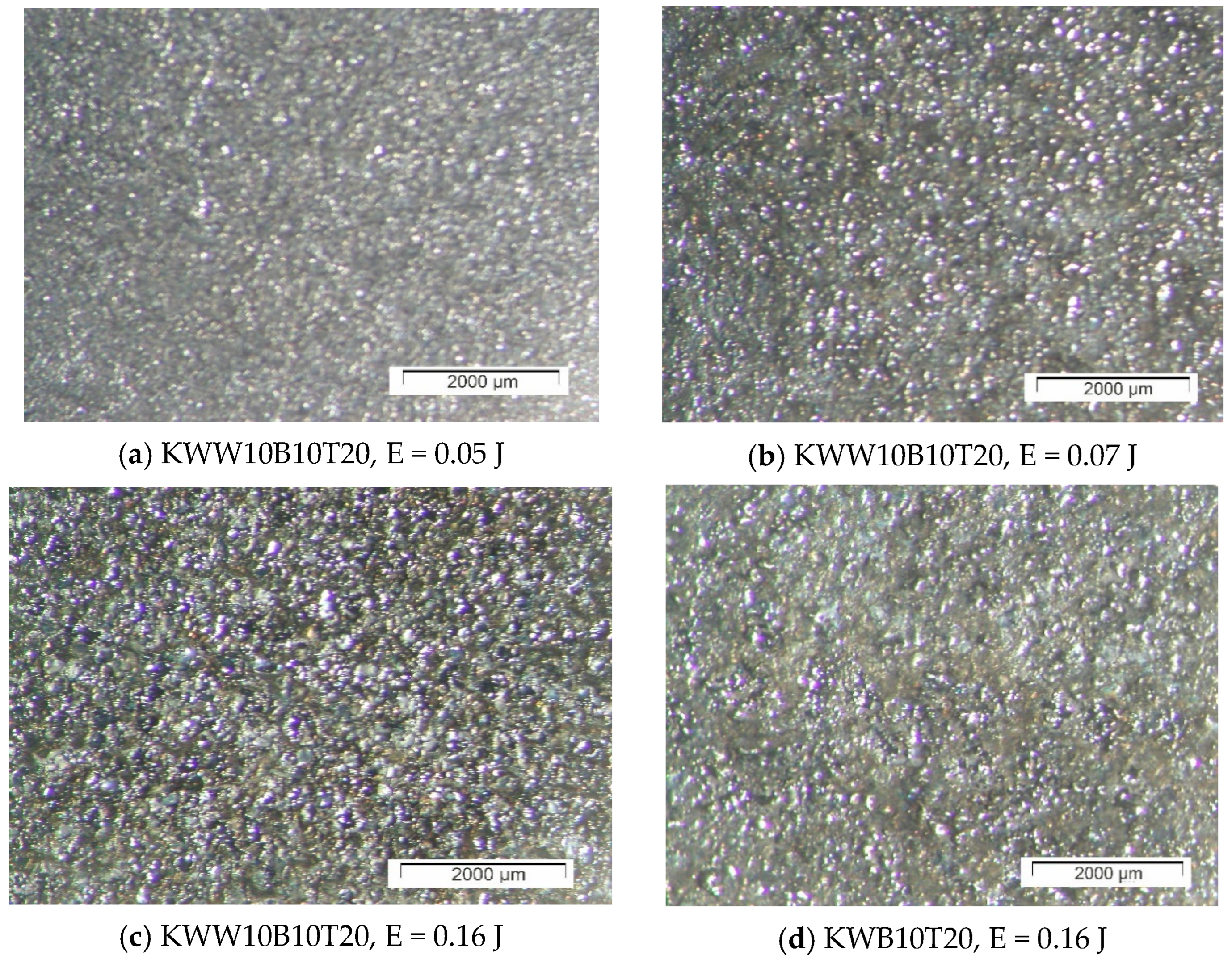
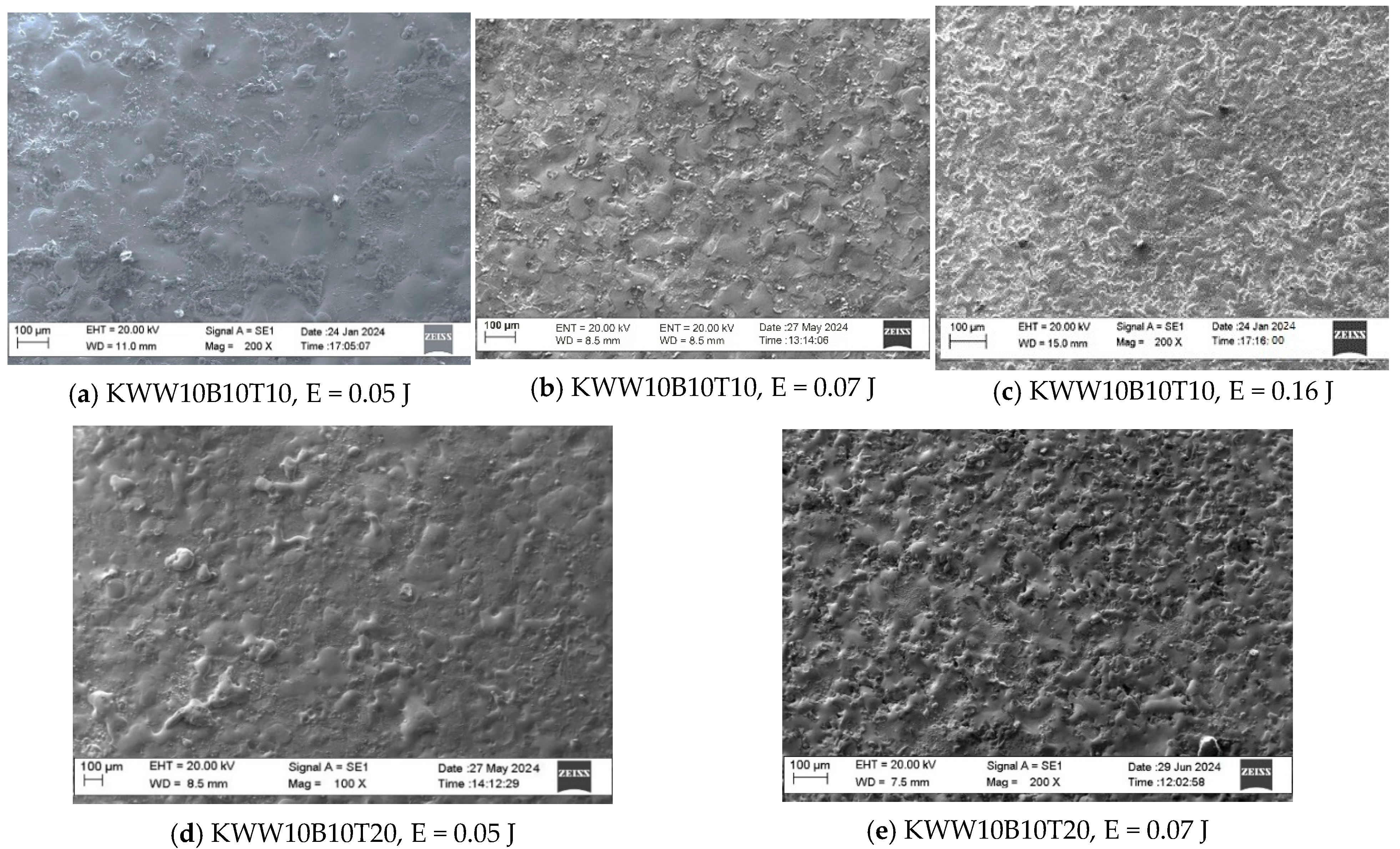
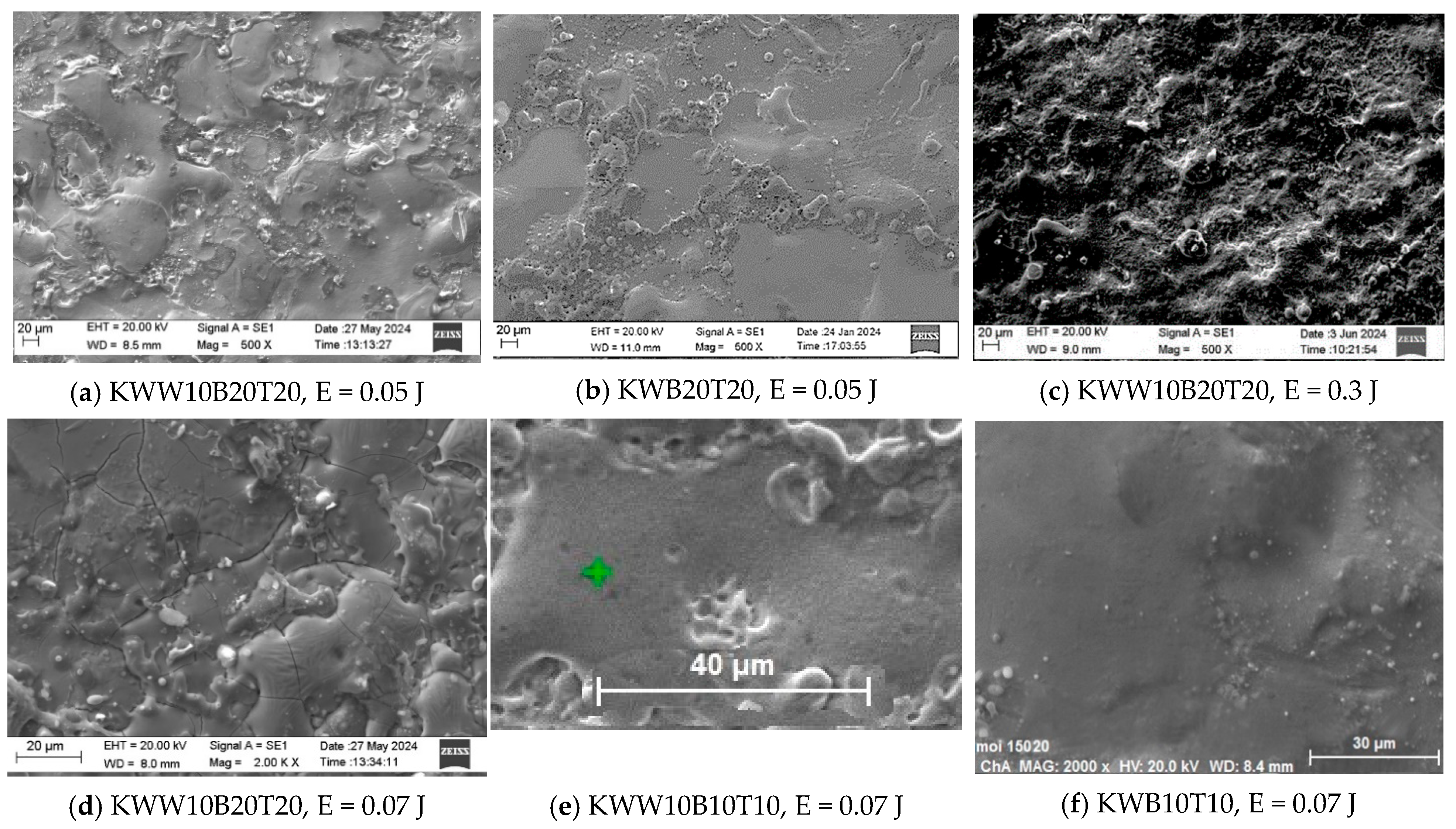
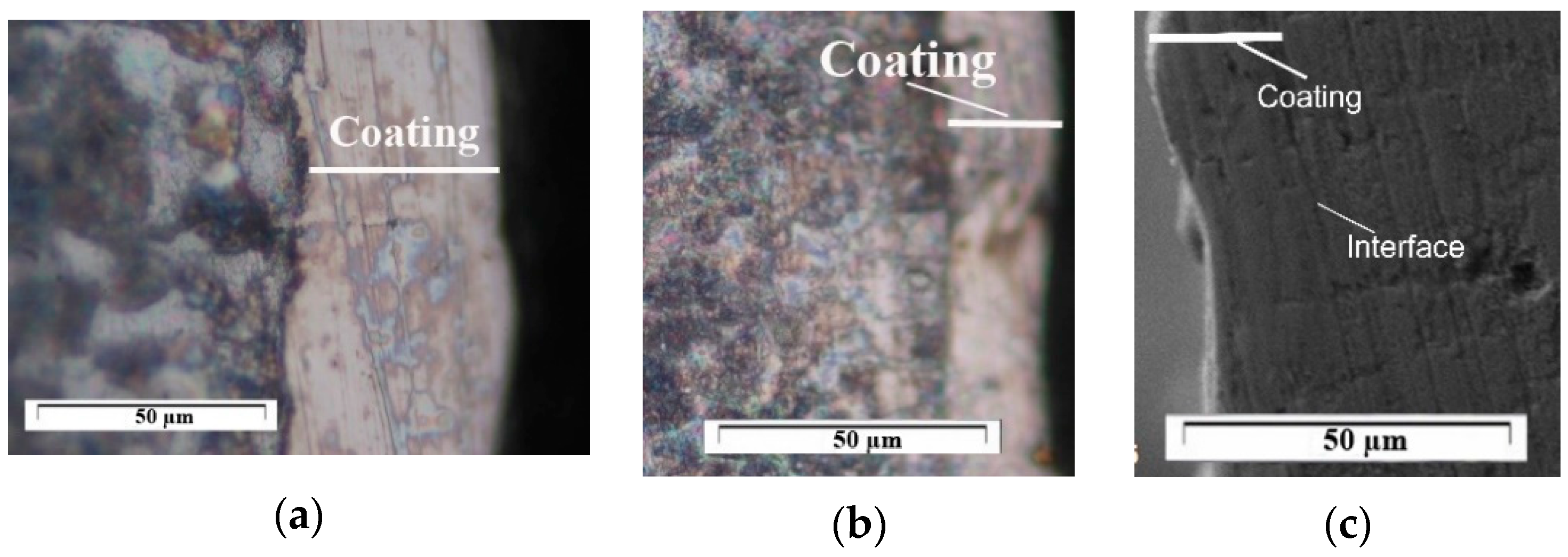
3.1. Coating Characterization—Roughness, Thickness and the Topography and Structure
- -
- In the coating with a minimum content of the solder mass (KWW10B20T20), at low pulse energies up to 0.03 J, the lowest roughness on the order of Ra ≈ 2–2.5 µm was recorded, and with an increase in energy above 0.07 J, the roughness increases almost abruptly; at the maximum energy of E = 0.3 J, it reaches Ra ≈ 7 µm. In the coatings from these electrodes, at E ≥ 0.05 J, the amount of material transferred from the brittle fracture of the electrode increases, as well as the unevenness and structural defects of the coatings, and accordingly, the roughness parameters also increase, and the gradient of thickness increase decreases.
- -
- For coatings with a maximum content of binding mass of 40%, when electrode KWT10 is at low energies of E = 0.02 and 0.03 J, the roughness of the coatings is comparable to, or higher than, that of KWW10B20T20 electrodes, but at E ≥ 0.05 J, the roughness parameters show lower values, and the thickness shows higher values than those of coatings from KWW10B20T20 and from the other electrodes. Therefore, the use of electrodes with a higher content of the solder metal mass makes it possible to obtain coatings with increased thickness at lower roughness.
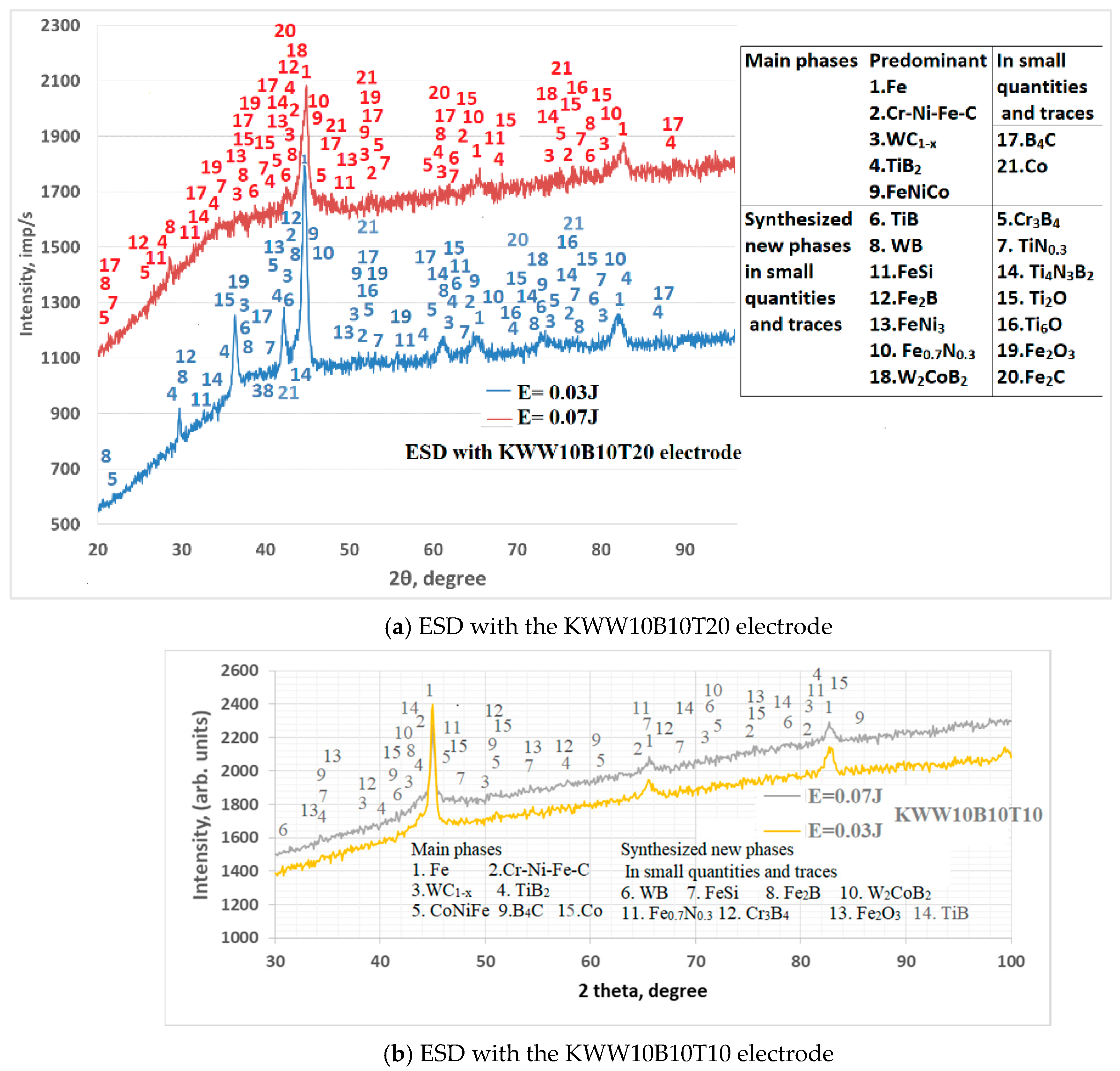
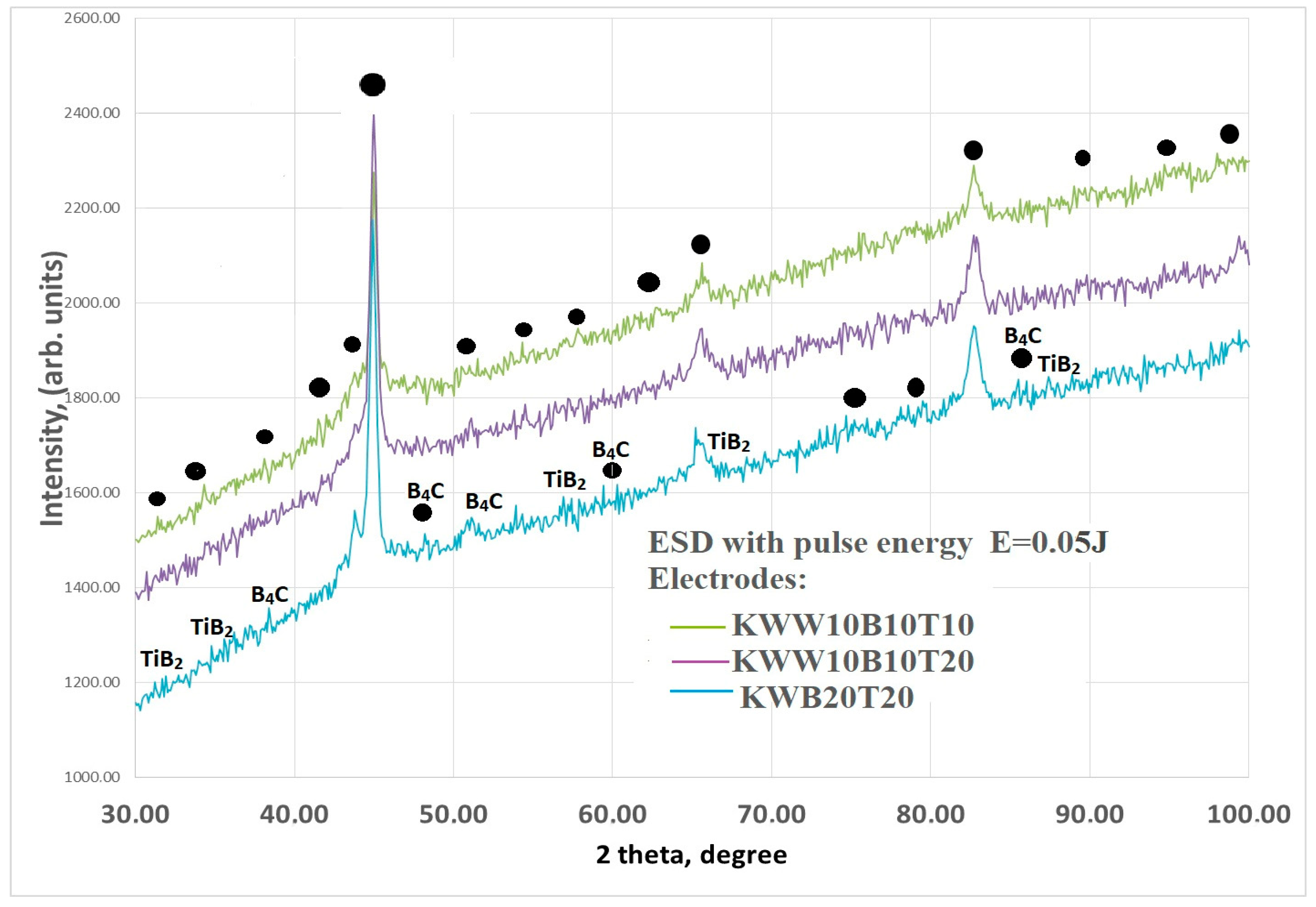
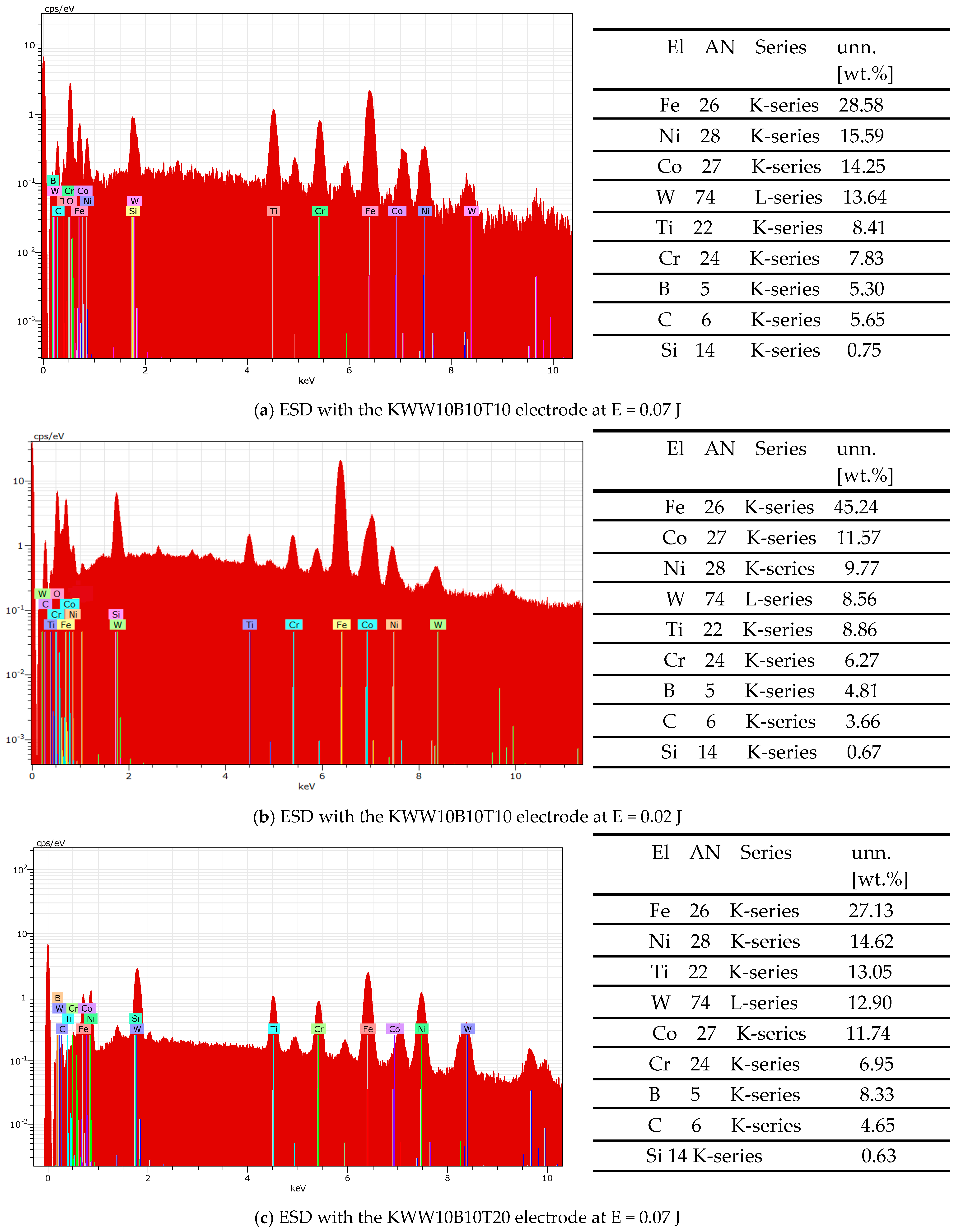
3.2. Phase Composition of Coatings
3.3. Tribological Tests
- -
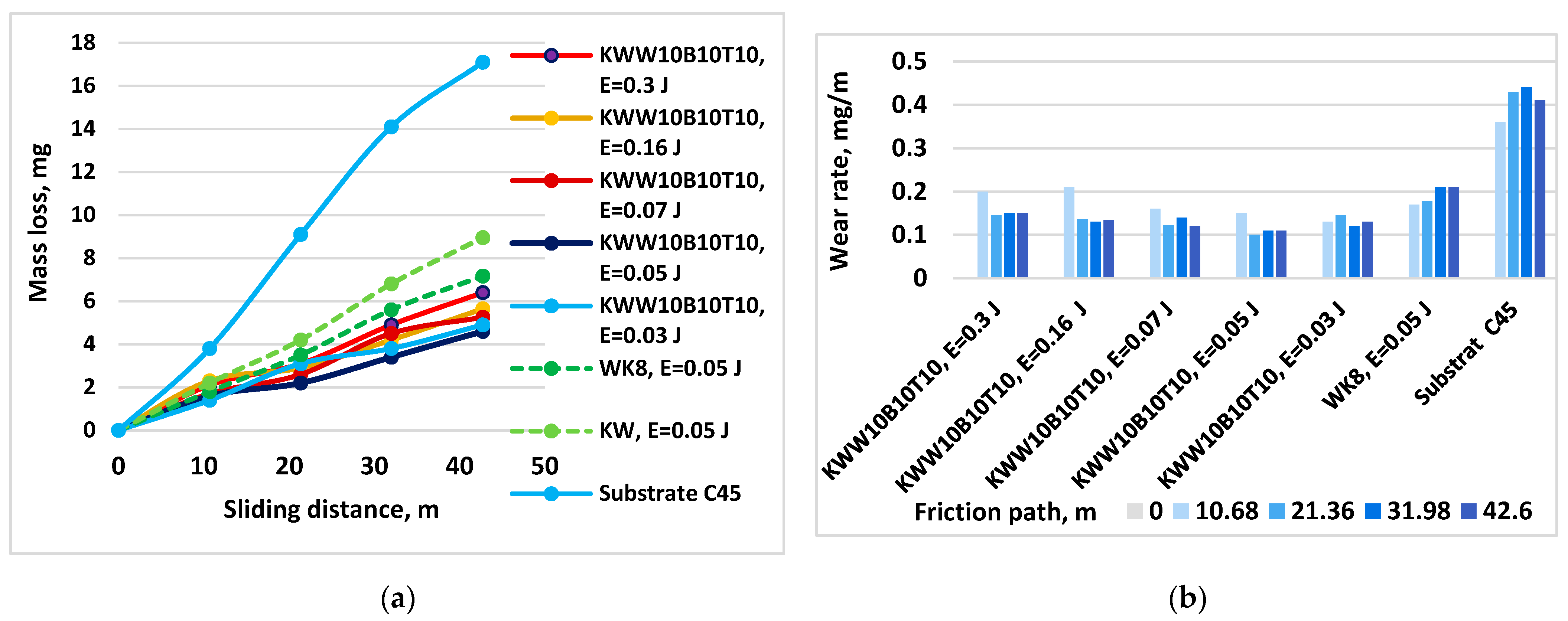
- The influence of pulse energy on wear can be clearly seen in Figure 13. With increasing energy, a tendency towards a decrease in the wear of KWW10B10T10 coatings is observed. At E = 0.05 J, wear reaches a minimum and then gradually increases. From Figure 13b, it is easy to determine the energy range in which the wear rate is minimal, E ≈ 0.03–0.16 J, with the least wear being recorded at E = 0.05 and 0.07 J. Within this range, the differences in the wear of the coated samples are relatively small (Figure 13a). From the metallographic, SEM and EDS analyses, it was established that in ESD with the KWW10B10T10 electrode at pulse energies of 0.03–0.07 J, the coatings achieve an optimal and most favorable balance in terms of wear resistance between, on the one hand, the roughness, uniformity and thickness of the coating, and, on the other hand, the amount of solid phases transferred to the cathode, newly formed wear-resistant phases and amorphous-crystalline structures, which play a decisive role in reducing wear. Apparently, at lower and medium energies up to 0.07 J, wear is mainly influenced by the content of wear-resistant phases and the higher microhardness and changes in the structure of the surface layer; the negative influence of increasing roughness with increasing energy in the direction of 0.03 to 0.07 J is compensated by the thickness, composition and structure of the coatings. With a further increase in the pulse energy above 0.07 J, the amount of material transferred from the brittle fracture in the solid phase increases, and the roughness, unevenness and structural defects of the coatings also increase (Table 4 and Table 5 and Figure 6 and Figure 7), and the amount of amorphous structures decreases, which causes a gradual increase in wear. Due to the fine-grained amorphous–crystalline structure and the presence of greater thickness and a large amount of phases with high hardness and wear resistance, at E = 0.07 and 0.16 J, the increase is very slight, with the wear values remaining comparable to those at E = 0.05 J, and even at E = 0.3 J, its values remain up to 2.5–3 times lower than those of uncoated steel but higher than those at E = 0.03–0.07 J. In the coatings from the other electrodes studied, the same tendency towards a decrease in wear is observed until certain values of pulse energy are reached, which are different for each specific electrode, after which an increasing trend is registered at E = 0.05 J. For the different electrodes with a further increase in energy, the effect of ESD decreases to 2–2.5 times lower wear than that of the substrate. Apparently, the roughness and unevenness of these coatings, which are significantly higher, gradually acquire a predominant influence on wear, which leads to its increase. This gives us a reason to recommend the use of pulse energy in the range of 0.03–0.07 J as a more favorable alternative for ESD with KWW10B10T10;
- -
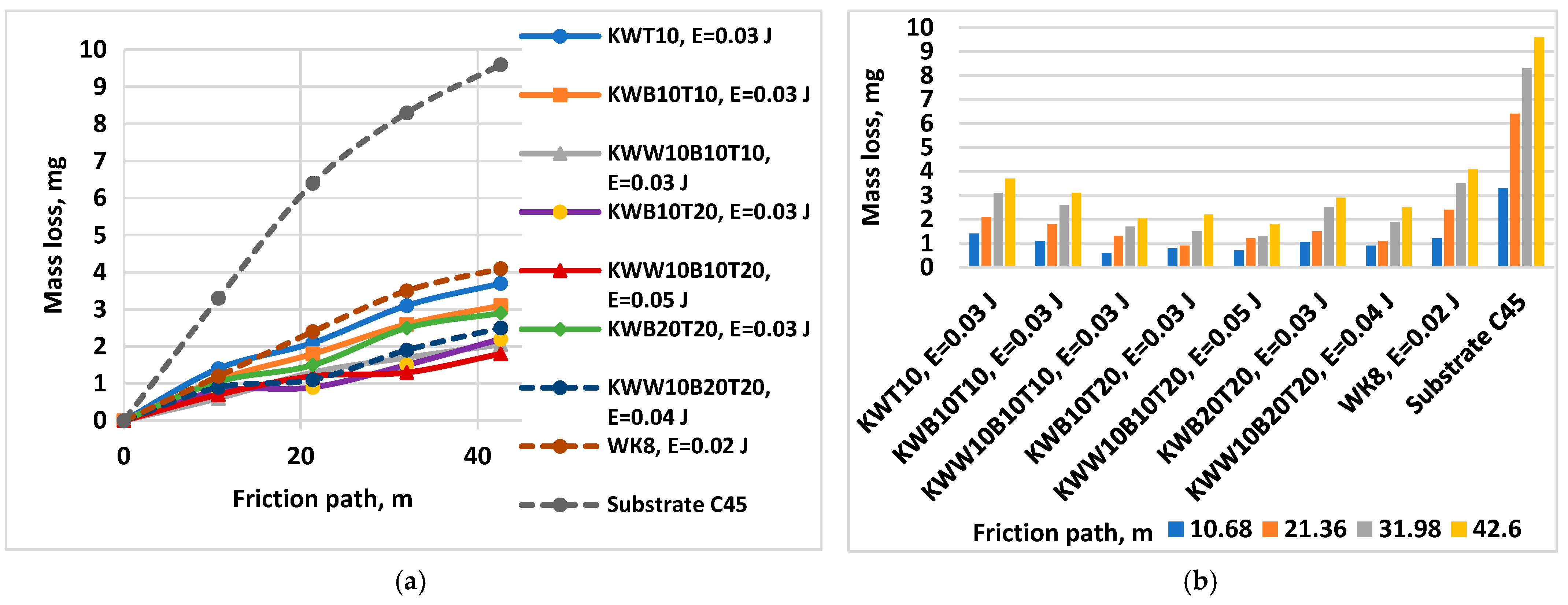
- Figure 14 shows the wear of the coatings applied with the studied electrodes at the “limit” pulse energy, with a further increase resulting in an increase in wear. The data from the figure show that the values of the limit pulse energy increase with increasing amounts of the binding metal mass in the electrode. However, for coatings from the KWT10, KWB10T10 and KWW10B10T10 electrodes, it can be assumed that the energy range at which the wear is lowest is E = 0.03–0.16 J (Figure 13a,b and Figure 14), and the range of the lowest wear of coatings from KWW10B10T20 is E = 0.03–0.07 J, and for that from electrode KWW10B20T20, the range is 0.03–0.05 J. As established above, the presence of higher amounts of the refractory high-hardness WC, B4C and TiB2 at the expense of the binding metal mass increases the hardness but also the brittleness by weakening the strength characteristics of the electrode, and as a result, the transfer of brittle fracture increases at lower pulse energies;
- -
- The analysis of the influence of the added 10% WC-Co shows that the coatings of the “KWW” electrodes demonstrate lower wear than that of the ESD with the “KW” electrodes. The comparison of the wear and wear rate (Figure 15) shows that the samples coated with KWW10T10B20 and KWW10T10B10 have at the very least wear at both load values of 5 and 10 N, up to 1.2 to 1.7 times lower than that of the samples coated with the other electrode compositions and more than 2 times lower than that of the samples coated with the classic hard alloy WK8. These results are consistent with the conclusions reached in the works of [15,29,31,32,35,44], where WC and NiCrBSi self-fluxing alloys were used for wear-resistant coatings. However, from the wear data (Figure 13, Figure 14 and Figure 15), it can be established that the differences in the wear of the samples coated with KWW10T10B20, KWW10T10B10 and KWW10T20B20 are relatively small. It is logical to expect that the increased content of B4C and TiB2 to 20% will demonstrate the lowest wear. However, in the ESD process, some of the transferred incompletely melted electrode particles of KWW10T20B20 do not adhere to the substrate, and those that do adhere do not fully embed and do not adhere well to the molten metal matrix (Figure 7a,c,d), worsening the roughness, uniformity, adhesion and structural characteristics of the coating. As a result, the wear of the KWW10T20B20-coated electrode increases by ≈10–30% compared to the minimum obtained in ESD with KWW10T10B20 but nevertheless remains up to 2.5–3.5 times lower than that of the uncoated substrate (Figure 14a and Figure 15a). The change in the wear rate is similar. Apparently, a higher concentration of high-hardness materials leads to a weakening of the bonds of the individual grains with the metal matrix in the electrode and in the coating, thereby resulting in an increase in wear; moreover, the broken-off high-hardness particles in the friction process act abrasively on the surface of the coating, contributing to its further increase.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Liu, G.; Konoplianchenko, I.E.; Tarelnyk, V.B. A review of the electro-spark deposition technology. Bull. Sumy Natl. Agrar. University. Ser. Mech. Autom. Prod. Process. 2021, 44, 45–53. [Google Scholar]
- Barile, C.; Casavola, C.; Pappalettera, G.; Renna, G.; Giovanni, P. Advancements in electrospark deposition (ESD) technique: A short review. Coatings 2022, 12, 1536. [Google Scholar] [CrossRef]
- Gitlevich, A.E.; Mikhailov, V.V.; Parkanskii, N.Y.; Revutskii, V.M. Electrospark Alloying of Metal Surfaces; Stinitsa: Chisinau, Moldova, 1985; 198p. (In Russian) [Google Scholar]
- Vizureanu, P.; Perju, M.C.; Achiţei, D.C.; Nejneru, C. Advanced Electro-Spark Deposition Process on Metallic Alloys. Adv. Surf. Eng. Res. 2008, 25, 45–68. [Google Scholar]
- Zhang, Y.; Li, L.; Chang, Q.; Wang, X.; Zhao, Y.; Zhu, S.; Xu, A.; Gao, X. Research Status and Prospect of ElectroSpark Deposition Technology. Surf. Technol. 2021, 50, 150. [Google Scholar]
- Mulin, Y.I.; Verkhoturov, A.D.; Vlasenko, V.D. Electrospark alloying of surfaces of titanium alloys. Perspect. Mater. 2006, 1, 79–85. [Google Scholar]
- Algodi, J.; Clare, A.T.; Brown, P.D. Modelling of single spark interactions during electrical discharge coating. J. Mater. Process. Technol. 2018, 252, 760–772. [Google Scholar] [CrossRef]
- Kuznetsov, I.S.; Kuznetsov, Y.A.; Kravchenko, I.N.; Kolomeichenko, A.V.; Labusova, T.A. Analytical Study of the Appearance of Heat Sources on the Surface of a Part during Electrospark Alloying. Russ. Metall. (Met.) 2020, 13, 1507–1512. (In Russian) [Google Scholar] [CrossRef]
- Liu, D.Y.; Gao, W.; Li, Z.W.; Zhang, H.F.; Hu, Z.Q. Electro-spark Deposition of Fe-based Amorphous Alloy Coatings. Mater. Lett. 2007, 61, 165–167. [Google Scholar] [CrossRef]
- Hasanabadi, F.M.; Ghaini, M.F.; Ebrahimnia, M.; Shahverdi, H.R. Production of amorphous and nanocrystalline iron based coatings by electro-spark deposition process. Surf. Coat. Technol. 2015, 270, 95–101. [Google Scholar] [CrossRef]
- Gould, J. Application of electro-spark deposition as a joining technology. Weld. J. 2011, 90, 191–197. [Google Scholar]
- Wei, X.; Chen, Z.; Zhong, J.; Xiang, Y. Feasibility of preparing Mo2FeB2-based cermet coating by electrospark deposition on high speed steel. Surf. Coat. Technol. 2016, 296, 58–64. [Google Scholar] [CrossRef]
- Petrzhik, M.; Molokanov, V.; Levashov, E. On conditions of bulk and surface glass formation of metallic alloys. J. Alloys Compd. 2017, 707, 68–72. [Google Scholar] [CrossRef]
- Cadney, S.; Brochu, M. Formation of amorphous Zr41.2Ti13.8Ni10Cu12.5Be22.5 coatings via the ElectroSpark Deposition process. Intermetallics 2008, 16, 518–523. [Google Scholar] [CrossRef]
- Nikolenko, S.V.; Kuz’menko, A.P.; Timakov, D.I.; Abakymov, P.V. Nanostructuring a steel surface by electrospark treatment with new electrode materials based on tungsten carbide. Surf. Eng. Appl. Electrochem. 2011, 47, 217–224. [Google Scholar] [CrossRef]
- Hong, X.; Tan, Y.; Zhou, C.; Xu, T.; Zhang, Z. Microstructure and tribological properties of Zr-based amorphous-nanocrystalline coatings deposited on the surface of titanium alloys by Electrospark Deposition. Appl. Surf. Sci. 2015, 356, 1244–1251. [Google Scholar] [CrossRef]
- Auchynnikau, E.; Kazak, N.; Mikhailov, V.; Ivashcu, S.; Shkurpelo, A. Tribotechnical characteristics of nanostructured coatings formed by eil method. In Proceedings of the BALTTRIB’ 2019 International scientific conference, Kaunas, Lithunia, 14–16 November 2019; pp. 114–118. [Google Scholar] [CrossRef]
- Kudryashov, A.E.; Potanin, A.Y.; Lebedev, D.N.; Sukhorukova, I.V.; Shtansky, D.V.; Levashov, E.A. Structure and properties of Cr–Al–Si–B coatings produced by pulsed electrospark deposition on a nickel alloy. Surf. Coat. Technol. 2016, 285, 278–288. [Google Scholar] [CrossRef]
- Rukanskis, M. Control of Metal Surface Mechanical and Tribological Characteristics Using Cost Effective Electro-Spark Deposition. Surf. Eng. Appl. Electrochem. 2019, 55, 607–619. [Google Scholar] [CrossRef]
- Padgurskas, J.; Kreivaitis, R.; Rukuiža, R.; Mihailov, V.; Agafii, V.; Kriukiene, R.; Baltušnikas, A. Tribological properties of coatings obtained by electro-spark alloying C45 steel surfaces. Surf. Coat. Technol. 2017, 311, 90–97. [Google Scholar] [CrossRef]
- Wang, R.J.; Qian, Y.Y.; Liu, J. Structural and Interfacial Analysis of WC92-Co8 Coating Deposited on Titanium Alloy by Electrospark Deposition. Appl. Surf. Sci. 2004, 228, 405–409. [Google Scholar] [CrossRef]
- Penyashki, T.; Kostadinov, G.; Mortev, I.; Dimitrova, E. Investigation of properties and wear of WC, TiC and TiN based multilayer coatings applied onto steels C45, 210CR12 and HS6-5-2 deposited by non-contact electrospark process. J. Balk. Tribol. Assoc. 2017, 23, 325–342. [Google Scholar]
- Aghajani, H.; Hadavand, E.; Peighambardoust, N.-S.; Khameneh-asl, S. Electro spark deposition of WC–TiC–Co–Ni cermet coatings on St52 steel. Surf. Interfaces 2020, 18, 100392. [Google Scholar] [CrossRef]
- Burkov, A.A.; Pyachin, S.A.; Zaytsev, A.V. Influence of Carbon Content of WC-Co Electrode Materials on the Wear Resistance of Electrospark Coatings. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 65–70. [Google Scholar] [CrossRef]
- Tarelnyk, V.B.; Paustovskii, A.V.; Tkachenko, Y.G.; Konoplianchenko, E.V.; Martsynkovskyi, V.S.; Antoszewski, B. Electrode Materials for Composite and Multilayer Electrospark-Deposited Coatings from Ni–Cr and WC–Co Alloys and Metals. Powder Metall. Met. Ceram. 2017, 55, 585–595. [Google Scholar] [CrossRef]
- Miller, T.; Pirolli, L.; Deng, F.; Ni, C.Y.; Teplyakov, A.V. Structurally different interfaces between electrospark deposited titanium carbonitride and tungsten carbide films on steel. Surf. Coat. Technol. 2014, 258, 814–821. [Google Scholar] [CrossRef]
- Zamulaeva, E.I.; Levashov, E.A.; Eremeeva, Z.B.; Kudryashov, A.E. Carbon-containing and nanostructured WC-Co electrodes for electrospark modification of the surface of titanium alloys. Met. Technol. 2008, 11, 24–31. [Google Scholar]
- Tarelnyk, V.; Gaponova, O.; Myslyvchenko, O.; Sarzhanov, B. Electrospark deposition of multilayer coatings. Powder Metall. Metal Ceram. 2020, 59, 76–88. [Google Scholar] [CrossRef]
- Kandeva, M.; Penyashki, T.; Kostadinov, G.; Nikolova, M.; Grozdanova, T. Wear resistance of multi-component composite coatings applied by concentrating energy flows. In Proceedings of the Engineering Sciences-PES Journal-16th International Conference on Tribology, Kragujevac, Serbia, 15–17 May 2019; Volume 1, pp. 197–207. [Google Scholar]
- Paustovskii, A.V.; Tkachenko, Y.G.; Alfintseva, R.A.; Kirilenko, S.N.; Yurchenko, D.Z. Optimization of the composition, structure and properties of electrode materials and electrospark coatings during the hardening and restoration of metal surfaces. Surf. Eng. Appl. Electrochem. 2013, 49, 4–13. [Google Scholar] [CrossRef]
- Mihailov, V.; Kazak, N.; Ivashcu, S.; Ovchinnikov, E.; Baciu, C.; Ianachevici, A.; Rukuiza, R.; Zunda, A. Synthesis of Multicomponent Coatings by Electrospark Alloying with Powder Materials. Coatings 2023, 13, 651. [Google Scholar] [CrossRef]
- Penyashki, T.G.; Radev, D.D.; Kandeva, M.K.; Kostadinov, G.D. Structural and Tribological Properties of Multicomponent Coatings on 45 and 210Cr12 Steels Obtained by Electrospark Deposition with WC-B4C-TiB2–Ni-Cr-Co-B-Si Electrodes. Surf. Eng. Appl. Electrochem. 2019, 55, 638–650. [Google Scholar] [CrossRef]
- Verkhoturov, A.D.; Nikolenko, S.V. Classifications. Development and creation of electrode materials for electrospark alloying. Hardening Technol. Coat. 2010, 2, 13–22. [Google Scholar]
- Penyashki, T.; Kostadinov, G.; Kandeva, M.; Kamburov, V.; Nikolov, A. Abrasive and Erosive Wear of TI6Al4V Alloy with Electrospark Deposited Coatings of Multicomponent Hard Alloys Materials Based of WC and TiB2. Coatings 2023, 13, 215. [Google Scholar] [CrossRef]
- Nikolenko, V.; Verkhoturov, A.D.; Syui, N. A Generation and study of new electrode materials with self-fluxing additives to improve the efficiency of mechanical electrospark alloying. Surf. Eng. Appl. Electrochem. 2015, 51, 38–45. [Google Scholar] [CrossRef]
- Kandeva, M.; Svoboda, P.; Kalitchin, Z.; Penyashki, T.; Kostadinov, G. Wear of Gas-flame Composite Coatings with Tungsten and Nickel Matrix. Part II. Erosive Wear. J. Environ. Prot. Ecol. 2019, 20, 1292–1302. [Google Scholar]
- Garrido, M.A.; Rico, A.; Gómez, M.T.; Fernández-Rico, J.E.; Rodríguez, J. Tribological and Oxidative Behavior of Thermally Sprayed NiCrBSi Coatings. J. Therm. Spray Technol. 2017, 26, 517–529. [Google Scholar] [CrossRef]
- Houdková, S.; Smazalová, E.; Vostřák, M.; Schubert, J. Properties of NiCrBSi Coating, as Sprayed and Remelted by Different Technologies. Surf. Coat. Technol. 2014, 253, 14–26. [Google Scholar] [CrossRef]
- Koga, G.Y.; Bolfarini, C.; Kiminami, C.S.; Jorge, A.M.; Botta, W.J. An Overview of Thermally Sprayed Fe-Cr-Nb-B Metallic Glass Coatings: From the Alloy Development to the Coating’s Performance Against Corrosion and Wear. J. Therm. Spray Technol. 2022, 31, 923–955. [Google Scholar] [CrossRef]
- Alontseva, D.; Missevra, S.; Russakova, A. Characteristics of Structure and Properties of Plasma—Detonated Ni-Cr and Co-Cr Based Powder Alloys Coatings. J. Mater. Sci. Eng. 2013, 1, 41–49. [Google Scholar]
- Schubert, J.; Houdková, J.Š.; Kašparová, M.; Česánek, Z. Effect of Co content on the properties of HVOF sprayed coatings based on tungsten carbide. In Proceedings of the 21st International Conference on Metallurgy and Materials–Metal 2012, Brno, Czech Republic, 23–25 May 2012; Volume 1086. [Google Scholar]
- Fanicchi, F.; Axinte, D.A.; Kell, J.; Brewster, G.; Norton, A.D. Combustion Flame Spray of CoNiCrAl coatings. Surf. Coat. Technol. 2017, 315, 546–557. [Google Scholar] [CrossRef]
- Vivek, D.; Kalyankar, P.; Wanare, S. Comparative Investigations on Microstructure and Slurry Abrasive Wear Resistance of NiCrBSi and NiCrBSi-WC Composite Hardfacings Deposited on 304 Stainless Steel. Tribol. Ind. 2022, 44, 199–211. [Google Scholar]
- Vencl, A.; Mrdak, M.; Hvizdos, P. Tribological Properties of WC-Co/NiCrBSi and Mo/NiCrBSi Plasma Spray Coatings under Boundary Lubrication Conditions. Tribol. Ind. 2017, 39, 183–191. [Google Scholar] [CrossRef]
- Umansky, A.P.; Storozhenko, M.S.; Akopyan, V.V.; Martsenyuk, I.S. Electrospark strengthening of steel by composite materials of the TiB2-(Fe-Mo) system. Aviat. Space Eng. Technol. 2012, 96, 214–218. [Google Scholar]
- Podchernyaeva, A.; Verkhoturov, A.D.; Panashenko, V.M.; Konevtsov, L.A. Electric spark surface hardening and integrated surface hardening of titanium. Study Notes 2014, 17, 73–87. [Google Scholar]
- Kazamer, N.; Valean, P.C.; Pascal, D.; Serban, V.A.; Muntean, R.; Margineal, G. Microstructure and phase composition of NiCrBSi–TiB2 vacuum furnace fused flame-sprayed coatings. IOP Conf. Ser. Mater. Sci. Eng. 2018, 416, 012001. [Google Scholar] [CrossRef]
- Umanskii, A.P.; Storozhenko, M.S.; Hussainova, I.; Terentiev, A.E.; Kovalchenko, A.M.; Antonov, M.M. Structure, phase composition and wear mechanisms of plasma-sprayed NiCrBSi–20 wt.% TiB2 coating. Powder Metall. Met. Ceram. 2015, 53, 663–671. [Google Scholar] [CrossRef]
- ISO 21920:2021; Geometrical Product Specifications (GPS)—Surface Texture: Profile—Part 1: Indication of Surface Texture. ISO: Geneva, Switzerland, 2021.
- Jönsson, B.; Hogmark, S. Hardness Measurements of Thin Films. Thin Solid Film. 1984, 114, 257. [Google Scholar] [CrossRef]
- Myasoedova, T.N.; Kalusulingam, R.; Mikhailova, T.S. Sol-Gel Materials for Electrochemical Applications: Recent Advances. Coatings 2022, 12, 1625. [Google Scholar] [CrossRef]





| Element, wt.% | C | Si | Cr | Fe | B | Co | Ni | Designation of Composition |
|---|---|---|---|---|---|---|---|---|
| CoNiCrBSi | 1.5 | 1.5 | 23 | 0.5 | 1.5 | 40 | balance | KW = 45%CoNiCrBSi + 55%WC |
| Designation/Composition, wt.% | KW | WC-Co8 | TiB2 | B4C |
|---|---|---|---|---|
| KWT10 | 90 | - | 10 | - |
| KWB10T10 | 80 | - | 10 | 10 |
| KWW10B10T10 | 70 | 10 | 10 | 10 |
| KWB10T20 | 70 | - | 20 | 10 |
| KWW10B10T20 | 50 | 10 | 20 | 20 |
| KWB20T20 | 60 | - | 20 | 20 |
| KWW10B20T20 | 50 | 10 | 20 | 20 |
| WK8 | 92/8 |
| № of Regimes | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Capacity, C, µF | 5 | 10 | 15 | 20 | 50 | 100 |
| Pulse energy E, J | 0.02 | 0.03 | ≈0.05 | ≈0.07 | ≈0.16 | ≈0.3 |
| № | Electrode, E = 0.03–0.3 J | Ra, μm | δ, μm | Hv, GPa | Coeff. of Hardening |
|---|---|---|---|---|---|
| 1 | WK8 | 1.8–5.5 | 14–42 | 7.8–12 | 3–5 |
| 2 | KWT10 | 2.5–5.3 | 19–66 | 8–12 | 3–5 |
| 3 | KWB10T10 | 2.5–5.3 | 18–63 | 8.3–12.5 | 3–5.3 |
| 4 | KWW10B10T10 | 2.3–5.6 | 16–59 | 8.8–13 | 3.5–5.6 |
| 5 | KWB10T20 | 2.2–5.5 | 16–56 | 8.8–13 | 3.5–5.6 |
| 6 | KWW10B10T20 | 2.2–5.9 | 20–53 | 9.5–14.5 | 3.6–6.2 |
| 7 | KWB20T20 | 2.1–5.8 | 18–55 | 9–14 | 3.8–6 |
| 8 | KWW10B20T20 | 2.1–6.8 | 18–55 | 9.5–15 | 3.8–6.4 |
| Electrode, Impulse Energy, J | Roughness and Thickness δ of the Coating After 3 Electrode Passes | Average Thickness | Cathode Growth, mg/cm2 | |||||
|---|---|---|---|---|---|---|---|---|
| Roughness, µm | Thickness δ, µm | µm | mg/cm2 | |||||
| Ra | Rz | Rq | Rt | min | max | |||
| Substrate | 2.38 | 9.56 | 2.7 | 9.65 | - | - | - | - |
| KWW10B20T20, E = 0.07 | 4.84 | 14.12 | 5.50 | 17.63 | 27.6 | 35.5 | 30 | 0.67 |
| Deviation, ±, µm | 0.88 | 2.53 | 0.82 | 3.72 | +5.5, −2.4 | 0.065 | ||
| KWB20T20, E = 0.07 | 4.46 | 12.72 | 5.16 | 15.58 | 30.2 | 38.1 | 33 | 0.83 |
| Deviation, ±, µm | 0.7 | 1.92 | 0.72 | 1.56 | +5.1, −2.8 | 0.067 | ||
| KWW10B10T10, E = 0.07 | 4.29 | 12.62 | 4.67 | 14.63 | 33.6 | 41.9 | 36 | 1.2 |
| Deviation, ±, µm | 0.53 | 1.22 | 0.65 | 1.31 | +5.9, −2.4 | 0.105 | ||
| KWW10B10T10, E = 0.05 | 4.04 | 11.8 | 4.44 | 13.48 | 23.7 | 30.3 | 28 | 0.88 |
| Deviation, ±, µm | 0.45 | 0.85 | 0.33 | 0.99 | +2.3, −4.3 | 0.077 | ||
| KWB10T10, E = 0.07 | 3.87 | 12.2 | 3.6 | 14.11 | 35.6 | 43.5 | 40 | 1.43 |
| Deviation, ±, µm | 0.49 | 1.22 | 0.53 | 1.43 | +3.5, −4.4 | 0.127 | ||
| KWB10T10, E = 0.05 | 3.38 | 9.56 | 3.7 | 11.65 | 26.5 | 35.9 | 32 | 1.05 |
| Deviation, ±, µm | 0.38 | 1.05 | 0.45 | 1.24 | +3.9, −5.5 | 0.091 | ||
| Phases in Coatings | 2θ Angle; ° |
|---|---|
| Predominant phases | |
| Fe | 44.5; 63; 65.18; 82.35 |
| Cr-Ni-Fe-C | 43.3; 50.6; 63; 74.06 |
| Cr-Ni-Fe | 44.45; 64.84 |
| WC1−x | 36.9; 43.08; 62.1; 64.85; 74.22; 78.34; 81.8 |
| TiB2 | 27.8; 34.1; 44.4; 61.16;68.1 |
| FeNiCo | 44.6; 52.4; 64.5; 74.5 |
| In small quantities and traces | |
| B4C | 22; 31.9; 34.9; 41.9; 50.6; 71.89; 88.6 |
| Co | 41.4; 44.2; 45.34; 47.3; 76.1 |
| FeNi | 44.1; 52.5; 64.5; 74.7; 85; 90.6 |
| Sintered new phases In small quantities and traces | |
| TiB | 36.65; 37.05; 42.95; 61.95; 73.7; 78.7; |
| WB | 21.25;29.9; 36.2; 42.24; 60.12; |
| FeSi | 27.5; 44.75; 49; 57.1; 65.1; |
| Fe1.88C0.14 | 43.4; 45; 60.7; 65.65; 81.88; |
| FeCr | 31.3; 44.8; 55.5; 65.25; 74.9; |
| Fe2B | 28.53; 29.86; 45.03 |
| FeB | 37.7; 41.2 |
| FeNi3 | 38.15; 44.12; 51.4 |
| Fe0.7N0.3 | 45.1; 65; 81.88 |
| Fe3W3C | 42.4; 46.5; 52.3; 59.5; 72.3 |
| W2CoB2 | 43.2; 74.1 |
| Cr3B4 | 23.55; 30.5; 36.87; 41.7; 46.1; 50.8; 62.8 |
| Co3Ti | 35.45; 43.55; 50.8; 63.2; 74.7 |
| TiN0.3 | 34.86; 37.65; 39.85; 42.78; 52.22; 62.5; 75.34 |
| Ti4N3B2 | 37.05; 42.95; 62.25; 74.55; 78.5 |
| Ti2O | 35.65; 37.05; 38.25; 40.6; 63.8; 70.50; 78.5 |
| Ti6O | 34.95; 38; 39.93; 52.5; 69.9 |
| Fe2O3 | 33.3; 35.7; 49.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penyashki, T.; Kostadinov, G.; Kandeva, M. Improvement of Surface Properties of Carbon Steel Through Electrospark Coatings from Multicomponent Hard Alloys. Materials 2025, 18, 2211. https://doi.org/10.3390/ma18102211
Penyashki T, Kostadinov G, Kandeva M. Improvement of Surface Properties of Carbon Steel Through Electrospark Coatings from Multicomponent Hard Alloys. Materials. 2025; 18(10):2211. https://doi.org/10.3390/ma18102211
Chicago/Turabian StylePenyashki, Todor, Georgi Kostadinov, and Mara Kandeva. 2025. "Improvement of Surface Properties of Carbon Steel Through Electrospark Coatings from Multicomponent Hard Alloys" Materials 18, no. 10: 2211. https://doi.org/10.3390/ma18102211
APA StylePenyashki, T., Kostadinov, G., & Kandeva, M. (2025). Improvement of Surface Properties of Carbon Steel Through Electrospark Coatings from Multicomponent Hard Alloys. Materials, 18(10), 2211. https://doi.org/10.3390/ma18102211






