Decarburization and Its Effects on the Properties of Plasma-Nitrided AISI 4140 Steel: A Review
Abstract
1. Introduction
2. Surface Decarburization in Plasma-Free Environments
2.1. Properties of Steel Surface and Near-Surface Regions
2.1.1. Hydrogen-Containing Environments
2.1.2. Oxygen-Containing Environments
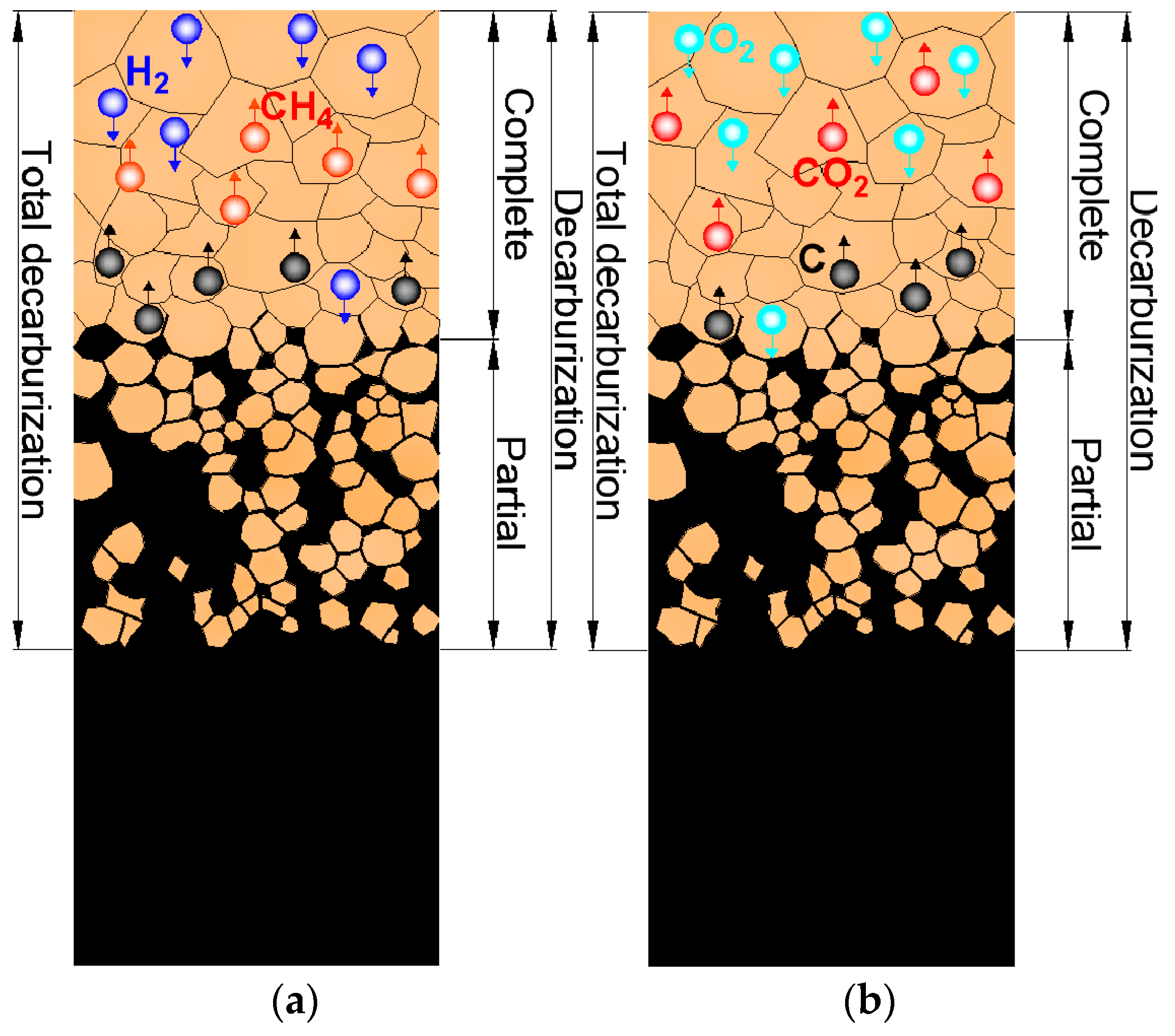
2.2. Complete and Partial Surface Decarburization
2.3. Prevention of the Surface Decarburization Effect
2.3.1. Packaging
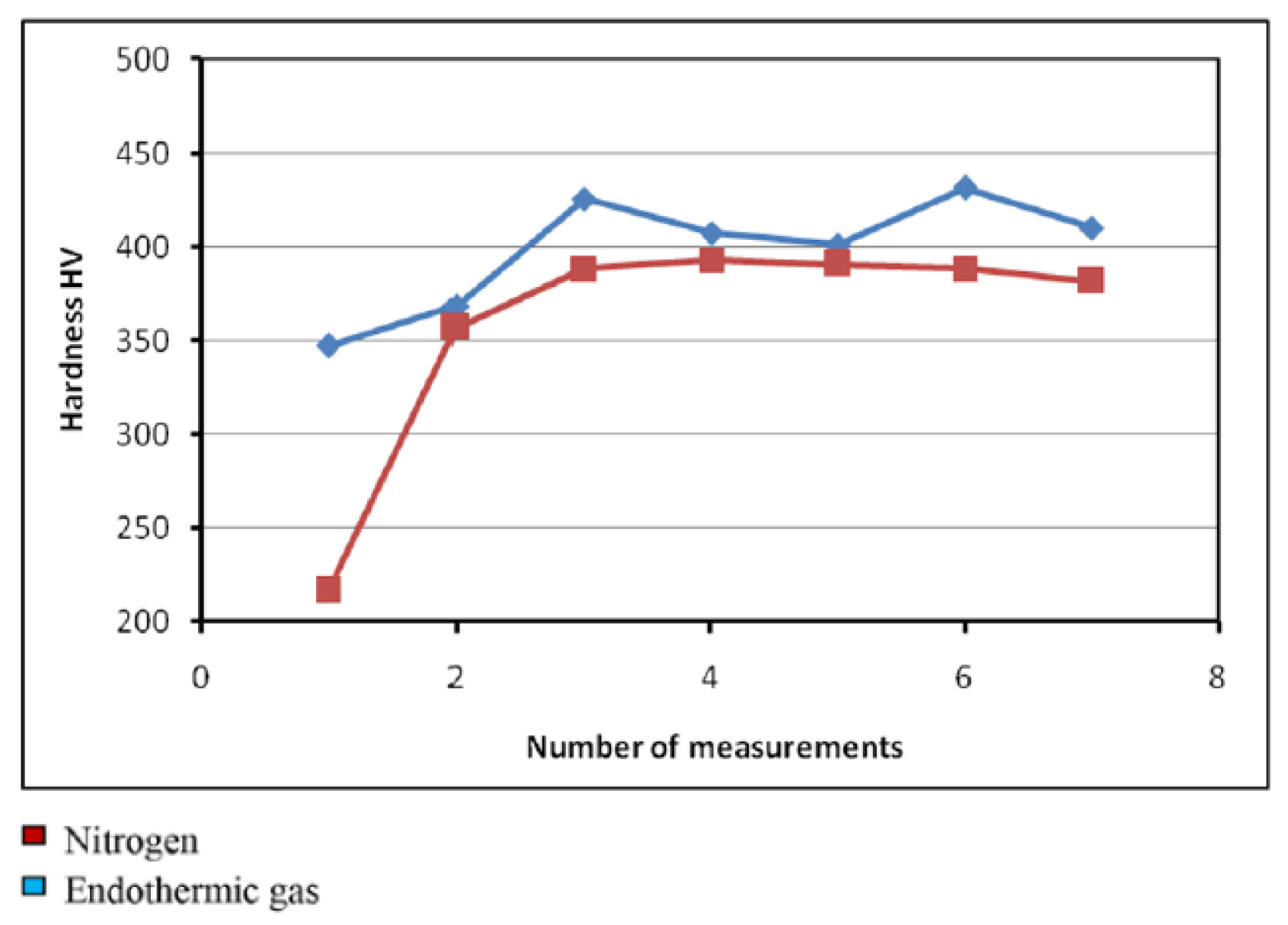
2.3.2. Protective Gases
3. Surface Decarburization in Plasma-Containing Environments
3.1. Plasma-Cleaning and Plasma-Nitriding Environments
3.1.1. Surface Decarburization in Plasma-Cleaning Environments
3.1.2. Surface Decarburization in Plasma Nitriding Environments
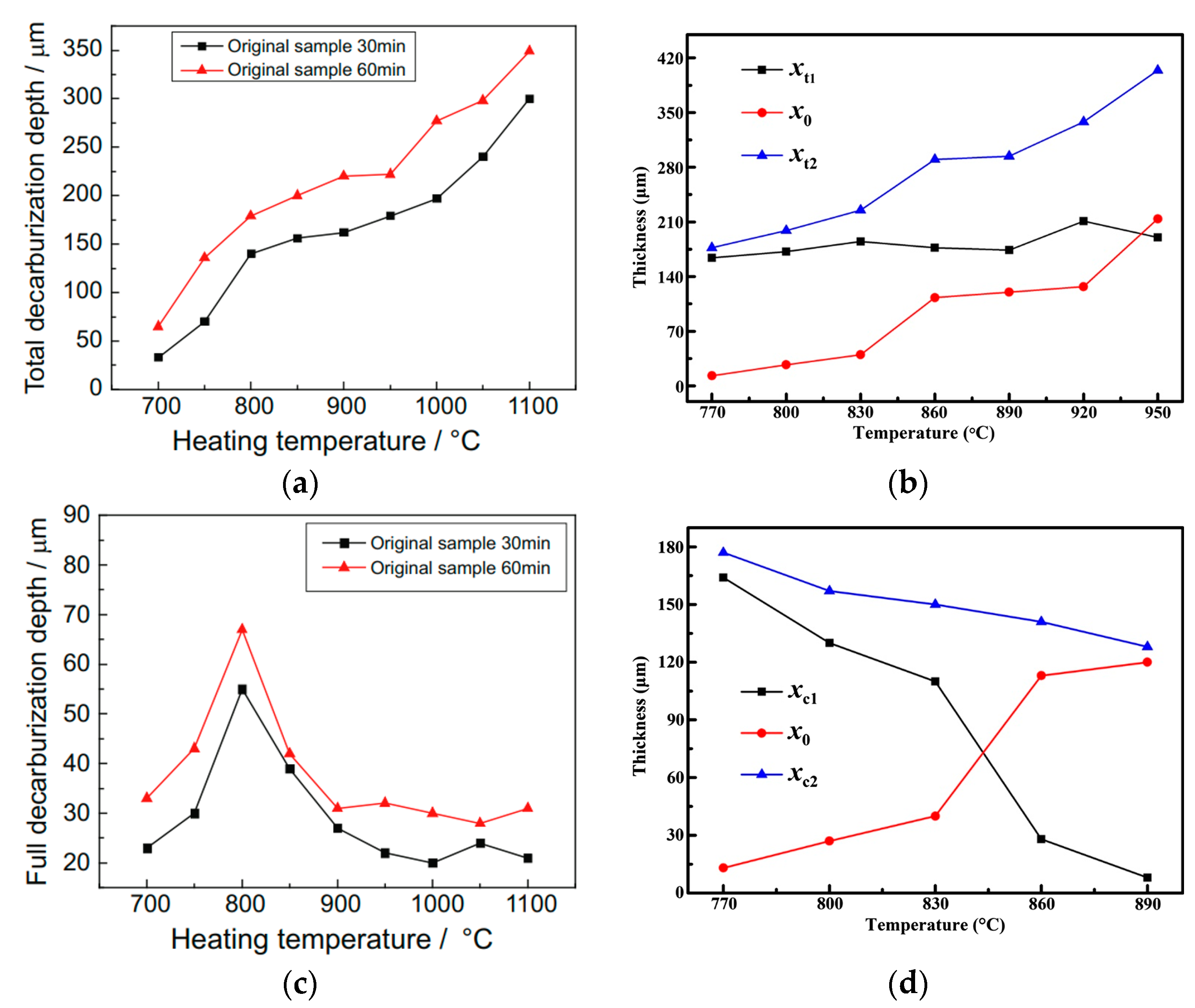
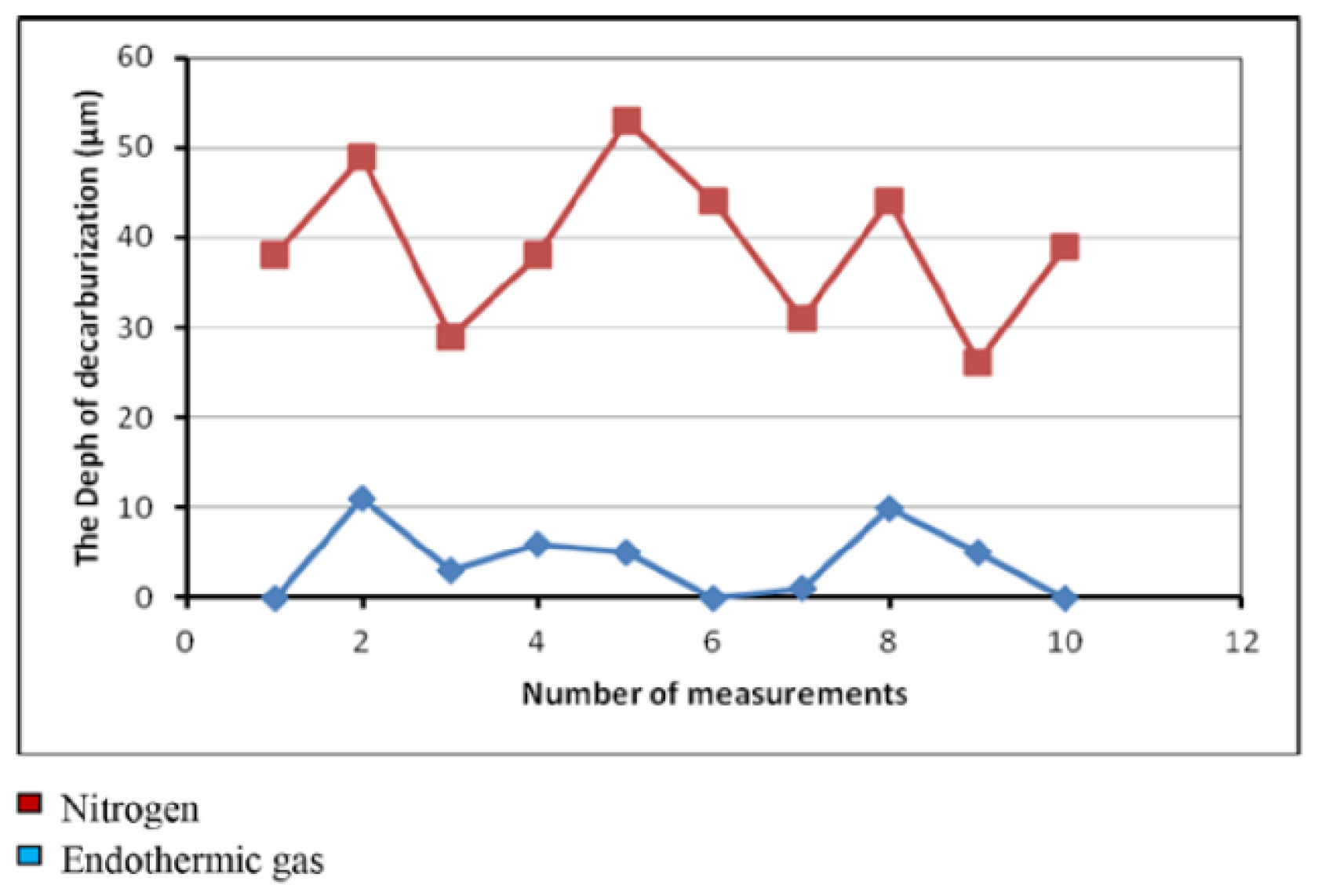
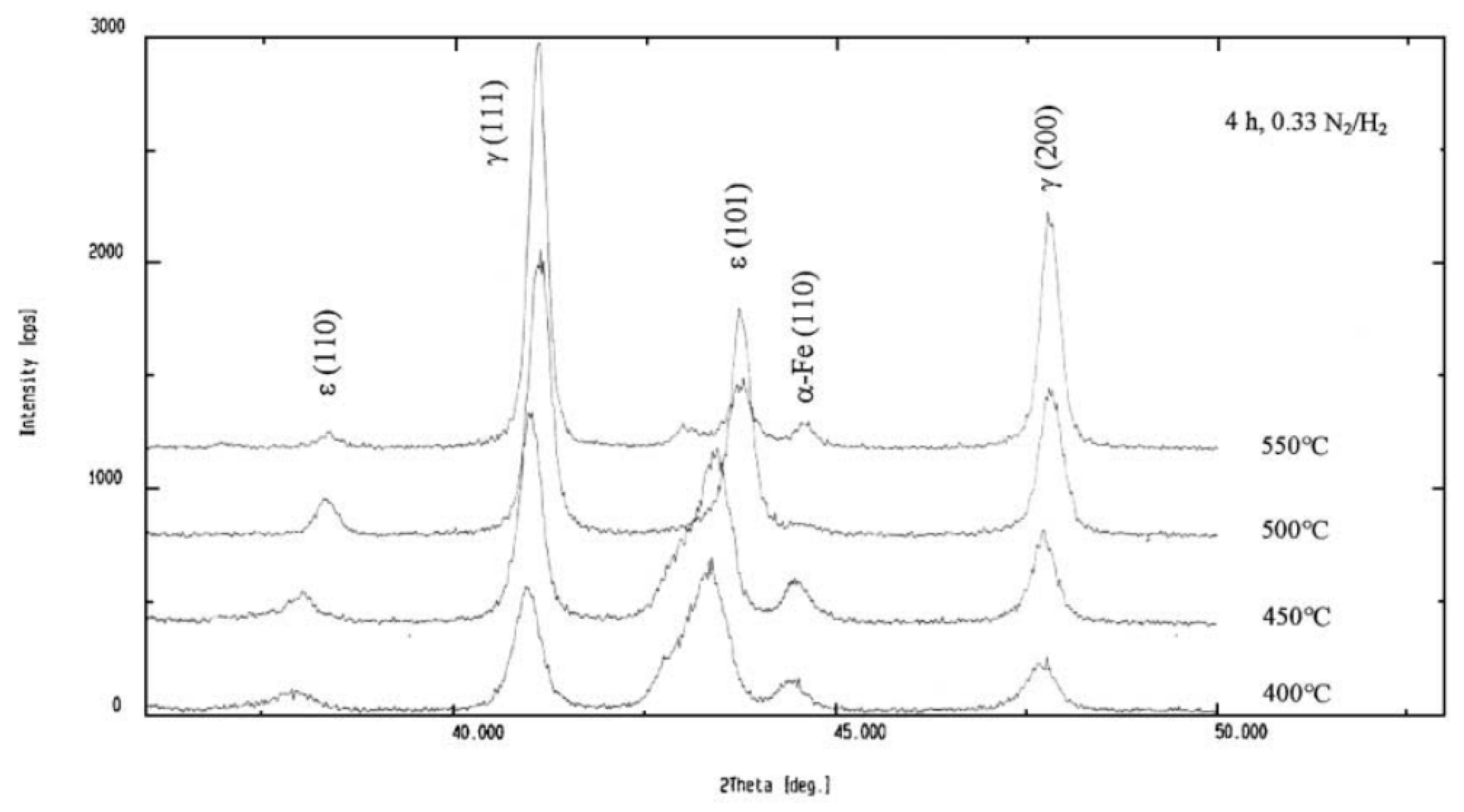
3.2. Effects of Temperature, Time, and Nitriding Potential on Surface Decarburization Phenomenon During Plasma Nitriding
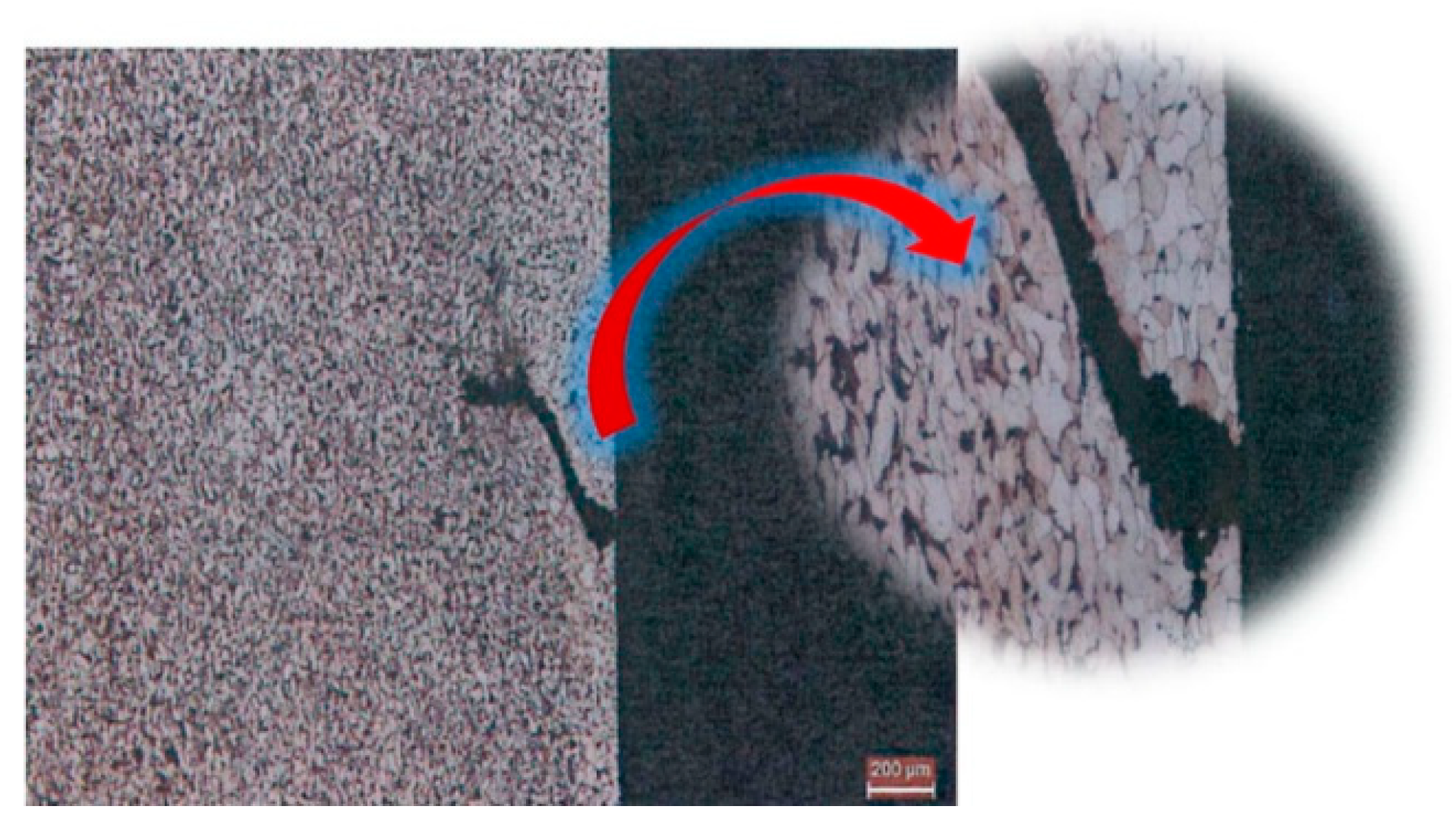
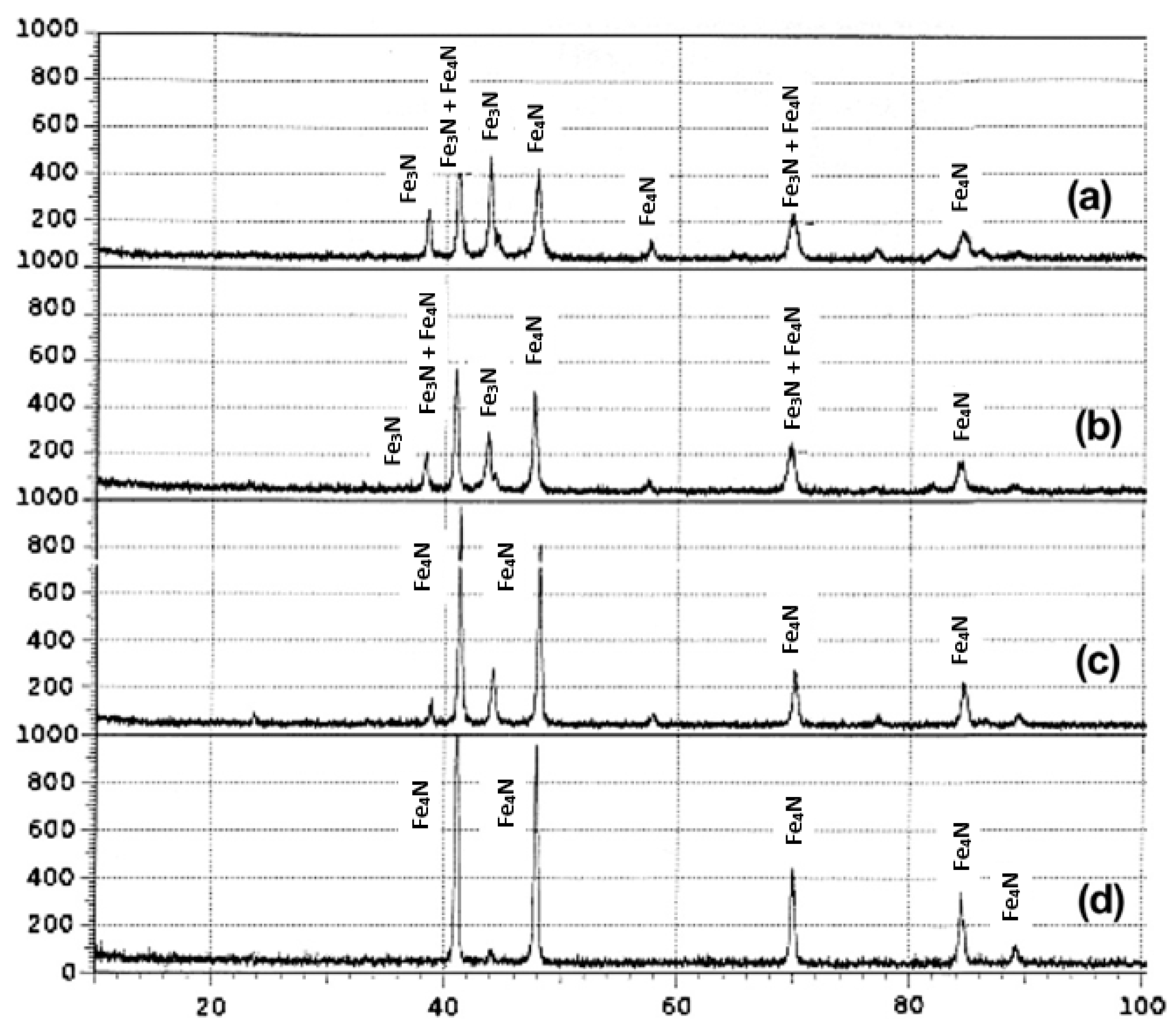
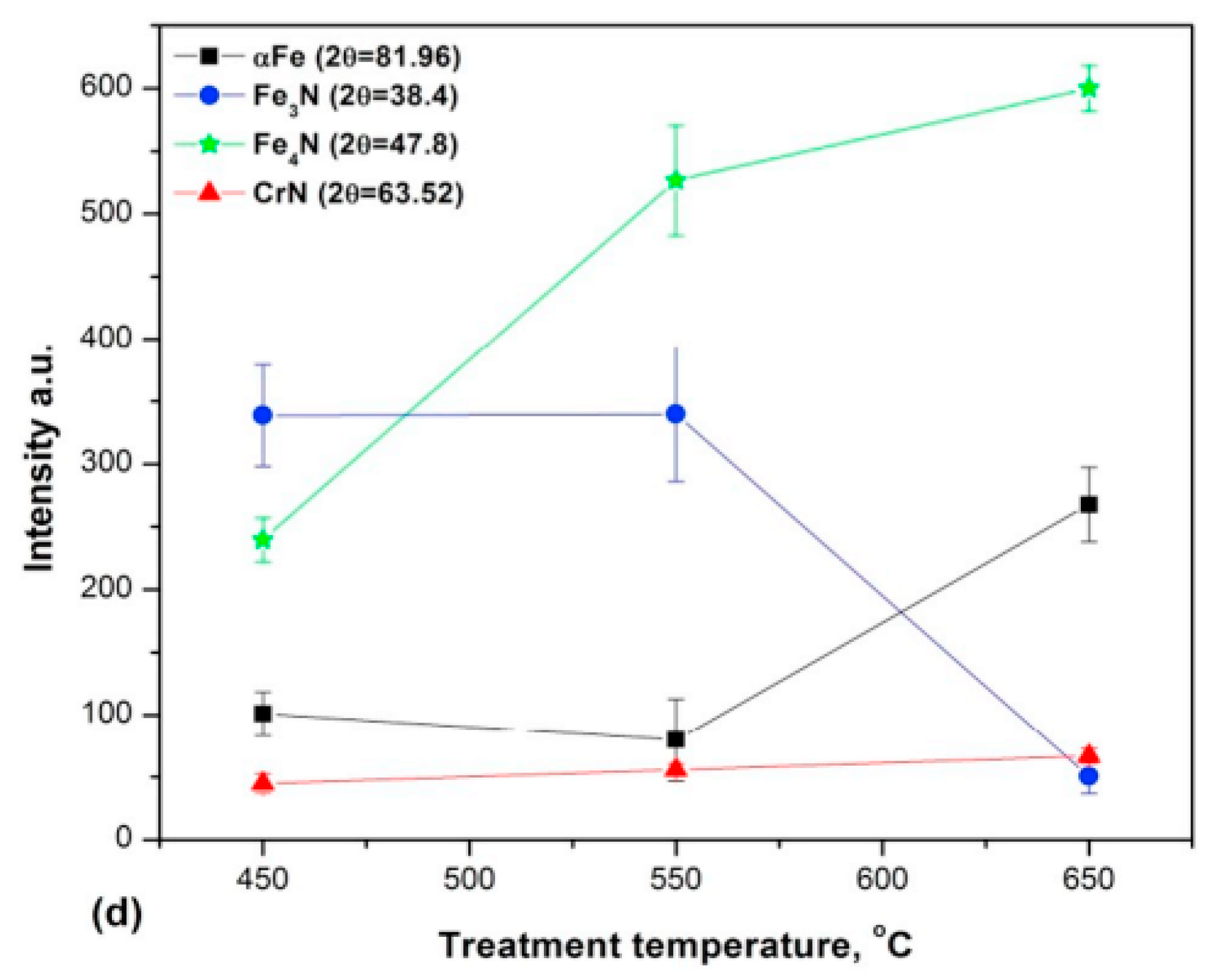
3.3. Carbon-Rich Zone at the Nitriding Front During Plasma Nitriding
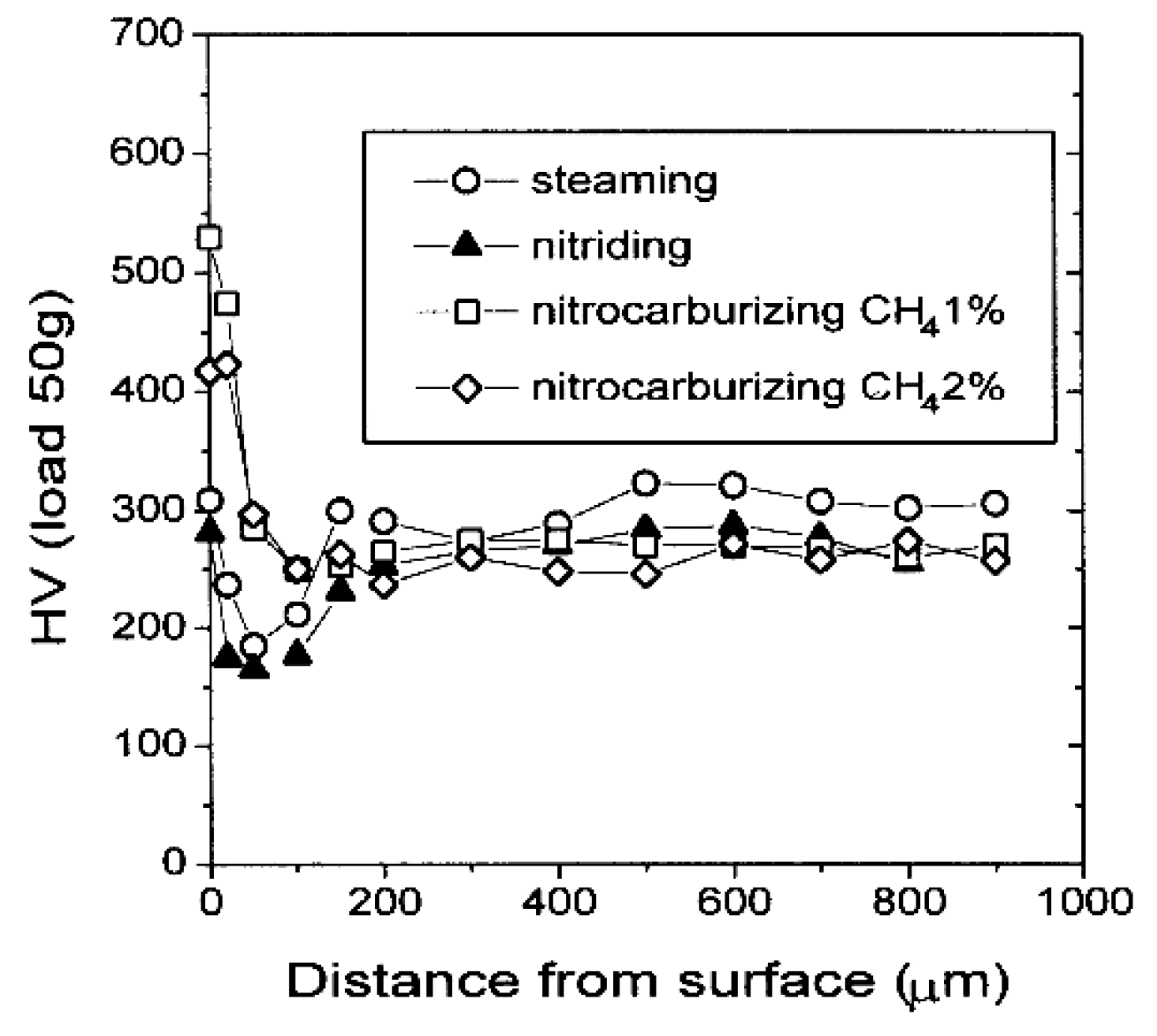
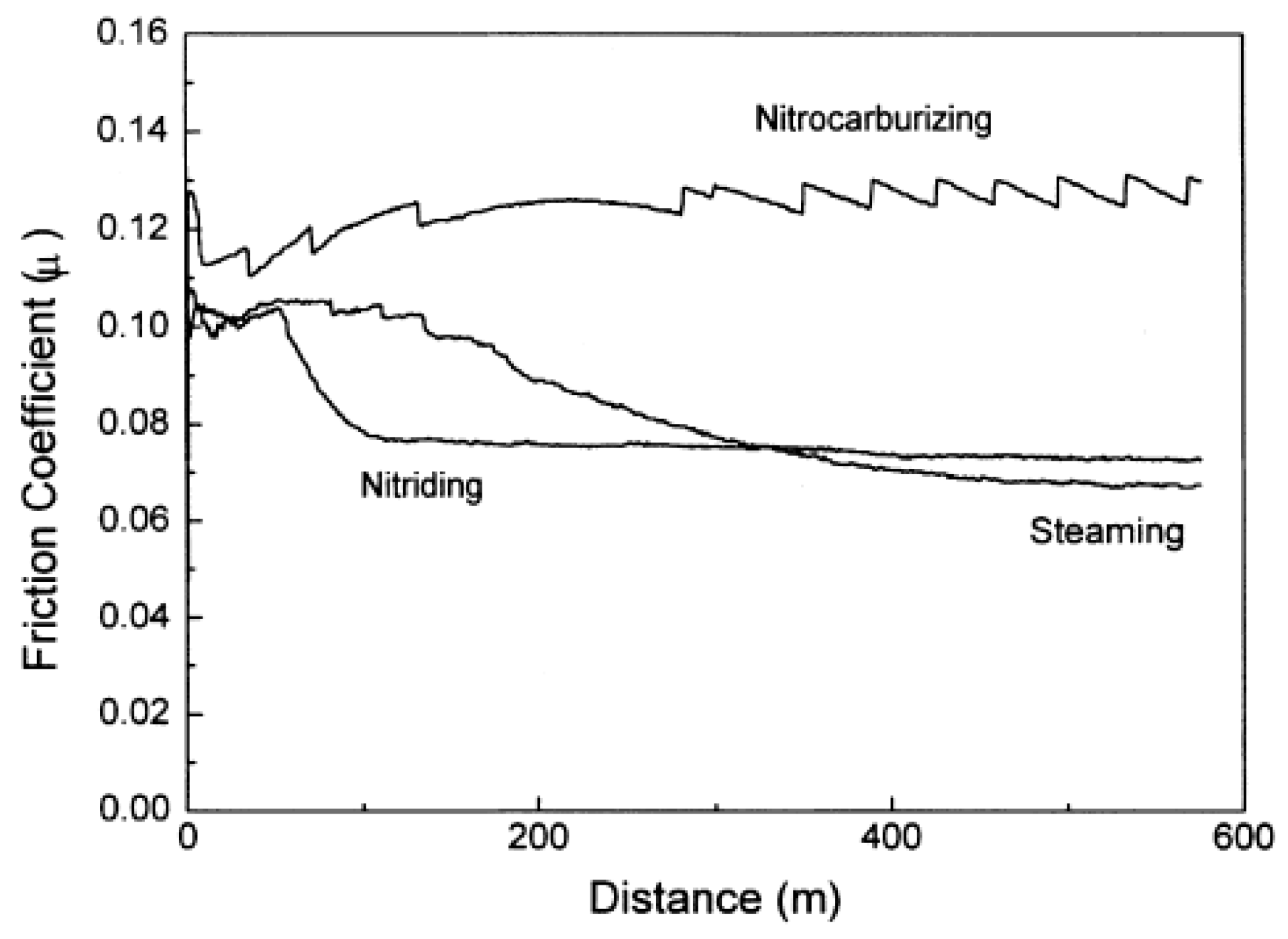
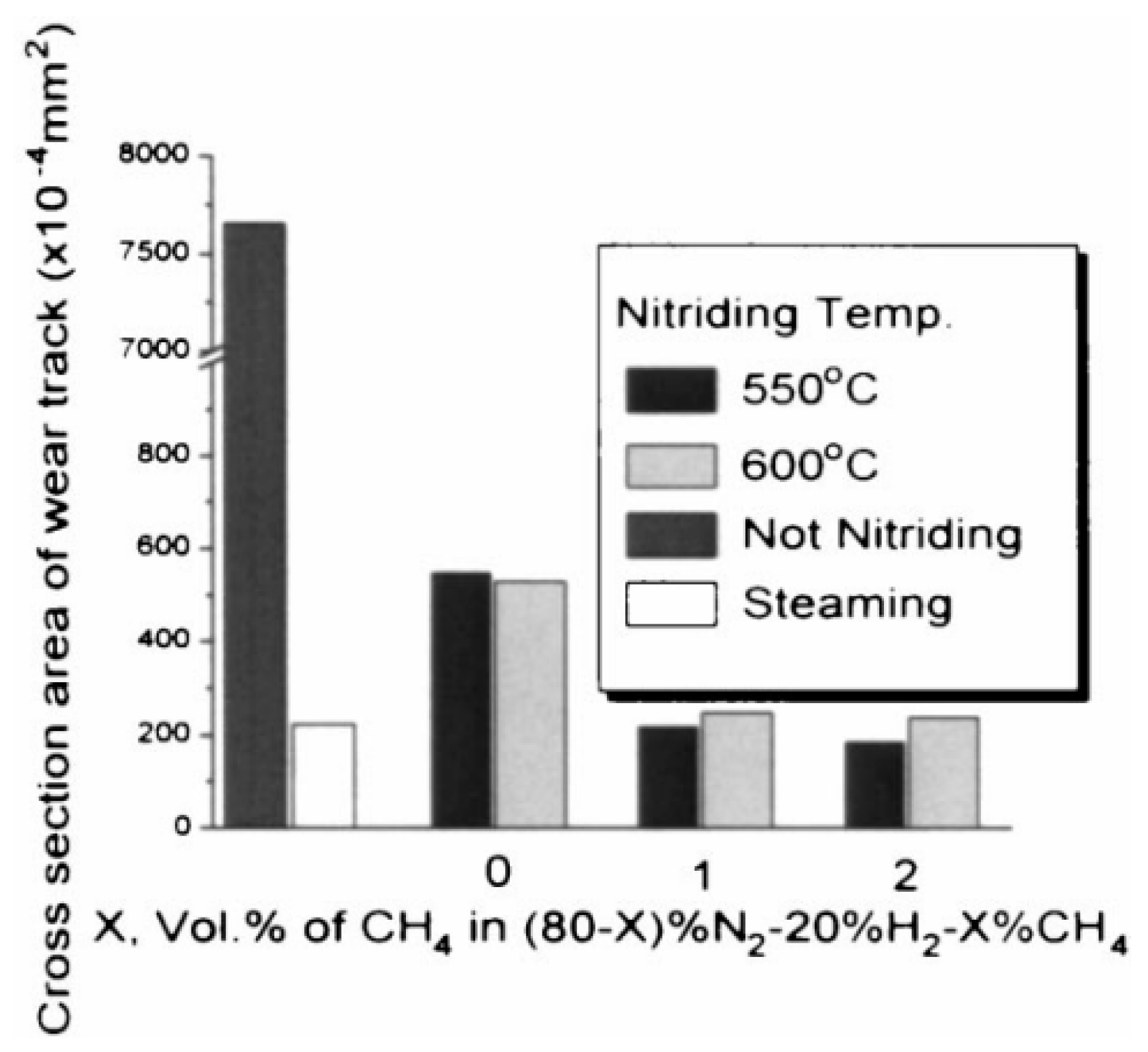
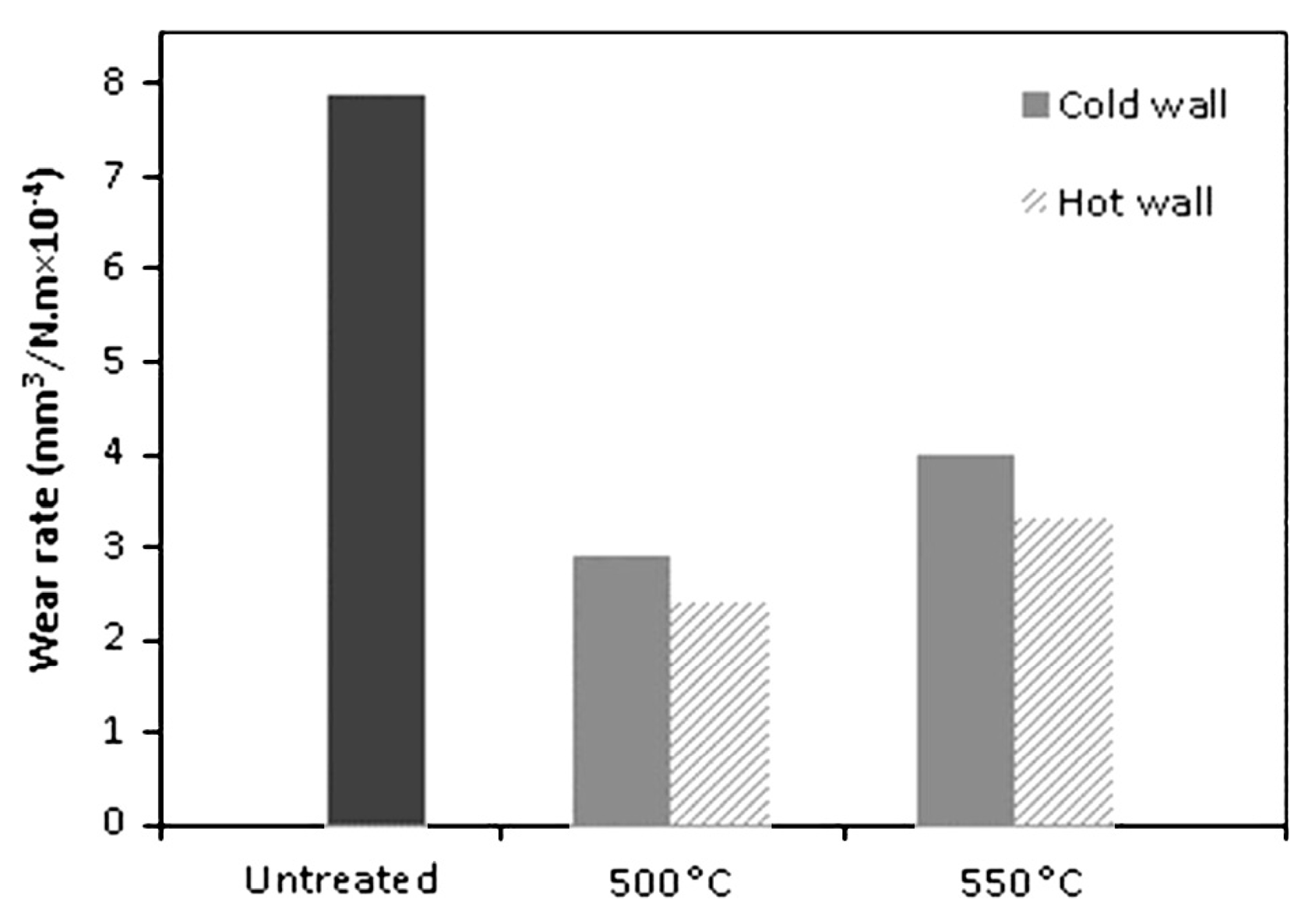
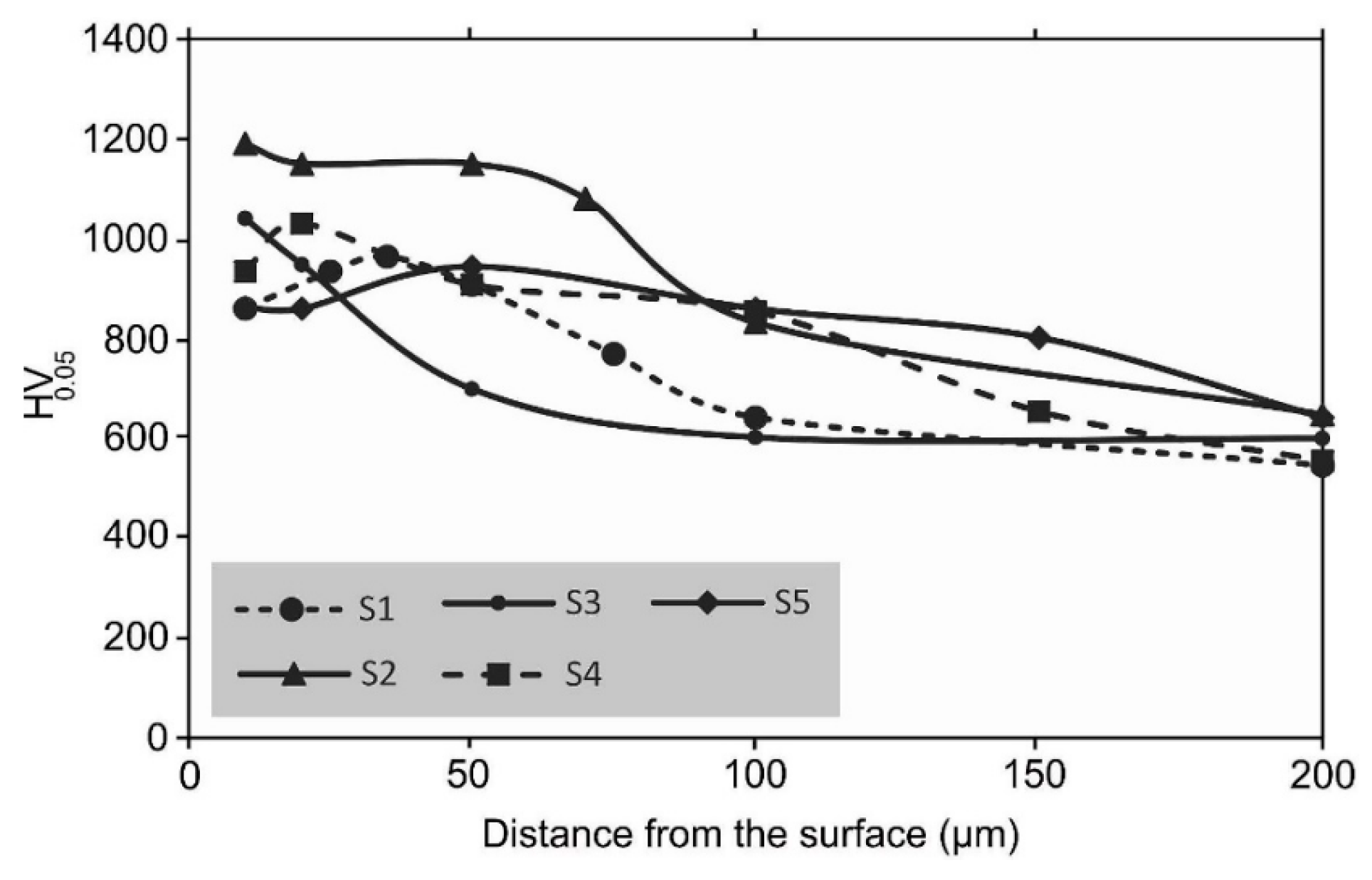
4. Influence of Plasma-Nitriding Parameters on Surface Hardness, Corrosion Performance, and Tribological Behavior of AISI 4140 and Similar Steel Grades
5. Future Directions
6. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.-S.; Yuan, W.-Q.; Lin, Y.C.; Li, H.-B.; Zou, Z.-H. Modeling and Simulation of Dynamic Recrystallization Behavior for 42CrMo Steel by an Extended Cellular Automaton Method. Vacuum 2017, 146, 142–151. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Fan, S.; Yan, G. Study on the Material Characteristic and Process Parameters of the Open-Die Warm Extrusion Process of Spline Shaft with 42CrMo Steel. J. Alloys Compd. 2013, 571, 12–20. [Google Scholar] [CrossRef]
- Quan, G.-Z.; Li, G.-S.; Chen, T.; Wang, Y.-X.; Zhang, Y.-W.; Zhou, J. Dynamic Recrystallization Kinetics of 42CrMo Steel during Compression at Different Temperatures and Strain Rates. Mater. Sci. Eng. A 2011, 528, 4643–4651. [Google Scholar] [CrossRef]
- Flori, M.; Gruzza, B.; Bideux, L.; Monier, G.; Robert-Goumet, C.; Benamara, Z. First Stages of Surface Steel Nitriding: X-Ray Photoelectron Spectroscopy and Electrical Measurements. Appl. Surf. Sci. 2009, 255, 9206–9210. [Google Scholar] [CrossRef]
- Li, C.X.; Dong, H.; Bell, T. A Feasibility Study of Plasma Nitriding of Steel with an Oxide Layer on the Surface. J. Mater. Sci. 2006, 41, 6116–6118. [Google Scholar] [CrossRef]
- Prawoto, Y.; Mat Yajid, M.A.; Lee, K.J. Microstructural Consideration on Quantitative Analysis of Thermal Treatment: Application to Decarburization of Steel. J. King Saud. Univ. Eng. Sci. 2013, 25, 141–147. [Google Scholar] [CrossRef]
- Wang, X.-W.; Hu, Q.-F.; Zhang, C.-L.; Chen, L.; Zhu, C.-Y.; Tao, B.; Jiang, B.; Liu, Y.-Z. Optimization of Heat Treatment for 38Si7 Spring Steel with Excellent Mechanical Properties and Controlled Decarburization. Materials 2022, 15, 3763. [Google Scholar] [CrossRef]
- Snoek, J.L. On the Decarburization of Steel and Related Questions. Physica 1941, 8, 734–744. [Google Scholar] [CrossRef]
- Ramezani, M.; Pasang, T.; Chen, Z.; Neitzert, T.; Au, D. Evaluation of Carbon Diffusion in Heat Treatment of H13 Tool Steel under Different Atmospheric Conditions. J. Mater. Res. Technol. 2015, 4, 114–125. [Google Scholar] [CrossRef]
- Taskan, Z.; Ozturk, E.; Tezcan, S.; Mindivan, H. Influence of plasma nitriding on tribological performance of HVOF sprayed AISI 316L and AISI 420 stainless steel coatings. Tribol. Mater. 2024, 3, 187–196. [Google Scholar] [CrossRef]
- Lampe, T.; Eisenberg, S.; Laudien, G. Compound Layer Formation during Plasma Nitriding and Plasma Nitrocarburising. Surf. Eng. 1993, 9, 69–76. [Google Scholar] [CrossRef]
- ASM International. ASM Handbook; ASM International: Materials Park, OH, USA, 1991. [Google Scholar]
- Maharjan, N.; Zhou, W.; Zhou, Y.; Wu, N. Decarburization during Laser Surface Processing of Steel. Appl. Phys. A 2018, 124, 682. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Zhang, H.; Li, D.; Li, J. Effect of the Oxide Layer Structure on the Decarburization Behavior of 60Si2Mn Spring Steel in Dry Air: Experimental and First-Principle Study. Mater. Charact. 2023, 196, 112651. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Sohn, I.R.; Pettit, F.S.; Meier, G.H.; Sridhar, S. Investigation of the Effect of Alloying Elements and Water Vapor Contents on the Oxidation and Decarburization of Transformation-Induced Plasticity Steels. Met. Mater. Trans. B 2009, 40, 567–584. [Google Scholar] [CrossRef]
- Ren, C.X.; Wang, D.Q.Q.; Wang, Q.; Guo, Y.S.; Zhang, Z.J.; Shao, C.W.; Yang, H.J.; Zhang, Z.F. Enhanced Bending Fatigue Resistance of a 50CrMnMoVNb Spring Steel with Decarburized Layer by Surface Spinning Strengthening. Int. J. Fatigue 2019, 124, 277–287. [Google Scholar] [CrossRef]
- Mercier, D.; Chicot, D. Combined Micro-Hardness and Eddy Currents Applied to the Study of Steel Decarburizing. Matéria 2006, 11, 88–100. [Google Scholar] [CrossRef]
- Dalley, A.M. Full-Thickness Decarburization of the Steel Shell of an Annealing Furnace. Metallogr. Microstruct. Anal. 2012, 1, 59–64. [Google Scholar] [CrossRef]
- Sergeev, N.N.; Chukanov, A.N.; Baranov, V.P.; Yakovenko, A.A. Development of Damage and Decarburization of High-Strength Low-Alloy Steels Under Hydrogen Embrittlement. Met. Sci. Heat. Treat. 2015, 57, 63–68. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zhao, X.; Li, J.; Wang, C.; Wang, M. Effect of Austenitization Process on Oxidative Decarbonization Behavior and Mechanical Properties of 22MnB5 Steel. J. Mater. Eng. Perform. 2024, 33, 213–226. [Google Scholar] [CrossRef]
- De Las Cuevas, F.; Gil Sevillano, J. Pérdida de Ductilidad Debido a La Descarburación y Pérdida de Mn de Un Acero TWIP de Tamaño de Grano Grosero. Rev. Metal. 2017, 53, 109. [Google Scholar] [CrossRef]
- Zhao, X.J.; Guo, J.; Wang, H.Y.; Wen, Z.F.; Liu, Q.Y.; Zhao, G.T.; Wang, W.J. Effects of Decarburization on the Wear Resistance and Damage Mechanisms of Rail Steels Subject to Contact Fatigue. Wear 2016, 364–365, 130–143. [Google Scholar] [CrossRef]
- Hou, J.; Han, F.-F.; Ye, X.-X.; Leng, B.; Liu, M.; Lu, Y.-L.; Zhou, X.-T. Effect of Surface Decarburization on Corrosion Behavior of GH3535 Alloy in Molten Fluoride Salts. Acta Metall. Sin. 2019, 32, 401–412. [Google Scholar] [CrossRef]
- Ganesh Kumar, M.; Kishore, A.; Kandhavel, P. A Comparison Study on Decarburization of Grey Cast Iron and En8 Steel. Int. J. Mech. Prod. Eng. 2019, 7, 39–46. [Google Scholar]
- Vander Voort, G.F. Measurement of Decarburization of Heat Treated Steel Surfaces. Microsc. Microanal. 2014, 20, 840–841. [Google Scholar] [CrossRef]
- Voort, G.F.V. Understanding and Measuring Decarburization. AMP Tech. Artic. 2015, 173, 22. [Google Scholar] [CrossRef]
- Hrybovs’ka, V.I.; Chepil’, R.V.; Ostash, O.P. Influence of Decarburization on the Durability of Steels for Rail Accessories. Mater. Sci. 2016, 52, 357–364. [Google Scholar] [CrossRef]
- Hao, X.J.; Yin, W.; Strangwood, M.; Peyton, A.J.; Morris, P.F.; Davis, C.L. Off-Line Measurement of Decarburization of Steels Using a Multifrequency Electromagnetic Sensor. Scr. Mater. 2008, 58, 1033–1036. [Google Scholar] [CrossRef]
- Hao, X.J.; Yin, W.; Strangwood, M.; Peyton, A.J.; Morris, P.F.; Davis, C.L. Characterization of Decarburization of Steels Using a Multifrequency Electromagnetic Sensor: Experiment and Modeling. Met. Mater. Trans. A 2009, 40, 745–756. [Google Scholar] [CrossRef]
- Mayer-Sidd, E.; Hutterer, F. Merkbuch für Fehler bei der Warmbearbeitung von Eisen und Stahl. Fehler des Stahls, Stahlauswahl, Schmieden, Glühen, Härten, Anlassen, Vergüten, Nieten usw; Union Deutsche Verlagsgesellschaft: Berlin, Germany, 1938. [Google Scholar]
- Wang, H.; Su, F.; Wen, Z. Study on Decarburization Mechanism and Law of GCr15 Bearing Steel during Heat Treatment. Adv. Mater. Sci. Eng. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Nagode, A.; Jerina, K.; Jerman, I.; Vella, D.; Bizjak, M.; Kosec, B.; Karpe, B.; Zorc, B. The Effect of Sol–Gel Boehmite Coatings on the Corrosion and Decarburization of C45 Steel. J. Sol-Gel Sci. Technol. 2018, 86, 568–579. [Google Scholar] [CrossRef]
- Zorc, M.; Nagode, A.; Bizjak, M.; Zorc, B. Decarburization of the Carbon Steel C45 During Annealing in Air. Mater. Geoenvironment 2018, 65, 167–178. [Google Scholar] [CrossRef]
- Jaason, K.; Peetsalu, P.; Saarna, M.; Kulu, P.; Beilmann, J. Decarburisation Effect on Hardened Strip Steel Fastening Components. Mater. Sci. 2016, 22, 148–152. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, J.; Huang, Y.; Zhang, C.; Yao, H.; Tian, Z.; Wang, S. Experimental and Modelling Research on the Influence of Different Pre-Decarburization Microstructures of Fe-0.6C-1.8Si-0.8Mn Spring Steel. J. Mater. Res. Technol. 2023, 23, 4766–4778. [Google Scholar] [CrossRef]
- Mayott, S. Analysis of the Effects of Reduced Oxygen Atmospheres on the Decarburization Depths of 300M Alloy Steel. Master’s Thesis, Rensselaer Polytechnic Institute, Troy, NY, USA, 2010. [Google Scholar]
- Zhao, F.; Wu, M.; Jiang, B.; Zhang, C.; Xie, J.; Liu, Y. The Effect of Carbon Contents on Intragranular Ferrite Formed in the V-Ti-N Microalloyed Steel with a Carbon Content Gradient Prepared by Controlling the Surface Decarburization. Mater. Charact. 2018, 140, 217–224. [Google Scholar] [CrossRef]
- Efremenko, V.G.; Chabak, Y.G.; Lekatou, A.; Karantzalis, A.E.; Efremenko, A.V. High-Temperature Oxidation and Decarburization of 14.55 Wt Pct Cr-Cast Iron in Dry Air Atmosphere. Met. Mater. Trans. A 2016, 47, 1529–1543. [Google Scholar] [CrossRef]
- Chen, Y.R.; Zhang, F.; Liu, Y. Decarburization of 60Si2MnA in 20 Pct H2O-N2 at 700 °C to 900 °C. Metall. Mater. Trans. A 2020, 51, 1808–1821. [Google Scholar] [CrossRef]
- Chen, Y.R.; Liu, Y.; Xu, X. Oxidation of 60Si2MnA Steel in Atmospheres Containing Different Levels of Oxygen, Water Vapour and Carbon Dioxide at 700–1000 °C. Oxid. Met. 2020, 93, 53–74. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Tong, Q.; Wang, L. Effects of Temperature and Oxygen Concentration on the Characteristics of Decarburization of 55SiCr Spring Steel. ISIJ Int. 2014, 54, 1920–1926. [Google Scholar] [CrossRef]
- Maria das Graças, M.M.; Mantel, M.J. Effect of the Temperature and Dew Point of the Decarburization Process on the Oxide Subscale of a 3% Silicon Steel. J. Magn. Magn. Mater. 2003, 254–255, 337–339. [Google Scholar] [CrossRef]
- Billings, G.J.; Smeltzer, W.W.; Kirkaldy, J.S. Oxidation and Decarburization Kinetics of Iron-Carbon Alloys. J. Electrochem. Soc. 1970, 117, 111. [Google Scholar] [CrossRef]
- Baud, J.; Ferrier, A.; Manenc, J.; Bénard, J. The Oxidation and Decarburizing of Fe-C Alloys in Air and the Influence of Relative Humidity. Oxid. Met. 1975, 9, 69–97. [Google Scholar] [CrossRef]
- Ge, X.; Ma, Q.; Wang, M.; Liu, H.; Guo, R.; Chen, Y. Surface Decarburization Behaviour of a Novel Die Steel. IOP Conf. Ser.: Mater. Sci. Eng. 2019, 688, 033031. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, J.; Qi, J.; Tian, Z.; Chen, H.; Zhang, H.; Wang, C. Modelling and Simulation of Isothermal and Continuous-Heating Surface Decarburization Behaviour of Fe-0.6C-1.8Si-0.8Mn Spring Steel. J. Mater. Res. Technol. 2021, 15, 1076–1089. [Google Scholar] [CrossRef]
- Li, S.; Feng, H.; Wang, S.; Gao, J.; Zhao, H.; Wu, H.; Xu, S.; Feng, Q.; Li, H.; Liu, X.; et al. Phase Transformation Behaviors of Medium Carbon Steels Produced by Twin Roll Casting and Compact Strip Production Processes. Materials 2023, 16, 1980. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Tong, Q.; Sun, Q. Effects of Si and Cr on Complete Decarburization Behavior of High Carbon Steels in Atmosphere of 2 Vol. % O2. J. Iron Steel Res. Int. 2016, 23, 1316–1322. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Zhou, L.; Jiang, C. Secondary Hardening, Austenite Grain Coarsening and Surface Decarburization Phenomenon in Nb-Bearing Spring Steel. J. Iron Steel Res. Int. 2012, 19, 47–51. [Google Scholar] [CrossRef]
- Zhu, W.; Cruchley, S.; Yin, W.; Hao, X.J.; Davis, C.L.; Peyton, A.J. Evaluation of Rail Decarburisation Depth Using a H-Shaped Electromagnetic Sensor. Ndt E Int. 2012, 46, 63–69. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, C.L.; Liu, Y.Z. Ferrite Decarburization of High Silicon Spring Steel in Three Temperature Ranges. Arch. Metall. Mater. 2016, 61, 1715–1722. [Google Scholar] [CrossRef]
- Hayashi, K.; Nishibata, T. Prediction of the Ferrite Layer Microstructure during Decarburization; Nippon Steel & SUMITOMO metal Technical Report No 120; Nippon Steel: Tokyo, Japan, 2018; pp. 22–29. [Google Scholar]
- Fletcher, E.E.; Elsea, A.R. The Effects of High-Pressure, High-Temperature Hydrogen on Steel; Technical Report 202; Defense Metals Information Center (DMIC), Battelle Memorial Institute Columbus: Columbus, OH, USA, 1964; pp. 1–64. [Google Scholar]
- Krishnan, G.N.; Scott, A.C.; Wood, B.J.; Cubicciotti, D. Effect of Transient Combustion Species on 4340 Steel; Technical Report; SRI International: Menlo Park, CA, USA, 1979; pp. 1–33. [Google Scholar]
- Egert, P.; Maliska, A.M.; Silva, H.R.T.; Speller, C.V. Decarburization during Plasma Nitriding. Surf. Coat. Technol. 1999, 122, 33–38. [Google Scholar] [CrossRef]
- Li, S.; Manory, R.R. Surface Morphology and Compound Layer Pores of Plasma Nitrocarburized Low Carbon Steel. Met. Mater. Trans. A 1996, 27, 135–143. [Google Scholar] [CrossRef]
- Deng, A.; Xue, S.; Guo, R. Failure Analysis of an Automotive Bushing. J. Fail. Anal. Preven. 2018, 18, 1379–1385. [Google Scholar] [CrossRef]
- Zwierzchowski, M. Analysis of the Possibilities of Using Forging Heat in Isothermal Annealing Processes for AISI 4140 Alloy. Arch. Metall. Mater. 2020, 65, 951–958. [Google Scholar] [CrossRef]
- García Navas, V.; Gonzalo, O.; Quintana, I.; Pirling, T. Residual Stresses and Structural Changes Generated at Different Steps of the Manufacturing of Gears: Effect of Banded Structures. Mater. Sci. Eng. A 2011, 528, 5146–5157. [Google Scholar] [CrossRef]
- Calliari, I.; Dabalà, M.; Ramous, E.; Zanesco, M.; Gianotti, E. Microstructure of a Nitrided Steel Previously Decarburized. J. Mater. Eng. Perform. 2006, 15, 693–698. [Google Scholar] [CrossRef]
- Hongfei, G.; Yan, J.; Zhang, R.; He, Z.; Zhao, Z.; Qu, T.; Wan, M.; Liu, J.; Li, C. Failure Analysis on 42CrMo Steel Bolt Fracture. Adv. Mater. Sci. Eng. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Gabrić, I.; Podrug, M. Surface Decarburization of 42crmo4 Steel in Chamber Furnaces without Protective Atmosphere. In Proceedings of the MATRIB 2013 Conference, Washington, DC, USA, 21–23 June 2013; Vela Luka, Croatia, 2013. Alar, Ž., Jakovljević, S., Šolić, S., Eds.; Croatian Society for Materials and Tribology: Zagreb, Croatia, 2013; pp. 156–166. [Google Scholar]
- Hu, Z.; Wu, M.; Hua, L.; Qin, X.; Ni, M. Influence of Graded Surface Decarburization of Automobile Forging Front Axle on the Bending Behavior Based on a Third-Order Shear Deformation Beam Theory. Machines 2022, 10, 139. [Google Scholar] [CrossRef]
- Marra, K.M.; Alvarenga, E.D.A.; Buono, V.T.L. Decarburization Kinetics during Annealing of a Semi-Processed Electrical Steel. ISIJ Int. 2004, 44, 618–622. [Google Scholar] [CrossRef]
- Jeong, W.C. Optimization of Spheroidizing Annealing Conditions in SCM440 Steel. J. Korean Soc. Heat Treat. 2006, 19, 270–279. [Google Scholar]
- Jing, Y.-A.; Yuan, Y.-M.; Yan, X.-L.; Zhang, L.; Sha, M.-H. Decarburization Mechanism during Hydrogen Reduction Descaling of Hot-Rolled Strip Steel. Int. J. Hydrog. Energy 2017, 42, 10611–10621. [Google Scholar] [CrossRef]
- Chen, J.; Feng, G.; Zheng, Y.; Ma, J.; Lin, P.; Wang, N.; Ma, H.; Zheng, J. Characterization of Surface Decarburization and Oxidation Behavior of Cr–Mo Cold Heading Steel. High Temp. Mater. Process. 2022, 41, 531–541. [Google Scholar] [CrossRef]
- Duan, X.; Yu, H.; Lu, J.; Huang, Z. Temperature Dependence and Formation Mechanism of Surface Decarburization Behavior in 35CrMo Steel. Steel Res. Int. 2019, 90, 1900188. [Google Scholar] [CrossRef]
- Totten, G.E.; Narazaki, M.; Blackwood, R.R.; Jarvis, L.M. Failures Related to Heat Treating Operations. In Failure Analysis and Prevention; Becker, W.T., Shipley, R.J., Eds.; ASM International: Materials Park, OH, USA, 2002; pp. 192–223. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Liu, Y. Surface Decarburization Characteristics and Relation between Decarburized Types and Heating Temperature of Spring Steel 60Si2MnA. Int. J. Miner. Metall. Mater. 2013, 20, 720–724. [Google Scholar] [CrossRef]
- ISO 3887:2023; Steels Determination of the Depth of Decarburization. International Organization for Standardization: Geneva, Switzerland, 2023.
- Alvarenga, H.D.; De Putte, T.V.; Van Steenberge, N.; Sietsma, J.; Terryn, H. Influence of Carbide Morphology and Microstructure on the Kinetics of Superficial Decarburization of C-Mn Steels. Met. Mater. Trans. A 2015, 46, 123–133. [Google Scholar] [CrossRef]
- Prathviraj, M.P.; Samuel, A.; Narayan Prabhu, K. Reprocessed Waste Sunflower Cooking Oil as Quenchant for Heat Treatment. J. Clean. Prod. 2020, 269, 122276. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, G.; Zhu, Y.; Xue, Z.; Xu, G. Effects of Heating Temperature and Atmosphere on Element Distribution and Microstructure in High-Mn/Al Austenitic Low-Density Steel. Int. J. Miner. Metall. Mater. 2024, 31, 2670–2680. [Google Scholar] [CrossRef]
- Pasang, T.; Chen, Z.; Ramezani, M.; Neitzert, T.; Au, D. The Effect of Heat Treatment Atmosphere on Hardening of Surface Region of H13 Tool Steel. J. Mater. Sci. Chem. Eng. 2013, 1, 20–29. [Google Scholar] [CrossRef][Green Version]
- Prabhudev, K.H. Handbook of Heat Treatment of Steels; Tata McGraw-Hill: New Delhi, India, 2011. [Google Scholar]
- Ambrosini, L.; Bahadur, S. Erosion of AISI 4140 Steel. Wear 1987, 117, 37–48. [Google Scholar] [CrossRef]
- Kwon, H.; Cha, J.C.; Kim, C.H. The Effect of Grain Size on Fracture Behaviour in Tempered Martensite Embrittlement for AISI 4340 Steel. Mater. Sci. Eng. 1988, 100, 121–128. [Google Scholar] [CrossRef]
- De Souza, M.F.; Serrão, L.F.; Pardal, J.M.; Tavares, S.S.M.; Fonseca, M.C. Tempering Influence on Residual Stresses and Mechanical Properties of AISI 4340 Steel. Int. J. Adv. Manuf. Technol. 2022, 120, 1123–1134. [Google Scholar] [CrossRef]
- Prijanovič Tonkovič, M.; Knez, M. The Effect of Protective Atmosphere on Decarburisation of the Heat-Treated Surface of Steel. Mater. Geoenviron. 2017, 64, 199–204. [Google Scholar] [CrossRef][Green Version]
- Belkind, A.; Gershman, S. Plasma Cleaning of Surfaces. Vac. Technol. Coat. 2008, 9, 46–57. [Google Scholar]
- Roliński, E.; Arner, J.; Sharp, G. Negative Effects of Reactive Sputtering in an Industrial Plasma Nitriding. J. Mater. Eng. Perform. 2005, 14, 343–350. [Google Scholar] [CrossRef]
- Sirin, S.Y.; Kaluc, E. Structural Surface Characterization of Ion Nitrided AISI 4340 Steel. Mater. Des. 2012, 36, 741–747. [Google Scholar] [CrossRef]
- Kurny, A.S.W.; Mallya, R.M.; Mohan Rao, M. A Study on the Nature of the Compound Layer Formed during the Ion Nitriding of En40B Steel. Mater. Sci. Eng. 1986, 78, 95–100. [Google Scholar] [CrossRef]
- Edenhofer, B. Physical and Metallurgical Aspects of Ionitriding. Heat Treat. Met. 1974, 2, 62. [Google Scholar]
- Miyamoto, J.; Tokuno, K.; Hagino, M. Effect of Sputtered Fe on the Plasma Nitriding Mechanism of AISI H13 Tool Steel Using Electron-Beam-Excited Plasma. ISIJ Int. 2021, 61, 2805–2812. [Google Scholar] [CrossRef]
- Tier, M.A.; Kieckow, F.; Strohaecker, R.; da Silva Rocha, A. A Study of Carbon Redistribution During Plasma Nitriding of Steel. In Proceedings of the 15th IFHTSE Conference, Vienna, Austria, 25–29 September 2006; pp. 192–198. [Google Scholar]
- Ruset, C.; Ciuca, S.; Grigore, E. The Influence of the Sputtering Process on the Constitution of the Compound Layers Obtained by Plasma Nitriding. Surf. Coat. Technol. 2003, 174–175, 1201–1205. [Google Scholar] [CrossRef]
- Kwietniewski, C.; Fontana, W.; Moraes, C.; Rocha, A.d.S.; Hirsch, T.; Reguly, A. Nitrided Layer Embrittlement Due to Edge Effect on Duplex Treated AISI M2 High-Speed Steel. Surf. Coat. Technol. 2004, 179, 27–32. [Google Scholar] [CrossRef]
- Forati Rad, H.; Amadeh, A.; Moradi, H. Wear Assessment of Plasma Nitrided AISI H11 Steel. Mater. Des. 2011, 32, 2635–2643. [Google Scholar] [CrossRef]
- Yao, J.; Yan, F.; Yang, Y.; Yan, M.; Zhang, Y. Comprehensive Analysis of Nitriding Behavior and Synergistic Strengthening Mechanism on Quenched M50 Steel. Surf. Coat. Technol. 2022, 436, 128307. [Google Scholar] [CrossRef]
- Krishnan, G.; Scott, A.C.; Wood, B.J.; Cubicciotti, D. Decarburization of 4340 Steel by Gaseous Atomic Hydrogen. Metall. Trans. A 1979, 10, 1798–1800. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Mahboubi, F.; Daviran, G. Plasma Nitriding Behavior of DIN 1.2344 Hot Work Tool Steel. Iran. J. Mater. Sci. Eng. 2020, 17, 1–9. [Google Scholar] [CrossRef]
- Ruset, C.; Grigore, E. Some Observations on Structure of Compound Layer Produced on Steels by Plasma Nitrocarburising. Surf. Eng. 2010, 26, 61–66. [Google Scholar] [CrossRef]
- Mozhi Varman, J.P.A.; Huchel, U. Effect of Pulse Repetition Time on Surface Properties of Pulsed Plasma Nitrided AISI 4340 Steel. Indian J. Sci. Technol. 2017, 10, 1–8. [Google Scholar] [CrossRef][Green Version]
- Alves Junior, C.; Freitas, N.V.D.S.; Morais, P.B.D.; Vitoriano, J.D.O. Changing the Characteristics of the Nitrided Layer Using Three Different Plasma Configurations. Matéria 2019, 24, e12371. [Google Scholar] [CrossRef]
- Israphilov, I.H.; Zvezdin, V.V.; Israphilov, D.I.; Chernova, M.A. Study on Parts Ion-Plasma Nitriding. J. Phys. Conf. Ser. 2014, 567, 012030. [Google Scholar] [CrossRef]
- Arul Mozhi Varman, J.P.; Uwe, H.; Balasubramanian, M. Effect of Pulse Repetition Time on Pulsed Plasma Nitriding of AISI 4340 Steel. J. Mater. Sci. Surf. Eng. 2016, 4, 386–391. [Google Scholar]
- Da Silva Rocha, A.; Strohaecker, T.; Tomala, V.; Hirsch, T. Microstructure and Residual Stresses of a Plasma-Nitrided M2 Tool Steel. Surf. Coat. Technol. 1999, 115, 24–31. [Google Scholar] [CrossRef]
- Nayebpashaee, N.; Soltanieh, M.; Kheirandish, S. A Study on Formation and Growth Mechanism of Nitride Layers During Plasma Nitriding Process of Plastic Injection Mold Steel. Mater. Manuf. Process. 2016, 31, 1192–1200. [Google Scholar] [CrossRef]
- Maniee, A.; Mahboubi, F.; Soleimani, R. The Study of Tribological and Corrosion Behavior of Plasma Nitrided 34CrNiMo6 Steel under Hot and Cold Wall Conditions. Mater. Des. 2014, 60, 599–604. [Google Scholar] [CrossRef]
- Skonieski, A.F.O.; Santos, G.R.D.; Hirsch, T.K.; Rocha, A.D.S. Metallurgical Response of an AISI 4140 Steel to Different Plasma Nitriding Gas Mixtures. Mat. Res. 2013, 16, 884–890. [Google Scholar] [CrossRef]
- Alsaran, A.; Çelik, A. Structural Characterization of Ion-Nitrided AISI 5140 Low-Alloy Steel. Mater. Charact. 2001, 47, 207–213. [Google Scholar] [CrossRef]
- Sousa, R.R.M.D.; Moura, Y.J.L.; Sousa, P.A.O.D.; Medeiros Neto, J.Q.; Costa, T.H.D.C.; Alves Junior, C. Nitriding of AISI 1020 Steel: Comparison between Conventional Nitriding and Nitriding with Cathodic Cage. Mat. Res. 2014, 17, 708–713. [Google Scholar] [CrossRef]
- Mohammadzadeh, R.; Akbari, A.; Drouet, M. Microstructure and Wear Properties of AISI M2 Tool Steel on RF Plasma Nitriding at Different N2–H2 Gas Compositions. Surf. Coat. Technol. 2014, 258, 566–573. [Google Scholar] [CrossRef]
- Medina, A.; Aguilar, C.; Béjar, L.; Oseguera, J.; Ruíz, A.; Huape, E. Effects of Post-Discharge Nitriding on the Structural and Corrosion Properties of 4140 Alloyed Steel. Surf. Coat. Technol. 2019, 366, 248–254. [Google Scholar] [CrossRef]
- Díaz-Guillén, J.C.; Díaz-Guillén, J.A.; Granda-Gutiérrez, E.E.; Díaz-Guillén, M.R.; González-Albarrán, M.A. Electrochemical Corrosion Performance of AISI D2 Tool Steel Surface Hardened by Pulsed Plasma Nitriding. Int. J. Electrochem. Sci. 2013, 8, 973–982. [Google Scholar] [CrossRef]
- Taherkhani, K.; Mahboubi, F. Investigation Nitride Layers and Properties Surfaces on Pulsed Plasma Nitrided Hot Working Steel AISI H13. Iran. J. Mater. Sci. Eng. 2013, 10, 29–36. [Google Scholar]
- Roliński, E.; Sharp, G. The Effect of Sputtering on Kinetics of Compound Zone Formation in the Plasma Nitriding of 3% Cr-Mo-V Steel. J. Mater. Eng. Perform. 2001, 10, 444–448. [Google Scholar] [CrossRef]
- Mashreghi, A.R.; Soleimani, S.M.Y.; Saberifar, S. The Investigation of Wear and Corrosion Behavior of Plasma Nitrided DIN 1.2210 Cold Work Tool Steel. Mater. Des. 2013, 46, 532–538. [Google Scholar] [CrossRef]
- Díaz-Guillén, J.C.; Naeem, M.; Hdz-García, H.M.; Acevedo-Davila, J.L.; Díaz-Guillén, M.R.; Khan, M.A.; Iqbal, J.; Mtz-Enriquez, A.I. Duplex Plasma Treatment of AISI D2 Tool Steel by Combining Plasma Nitriding (with and without White Layer) and Post-Oxidation. Surf. Coat. Technol. 2020, 385, 125420. [Google Scholar] [CrossRef]
- Alves, C.; De Araújo, F.O.; Ribeiro, K.J.B.; Da Costa, J.A.P.; Sousa, R.R.M.; De Sousa, R.S. Use of Cathodic Cage in Plasma Nitriding. Surf. Coat. Technol. 2006, 201, 2450–2454. [Google Scholar] [CrossRef]
- Slycke, J.; Sproge, L.; Agren, J. Nitrocarburizing and the Ternary Fe-N-C Phase Diagram. Scand. J. Metall. 1988, 17, 122–126. [Google Scholar]
- Corengia, P.; Ybarra, G.; Moina, C.; Cabo, A.; Broitman, E. Microstructural and Topographical Studies of DC-Pulsed Plasma Nitrided AISI 4140 Low-Alloy Steel. Surf. Coat. Technol. 2005, 200, 2391–2397. [Google Scholar] [CrossRef]
- Wen, D.-C. Plasma Nitriding of Plastic Mold Steel to Increase Wear- and Corrosion Properties. Surf. Coat. Technol. 2009, 204, 511–519. [Google Scholar] [CrossRef]
- Basso, R.L.O.; Pastore, H.O.; Schmidt, V.; Baumvol, I.J.R.; Abarca, S.A.C.; De Souza, F.S.; Spinelli, A.; Figueroa, C.A.; Giacomelli, C. Microstructure and Corrosion Behaviour of Pulsed Plasma-Nitrided AISI H13 Tool Steel. Corros. Sci. 2010, 52, 3133–3139. [Google Scholar] [CrossRef]
- Fernandes, F.A.P.; Picone, C.A.; Totten, G.E.; Casteletti, L.C. Corrosion Behavior of Plasma Nitrided and Nitrocarburised Supermartensitic Stainless Steel. Mat. Res. 2018, 21, 1–9. [Google Scholar] [CrossRef]
- Fernandes, F.A.P.; Heck, S.C.; Picone, C.A.; Casteletti, L.C. On the Wear and Corrosion of Plasma Nitrided AISI H13. Surf. Coat. Technol. 2020, 381, 125216. [Google Scholar] [CrossRef]
- Zagonel, L.F.; Basso, R.L.O.; Alvarez, F. Precipitates Temperature Dependence in Ion Beam Nitrited AISI H13 Tool Steel. Plasma Process. Polym. 2007, 4, S736–S740. [Google Scholar] [CrossRef]
- Pye, D. Practical Nitriding and Ferritic Nitrocarburizing; ASM International, 2003. ASM International: Almere, The Netherlands, 2003. [Google Scholar] [CrossRef]
- Zdravecká, E.; Slota, J.; Solfronk, P.; Kolnerová, M. Evaluation of the Effect of Different Plasma-Nitriding Parameters on the Properties of Low-Alloy Steel. J. Mater. Eng. Perform. 2017, 26, 3588–3596. [Google Scholar] [CrossRef]
- Mahboubi, F.; Samandi, M.; Dunne, D.; Bloyce, A.; Bell, T. Plasma Nitriding of Microalloyed Steel. Surf. Coat. Technol. 1995, 71, 135–141. [Google Scholar] [CrossRef]
- Sun, Y.; Bell, T. Plasma Surface Engineering of Low Alloy Steel. Mater. Sci. Eng. A 1991, 140, 419–434. [Google Scholar] [CrossRef]
- Nayal, G.; Lewis, D.B.; Lembke, M.; Münz, W.-D.; Cockrem, J.E. Influence of Sample Geometry on the Effect of Pulse Plasma Nitriding of M2 Steel. Surf. Coat. Technol. 1999, 111, 148–157. [Google Scholar] [CrossRef]
- Zlatanović, M.; Gredić, T.; Popović, N.; Bogdanov, Ž. Matching of TiN Coating Structures by Plasma Nitriding of Substrates. Vacuum 1993, 44, 83–88. [Google Scholar] [CrossRef]
- Gredić, T.; Zlatanović, M.; Münz, W.-D. Plasma Nitriding of Ti and Ti-Al Coatings. Surf. Coat. Technol. 1993, 61, 338–345. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, S.Z.; Kim, J.H.; Lee, K.N. Tribological Characteristics of Ion Nitrided Sintered Steels. Surf. Coat. Technol. 1999, 114, 169–173. [Google Scholar] [CrossRef]
- Kandeva, M.; Kalitchin, Z.; Zadorozhnaya, E.; Vencl, A. Performance Characteristics of Lubricant Based on Rapeseed Oil Containing Different Amounts of Metal-Containing Additive. Ind. Lubr. Tribol. 2022, 74, 309–315. [Google Scholar] [CrossRef]
- Landgraf, P.; Bergelt, T.; Rymer, L.-M.; Kipp, C.; Grund, T.; Bräuer, G.; Lampke, T. Evolution of Microstructure and Hardness of the Nitrided Zone during Plasma Nitriding of High-Alloy Tool Steel. Metals 2022, 12, 866. [Google Scholar] [CrossRef]
- Cesconetto, M.D.C.R.L.; Franco, A.R., Jr.; Vieira, E.A. Improving the Abrasive Wear Resistance of a Microalloyed Steel by Plasma Nitriding. Mat. Res. 2015, 18, 334–340. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Sohi, M.H.; Raouf, A.H.; Mahboubi, F. Effect of Plasma Nitriding Temperature on the Corrosion Behavior of AISI 4140 Steel before and after Oxidation. Surf. Coat. Technol. 2010, 205, S261–S266. [Google Scholar] [CrossRef]
- Oliveira, S.D.; Tschiptschin, A.P.; Pinedo, C.E. Simultaneous Plasma Nitriding and Ageing Treatments of Precipitation Hardenable Plastic Mould Steel. Mater. Des. 2007, 28, 1714–1718. [Google Scholar] [CrossRef]
- Çelik, A.; Karadeniz, S. Investigation of Compound Layer Formed during Ion Nitriding of AISI 4140 Steel. Surf. Coat. Technol. 1996, 80, 283–286. [Google Scholar] [CrossRef]
- Molinari, A.; Tesi, B.; Bacci, T.; Pradelli, G. Low Temperature Ion-Nitriding of Fe-Mo-C Sintered Steels. J. Phys. IV Fr. 1993, 3, C7-949–C7-954. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Lu, L.; Cui, G.; Shen, X.; Yi, H.; Zhang, W. Effect of Process Temperature on the Microstructure and Properties of Gas Oxynitrocarburized 35CrMo Alloy Steel. Mater. Des. 2010, 31, 2654–2658. [Google Scholar] [CrossRef]
- Abdalla, A.J.; Baggio-Scheid, V.H.; Reis Pereira Baptista, C.A.; Ribeiro Barbosa, M.J. Fatigue Life Changing in Rolled Carbon Steel after Plasma Termochemical Treatments. Procedia Eng. 2010, 2, 1653–1661. [Google Scholar] [CrossRef][Green Version]
- Gecu, R. Combined effects of cryogenic treatment and tempering on microstructural and tribological features of AISI H13 steel. Mater. Chem. Phys. 2022, 292, 1–10. [Google Scholar] [CrossRef]
- Matarneh, E.M. The Effect of Austenitization Treatment Temperature on H-13 Tool Steel’s Mechanical Properties. Int. J. Mech. Appl. 2016, 6, 77–82. [Google Scholar] [CrossRef]
- Sunwoo, J.A. Exploratory Study on H13 Steel Dies. Off. Sci. Tech. Inf. (OSTI) 1994, 1–5. [Google Scholar] [CrossRef][Green Version]
- Conci, M.D.; Bozzi, A.C.; Franco, A.R., Jr. Effect of Plasma Nitriding Potential on Tribological Behaviour of AISI D2 Cold-Worked Tool Steel. Wear 2014, 317, 188–193. [Google Scholar] [CrossRef]
- Soenen, B.; Jacobs, S.; De Wulf, M. Modelling Decarburization in Electrical Steels. Steel Res. Int. 2005, 76, 425–428. [Google Scholar] [CrossRef]
- Purkert, F.E. Prevention of decarburization in Annealing of high carbon steel. J. Heat Treat. 1982, 2, 225–231. [Google Scholar] [CrossRef]
- Choi, S.; van der Zwaag, S. Prediction of Decarburized Ferrite Depth of Hypoeutectoid Steel with Simultaneous Oxidation. ISIJ Int. 2012, 52, 549–558. [Google Scholar] [CrossRef]











| Element | C | Si | Cr | Mn | Mo | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| wt.% | 0.38–0.45 | 0.20–0.40 | 0.90–1.20 | 0.50–0.80 | 0.15–0.25 | ≤0.04 | ≤0.04 | Bal. |
| 1. Material | 2. State of the Material Before Nitriding | 3. Nitriding Condition | 4. Chemico-Structural Changes |
| API 5L X-70 | Pearlite in a matrix of ferrite was wet-grounded using SiC papers and mechanically polished to a mirror-like finish | Plasma cleaning: temperature of 200–250 °C, Ar atmosphere, pressure of 100 Pa, duration of 15 min. Plasma nitriding: temperatures of 410, 440 and 470 °C, nitriding times of 1, 3 and 5 h, pressure of 533 Pa, gas composition of 10% N2 and 90% H2 | During 1 h nitriding of the sample, both ε-Fe2-3N and γ’-Fe4N nitrides were detected, and during 3 and 5 h nitriding of the samples only γ’-Fe4N nitride was evidenced |
| 5. Methods for monitoring chemical–structural changes | 6. Tribology | 7. The most favorable structure from the aspect of tribology | |
| SEM, XRD, “free ball” micro-abrasion tester, micro-HV | Ball-on-disc tribometer was used. Counter-body was an AISI 52100 steel ball with 25.4 mm in diameter. Abrasive slurry was 4.5 μm SiC particles. Normal load was 0.24 N. Test samples rotated at 150 rpm | The maximum wear resistance was achieved when the compound layer consisted mainly of ε-Fe2-3N nitride and a diffusion zone with large needle-like γ’-Fe4N nitride at 440 °C for 1 h |
| 1. Material | 2. State of the Material Before Nitriding | 3. Oxynitro-Carburization Condition | 4. Chemico-Structural Changes | 5. Methods for Monitoring Chemico-Structural Changes |
| 35CrMo | Austenitized at 860 °C for 1800 s, quenched in oil, tempered at 580 °C for 1800 s, and air-cooled | Oxynitro-carburization was carried out at 550, 570 and 610 °C for 2 h, with a gas mixture of NH3, O2, and additive organic gas at 0.11 MPa in a low-temperature gas multi-element penetrating system. The samples were cooled in air | Microstructure, surface composition, case depth, microhardness, wear, and corrosion resistance of the γ’-Fe4N, ε-Fe3N, Fe3O4, and Fe2O3 | XRD, wear testing, corrosion test, and microhardness |
| 6. Corrosion | 7. Tribology | 8. The most favorable structure from the aspect of corrosion | 9. The most favorable structure from the aspect of tribology | |
| Salt spray test system was used to evaluate the corrosion resistant behavior after surface treatment | Block-on-ring tribometer was employed. Counter-body was a GCr15 steel. Normal load was 200 N, sliding speed was 0.4 m/s, and a sliding distance was 251 m | The properties of samples treated at 570 °C were proved to be the best | The best wear resistance property is obtained from the 570 °C treated sample. Resistance increases with increasing ε-Fe3N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, Ž.; Gligorijević, B.; Prvulović, S.; But, A.; Svoboda, P.; Piteľ, J.; Vencl, A. Decarburization and Its Effects on the Properties of Plasma-Nitrided AISI 4140 Steel: A Review. Materials 2025, 18, 2207. https://doi.org/10.3390/ma18102207
Stojanović Ž, Gligorijević B, Prvulović S, But A, Svoboda P, Piteľ J, Vencl A. Decarburization and Its Effects on the Properties of Plasma-Nitrided AISI 4140 Steel: A Review. Materials. 2025; 18(10):2207. https://doi.org/10.3390/ma18102207
Chicago/Turabian StyleStojanović, Željko, Bojan Gligorijević, Slavica Prvulović, Adrian But, Petr Svoboda, Ján Piteľ, and Aleksandar Vencl. 2025. "Decarburization and Its Effects on the Properties of Plasma-Nitrided AISI 4140 Steel: A Review" Materials 18, no. 10: 2207. https://doi.org/10.3390/ma18102207
APA StyleStojanović, Ž., Gligorijević, B., Prvulović, S., But, A., Svoboda, P., Piteľ, J., & Vencl, A. (2025). Decarburization and Its Effects on the Properties of Plasma-Nitrided AISI 4140 Steel: A Review. Materials, 18(10), 2207. https://doi.org/10.3390/ma18102207








