Research on the Corrosion Resistance and Mechanical Properties of Graphene Oxide–Modified AT13 Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Raw Materials
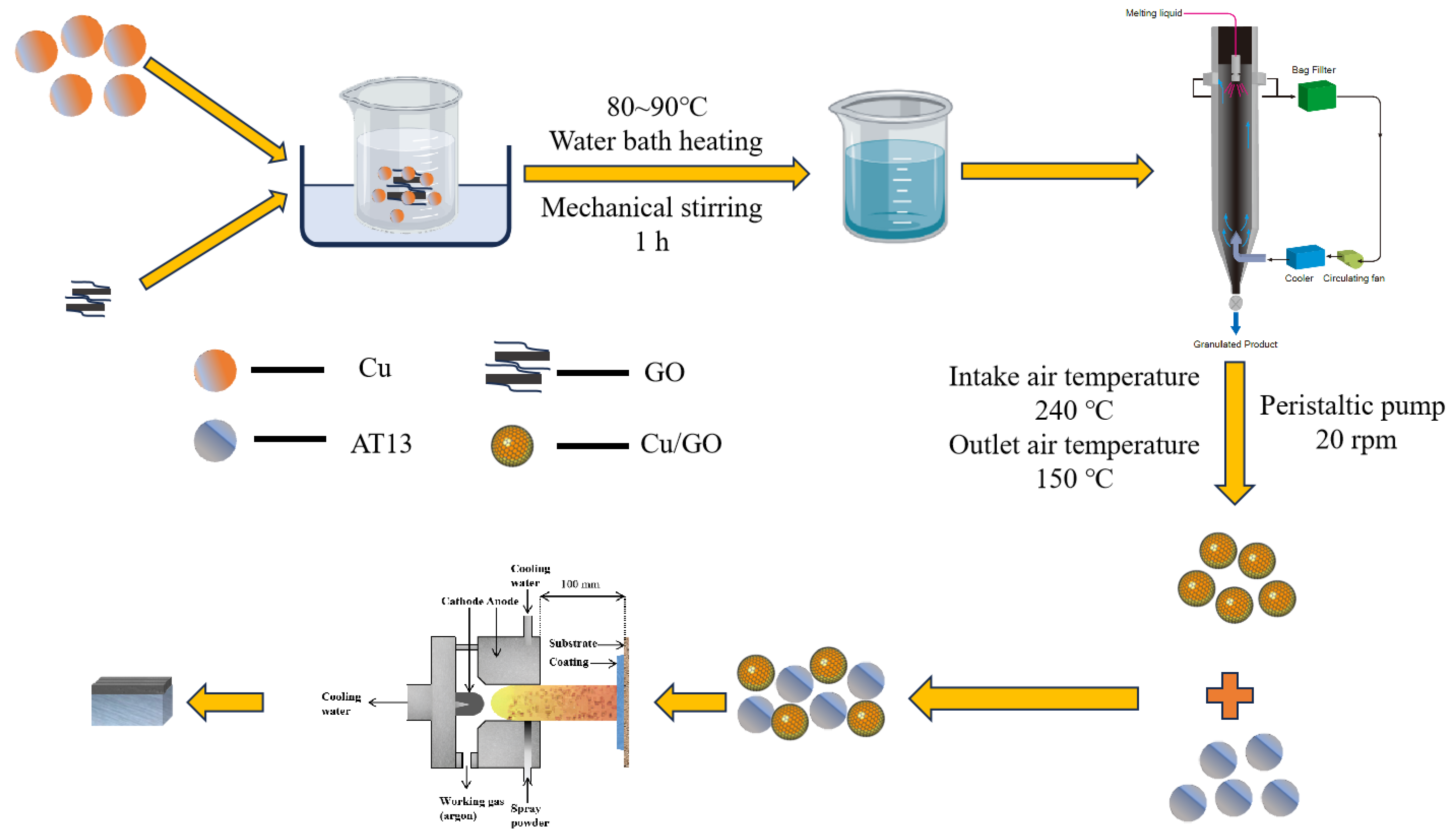
2.2. Preparation of Coatings
2.3. Characterization Analysis Methods
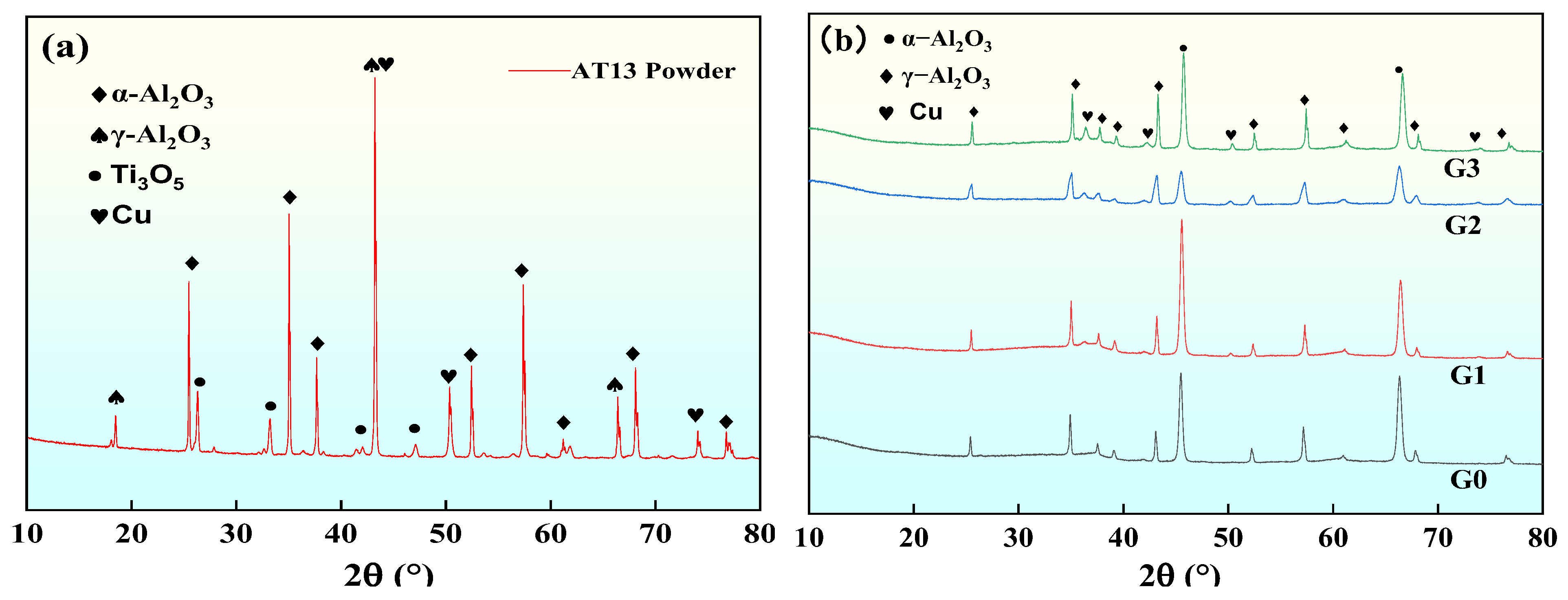
3. Results and Discussion
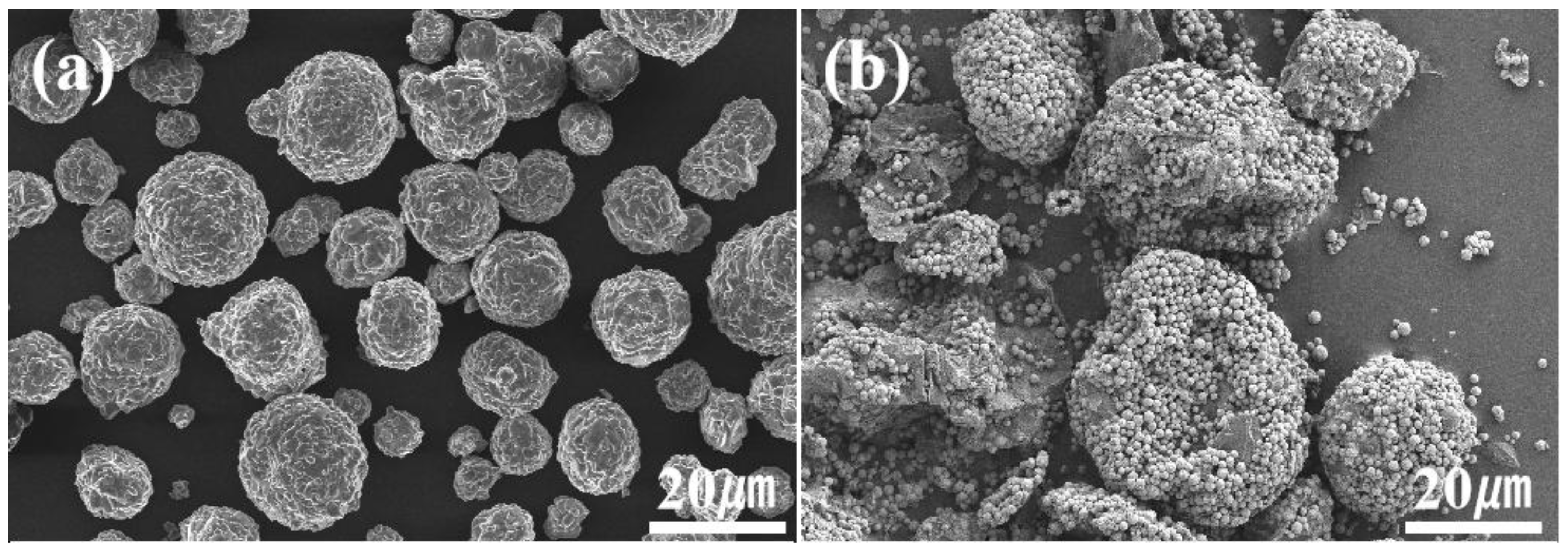
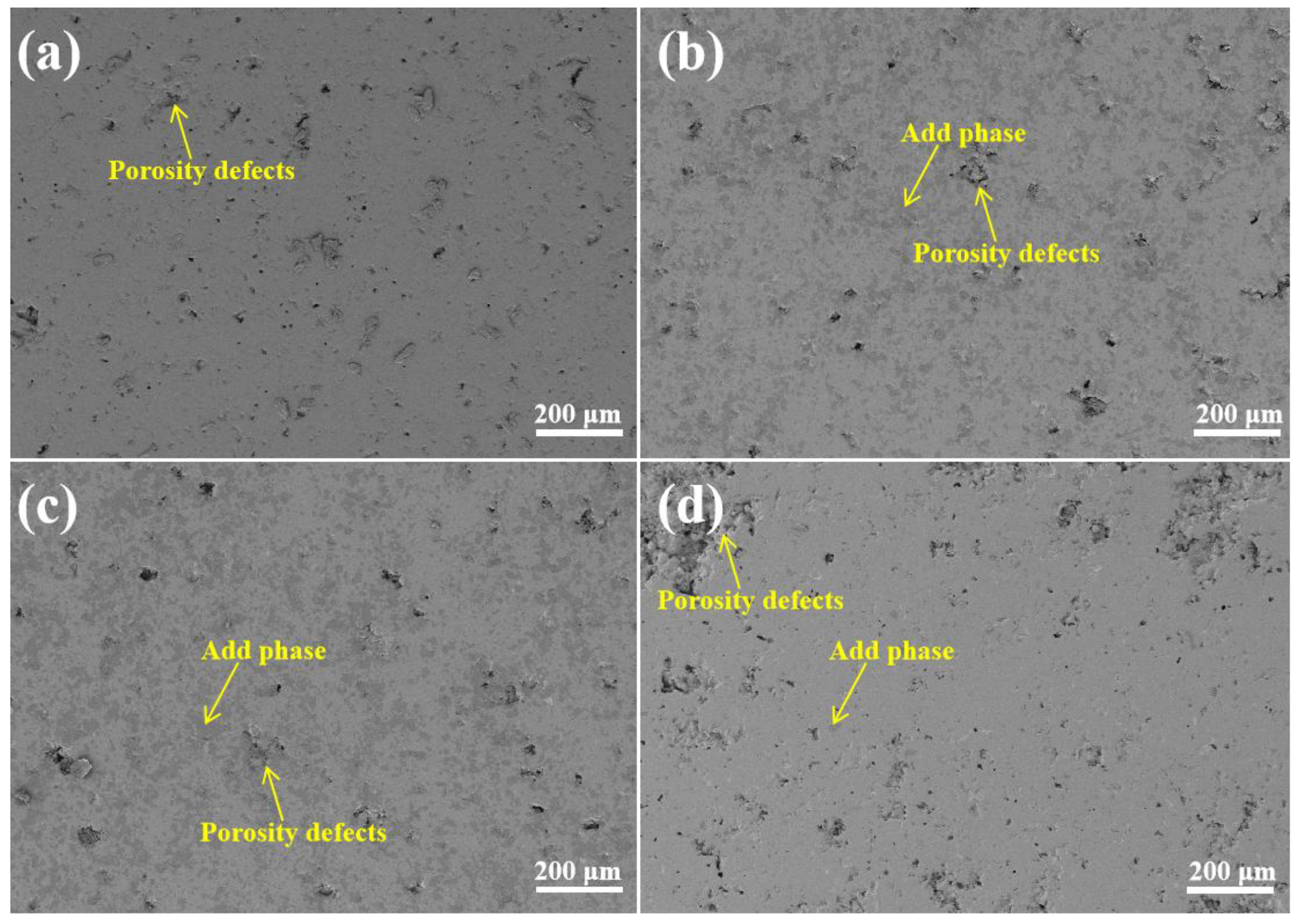
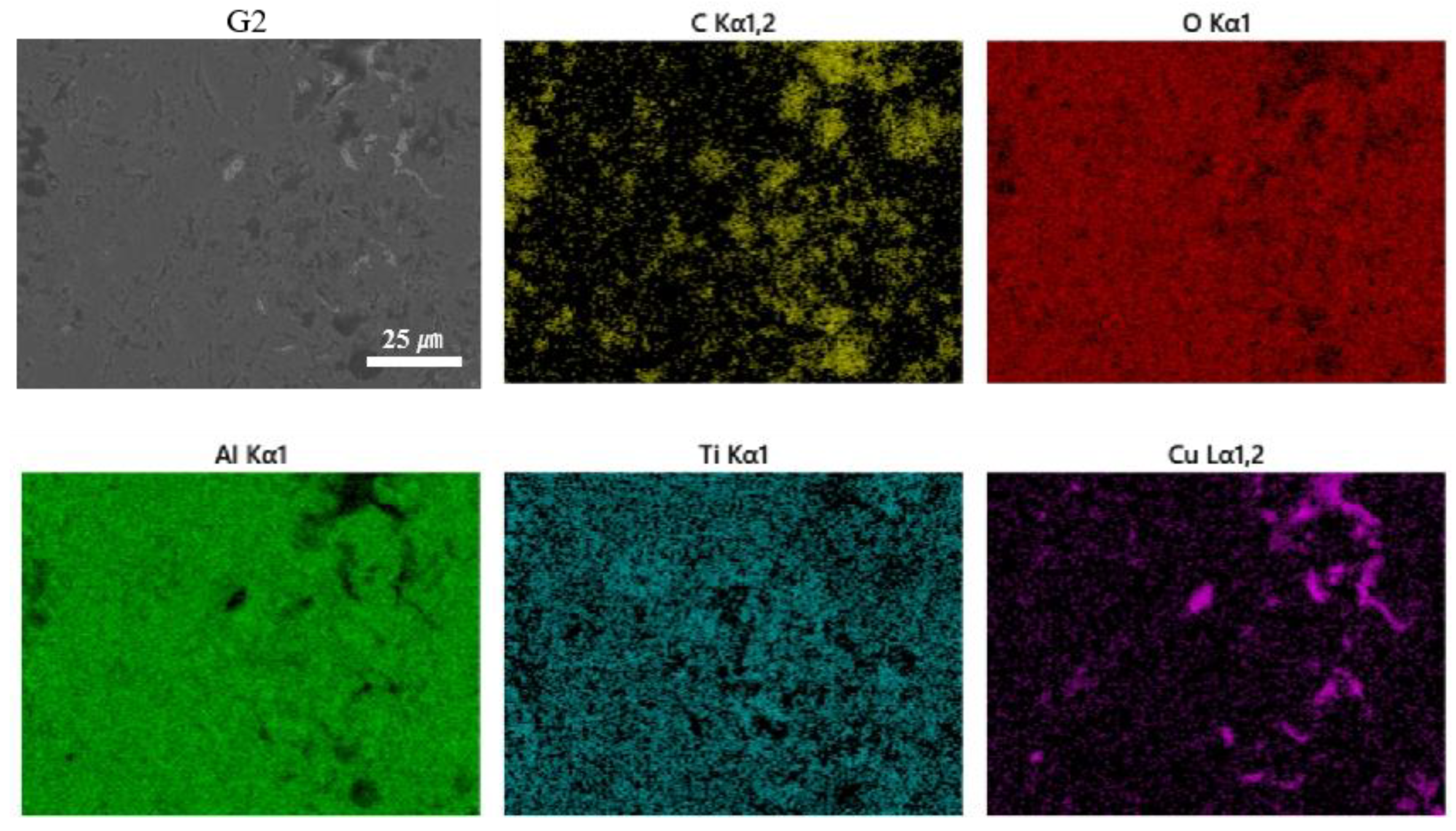
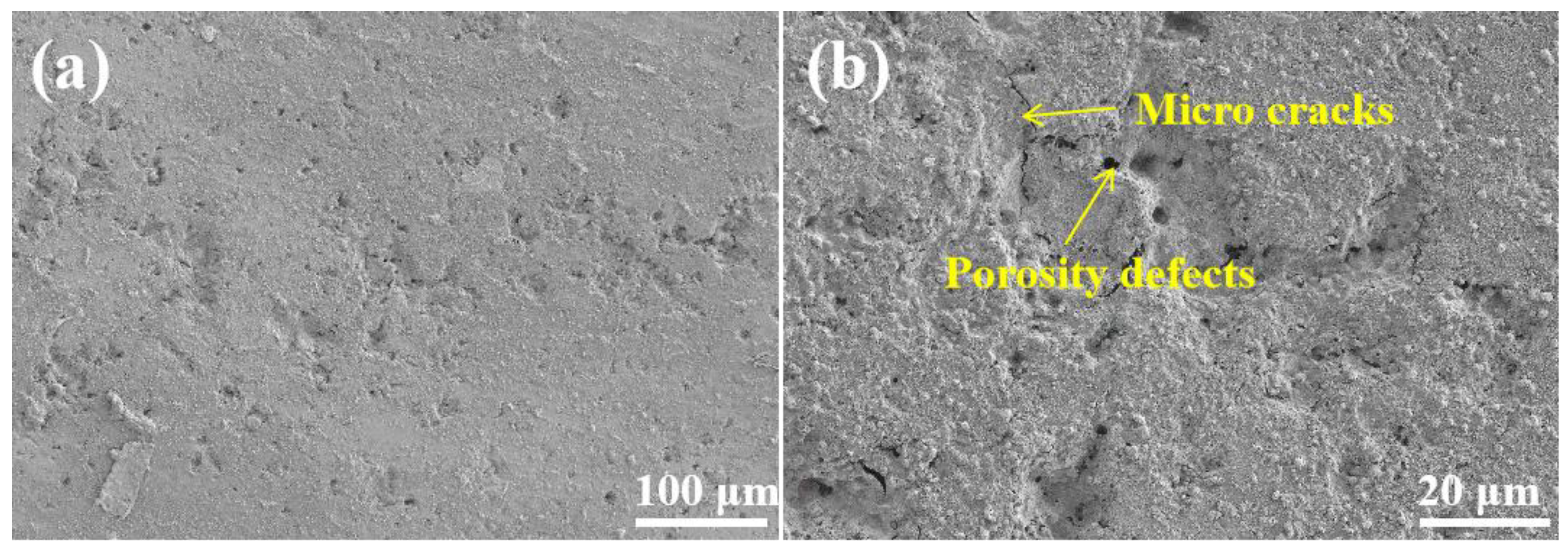
3.1. Morphology and Porosity
3.2. Vickers Hardness and Fracture Toughness
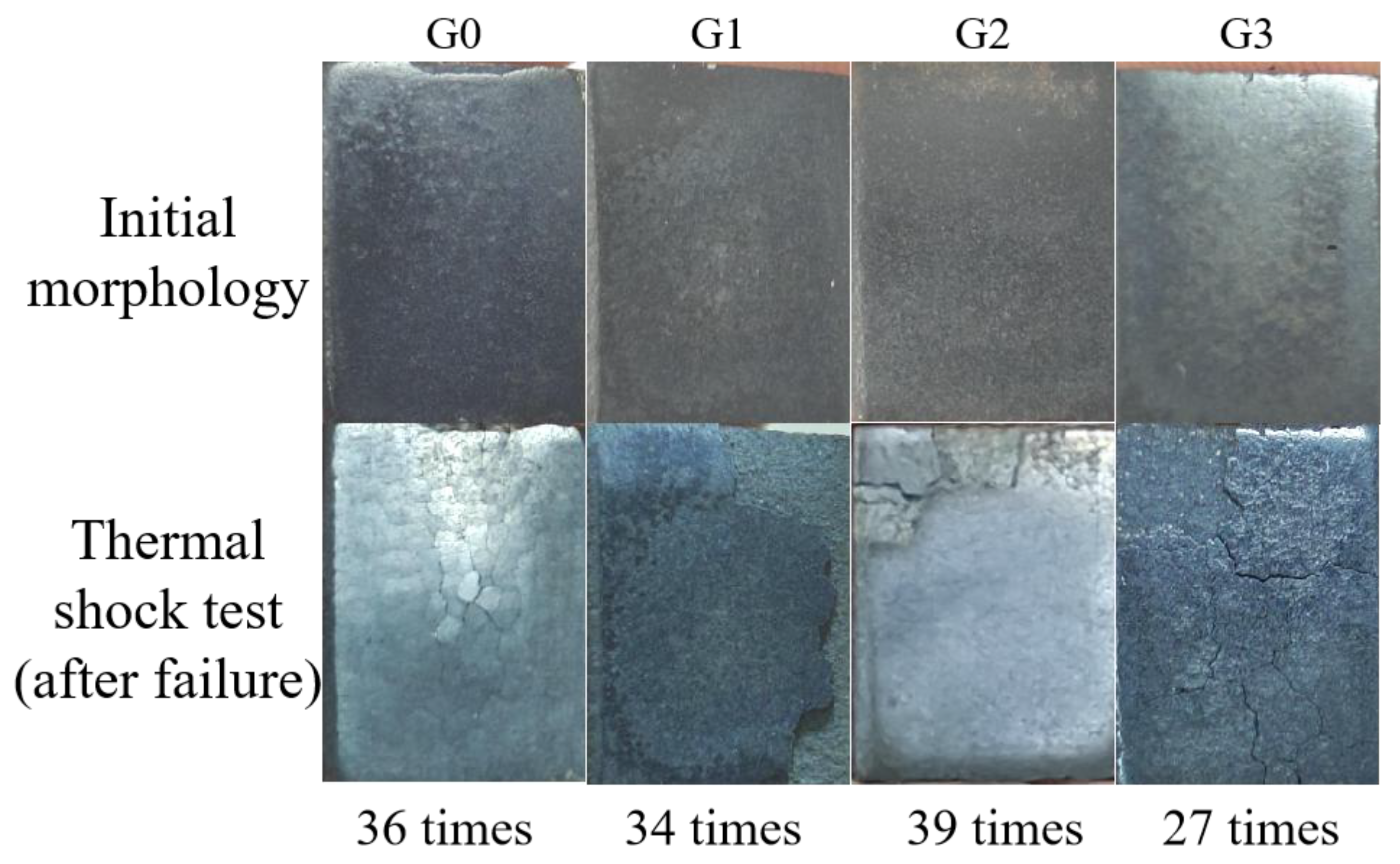
3.3. Thermal Shock Performance
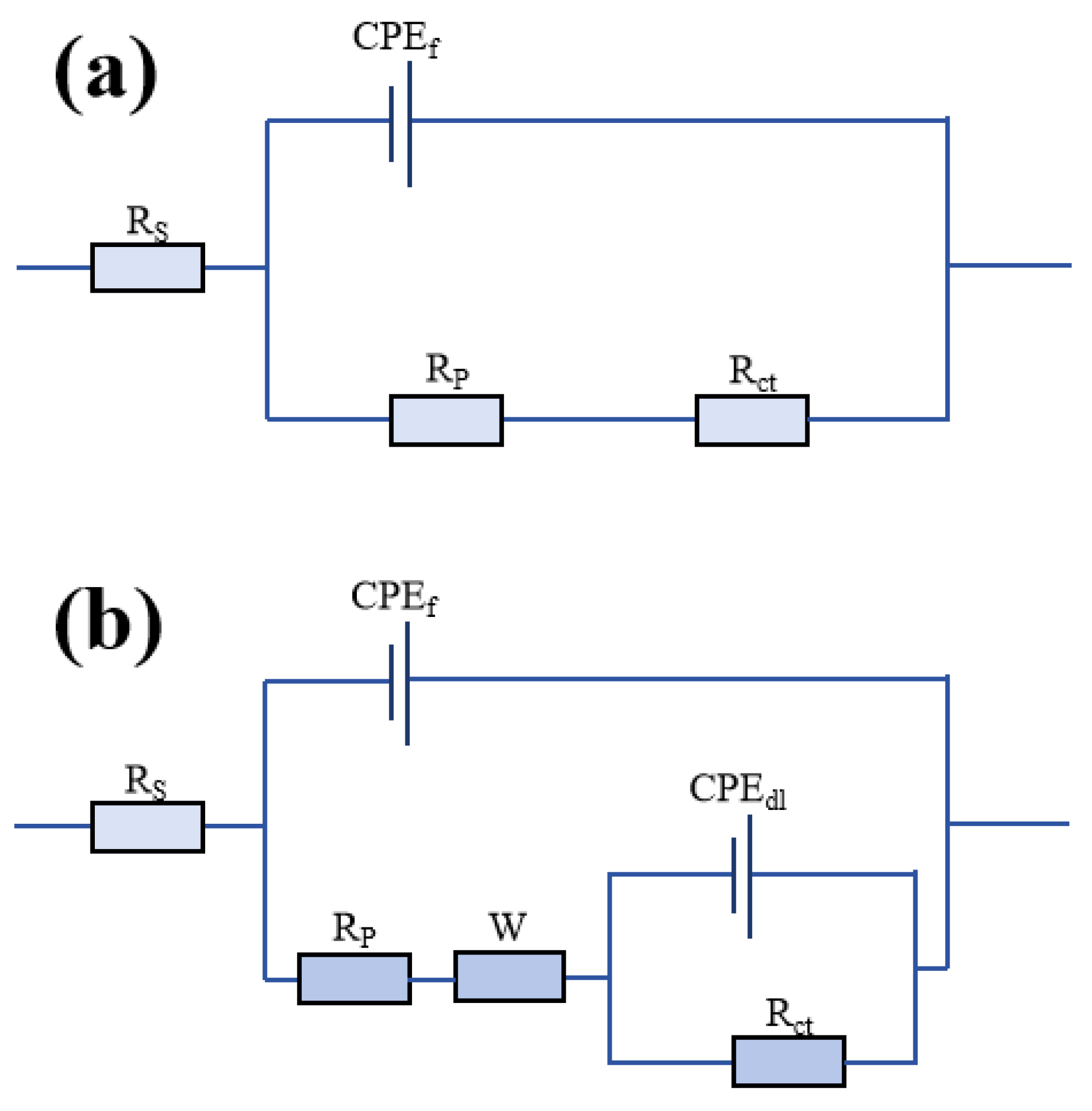
3.4. Corrosion Resistance
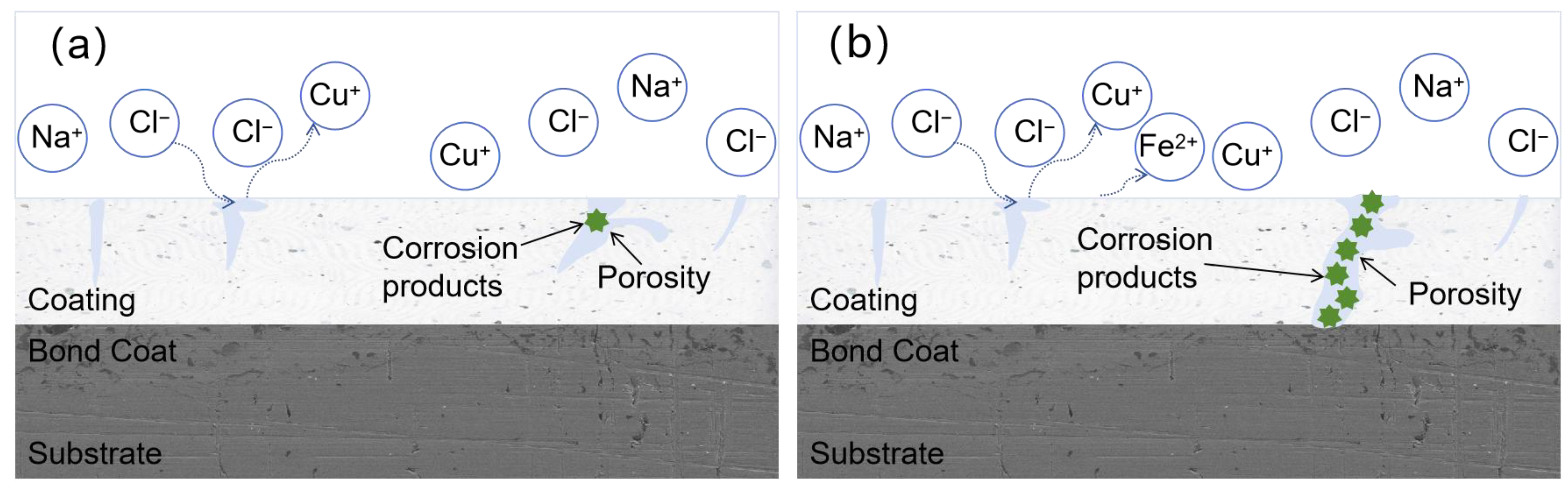
3.5. Corrosion Pattern of an AT13/Cu/GO Ceramic Coating in Artificial Seawater
3.6. Corrosion Failure Mechanism
4. Conclusions
- (1)
- Co-doping with GO/Cu promotes densification of the AT13 ceramic coating by filling the pore structure, resulting in a significant increase in its hardness. The coating G2, which was doped with 10 wt.% GO/Cu, had the lowest porosity (0.76% on the front side and 1.63% on the cross-section) and the highest Vickers hardness of 1152.28 HV0.3.
- (2)
- The incorporation of GO effectively increased the coating’s toughness and inhibited crack initiation and extension under sudden temperature changes. Coating G2 had the best fracture toughness of 3.56 MPa⋅m1/2 and the best thermal shock performance, failing after 39 thermal shock cycles.
- (3)
- As the GO/Cu doping ratio increases, the corrosion resistance of the coatings first rises and then decreases, and the G2 coating with a doping ratio of 10 wt.% had the best effect, with its self-corrosion current density decreasing by one order of magnitude compared to the original coating. At the same time, it had the lowest self-corrosion rate of 0.057 mm⋅a−1.
- (4)
- In the 31 d artificial seawater immersion experiment, the corrosion resistance of coating G2 decreased by an order of magnitude in 0–10 d due to erosion by corrosive media; during 10–18 d, the corrosion resistance recovered due to the accumulation of corrosion products; by 18 d, the corrosive media had diffused to the coating–substrate interface through pore defects, and the corrosion resistance further decreased; during 24–31 d, the generation and dissolution of corrosion products reached an equilibrium, and the corrosion resistance recovered; after 31 d, only a small number of pits and cracks were observed on the coating’s surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robey, H.N. Application of Variable Speed Constant Frequency Generators to Propulsion-Derived Ship Service. Nav. Eng. J. 1985, 97, 296–305. [Google Scholar] [CrossRef]
- Carter, T.J. Common failures in gas turbine blades. Eng. Fail. Anal. 2005, 12, 237–247. [Google Scholar] [CrossRef]
- Wang, J.; Chen, M.; Yang, L.; Sun, W.; Zhu, S.; Wang, F. Nanocrystalline coatings on superalloys against high temperature oxidation: A review. Corros. Commun. 2021, 1, 58–69. [Google Scholar] [CrossRef]
- Pan, W.; Liu, G.; Wang, X.; Zhao, M.; Zhao, C.; Ren, X.; Wang, Z.; Zheng, Y.; Yang, J.; Wan, C. Review of thermal barrier coating materials and technology development. Therm. Spray Technol. 2025, 17, 1–17. [Google Scholar]
- Yang, Z.; Fu, Y.; Wang, Y.; Gu, Y.; Huang, S.; Meng, F. Research progress of YSZ-based thermal barrier coatings. Surf. Technol. 2025, 54, 27–43. [Google Scholar]
- Li, D.; Li, X.; Zhu, Y.; Chen, P.; Zhu, B. Research progress of rare earth zirconate thermal barrier coating materials. Refractories 2021, 55, 258–263. [Google Scholar]
- Bogdan, M.; Peter, I. A comprehensive understanding of thermal barrier coatings (TBCs): Applications, materials, coating design and failure mechanisms. Metals 2024, 14, 575. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, T.; Guo, X. Diffusion barrier effect of Al2O3 layer at the interface between Mo-Si-B coating and Nb-Si based alloy. Corros. Commun. 2021, 4, 45–56. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Wang, W.; Tian, L.; Zhao, J.; Sun, J. Synergistic damage mechanisms of high-temperature metal corrosion in marine environments: A review. Progress Org. Coat. 2024, 197, 108765. [Google Scholar] [CrossRef]
- Cao, H.; Hou, G.; Xu, T.; Ma, J.; Wan, H.; An, Y.; Zhou, H.; Chen, J. Effect of seawater temperature on the corrosion and cavitation erosion-corrosion resistance of Al10Cr28Co28Ni34 high-entropy alloy coating. Corros. Sci. 2024, 228, 111822. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Lü, Z. Research progress of ceramic fibers for composite thermal barrier coatings. J. Liaoning Univ. Sci. Technol. 2023, 46, 254–261+307. [Google Scholar]
- Wei, X.; Yu, Q.; Yuan, P.; Jin, P.; Li, J.; Zhu, Q.; Sun, C. Influence of TiO2 dopingand spraying power on the microstructure, mechanical properties and cavitation erosion resistance of YSZ coatings. Ceram. Int. 2024, 50, 29244–29255. [Google Scholar] [CrossRef]
- Mehar, S.; Sapate, S.G. Contact severity maps for plasma sprayed YSZ modified Al2O3-3% TiO2 coatings. Ceram. Int. 2024, 50, 13003–13013. [Google Scholar] [CrossRef]
- Pandey, U.; Sharma, C. Comparative study of graphene oxide-multifunctional oxide doping on corrosion resistance of electrodeposited nickel coatings in saline environments. Int. J. Hydrogen Energy 2024, 60, 165–179. [Google Scholar] [CrossRef]
- Amudha, A.; Nagaraja, H.S.; Shashikala, H.D. Plasma-sprayed graphene oxide reinforced alumina composite coatings on low carbon steel with improved fracture toughness, brittleness index, and microhardness. Mater. Today Proc. 2021, 39, 1503–1508. [Google Scholar] [CrossRef]
- IS 2062; Hot Rolled Medium and High Tensile Structural Steel. Bureau of Indian Standards (BIS): New Delhi, India, 2013.
- Zhang, C.; Wu, W.; Hu, H.; Rui, Z.; Ye, J.; Shen, H. Effect of multidimensional filler hybridization on the mechanical properties of thermoplastic polyurethane composites prepared by selective laser sintering. J. Appl. Polym. Sci. 2023, 140, e54041. [Google Scholar] [CrossRef]
- Li, Y.; Qu, S.; Liu, T.; Lu, B.; Feng, X.; Yang, Y. High temperature oxidation behavior of γ′-strengthened CoNi-base superalloys. Corros. Commun. 2024, 16, 61–70. [Google Scholar] [CrossRef]
- Shi, S.; Ye, Z.; Li, X.; Xu, X.; Zhao, W.; Sun, J. Corrosion damages of nickel-based alloy G3 in 600 °C H2OCO2H2SH2 steam. Corros. Commun. 2024, 15, 42–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Chen, S.; Liu, J.; Li, T.; Wang, L.; Wu, K. Corrosion behavior and passive film properties of nickel-based alloy in phosphoric acid. Corros. Commun. 2023, 9, 77–88. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, H.; Guo, L. Crystallization behavior of calcium–magnesium–alumina–silicate coupled with NaCl/Na2SO4. Corros. Commun. 2023, 10, 1–9. [Google Scholar] [CrossRef]
- Gong, L.; Alamarguy, D.; Ruscassier, N.; Haghi-Ashtiani, P.; Giorgi, M.L. Understanding of selective oxidation of Fe-Mn binary alloys during continuous annealing through X-ray photoelectron spectroscopy. Corros. Commun. 2023, 11, 72–82. [Google Scholar] [CrossRef]
- Wang, P.; Du, K.; Yin, H.; Wang, D. Corrosion and protection of metallic materials in molten carbonates for concentrating solar power and molten carbonate electrolysis applications. Corros. Commun. 2023, 11, 58–71. [Google Scholar] [CrossRef]
- Evans, A.G.; Charles, E.A. Fracture toughness determinations by indentation. J. Am. Ceram. Soc. 1976, 59, 371–372. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Huang, Q.; Zhang, C.; Zhang, L. Characteristics and thermal shock resistance of HVOF-sprayed TiAlNb coatings. J. Therm. Spray Technol. 2020, 29, 1752–1762. [Google Scholar] [CrossRef]
- Hahn, T.A. Thermal expansion of copper from 20 to 800 K—Standard reference material 736. J. Appl. Phys. 1970, 41, 5096–5101. [Google Scholar] [CrossRef]
- Chen, M.; Xu, Z.; Kim, J.H.; Seol, S.K.; Kim, J.T. Meniscus-on-demand parallel 3D nanoprinting. ACS Nano 2018, 12, 4172–4177. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z.; Yang, Z.; Zhao, Q.; Zhang, L.; Xu, Y.; Hao, J.; Zhan, H.; Zhao, Y. Study on self-sealing effect of GO/TiO2 ceramic coating regulated by graphene oxide. Rare Metal. Mater. Eng. 2021, 50, 3621–3626. (In Chinese) [Google Scholar]
- Song, R.G.; Wang, C.; Jiang, Y.; Li, H.; Lu, G.; Wang, Z.X. Microstructure and properties of Al2O3/TiO2 nanostructured ceramic composite coatings prepared by plasma spraying. J. Alloys Compd. 2012, 544, 13–18. [Google Scholar] [CrossRef]
- Dejang, N.; Watcharapasorn, A.; Wirojupatump, S.; Niranatlumpong, P.; Jiansirisomboon, S. Fabrication and properties of plasma-sprayed Al2O3/TiO2 composite coatings: A role of nano-sized TiO2 addition. Surf. Coat. Technol. 2010, 204, 1651–1657. [Google Scholar] [CrossRef]
- Mei, D.; Lamaka, S.V.; Feiler, C.; Zheludkevich, M.L. The effect of small-molecule bio-relevant organic components at low concentration on the corrosion of commercially pure Mg and Mg-0.8 Ca alloy: An overall perspective. Corros. Sci. 2019, 153, 258–271. [Google Scholar] [CrossRef]
- Otterstein, E.; Karapetyan, G.; Nicula, R.; Stir, M.; Schick, C.; Burkel, E. Sol–gel synthesis and characterization of fine-grained ceramics in the alumina–titania system. Thermochim. Acta 2008, 468, 10–14. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, R.; Peng, C.; Yan, F.; Zhang, X. Corrosion behavior of Mg-Al-Pb and Mg-Al-Pb-Zn-Mn alloys in 3.5% NaCl solution. Trans. Nonferrous Met. Soc. China 2010, 20, 1936–1943. [Google Scholar] [CrossRef]
- Feng, Y.; Fang, J.; Wu, J.; Ou, Z.; Liu, P.; Sun, Z. Preparation and performance study of graphene/Al2O3 + 13%TiO2 self-lubricating coating by plasma spraying. Surf. Technol. 2019, 48, 189–195. (In Chinese) [Google Scholar]
- Hu, Y.; Xu, C.; Xiao, G.; Yi, M.; Guo, R. Preparation and properties of alumina/graphene oxide composite ceramics. J. Chin. Ceram. Soc. 2016, 44, 432–437. [Google Scholar]
- Deng, W.; Li, T.; Li, H.; Dang, A. Research progress of graphene/ceramic composites. Solid Rocket Technol. 2022, 45, 13–22. (In Chinese) [Google Scholar]
- Shozib, I.A.; Ahmad, A.; Abdul-Rani, A.M.; Beheshti, M.; Aliyu, A.A.A. A review on the corrosion resistance of electroless Ni-P based composite coatings and electrochemical corrosion testing methods. Corros. Rev. 2022, 40, 1–37. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, R.; Fu, X.; Wang, X.; Zhou, J.; Yu, L. Influence of the Cu content on the electrochemical corrosion performances of Ni60 coating. Corros. Sci. 2022, 205, 110408. [Google Scholar] [CrossRef]
- Chen, C.; Yu, M.; Tong, J.; Xiong, L.; Li, Y.; Kong, X.; Liu, J.; Li, S. A review of fluorescence based corrosion detection of metals. Corros. Commun. 2022, 6, 1–15. [Google Scholar] [CrossRef]
- Isakhani-Zakaria, M.; Allahkaram, S.R.; Ramezani-Varzaneh, H.A. Evaluation of corrosion behaviour of Pb-Co3O4 electrodeposited coating using EIS method. Corros. Sci. 2019, 157, 472–480. [Google Scholar] [CrossRef]
- Wang, R.; Satake, Y.; Li, Y.; Xiao, T.; Honda, M.; Fukuhara, H.; Hiramatsu, S. Microstructure of Ni-based hard facing alloy and its polarization behavior in aqueous solution with different pH and chloride concentration. Corros. Commun. 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Song, G.L.; Zhang, C.; Chen, X.; Zheng, D. Galvanic activity of carbon fiber reinforced polymers and electrochemical behavior of carbon fiber. Corros. Commun. 2021, 1, 26–39. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Chen, Y.; Zhong, Z.; Ci, W.; Wu, J.; Xie, Z.; Yuan, Y.; Pan, F. A self-healing corrosion protection coating with graphene oxide carrying 8-hydroxyquinoline doped in layered double hydroxide on a micro-arc oxidation coating. Corros. Sci. 2022, 194, 109941. [Google Scholar] [CrossRef]
- Cui, J.; Bao, Y.; Sun, Y.; Wang, H.; Li, J. Critical factors on corrosion protective waterborne coatings containing functionalized graphene oxide: A review. Compos. Part A Appl. Sci. Manuf. 2023, 174, 107729. [Google Scholar] [CrossRef]






| Powder | Particle Size (µm) | Purity | Morphology |
|---|---|---|---|
| AT13 | 15~45 | 99.9% | Near-spherical |
| NiCrAlY | 15~53 | 99.9% | Near-spherical |
| Cu | 1~5 | 99.9% | Spherical |
| GO | 0.2~10 | 98% | Flaky |
| Coating | NiCrAlY Bonding Layer | Composite AT13 Coating |
|---|---|---|
| Spraying Voltage | 70 v | 70 v |
| Spraying Current | 500 A | 600 A |
| Spraying Distance | 100 mm | 100 mm |
| Argon Gas Flow Rate | 40 L/min | 40 L/min |
| Gun Movement Speed | 800 mm/s | 600 mm/s |
| Powder Feeding Rate | 40 g/min | 40 g/min |
| Deposition Thickness | 100 μm | 250~300 μm |
| Reagents | NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | SrCl2 | NaHCO3 | KBr | H3BO3 | NaF |
|---|---|---|---|---|---|---|---|---|---|---|
| Dose (g/L) | 24.53 | 5.20 | 4.09 | 1.160 | 0.695 | 0.025 | 0.201 | 0.101 | 0.027 | 0.003 |
| Ecorr (mv) | Icorr (μA·cm−2) | corr Rate (mm/a) | |
|---|---|---|---|
| G0 | −841.2 | 28.47 | 0.331 |
| G1 | −710.3 | 7.905 | 0.091 |
| G2 | −717.9 | 4.958 | 0.057 |
| G3 | −738 | 6.97 | 0.081 |
| Time | CPEf (F) | Rp (Ω·cm2) | Rct (Ω·cm2) | CPEdl (F) | Goodness of Fit |
|---|---|---|---|---|---|
| 1 d | 1.64 × 10−5 | 3.74 × 103 | 1.60 × 104 | - | 1.47 × 10−5 |
| 4 d | 2.41 × 10−6 | 7.47 × 102 | 7.23 × 103 | - | 1.57 × 10−5 |
| 10 d | 1.79 × 10−6 | 4.53 × 102 | 3.67 × 103 | - | 1.13 × 10−5 |
| 18 d | 1.85 × 10−6 | 6.58 × 102 | 3.31 × 103 | 1.44 × 10−5 | 4.71 × 10−4 |
| 24 d | 5.81 × 10−7 | 4.79 × 102 | 7.5 × 102 | 1.57 × 10−5 | 2.96 × 10−4 |
| 31 d | 9.82 × 10−6 | 8.56 × 102 | 9.56 × 103 | 5.17 × 10−6 | 3.71 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Chu, Z.; Xu, J.; Tang, W.; Gao, L. Research on the Corrosion Resistance and Mechanical Properties of Graphene Oxide–Modified AT13 Coatings. Materials 2025, 18, 2168. https://doi.org/10.3390/ma18102168
Xu Y, Chu Z, Xu J, Tang W, Gao L. Research on the Corrosion Resistance and Mechanical Properties of Graphene Oxide–Modified AT13 Coatings. Materials. 2025; 18(10):2168. https://doi.org/10.3390/ma18102168
Chicago/Turabian StyleXu, Yuchen, Zhenhua Chu, Jingxiang Xu, Wan Tang, and Li Gao. 2025. "Research on the Corrosion Resistance and Mechanical Properties of Graphene Oxide–Modified AT13 Coatings" Materials 18, no. 10: 2168. https://doi.org/10.3390/ma18102168
APA StyleXu, Y., Chu, Z., Xu, J., Tang, W., & Gao, L. (2025). Research on the Corrosion Resistance and Mechanical Properties of Graphene Oxide–Modified AT13 Coatings. Materials, 18(10), 2168. https://doi.org/10.3390/ma18102168







