1. Introduction
Zirconia (ZrO
2) has been widely studied due to its excellent mechanical, thermal, and chemical properties, even under extremely harsh conditions. Notably, zirconia exhibits high toughness on par with metals, despite being a ceramic material, which makes it an ideal candidate for a wide range of material development applications, both structural and functional [
1,
2,
3,
4]. Molten slag in steelmaking processes is a highly basic and alkaline substance that causes significant chemical erosion of refractory materials. Therefore, zirconia (ZrO
2), with high mechanical properties and chemical durability under extreme conditions, is used as the refractory material in areas that come into contact with molten slag. Zirconia can exhibit significant changes in crystal structure and mechanical properties depending on the temperature. The monoclinic phase is stable below 1170 °C, the tetragonal phase is stable between 1170 °C and 2370 °C, and the cubic phase is stable above 2370 °C. The transition from the tetragonal to the monoclinic phase involves volume expansion, which causes cracks and grain boundary fractures, leading to a rapid decrease in strength [
5,
6,
7]. Therefore, various research projects have been conducted to improve the mechanical and thermal properties of zirconia used in high-temperature environments by using compounds such as Y
2O
3, MgO, Al
2O
3, Bi2O
3, and CaO as phase stabilizers [
8,
9]. Doping with these stabilizers reduces the transformation of the tetragonal phase, resulting in a structure with lower residual stress within the lattice [
10,
11]. The type and concentration of stabilizers determine whether zirconia becomes partially stabilized zirconia (PSZ) or fully stabilized zirconia (FSZ) at high temperatures. PSZ and FSZ absorb the stress generated when cracks occur by transforming into the monoclinic phase, which enhances mechanical properties. Therefore, the type and amount of stabilizers affect the phase stabilization of zirconia [
12,
13,
14]. CaO-doped zirconia exhibits superior mechanical properties under high-temperature conditions, such as higher hardness and better high-temperature oxidation resistance, compared to zirconia doped with other stabilizers. Due to these properties and relatively low cost, CaO-doped zirconia is primarily used in structural ceramics like refractories [
15,
16,
17]. CaO-PSZ demonstrates outstanding mechanical and thermal properties, but prolonged use at temperatures exceeding 1500 °C can cause destabilization due to the instability of Ca. Therefore, research is necessary to prevent the destabilization of CaO-PSZ and to improve its mechanical and thermal properties at high temperatures.
Previous research confirmed the trend in the mechanical properties of CaO-PSZ with an increase in CaO doping from 5 to 10 mol%, identifying that 9 mol% CSZ exhibited the most superior mechanical properties [
18]. K.H. Heussner demonstrated that doping ZrO
2 with CeO
2 in nitrogen or oxygen atmospheres can enhance its mechanical strength [
19]. Y. Nigara confirmed that doping CSZ with CeO
2 improves oxygen permeability [
20]. However, studies have not yet explored the changes in mechanical and chemical properties resulting from the additional doping of CeO
2 in zirconia that has been enhanced through CaO doping.
To further improve the properties and chemical stability of CSZ, we doped 9 mol% CSZ with CeO2, which is chemically more stable than CaO and can substitute larger Ce4+ ions (97 pm) for Zr4+ ions (84 pm) in the lattice, investigating the resulting changes in properties and chemical stability against molten slag.
2. Materials and Methods
The 9 mol% CSZ was synthesized using ZrO
2 (99%, Daejung Chemicals & Metals Co., Siheung-si, Republic of Korea) and CaO (98%, Junsei Chemical Co., Tokyo, Japan) as starting materials through a solid-state method. The synthesized 9CSZ was then doped with CeO
2 (99.9%, Junsei Chemical Co., Tokyo, Japan) according to the ratios specified in
Table 1. The mixture was ball-milled with zirconia balls and ethanol for 24 h, dried at 90 °C for 24 h, and then calcined at 1200 °C with a heating rate of 5 °C per minute for 2 h.
The test specimens were prepared by uniaxially pressing the synthesized powder at a pressure of 1 ton/cm3 to form cylindrical (20 mm diameter) and rod-shaped (5 mm width, 35 mm length) bodies. These formed bodies were then sintered in a box-type furnace, with a heating rate of 5 °C per minute, at 1600 °C for 6 h, followed by furnace cooling. The thermal shock test for the specimens involved heating the sintered samples in an air atmosphere to 1300 °C at a rate of 5 °C per minute, holding them at that temperature for 5 min, and then allowing them to cool naturally to 900 °C. This cycle was repeated 40 times to apply thermal shock.
Erosion tests were performed to evaluate the chemical stability of each specimen in the slag. Five grams of CSZ powder was pressed into pellets using a cylindrical mold with a diameter of 15 mm under a pressure of 1 ton/m
2. The formed CSZ specimens were placed in a graphite crucible, and 500 g of slag, as specified in
Table 2, was added. The crucible was then maintained at 1550 °C for 3 h and subsequently cooled in the furnace. Since the slag dissolves in Al
2O
3, SiO
2, and MgO, a graphite crucible, which does not react with the slag, was used for the reactivity test. After cooling was completed following the melting process, the crucible was broken to retrieve the CSZ specimens from within the slag. The inner parts of the specimens, which were free from slag contamination, were then crushed and subjected to XRD analysis.
To evaluate the chemical stability of each specimen, we conducted reactivity tests with slag. The pellet-shaped specimens were placed in a graphite crucible, and the slag specified in
Table 2 was added. The samples were then maintained at 1550 °C for 3 h, followed by furnace cooling. Since the slag has solubility in Al
2O
3, SiO
2, and MgO, a graphite crucible, which does not react with the slag, was used for the reactivity tests.
A crystal structure analysis was performed using X-ray diffraction (XRD, Rigaku, Ultima-IV, Japan) with measurements taken from 20° to 80° at a step size of 0.02°/2θ and a scanning speed of 2°/min. The XRD peaks and phase fractions were analyzed using the Rietveld refinement method with Highscore Plus software (version 3.0c) with a reference pattern for monoclinic ZrO
2 (m-ZrO
2, ICSD 98-006-0900) and tetragonal ZrO
2 (t-ZrO
2, ICSD 98-007-0014). And the monoclinic phase fraction was double-checked for integrated intensity by using ISO 5803 [
21].
Equation (1) is for a two-phase system (monoclinic and tetragonal phases), and Equation (2) is for a multi-phase system (a mixture of monoclinic, tetragonal, and cubic).
X is the integrated intensity ratio, where
and
refer to the integral intensity of the monoclinic X-ray diffraction pattern.
refers to the integral intensity from the (101) plane of the tetragonal and
is the total integrated intensity of the tetragonal phase (101) and cubic phase (111) reflections. The volume fraction of the monoclinic phase was calculated using Equation (3):
is the volume fraction of the monoclinic phase and P is the intensity factor. In the monoclinic-tetragonal ZrO2 system, P = 1.219 was used, while in the multiphase system, P = 1.265 was used.
We used a high-resolution scanning electron microscope (HR-SEM, SU8230, Hitachi, Tokyo, Japan) to observe the microstructural changes on the specimen surfaces. The mechanical properties were assessed using a micro Vickers hardness tester (Wilson, VH1102, Lake Bluff, IL, USA) under test conditions of 20 N load and a dwell time of 15 s. Each measurement was repeated 10 times, and the Vickers hardness was calculated using the average value. Wear resistance was measured by determining the specific wear rate (m
3/N) under the conditions of a sliding distance of 1000 m, a load of 9.6 N, a velocity of 0.1 m/s, and an operating type of ball-on-disk. The measurements were conducted in accordance with the ISO 20808 testing method [
22]. The flexural strength of each specimen was measured using a universal testing machine from the United States, with a lower support-point distance of 20 mm and a crosshead speed of 0.5 mm/min in a three-point bending test.
3. Results and Discussion
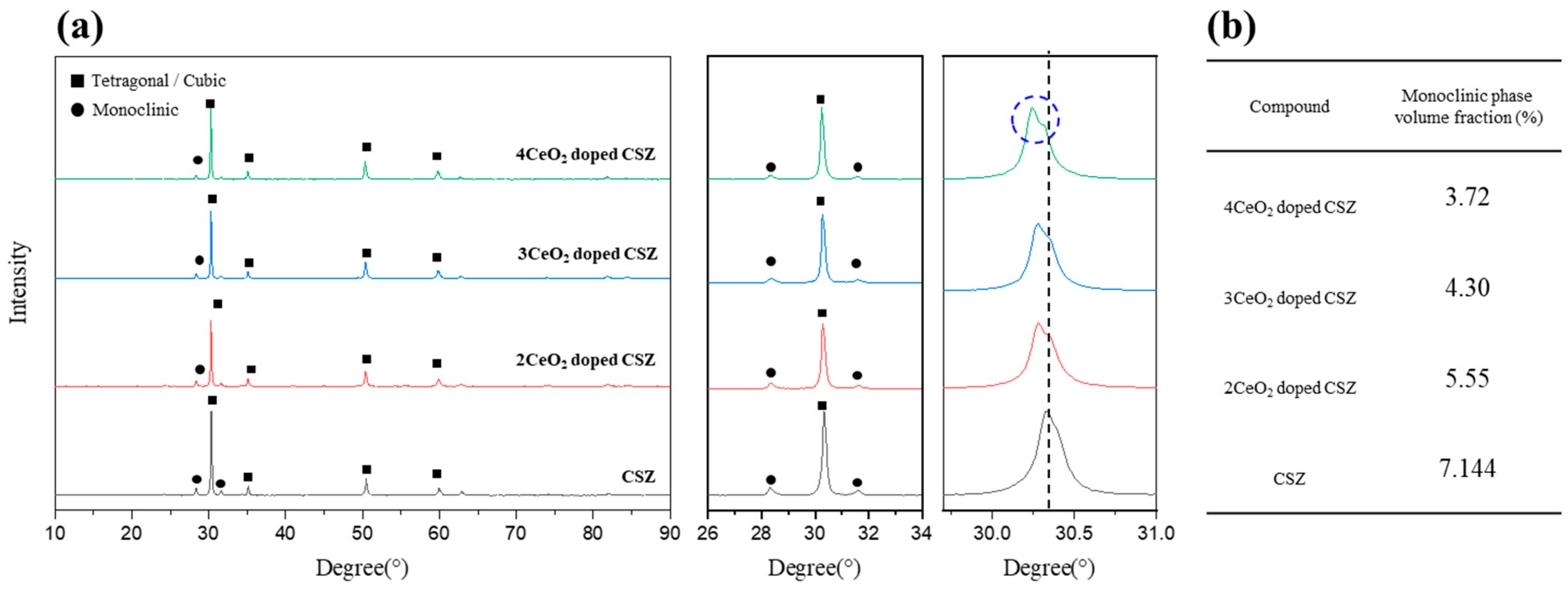
The results of the phase analysis after doping CeO
2 at 2–4 mol% into 9 mol% CSZ are shown in
Figure 1. The monoclinic fraction (V
m) of zirconia was 7.14% for 9CSZ, while under doping conditions of 2–4 mol% CeO
2, it decreased slightly to 5.55% and 3.72%, respectively, compared to CSZ, without showing any abrupt change. CeO
2 doping has a lesser effect on improving phase stability at room temperature because the Ce
4+ ion has the same charge state as the Zr
4+ ion, resulting in no formation of oxygen vacancies due to charge compensation. Phase stabilization is induced by lattice stress caused by the ionic radius difference between Zr
4+ (84 pm) and Ce⁴⁺ (97 pm). The doping of Ce
4+, which has a larger ionic radius than Zr
4+, leads to lattice contraction, which is observed as a low-angle shift in the XRD pattern. In particular, the peak splitting between 30° and 30.5° observed in 4 mol% CeO₂-doped CSZ suggests the formation of a cubic phase.
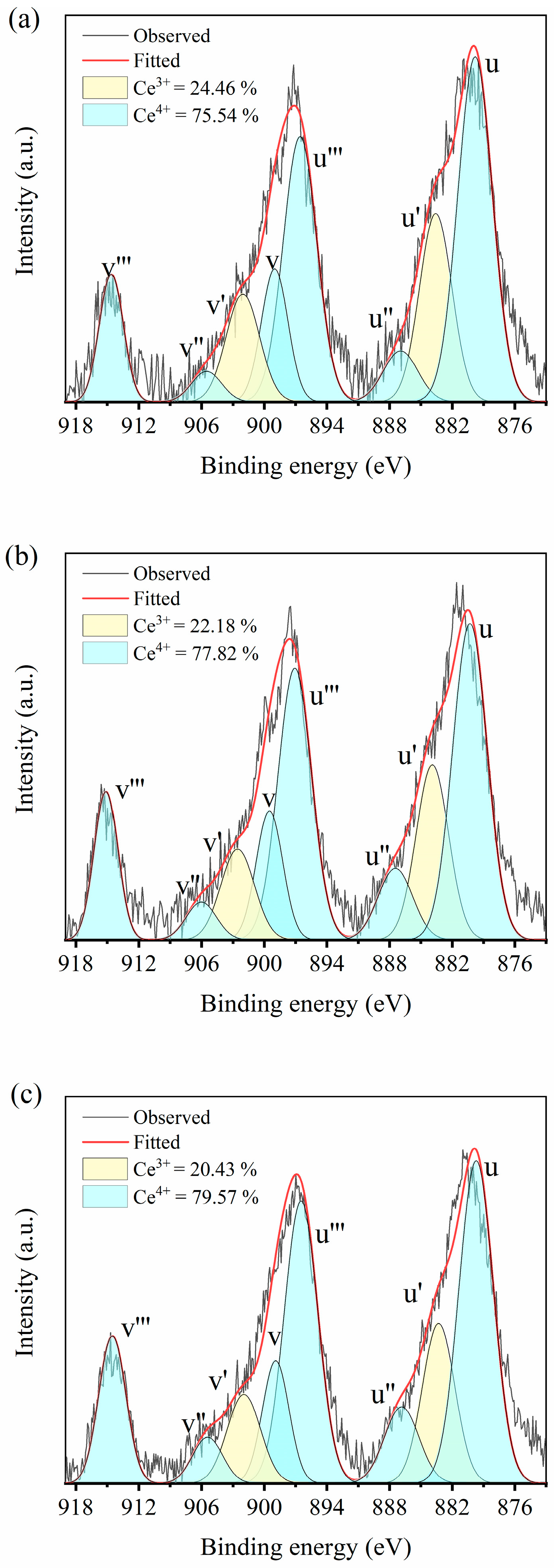
Figure 2 shows the Ce 3d XPS spectrum of CeO
2-doped CSZ after wet ball milling and calcination at 1200 °C in an air atmosphere for 2 h. The sub-bands denoted as u′ and v′ in the XPS spectrum corresponded to the initial electron state of 3d
104f
1 of Ce
3+, while u, u′′, u′′′, v, v′′, and v′′′ represented the 3d
104f
0 state of Ce
4+ [
23]. It is evident that Ce predominantly existed in the form of Ce
4+ within the zirconia lattice, and with increasing CeO
2 doping, the proportion of Ce
4+ increased from 73.98% in 1Ce_CSZ to 79.57% in 4Ce_CSZ. This trend indicates that local lattice tension increased with increased CeO
2 doping due to the ionic radius differences among Ce
3+ (114 Å), Ce
4+ (97 Å), and Zr
4+ (86 Å). The proportion of Ce
3+ decreased to alleviate local lattice tension induced by the difference in ionic size [
24]. The stabilizing effect of CeO
2 doping is primarily due to lattice stress caused by the ionic radius difference between Zr
4+ and Ce
4+, not due to oxygen vacancy formation by charge compensation of Ce
3+ [
25]. CeO
2 doping leads to an increase in the tetragonal phase, which can lead to improving mechanical properties by transformation toughening under external mechanical stress [
26,
27].
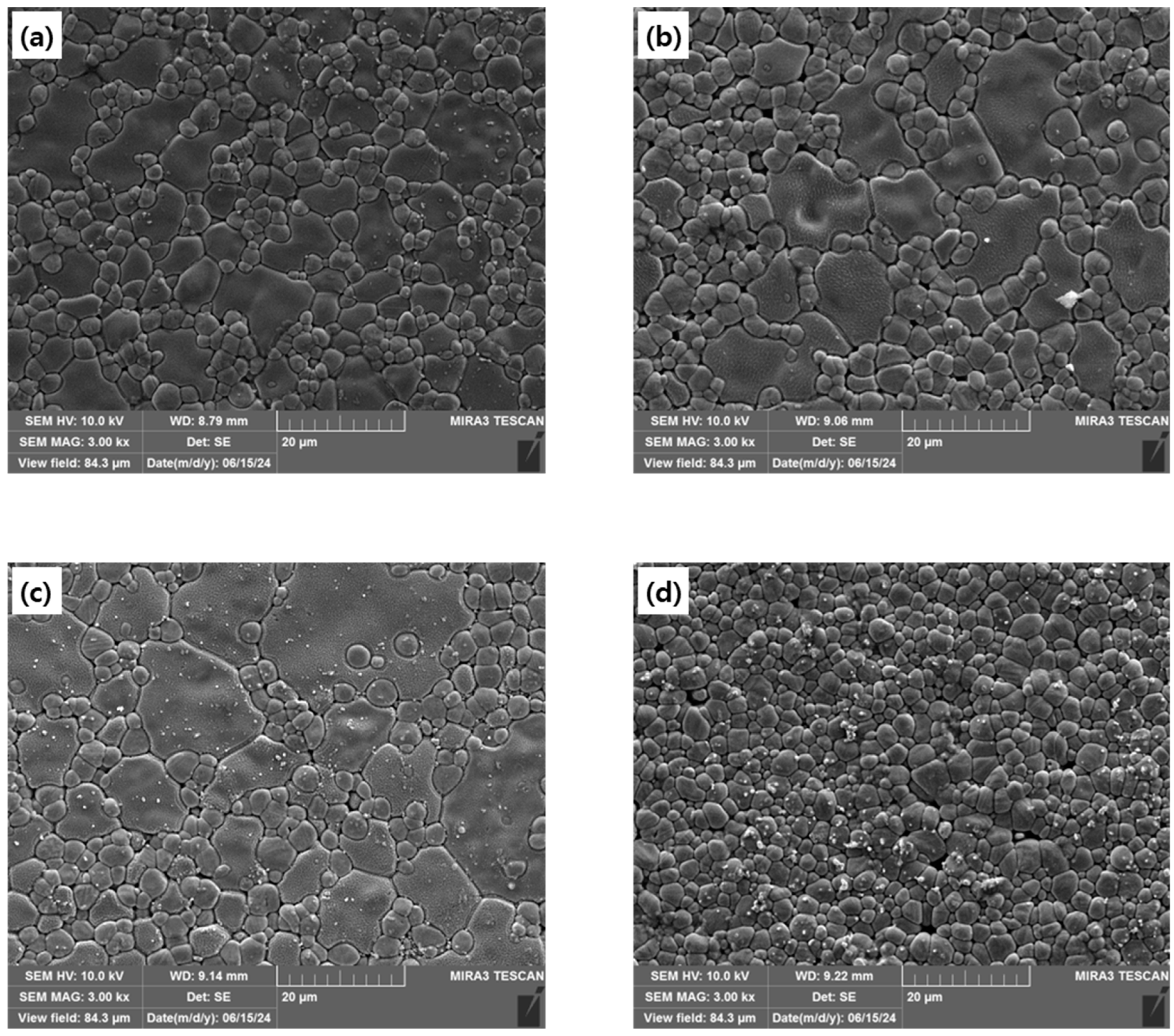
Figure 3 shows the microstructures of each specimen. The grain size of 9CSZ doped with 2–3 mol% CeO
2 was comparable to that of 9CSZ, exhibiting a mixture of large grains ranging from 11.9 to 18 μm and small grains below 6 μm. In contrast, 9CSZ doped with 4 mol% CeO
2 showed only small grains below 5.8 μm. The changes in the microstructural distribution in 4 mol% CeO
2-doped CSZ are thought to occur because the increased amount of CeO₂ doping, which does not sinter well, prevents grain growth during heat treatment.
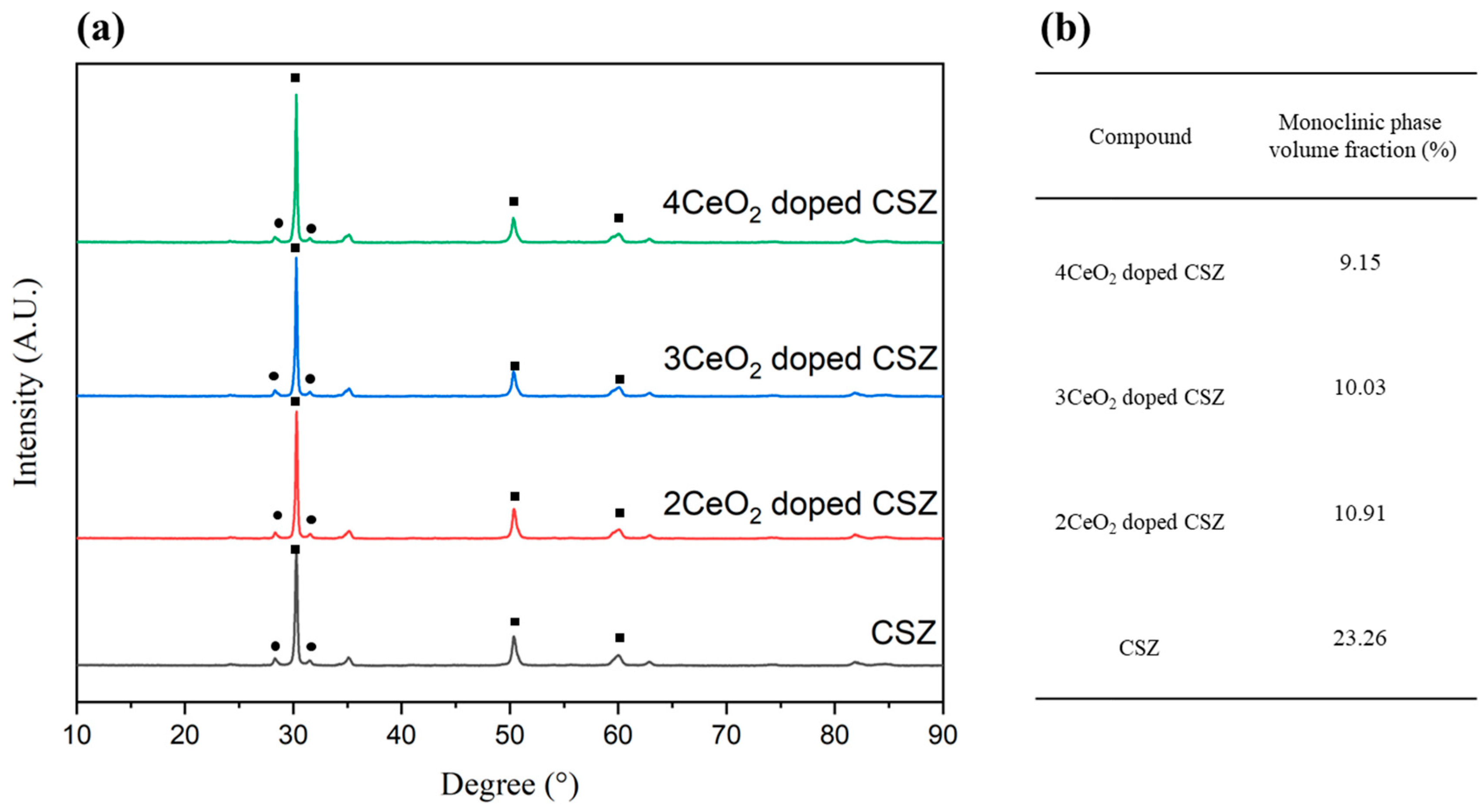
The phase analysis results after applying thermal stress to 2–4 mol% CeO
2-doped CSZ are shown in
Figure 4. The monoclinic phase fraction (V
m) of zirconia was significantly increased from 7.14% to 23.26% in the case of 9CSZ, whereas under the 2–4 mol% CeO
2 doping conditions, it was relatively less changed to 10.91%, 10.03%, and 9.15%, respectively. These results indicate that the phase stability of 9CSZ with CeO
2 improved under thermal stress. The reduction in oxygen vacancies and the minimization of lattice structure distortion are believed to be caused by the substitution of Ce
4+, which has the same electric valence state as Zr
4+ and a similar ionic size.
The mechanical properties of each specimen were observed after subjecting 2–4 mol% CeO
2-doped CSZ to thermal shock under Δ400 °C (1300 and 900 °C) conditions.
Figure 5 shows the changes in Vickers hardness before and after thermal shock, indicating that the Vickers hardness gradually increased with an increasing amount of CeO
2 doping. The Vickers hardness of all specimens decreased after applying thermal shock. Specifically, 2–4 mol% CeO
2-doped CSZ showed a decrease of 20.3% (1254.9→1000.1 HV), 17.5% (1303.4→1077.9 HV), and 22.8% (1497.6→1156.7 HV), respectively. In contrast, 9CSZ exhibited a relatively larger decrease of approximately 41.5% (1088.4→637.0 HV).
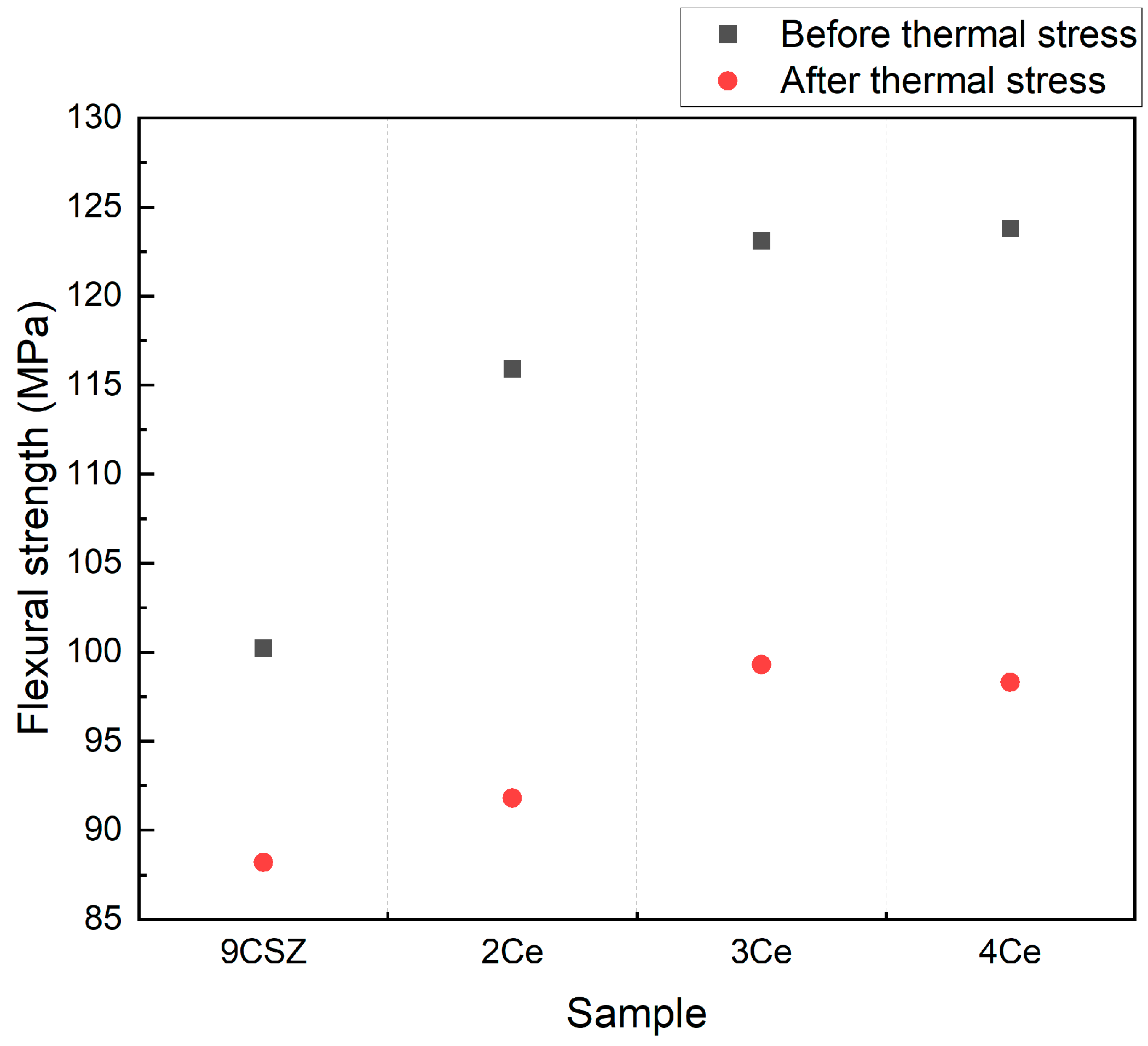
The flexural strength before and after thermal shock is shown in
Figure 6. The flexural strength gradually increased with an increase in CeO
2 doping content, and the flexural strength of all specimens decreased after thermal shock. Specifically, the flexural strength of 9 mol% CSZ before the thermal shock test was 100.23 MPa, while the flexural strength of 2–4 mol% CeO
2-doped CSZ was 115.9 MPa, 123.1 MPa, and 123.8 MPa, respectively. After thermal shock, the flexural strength of 9 mol% CSZ was 88.2 MPa, and the flexural strength of 2–4 mol% CeO
2-doped CSZ was 91.38 MPa, 99.3 MPa, and 98.3 MPa, respectively. The decrease in flexural strength of CeO
2-doped CSZ after the thermal shock was greater compared to 9 mol% CSZ, but it still exhibited higher values than 9 mol% CSZ even after the thermal shock. The increase in flexural strength for 4 mol% CeO
2-doped CSZ was smaller compared to 3 mol%-doped CSZ, which was consistent with the trends observed in the Vickers hardness results.
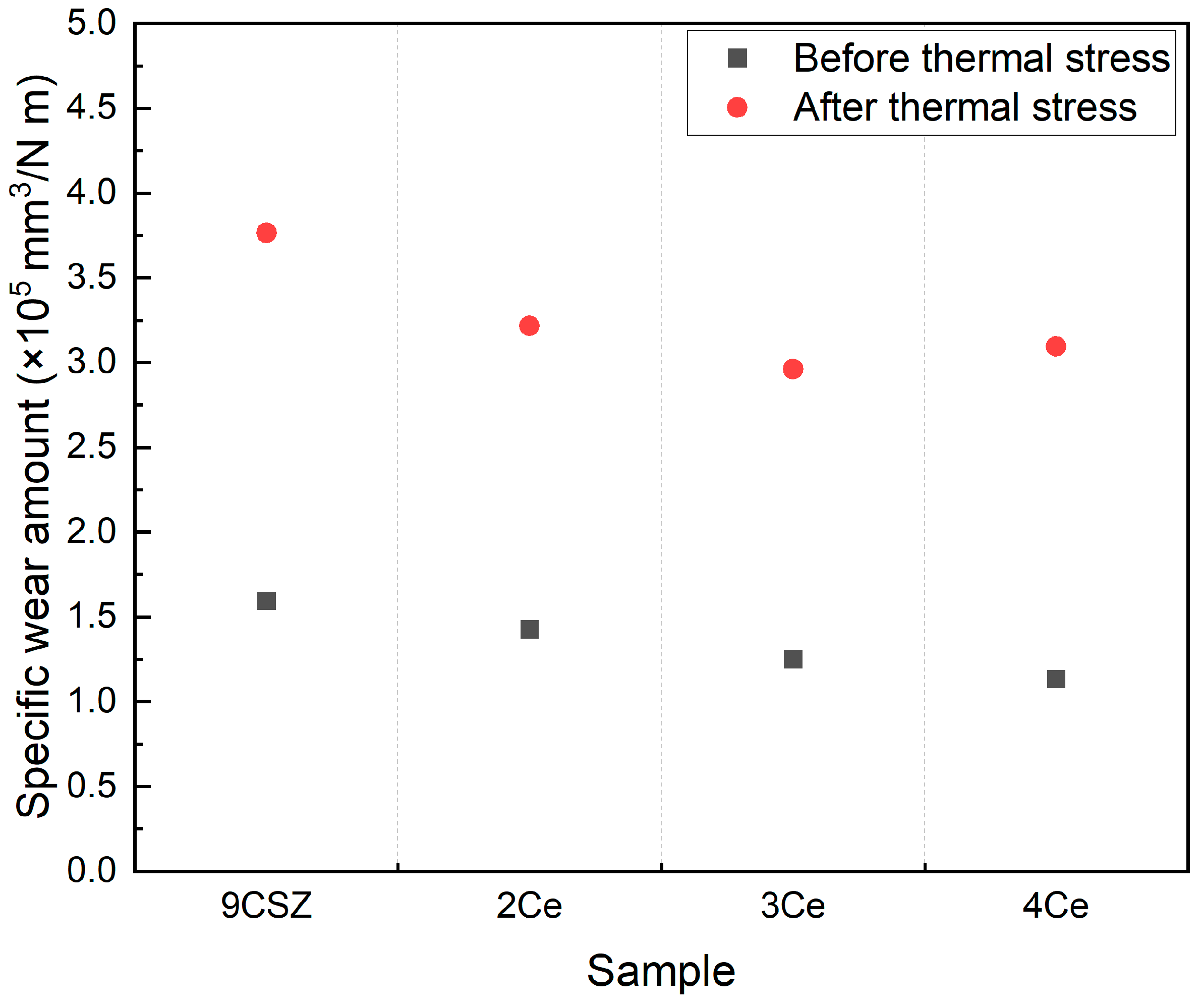
The results of the wear resistance evaluation of the specimens before and after thermal shock are shown in
Figure 7. The wear amount of the specimens decreased with increasing CeO
2 doping levels, and all specimens exhibited an increase in wear amount after thermal shock, which was consistent with the trend observed in Vickers hardness. The specific wear amount of 9CSZ increased by 136.2% (1.5941→3.7650 × 10
5 mm
3/Nm) before and after thermal stress. CeO
2-doped 9CSZ exhibited specific wear amount changes of 125.4% (1.4265→3.2160 × 10
5 mm
3/Nm), 136.7% (1.2512→2.9610 × 10
5 mm
3/Nm), and 173.4% (1.1320→3.0951 × 10
5 mm
3/Nm) before and after thermal stress as the CeO
2 doping level increased from 2 to 4 mol%. As the CeO
2 doping level increased, the increase in the wear amount before and after thermal stress also increased, with a particularly sharp change observed in the 4 mol% CeO
2-doped CSZ. The sharp increase in the wear amount before and after thermal stress in the 4 mol% CeO
2-doped CSZ is believed to be related to the formation of the cubic phase, as indicated by XRD analysis.
The results of
Figure 5,
Figure 6 and
Figure 7 show that doping 9CSZ with CeO
2 can further enhance the mechanical properties of CSZ. However, it was identified that doping with more than 4 mol% CeO
2 leads to a decrease in the property stability of CSZ after thermal stress.
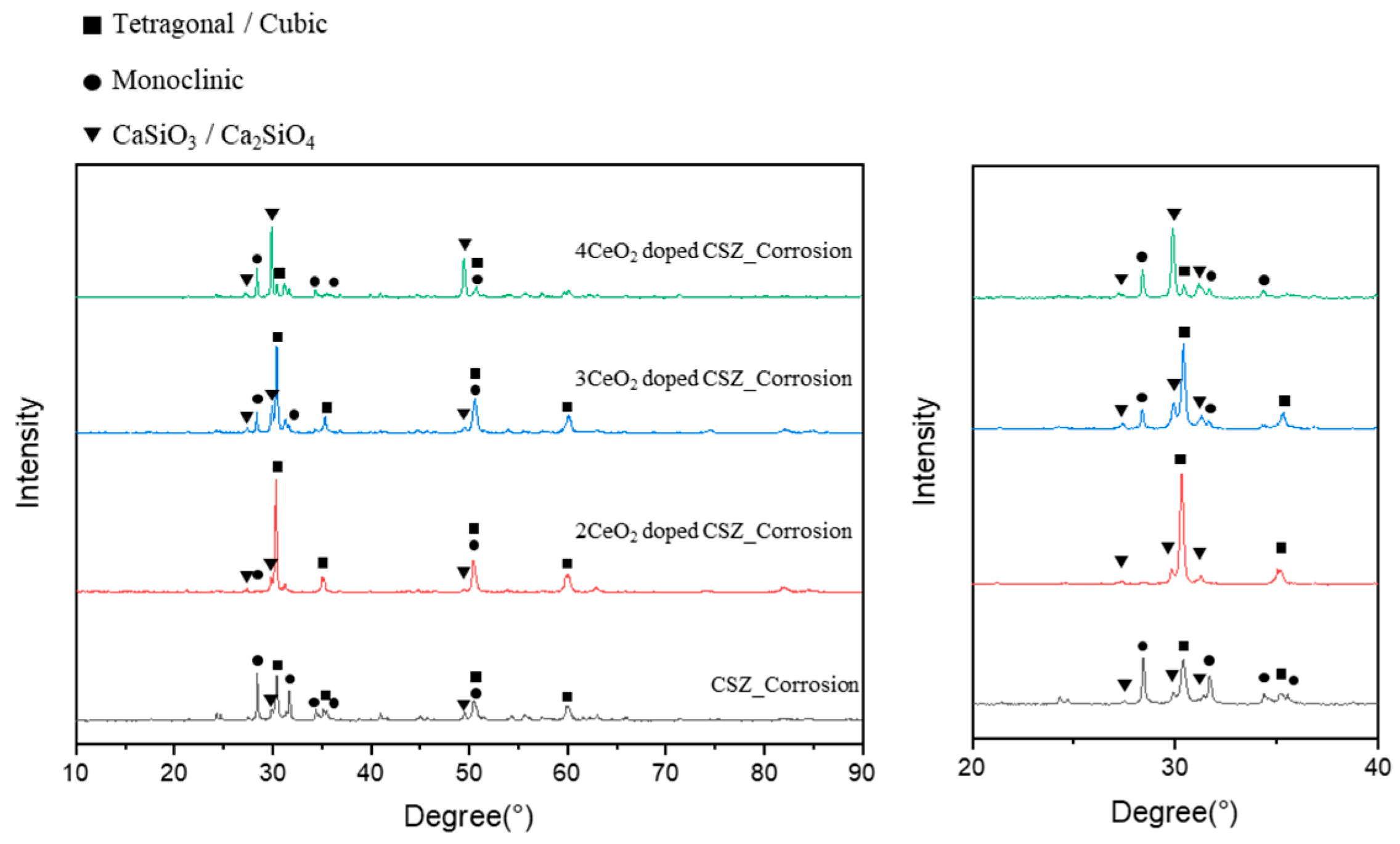
Figure 8 shows the phase transition of the CeO
2-doped CSZ after an erosion test with a highly basic CaF
2-based slag to examine the effect of CeO
2 doping on the erosion resistance of CSZ. The monoclinic phase fraction of 9CSZ increased significantly from 7.144% to 67.71% after the erosion test, while 2Ce_CSZ and 3 Ce_CSZ showed a relatively lower monoclinic phase fraction of 4.07% and 30.85%. The 4Ce_CSZ showed a higher monoclinic phase faction of 77.12%. As the CeO
2 content increased, the monoclinic phase fraction also tended to increase, with the 2 mol% CeO
2 doping condition showing the lowest monoclinic phase fraction.
The higher phase stability of CeO2-doped CSZ after chemical erosion with CaF2-based slag is considered to be due to the very low solubility of CeO2 in the slag, unlike CaO, which prevents the dopant from chemically dissolving into the slag at high temperatures. However, excessive CeO2 doping can lead to structural instability in CSZ, so it is important to investigate the optimal doping concentration.
4. Conclusions
We doped 9 mol% CaO-stabilized ZrO2 with 2–4 mol% CeO2 to investigate the mechanical and chemical changes due to CeO2 doping. The mechanical property changes under thermal stress and the behavior of the CSZ composition and phase changes after an erosion test in CaF2-based slag were then observed. The monoclinic phase fraction (Vm) of zirconia was 7.14% for 9CSZ, while under doping conditions of 2–4 mol% CeO2, it decreased slightly to 5.55% and 3.72%, respectively, compared to CSZ, without showing any abrupt change. The Ce4+ ion had the same charge state as the Zr4+ ion, resulting in no formation of oxygen vacancies due to charge compensation. Phase stabilization was induced solely by lattice stress caused by the difference in the ionic radii between Zr4+ (84 pm) and Ce4+ (97 pm). Therefore, doping with a small amount of CeO2 is presumed to have a lesser effect on improving phase stability at room temperature. In particular, the peak splitting between 30° and 30.5° observed in 4 mol% CeO₂-doped CSZ suggested the formation of a cubic phase. The grain size of 9CSZ doped with 2–3 mol% CeO2 was comparable to that of 9CSZ, while 9CSZ doped with 4 mol% CeO2 exhibited finer microstructure. This is presumed to be due to the cubic phase formed at 4 mol% doping.
The Vickers hardness increased from 1088.4 HV to 1497.6 HV as the CeO2 doping amount in 9CSZ increased. Even after applying a thermal shock of △400 °C (1300→900 °C), the CeO2-doped 9CSZ showed relatively higher values, increasing from 637.0 HV to 1156.7 HV, compared to 9CSZ. The specific wear amount before thermal stress decreased to 1.4265 × 105 mm3/Nm, 1.2512 × 105 mm3/Nm, and 1.1320 × 105 mm3/Nm with the addition of 2–4 mol% CeO2 doping, compared to the specific wear amount of CSZ, which was 1.5941 × 105 mm3/Nm. The specific wear amount of 9CSZ increased by 136.2% (1.5941→3.7650 × 105 mm3/Nm) before and after thermal stress. CeO2-doped 9CSZ exhibited specific wear amount changes of 125.4% (1.4265→3.2160 × 105 mm3/Nm), 136.7% (1.2512→2.9610 × 105 mm3/Nm), and 173.4%(1.1320→3.0951 × 105 mm3/Nm) before and after thermal stress as the CeO2 doping level increased from 2 to 4 mol%. As the level of CeO2 doping increased, the increase in wear amount before and after thermal stress also increased, with a particularly sharp change observed in CSZ doped with 4 mol% CeO2. The sharp increase in wear amount before and after thermal stress in CSZ doped with 4 mol% CeO2 is believed to be related to the formation of the cubic phase generated in 4 mol% CeO2-doped CSZ.
Doping CeO2 into 9CSZ resulted in a lower formation of the monoclinic phase compared to 9CSZ, even under the chemical reaction conditions with CaF2-based slag at 1550 °C. These results suggested that CeO2 doping in CSZ can enhance phase stability under high temperature and high alkalinity conditions. However, the monoclinic phase fraction was lowest at 2 mol% CeO2 doping, and it showed an increasing trend as the doping concentration increased beyond that.
As a result, CeO2 doping was found to improve the mechanical properties of 9 mol% CSZ and enhance its stability against CaF2-based slag at high temperatures. However, doping with more than 4 mol% CeO2 can cause a decrease in mechanical properties under thermal stress conditions of 1300–900 °C due to the formation of the cubic phase. Therefore, it is important to optimize the CeO2 doping concentration according to the operating conditions.