Typical Case of Converter Smelting with High Cooling Ratio in Chinese Iron and Steel Enterprises: CO2 Emission Analysis
Abstract
1. Introduction

2. Methodology and Analysis of Carbon Dioxide Emissions
2.1. Methodology for Estimating Carbon Dioxide Emissions Within China’s Steel Sector
2.1.1. Direct CO2 Emissions
2.1.2. CO2 Emissions from Energy Consumption
2.1.3. Life Cycle Assessment (LCA)-Based CO2 Emissions
2.2. Determination of CO2 Emissions from Converter Steelmaking Operation
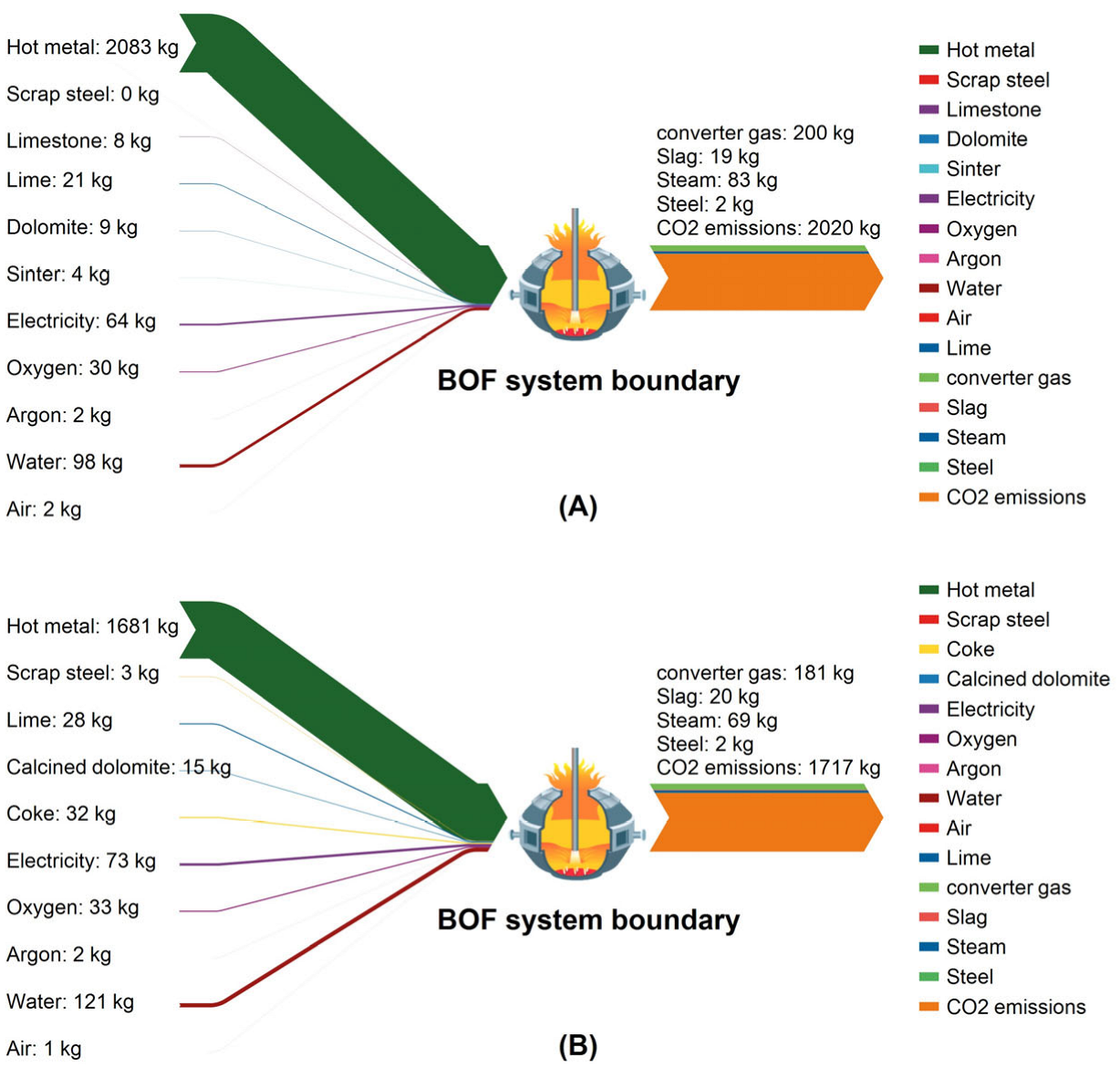
2.3. System Boundary and Parameterization
2.4. Determination of CO2 Emission Factors
2.4.1. Determination of Direct CO2 Emission Factors
2.4.2. Determination of Indirect CO2 Emission Factors
3. Calculation and Assessment of CO2 Emissions for Converter Smelting Processes
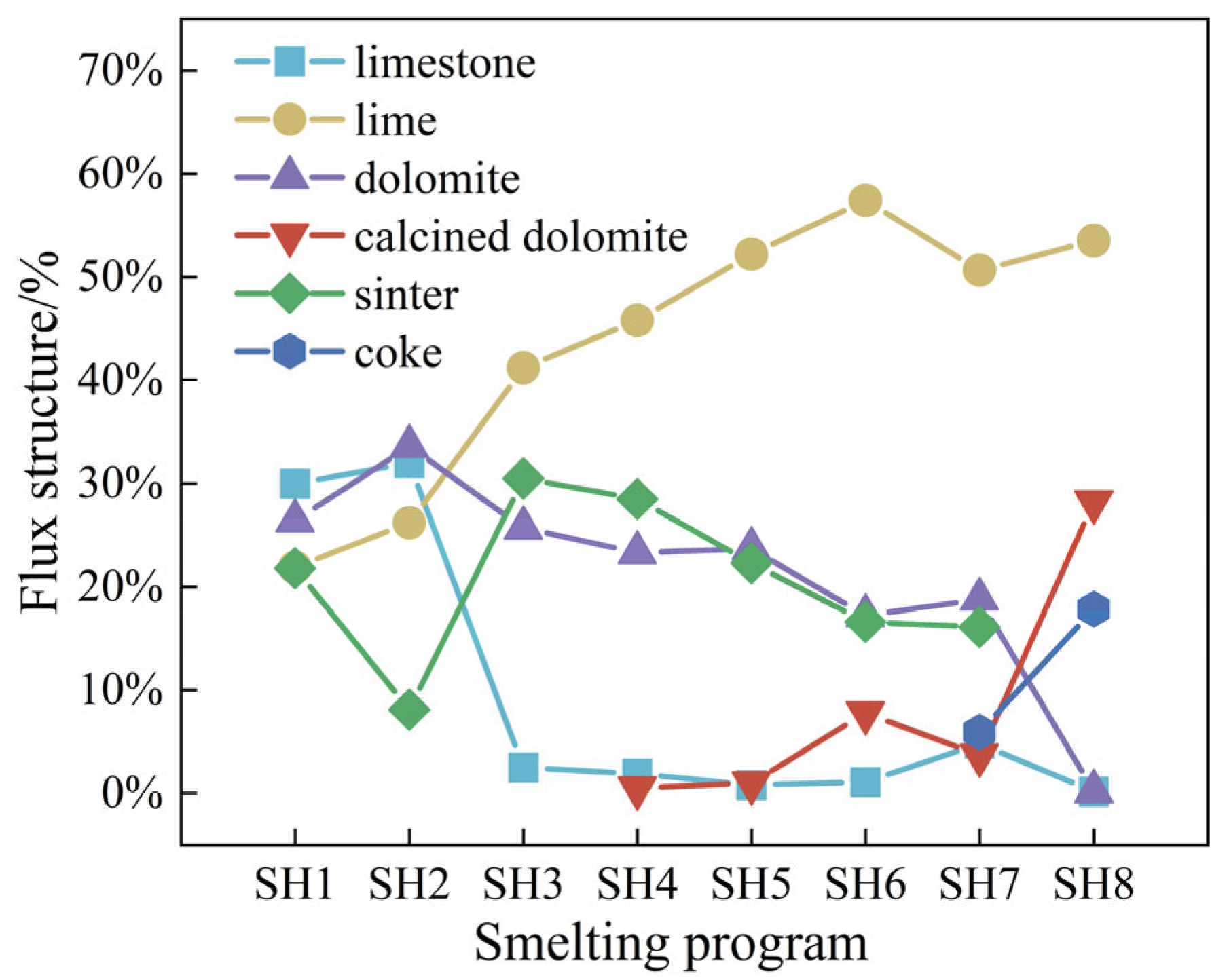
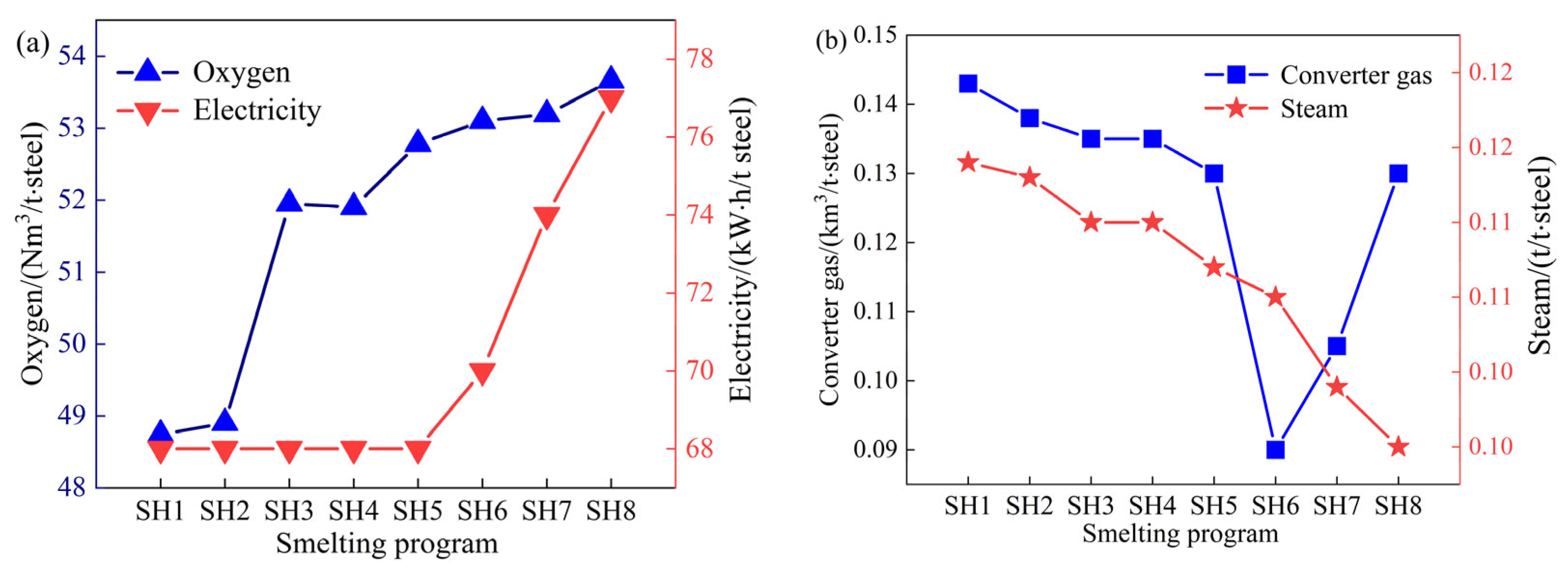
3.1. Industrial Applications of Converter Smelting with Different Scrap Ratios
3.2. Modeling the Utilization of the Converter Heating Agent
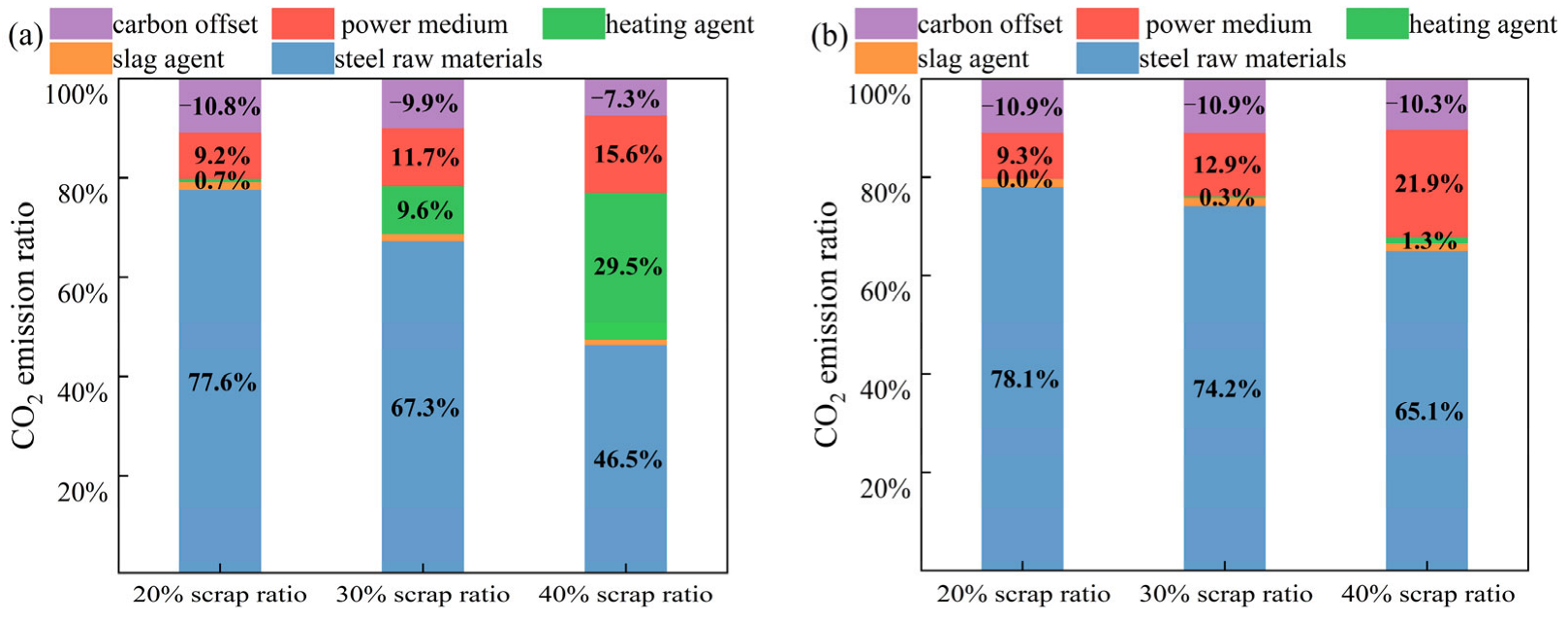
3.3. CO2 Emission Analysis for Smelting with Different Scrap Ratios
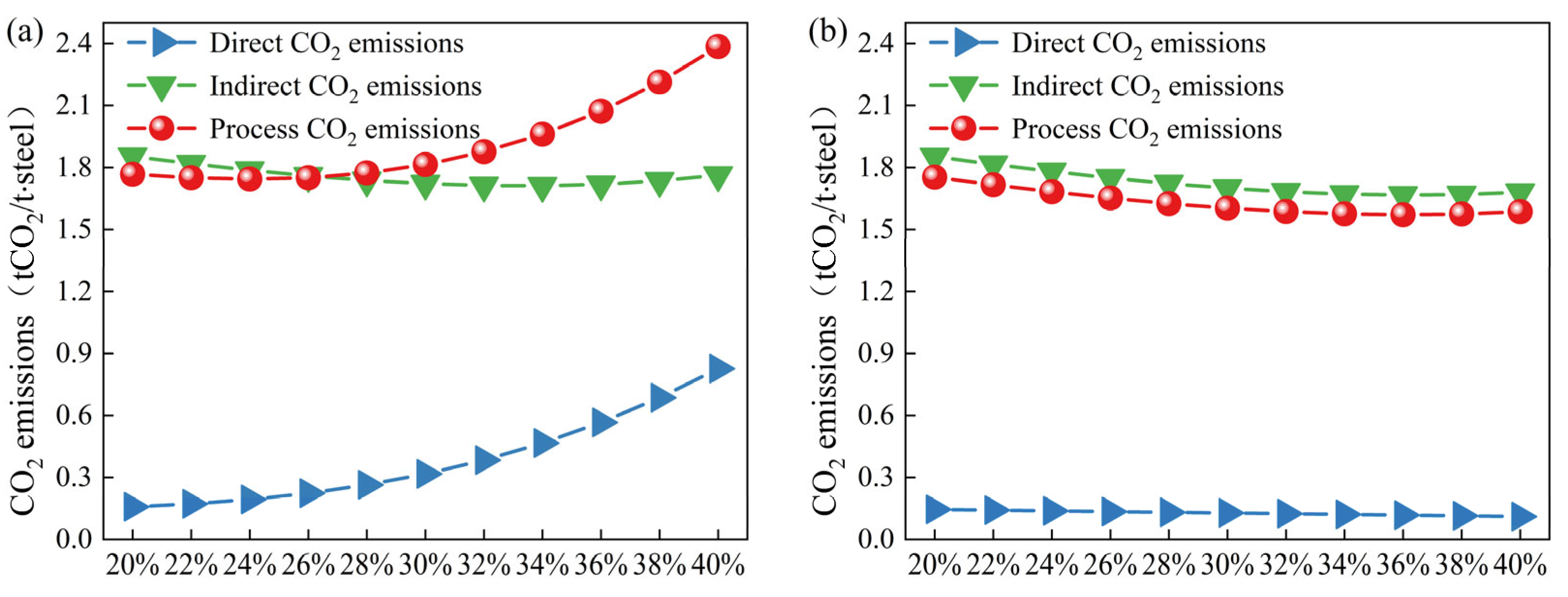
3.4. CO2 Emissions Analysis for High-Scrap-Ratio Smelting
4. Conclusions
- (1)
- Based on statistical data for the coke dosage under high scrap ratios, the utilization rate of the charcoal heating agent decreases gradually as the scrap ratio is raised, and the relationship between the utilization rate of the charcoal heating agent and the scrap ratio can be expressed as .
- (2)
- As the scrap ratio is raised, the dosages of lime and calcined dolomite in the flux increase, while the dosages of limestone and dolomite decrease; the consumption of oxygen and electricity increases, and the amounts of recovered converter gas and steam decrease. The overall carbon emissions from the converter process decrease with the increasing scrap ratio; every 1% increase in the scrap ratio results in a reduction of 14.09 kgCO2/t·steel for the converter process.
- (3)
- The use of biochar as a heating agent in converter smelting with a high scrap ratio can effectively increase the scrap ratio of converter smelting and reduce carbon emissions. The use of biochar as a heating agent can increase the scrap ratio to 36%, and the CO2 emissions for a ton of steel are 1.571 tCO2, which represents a reduction in emissions of 172 kgCO2/t·steel compared with the use of coke. The annual output of blast furnace–converter long-process steelmaking in China is approximately 917 million tons; therefore, up to 330 million tons of scrap can be consumed, yielding a reduction in CO2 emissions of 158 million tons.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| LCA | Life cycle assessment |
| IPCC | United Nations Intergovernmental Panel on Climate Change |
| JlSF | The Japan Iron and Steel Federation |
| WSA | World Steel Association |
| BOF | Basic oxygen furnace |
| BOFG | Converter gas |
| CA | Compressed air |
| SH1~8 | Smelting scheme 1~8 |
References
- He, K.; Wang, L. A review of energy use and energy-efficient technologies for the iron and steel industry. Renew. Sustain. Energy Rev. 2017, 70, 1022–1039. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, J.; Zhao, X.; Wang, Y. Energy and exergy analyses of an integrated iron and steel making process. Int. J. Exergy 2018, 26, 454–480. [Google Scholar] [CrossRef]
- Ariyama, T.; Takahashi, K.; Kawashiri, Y.; Nouchi, T. Diversification of the ironmaking process toward the long-term global goal for carbon dioxide mitigation. J. Sustain. Metall. 2019, 5, 26–94. [Google Scholar] [CrossRef]
- Jahanshahi, S.; Mathieson, J.G.; Somerville, M.A.; Haque, N.; Norgate, T.E.; Deev, A.; Pan, Y.; Xie, D.; Ridgeway, P.; Zulli, P. Development of low-emission integrated steelmaking process. J. Sustain. Metall. 2015, 1, 94–114. [Google Scholar] [CrossRef]
- Zhang, L. Development history and future prospects of steelmaking (II)—Future prospects. Iron Steel 2023, 58, 1–12. [Google Scholar] [CrossRef]
- Lin, Y.; Gunawan, T.A.; Isaac, C.; Luo, H.; Cheng, F.; Larson, E.D.; Greig, C.; Ma, L.; Li, Z. A preliminary assessment of CO2 capture, transport, and storage network for China’s steel sector. J. Clean. Prod. 2024, 454, 142280. [Google Scholar] [CrossRef]
- Shamsuddin, M. Metal recovery from scrap and waste. JOM 1986, 38, 24–31. [Google Scholar] [CrossRef]
- Wang, C.; Brämming, M.; Larsson, M. Numerical model of scrap blending in BOF with simultaneous consideration of steel quality, production cost, and energy use. Steel Res. Int. 2013, 84, 387–394. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y. Can the pilot emission trading system coordinate the relationship between emission reduction and economic development goals in China? J. Clean. Prod. 2022, 363, 132629. [Google Scholar] [CrossRef]
- Yao, J.; Han, H.D.; Yang, Y.; Song, Y.M.; Li, G.H. A Review of Recent Progress of Carbon Capture, Utilization, and Storage (CCUS) in China. Appl. Sci. 2023, 13, 1169. [Google Scholar] [CrossRef]
- Wang, D.Z.; Bao, Y.P.; Gao, F.; Xing, L.D. Effect of Converter Scrap Ratio on Carbon Emission in BF-LD Process. J. Sustain. Metall. 2022, 8, 1975–1987. [Google Scholar] [CrossRef]
- Wübbeke, J.; Heroth, T. Challenges and political solutions for steel recycling in China. Resour. Conserv. Recycl. 2014, 87, 1–7. [Google Scholar] [CrossRef]
- Williams Syndrome Association. World Steel Statistics. Available online: https://worldsteel.org/zh-hans/data/world-steel-in-figures/ (accessed on 12 June 2024).
- Zhang, J.; Chen, D.F. Study and Test of Carbonaceous Heat Agent. Adv. Sci. Lett. 2011, 4, 1847–1850. [Google Scholar] [CrossRef]
- Wei, G.S.; Zhu, R.; Tang, T.P.; Dong, K. Study on the melting characteristics of steel scrap in molten steel. Ironmak. Steelmak. 2019, 46, 609–617. [Google Scholar] [CrossRef]
- Deng, S.; Xu, A.J. Steel scrap melting model for a dephosphorisation basic oxygen furnace. J. Iron Steel Res. Int. 2020, 27, 972–980. [Google Scholar] [CrossRef]
- Dong, P.; Zheng, S.; Zhu, M. Numerical Study on Gas-Metal-Slag Interaction with Single-Flow Postcombustion Oxygen Lance in the Steelmaking Process of a Top-Blown Converter. JOM 2022, 74, 1509–1520. [Google Scholar] [CrossRef]
- Dong, P.-Y.; Zheng, S.-G.; Zhu, M.-Y. Simulation and application of post-combustion oxygen lance in a top-blown converter. Ironmak. Steelmak. 2023, 50, 55–66. [Google Scholar] [CrossRef]
- Jeffry, L.; Ong, M.Y.; Nomanbhay, S.; Mofijur, M.; Mubashir, M.; Show, P.L. Greenhouse gases utilization: A review. Fuel 2021, 301, 121017. [Google Scholar] [CrossRef]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass applications in iron and steel industry: An overview of challenges and opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Papa, A.; Di Carlo, A.; Bocci, E.; Taglieri, L.; Del Zotto, L.; Gallifuoco, A. Energy analysis of an integrated plant: Fluidized bed steam gasification of hydrothermally treated biomass coupled to solid oxide fuel cells. Energies 2021, 14, 7331. [Google Scholar] [CrossRef]
- Maitlo, G.; Ali, I.; Mangi, K.H.; Ali, S.; Maitlo, H.A.; Unar, I.N.; Pirzada, A.M. Thermochemical conversion of biomass for syngas production: Current status and future trends. Sustainability 2022, 14, 2596. [Google Scholar] [CrossRef]
- Wang, K.; Kong, G.; Zhang, G.; Zhang, X.; Han, L.; Zhang, X. Steam gasification of torrefied/carbonized wheat straw for H2-enriched syngas production and tar reduction. Int. J. Environ. Res. Public Health 2022, 19, 10475. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhao, H.; Li, Z.; Tan, X.; Lu, X. Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria. Energy Environ. Sci. 2012, 5, 9857–9865. [Google Scholar] [CrossRef]
- Mesa, L.; Martínez, Y.; Barrio, E.; González, E. Desirability function for optimization of Dilute Acid pretreatment of sugarcane straw for ethanol production and preliminary economic analysis based in three fermentation configurations. Appl. Energy 2017, 198, 299–311. [Google Scholar] [CrossRef]
- Westensee, D.K.; Rumbold, K.; Harding, K.G.; Sheridan, C.M.; van Dyk, L.D.; Simate, G.S.; Postma, F. The availability of second generation feedstocks for the treatment of acid mine drainage and to improve South Africa’s bio-based economy. Sci. Total Environ. 2018, 637, 132–136. [Google Scholar] [CrossRef]
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef]
- Chol, C.G.; Dhabhai, R.; Dalai, A.K.; Reaney, M. Purification of crude glycerol derived from biodiesel production process: Experimental studies and techno-economic analyses. Fuel Process. Technol. 2018, 178, 78–87. [Google Scholar] [CrossRef]
- Ningaraju, C.; Yatish, K.V.; Sakar, M. Simultaneous refining of biodiesel-derived crude glycerol and synthesis of value-added powdered catalysts for biodiesel production: A green chemistry approach for sustainable biodiesel industries. J. Clean. Prod. 2022, 363, 132448. [Google Scholar] [CrossRef]
- Polman, E.M.N.; Gruter, G.-J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew. Sustain. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Yogalakshmi, K.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, J.; Zhou, L.; Liu, Y.; Guo, Z.; Wang, Q. Experimental study of commercial charcoal as alternative fuel for coke breeze in iron ore sintering process. Energy Convers. Manag. 2016, 125, 254–263. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, J.; Zhou, L.; Liu, Y.; Wang, Q. Characteristics of charcoal combustion and its effects on iron-ore sintering performance. Appl. Energy 2016, 161, 364–374. [Google Scholar] [CrossRef]
- Yagi, J.-I.; Muchi, I. Theoretical estimations on the longitudinal distributions of process variables in blast furnace and on its productivity. Trans. Iron Steel Inst. Jpn. 1970, 10, 392–405. [Google Scholar] [CrossRef]
- Mousa, E.; Sjöblom, K. Modeling and optimization of biochar injection into blast furnace to mitigate the fossil CO2 emission. Sustainability 2022, 14, 2393. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Y.; Gao, P.; Han, Y.; Li, Y. Mechanism for suspension magnetization roasting of iron ore using straw-type biomass reductant. Int. J. Min. Sci. Technol. 2021, 31, 1075–1083. [Google Scholar] [CrossRef]
- Baharin, N.S.K.; Koesoemadinata, V.C.; Nakamura, S.; Yahya, W.J.; Yuzir, M.A.M.; Akhir, F.N.M.; Iwamoto, K.; Othman, N.; Ida, T.; Hara, H. Conversion and characterization of Bio-Coke from abundant biomass waste in Malaysia. Renew. Energy 2020, 162, 1017–1025. [Google Scholar] [CrossRef]
- Fidalgo, B.; Berrueco, C.; Millan, M. Chars from agricultural wastes as greener fuels for electric arc furnaces. J. Anal. Appl. Pyrolysis 2015, 113, 274–280. [Google Scholar] [CrossRef]
- Bianco, L.; Baracchini, G.; Cirilli, F.; Di Sante, L.; Moriconi, A.; Moriconi, E.; Agorio, M.M.; Pfeifer, H.; Echterhof, T.; Demus, T.; et al. Sustainable Electric Arc Furnace Steel Production: GreenEAF. BHM Berg Hüttenmännische Monatshefte 2013, 158, 17–23. [Google Scholar] [CrossRef]
- de Kleijne, K.; Hanssen, S.V.; van Dinteren, L.; Huijbregts, M.A.J.; van Zelm, R.; de Coninck, H. Limits to Paris compatibility of CO2 capture and utilization. One Earth 2022, 5, 168–185. [Google Scholar] [CrossRef]
- Morrow, W.R.; Hasanbeigi, A.; Sathaye, J.; Xu, T. Assessment of energy efficiency improvement and CO2 emission reduction potentials in India’s cement and iron & steel industries. J. Clean. Prod. 2014, 65, 131–141. [Google Scholar] [CrossRef]
- Kim, J.; Sovacool, B.K.; Bazilian, M.; Griffiths, S.; Lee, J.; Yang, M.Y.; Lee, J. Decarbonizing the iron and steel industry: A systematic review of sociotechnical systems, technological innovations, and policy options. Energy Res. Soc. Sci. 2022, 89, 102565. [Google Scholar] [CrossRef]
- Feng, C.; Zhu, R.; Wei, G.; Dong, K.; Dong, J. Typical case of carbon capture and utilization in Chinese iron and steel enterprises: CO2 emission analysis. J. Clean. Prod. 2022, 363, 132528. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of biomass in integrated steelmaking–Status quo, future needs and comparison to other low-CO2 steel production technologies. Appl. Energy 2018, 213, 384–407. [Google Scholar] [CrossRef]
- Shan, Y.; Guan, D.; Liu, J.; Mi, Z.; Liu, Z.; Liu, J.; Schroeder, H.; Cai, B.; Chen, Y.; Shao, S.; et al. Methodology and applications of city level CO2 emission accounts in China. J. Clean. Prod. 2017, 161, 1215–1225. [Google Scholar] [CrossRef]
- Zheng, Z.; Lian, X.; Shen, X.; Feng, L.; Gao, X. Evaluation indexes of material transformation and energy conversion in iron and steel production. Iron Steel 2021, 56, 120–128. [Google Scholar] [CrossRef]
- GB/T 34194-2017; Guides for Energy Efficiency Assessment of Converter Process. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- GB/T 2589-2020; General Rules for Calculation of the Comprehensive Energy Consumption. Standardization Administration of the People’s Republic of China: Beijing, China, 2021.
- ISO 14404-1; Calculation Method of Carbon Dioxide Emission Intensity from Iron and Steel Production—Part 1: Steel Plant with Blast Furnace. International Organization for Standardization: Geneva, Switzerland, 2013.
- Guerrero-Lemus, R.; Martínez-Duart, J.M.; Guerrero-Lemus, R.; Martínez-Duart, J.M. Biomass. In Renewable Energies and CO2; Lecture Notes in Energy (LNEN); Springer: Berlin/Heidelberg, Germany, 2013; Volume 3, pp. 37–62. [Google Scholar] [CrossRef]
- Norgate, T.; Haque, N.; Somerville, M.; Jahanshahi, S. Biomass as a source of renewable carbon for iron and steelmaking. ISIJ Int. 2012, 52, 1472–1481. [Google Scholar] [CrossRef]
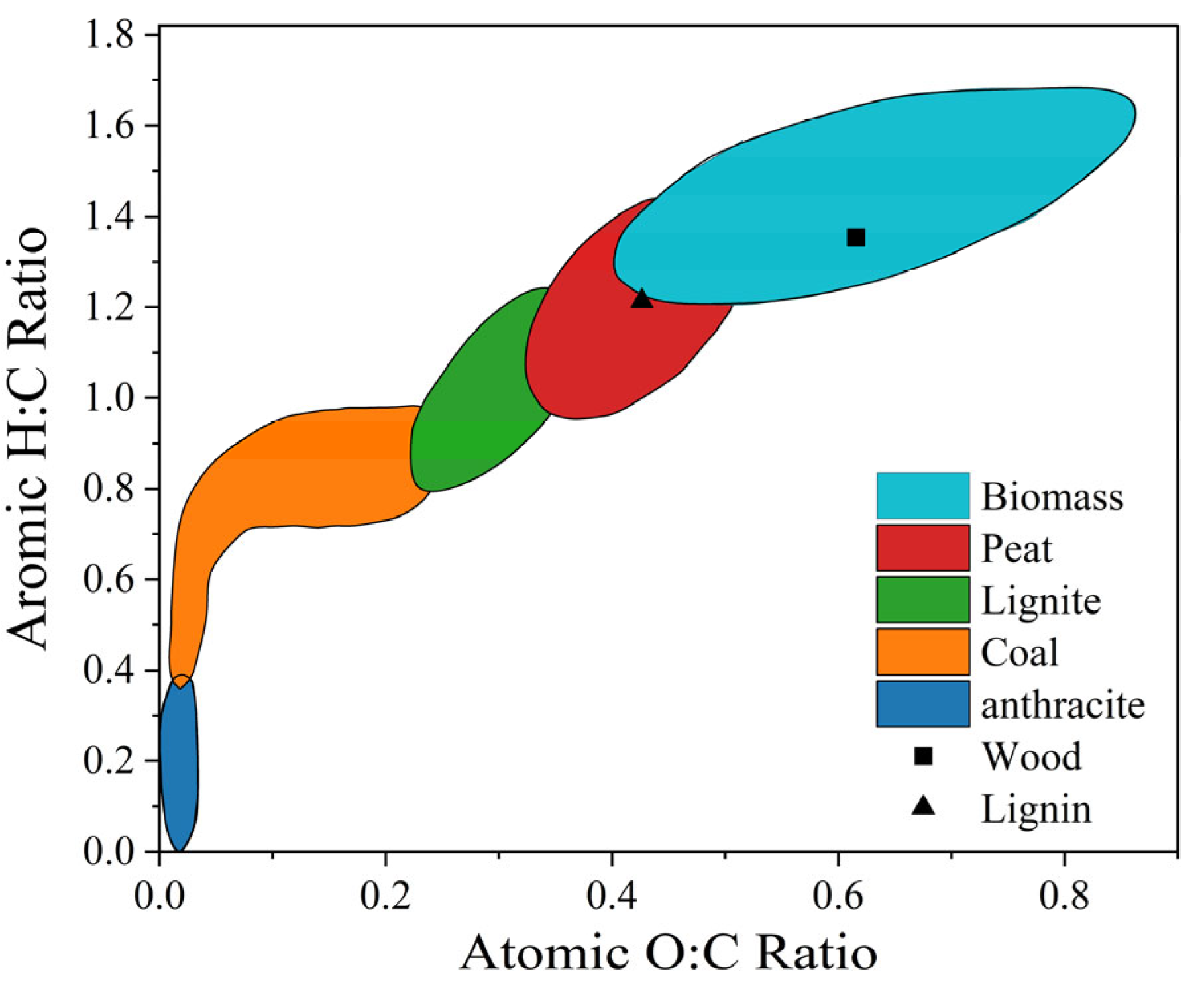
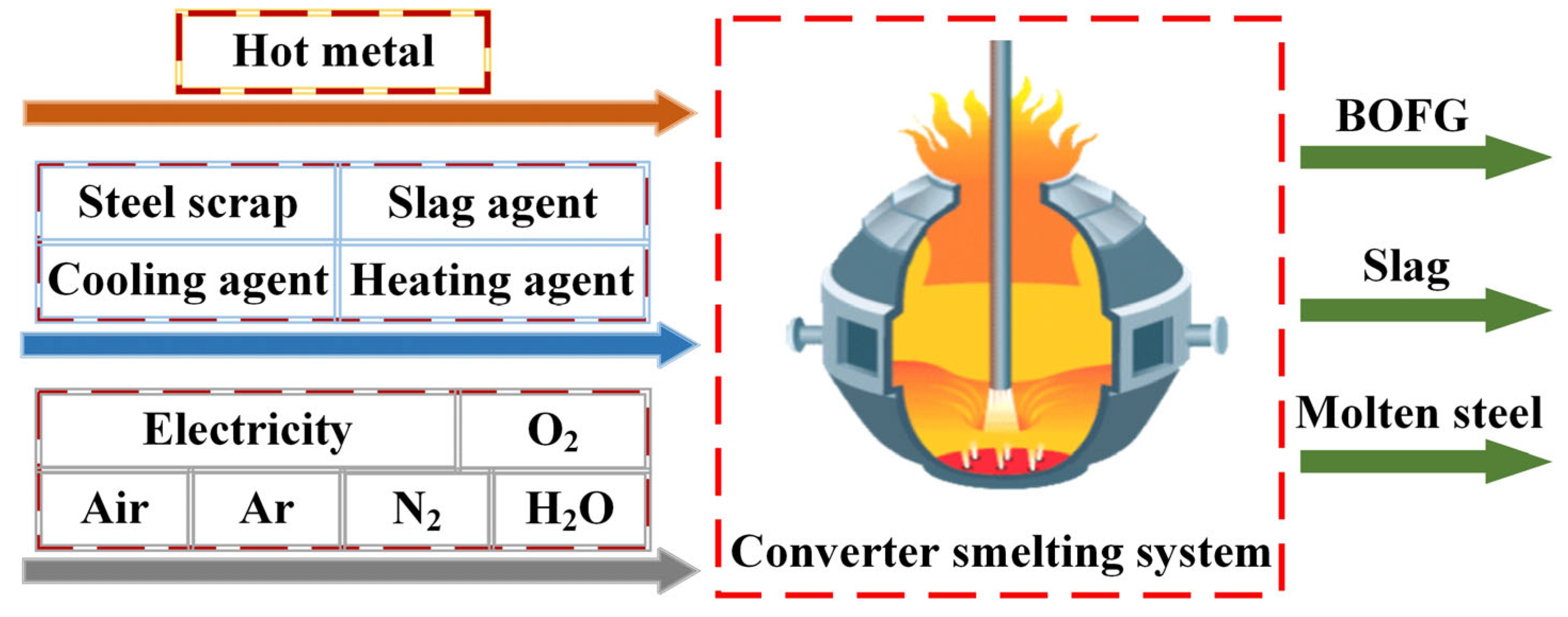
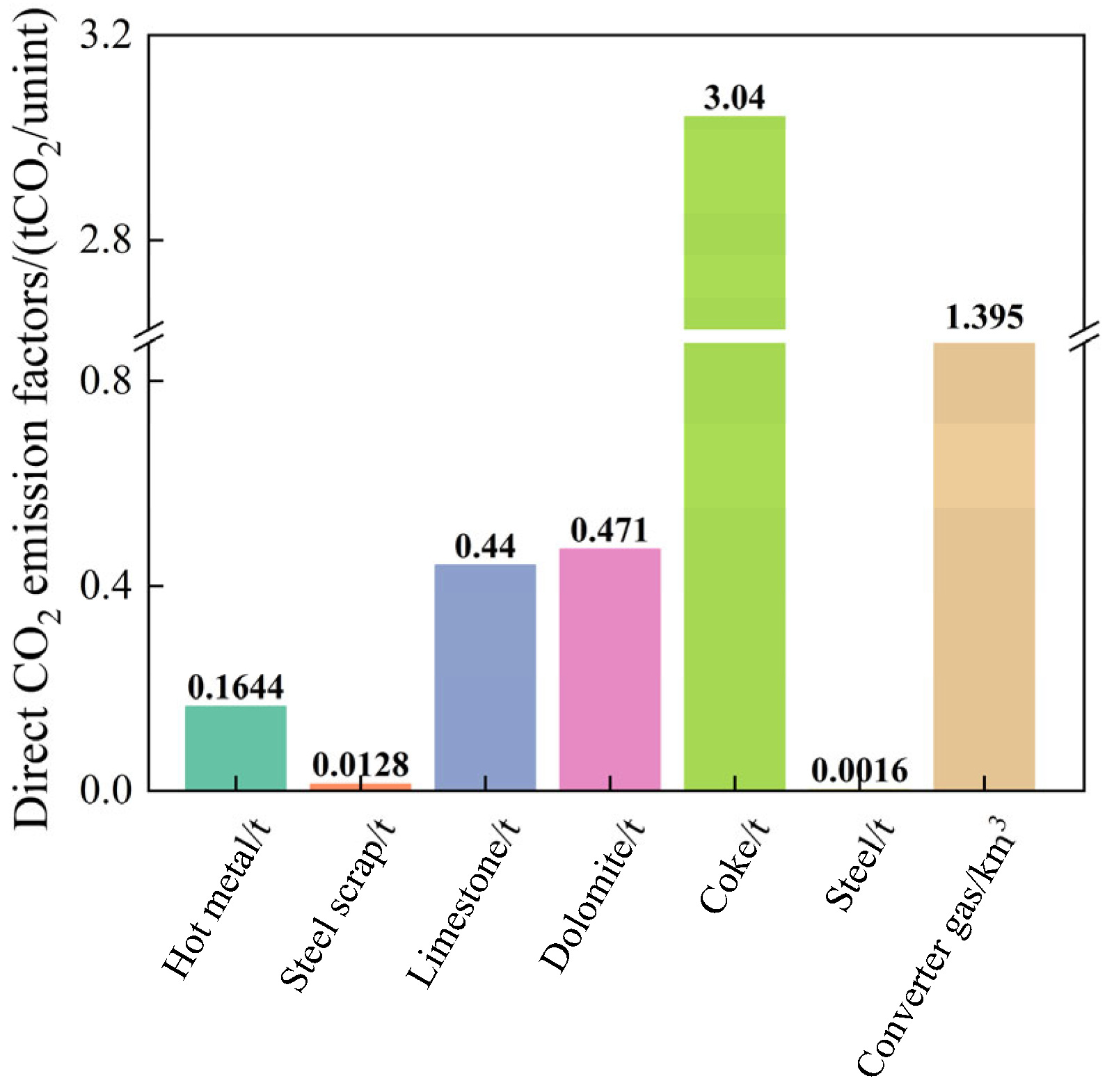

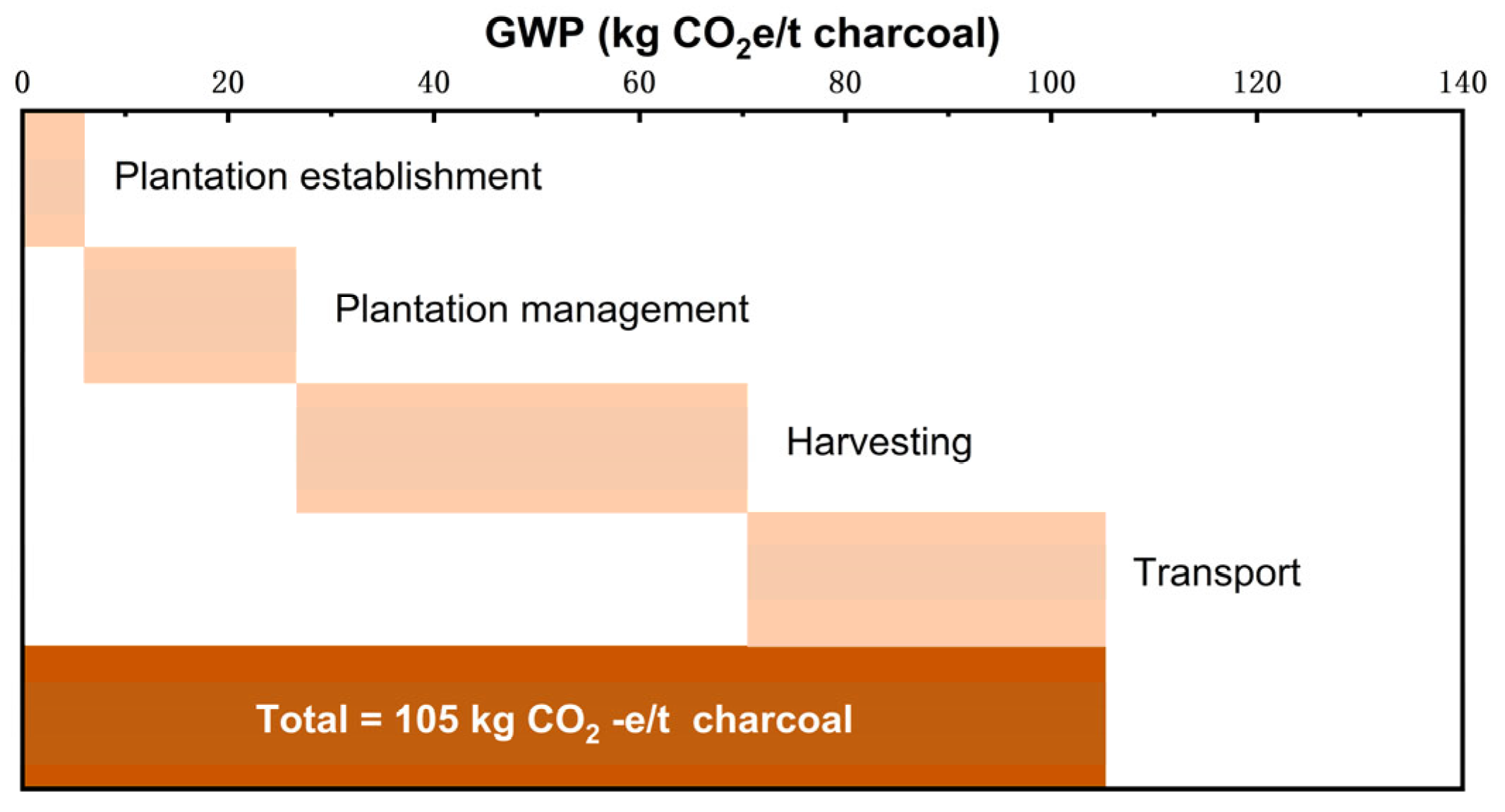



| Treatment | Raw Material | Process Method | Main Products | Ref. |
|---|---|---|---|---|
| Thermochemical treatment | Lignocellulosic biomass | Combustion, gasification, pyrolysis, and liquefaction | Biomass charcoal, biomass oil | [22,23,24] |
| Chemical transformation | Low lignocellulosic biomass | Hydrolysis, organic melt dissolution, ionic liquid pretreatment | Chemicals such as acetic acid, polysaccharides | [25,26,27] |
| Physicochemical transformation | Vegetable oils, animal fats | Ester exchange and esterification reactions | Biodiesel | [28,29,30] |
| Biological treatment | Biomass, microorganisms, and enzymes | Biodegradation | Biofuels, aldehydes, and phenolic acids | [31,32] |
| Project | Units (Unit) | Direct Emission Factor (tCO2/Unit) | Indirect Emission Factor (tCO2/Unit) |
|---|---|---|---|
| Hot metal | t | 0.1644 | 1.8550 |
| Steel scrap | t | 0.0128 | 0 |
| Limestone | t | 0.4400 | 0.0030 |
| Lime | t | 0 | 1.0350 |
| Dolomite | t | 0.4710 | 0.0620 |
| Calcined dolomite | t | 0 | 1.1000 |
| Sinter | t | 0 | 0.3290 |
| Coke | t | 3.0400 | 0.4630 |
| Biochar | t | 0 | 0.1050 |
| Electricity | MW-h | 0 | 0.9419 |
| O2 | km3 | 0 | 0.6146 |
| N2 | km3 | 0 | 0.1295 |
| Ar | km3 | 0 | 4.7057 |
| Water | t | 0 | 0.3173 |
| Air | km3 | 0 | 0.1165 |
| Steel | t | 0.0016 | 0 |
| Slag | t | 0 | 0.3000 |
| Converter gas | km3 | 1.3954 | 0 |
| Steam | t | 0 | 0.7273 |
| Smelting Program | Scrap Ratio/% | Scrap Usage/t | Data Volume |
|---|---|---|---|
| SH1 | 2.70 | 0~10 | 1806 |
| SH2 | 4.63 | 10~20 | 92 |
| SH3 | 9.35 | 20~30 | 954 |
| SH4 | 11.97 | 30~40 | 701 |
| SH5 | 16.12 | 40~50 | 618 |
| SH6 | 19.01 | 50~60 | 426 |
| SH7 | 22.14 | 60~70 | 2413 |
| SH8 | 24.21 | 70~80 | 488 |
| Project | 20% | 22% | 24% | 26% | 28% | 30% | 32% | 34% | 36% | 38% | 40% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| heating agent/kg | 4.24 | 9.93 | 18.08 | 29.16 | 43.69 | 62.22 | 85.32 | 113.56 | 147.58 | 188.00 | 235.50 |
| utilization factor/% | 32.28 | 25.20 | 20.11 | 16.33 | 13.48 | 11.27 | 9.53 | 8.14 | 7.02 | 6.10 | 5.34 |
| oxygen/Nm3 | 55.61 | 63.83 | 76.10 | 93.23 | 116.07 | 145.53 | 182.52 | 228.04 | 283.11 | 348.77 | 426.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Feng, C.; Li, Y.; Guo, F.; Zhu, R.; Zhang, M.; Wang, X.; Du, X.; Huo, L.; Wen, F.; et al. Typical Case of Converter Smelting with High Cooling Ratio in Chinese Iron and Steel Enterprises: CO2 Emission Analysis. Materials 2025, 18, 65. https://doi.org/10.3390/ma18010065
Yang H, Feng C, Li Y, Guo F, Zhu R, Zhang M, Wang X, Du X, Huo L, Wen F, et al. Typical Case of Converter Smelting with High Cooling Ratio in Chinese Iron and Steel Enterprises: CO2 Emission Analysis. Materials. 2025; 18(1):65. https://doi.org/10.3390/ma18010065
Chicago/Turabian StyleYang, Huapeng, Chao Feng, Yubin Li, Feihong Guo, Rong Zhu, Minke Zhang, Xing Wang, Xin Du, Liyun Huo, Fuxin Wen, and et al. 2025. "Typical Case of Converter Smelting with High Cooling Ratio in Chinese Iron and Steel Enterprises: CO2 Emission Analysis" Materials 18, no. 1: 65. https://doi.org/10.3390/ma18010065
APA StyleYang, H., Feng, C., Li, Y., Guo, F., Zhu, R., Zhang, M., Wang, X., Du, X., Huo, L., Wen, F., Ren, T., Wei, G., & Liu, F. (2025). Typical Case of Converter Smelting with High Cooling Ratio in Chinese Iron and Steel Enterprises: CO2 Emission Analysis. Materials, 18(1), 65. https://doi.org/10.3390/ma18010065






