Shape-Persistent Tetraphenylethylene Macrocycle: Highly Efficient Synthesis and Circularly Polarized Luminescence
Abstract
1. Introduction
2. Results and Discussion
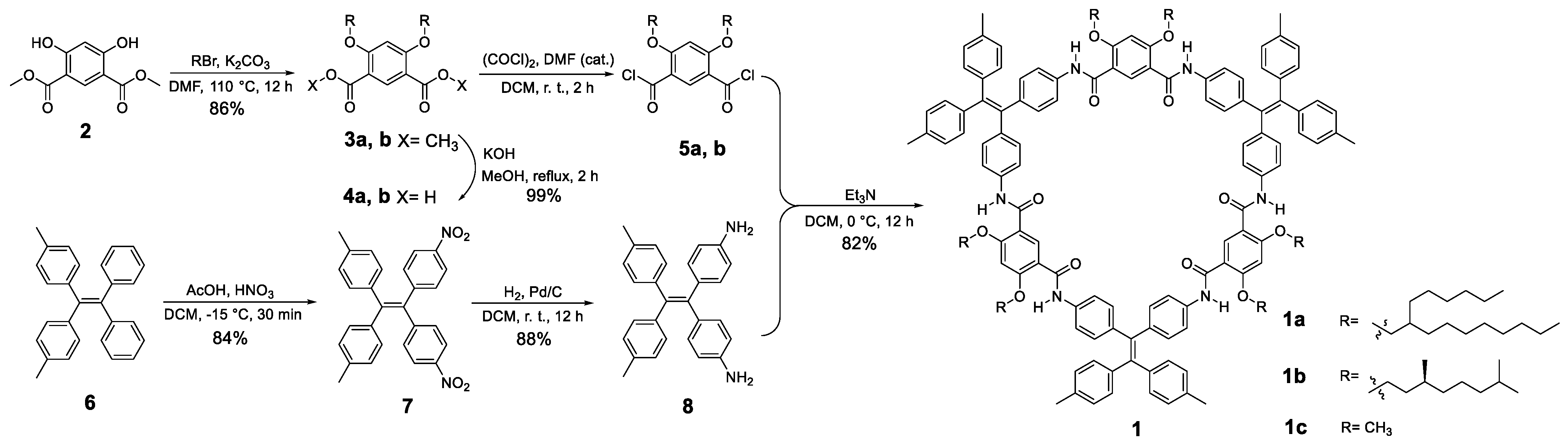
2.1. Synthesis
2.2. Molecular Conformation
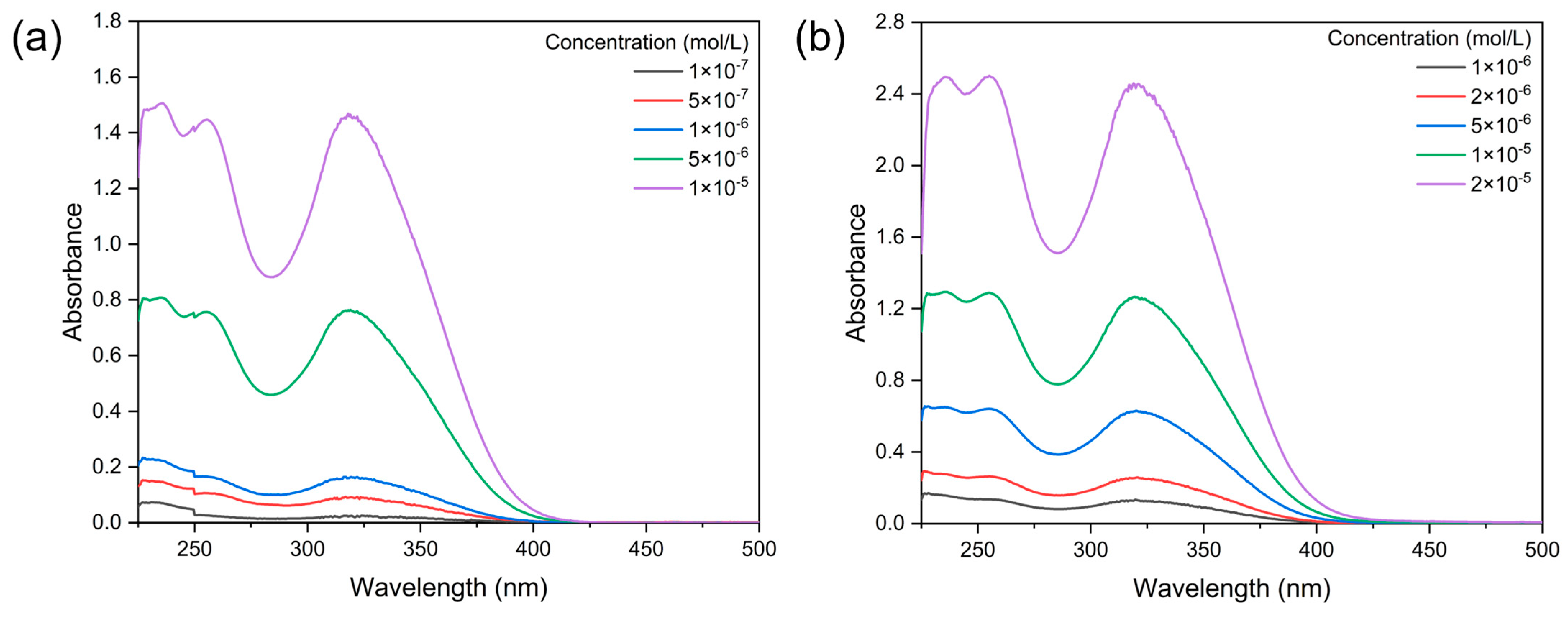
2.3. Photophysical Property
2.4. Chiral Optical Properties
2.5. Morphology of Aggregate State
3. Materials and Methods
3.1. Materials and Reagents
3.2. Experimental Methods
3.3. DFT Calculations
3.4. Synthesis of Shape-Persistent Tetraphenylethylene Macrocycle
3.4.1. Synthesis of Compound 3
3.4.2. Synthesis of Compound 4
3.4.3. Synthesis of Compound 5
3.4.4. Synthesis of Compound 7
3.4.5. Synthesis of Compound 8
3.4.6. Synthesis of Compound 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Burton, A.S.; Elsila, J.E.; Aponte, J.C.; Dworkin, J.P. The Search for Chiral Asymmetry as a Potential Biosignature in our Solar System. Chem. Rev. 2020, 120, 4660. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Dong, X.Y.; Zang, S.Q. Circularly Polarized Luminescence of Agglomerate Emitters. Aggregate 2021, 2, e48. [Google Scholar] [CrossRef]
- Han, J.; Guo, S.; Lu, H.; Liu, S.; Zhao, Q.; Huang, W. Recent Progress on Circularly Polarized Luminescent Materials for Organic Optoelectronic Devices. Adv. Opt. Mater. 2018, 6, 1800538. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, C.-C.; Murata, K.; Kamal, A.S.A.; Lin, B.-W.; Chen, M.-H.; Tang, S.; Ho, Y.-L.; Chen, C.-C.; Chen, C.-W.; et al. Chiroptical Response Inversion and Enhancement of Room-Temperature Exciton-Polaritons Using 2D Chirality in Perovskites. Adv. Mater. 2023, 35, 2303203. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.; Evans, N.H.; Parker, D. Lanthanide Complexes as Chiral Probes Exploiting Circularly Polarized Luminescence. Chem. Soc. Rev. 2012, 41, 7673. [Google Scholar] [CrossRef]
- He, C.; Li, Y. Absolute Asymmetric Synthesis Driven by Circularly Polarized Light. Chin. Chem. Lett. 2023, 34, 108077. [Google Scholar] [CrossRef]
- Song, F.; Zhao, Z.; Liu, Z.; Lam, J.W.Y.; Tang, B.Z. Circularly Polarized Luminescence from AIEgens. J. Mater. Chem. C 2020, 8, 3284. [Google Scholar] [CrossRef]
- Li, T.; Zhu, X.; Ouyang, G.; Liu, M. Circularly Polarized Luminescence from Chiral Macrocycles and Their Supramolecular Assemblies. Mater. Chem. Front. 2023, 7, 3879. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Han, X.-N.; Han, Y.; Chen, C.-F. Advances in Circularly Polarized Luminescence Materials Based on Chiral Macrocycles. Chem. Commun. 2023, 59, 13089. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Feng, H.-T.; Yuan, Y.-X.; Zheng, Y.-S.; Tang, B.Z. Chiral AIEgens—Chiral Recognition, CPL Materials and Other Chiral Applications. Coord. Chem. Rev. 2020, 416, 213329. [Google Scholar] [CrossRef]
- Sang, Y.; Han, J.; Zhao, T.; Duan, P.; Liu, M. Circularly Polarized Luminescence in Nanoassemblies: Generation, Amplification, and Application. Adv. Mater. 2020, 32, 1900110. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, J.-C.; Lam, J.W.Y.; Feng, H.-T.; Tang, B.Z. Chiral Assembly of Organic Luminogens with Aggregation-Induced Emission. Chem. Sci. 2022, 13, 611. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.-T.; Yuan, Y.-X.; Xiong, J.-B.; Zheng, Y.-S.; Tang, B.Z. Macrocycles and Cages Based on Tetraphenylethylene with Aggregation-Induced Emission Effect. Chem. Soc. Rev. 2018, 47, 7452. [Google Scholar] [CrossRef] [PubMed]
- Saikawa, M.; Nakamura, T.; Uchida, J.; Yamamura, M.; Nabeshima, T. Synthesis of Figure-of-Eight Helical BisBODIPY Macrocycles and Their Chiroptical Properties. Chem. Commun. 2016, 52, 10727. [Google Scholar] [CrossRef]
- Shang, W.; Zhu, X.; Jiang, Y.; Cui, J.; Liu, K.; Li, T.; Liu, M. Self-Assembly of Macrocyclic Triangles into Helicity-Opposite Nanotwists by Competitive Planar over Point Chirality. Angew. Chem. Int. Ed. 2022, 61, e202210604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, L.; Wang, Y.; Li, F.; Li, Y.; Meng, Q. Chiral TPE Foldamers in Macrocycles: Aggregation Enhanced Emission and Circularly Polarized Luminescence. Chem. Eur. J. 2023, 29, e202302373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Y.; Yuan, L. Hydrogen-Bonded Aromatic Amide Macrocycles: Synthesis, Properties and Functions. Org. Biomol. Chem. 2022, 20, 9023. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, Z.; Wang, L.; Chen, L.; Cai, Y.; Deng, P.; Feng, W.; Li, X.; Yuan, L. A Dynamic Hydrogen-Bonded Azo-Macrocycle for Precisely Photo-Controlled Molecular Encapsulation and Release. Angew. Chem. Int. Ed. 2019, 58, 12519. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, B.; Chen, L.; Hu, J.; Wen, C.; Zheng, Q.; Wu, L.; Zeng, H.; Gong, B.; Yuan, L. Liquid-Crystalline Mesogens Based on Cyclo[6]aramides: Distinctive Phase Transitions in Response to Macrocyclic Host–Guest Interactions. Angew. Chem. Int. Ed. 2015, 54, 11147. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Luo, Y.; Chen, L.; Sun, X.; Chen, X.; Qin, S.; Feng, W.; Li, X.; Yuan, L. A Host–Guest Interaction Activated Bobbitt Oxidant for Highly Efficient Oxidation of Alcohols. Chem. Commun. 2022, 58, 12867. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, X.; Penocchio, E.; Ragazzon, G.; Chen, X.; Lu, S.; Zhou, Y.; Fu, K.; Liu, Z.; Cai, Y.; et al. Beyond Single-Cycle Autonomous Molecular Machines: Light-Powered Shuttling in a Multi-Cycle Reaction Network. Angew. Chem. Int. Ed. 2024, e202414072. [Google Scholar] [CrossRef]
- Xiong, J.-B.; Wang, J.-H.; Li, B.; Zhang, C.; Tan, B.; Zheng, Y.-S. Porous Interdigitation Molecular Cage from Tetraphenylethylene Trimeric Macrocycles That Showed Highly Selective Adsorption of CO2 and TNT Vapor in Air. Org. Lett. 2018, 20, 321. [Google Scholar] [CrossRef]
- Feng, W.; Yamato, K.; Yang, L.; Ferguson, J.S.; Zhong, L.; Zou, S.; Yuan, L.; Zeng, X.-C.; Gong, B. Efficient Kinetic Macrocyclization. J. Am. Chem. Soc. 2009, 131, 2629. [Google Scholar] [CrossRef] [PubMed]
- Spano, F.C.; Silva, C. H- and J-Aggregate Behavior in Polymeric Semiconductors. Annu. Rev. Phys. Chem. 2014, 65, 477. [Google Scholar] [CrossRef]
- Xiong, J.-B.; Feng, H.-T.; Sun, J.-P.; Xie, W.-Z.; Yang, D.; Liu, M.; Zheng, Y.-S. The Fixed Propeller-Like Conformation of Tetraphenylethylene that Reveals Aggregation-Induced Emission Effect, Chiral Recognition, and Enhanced Chiroptical Property. J. Am. Chem. Soc. 2016, 138, 11469. [Google Scholar] [CrossRef]
- Frisch, G.W.; Trucks, H.B.; Schlegel, G.E.; Scuseria, M.A.; Robb, J.R.; Cheeseman, G.; Scalmani, V.; Barone, B.; Mennucci, G.A.; Petersson, H.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Yuan, L.; Feng, W.; Yamato, K.; Sanford, A.R.; Xu, D.; Guo, H.; Gong, B. Highly Efficient, One-Step Macrocyclizations Assisted by the Folding and Preorganization of Precursor Oligomers. J. Am. Chem. Soc. 2004, 126, 11120. [Google Scholar] [CrossRef]
- Kulkarni, C.; Bejagam, K.K.; Senanayak, S.P.; Narayan, K.S.; Balasubramanian, S.; George, S.J. Dipole-Moment-Driven Cooperative Supramolecular Polymerization. J. Am. Chem. Soc. 2015, 137, 3924. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Zheng, Y.; Liu, Z.; Yang, Z.; Lu, Z.; Ai, X.; Ye, Z.; Yang, C.; Li, X.; Yuan, L. Shape-Persistent Tetraphenylethylene Macrocycle: Highly Efficient Synthesis and Circularly Polarized Luminescence. Materials 2025, 18, 200. https://doi.org/10.3390/ma18010200
Liu P, Zheng Y, Liu Z, Yang Z, Lu Z, Ai X, Ye Z, Yang C, Li X, Yuan L. Shape-Persistent Tetraphenylethylene Macrocycle: Highly Efficient Synthesis and Circularly Polarized Luminescence. Materials. 2025; 18(1):200. https://doi.org/10.3390/ma18010200
Chicago/Turabian StyleLiu, Peixin, Yuexuan Zheng, Zejiang Liu, Zhiyao Yang, Ziying Lu, Xiongrui Ai, Zecong Ye, Cheng Yang, Xiaowei Li, and Lihua Yuan. 2025. "Shape-Persistent Tetraphenylethylene Macrocycle: Highly Efficient Synthesis and Circularly Polarized Luminescence" Materials 18, no. 1: 200. https://doi.org/10.3390/ma18010200
APA StyleLiu, P., Zheng, Y., Liu, Z., Yang, Z., Lu, Z., Ai, X., Ye, Z., Yang, C., Li, X., & Yuan, L. (2025). Shape-Persistent Tetraphenylethylene Macrocycle: Highly Efficient Synthesis and Circularly Polarized Luminescence. Materials, 18(1), 200. https://doi.org/10.3390/ma18010200








