Memory Effect of Double Oxides Compared to Simple Ion Exchange for Controlled Fluoride Ion Capture and Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
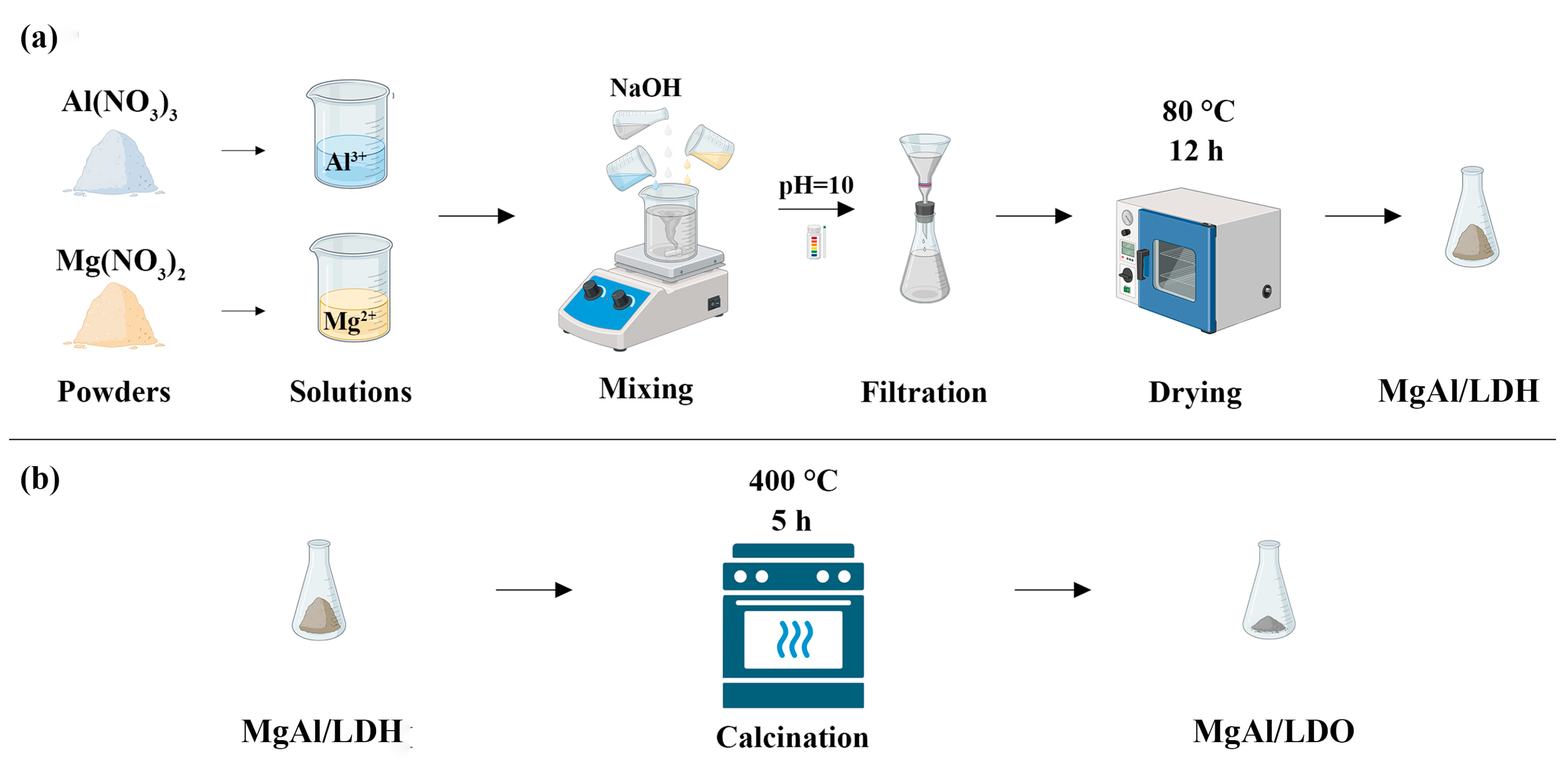
2.2. Preparation of Layered Structures
Coprecipitation Preparation of MgAl/LDH
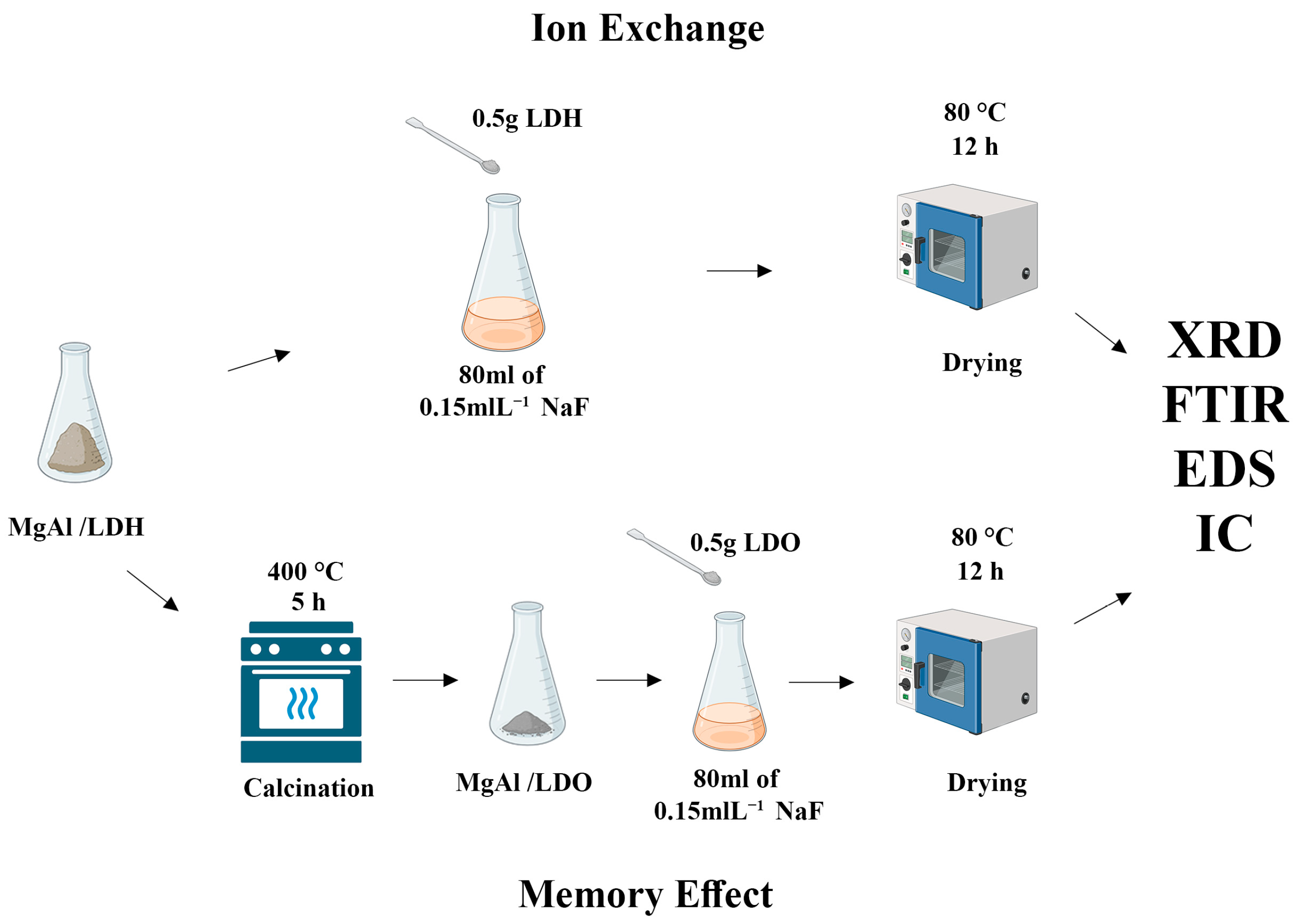
2.3. Ion Exchange for MgAl/LDH
2.4. Memory Effect Testing for MgAl/LDO
2.5. Characterization Methods
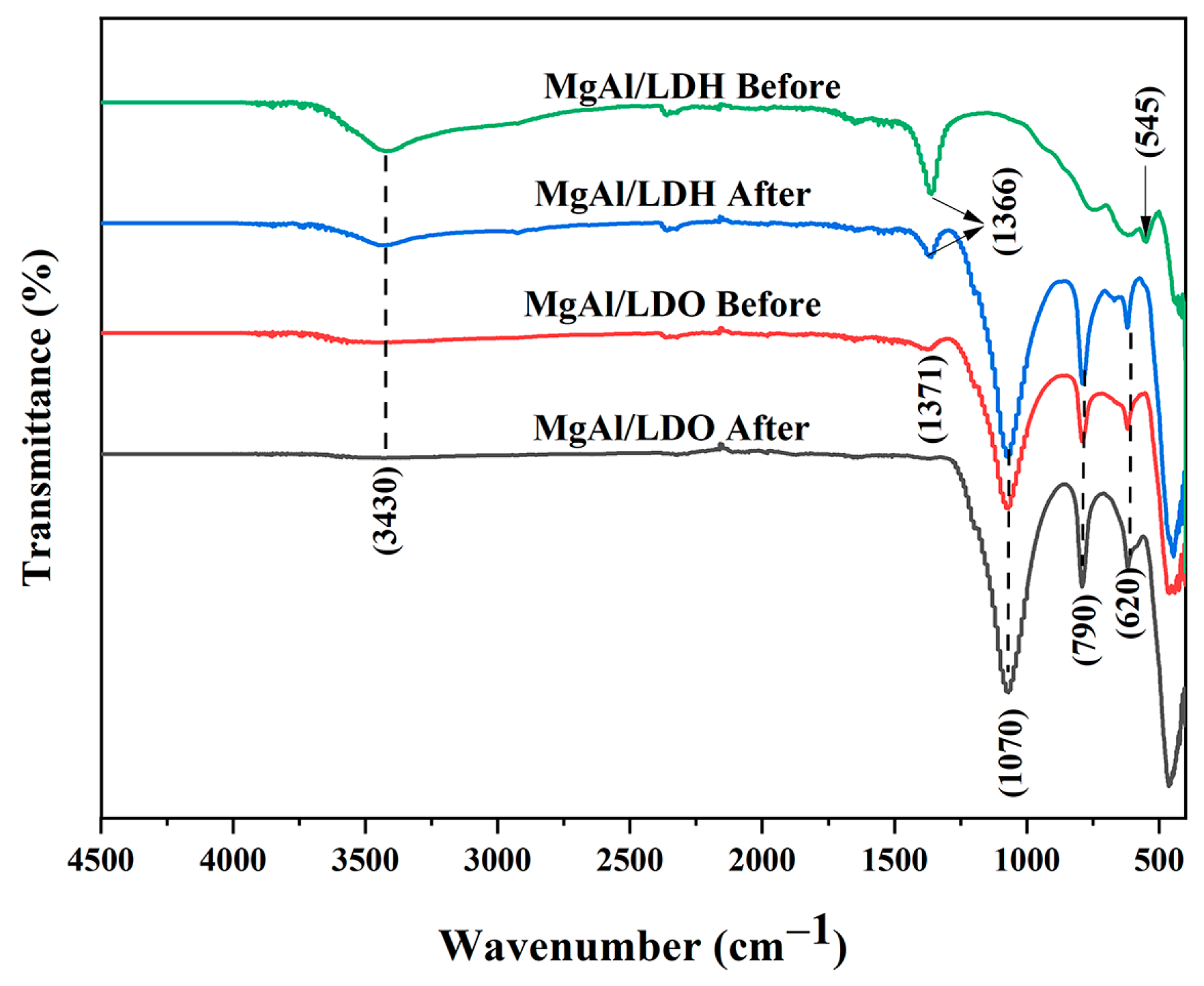
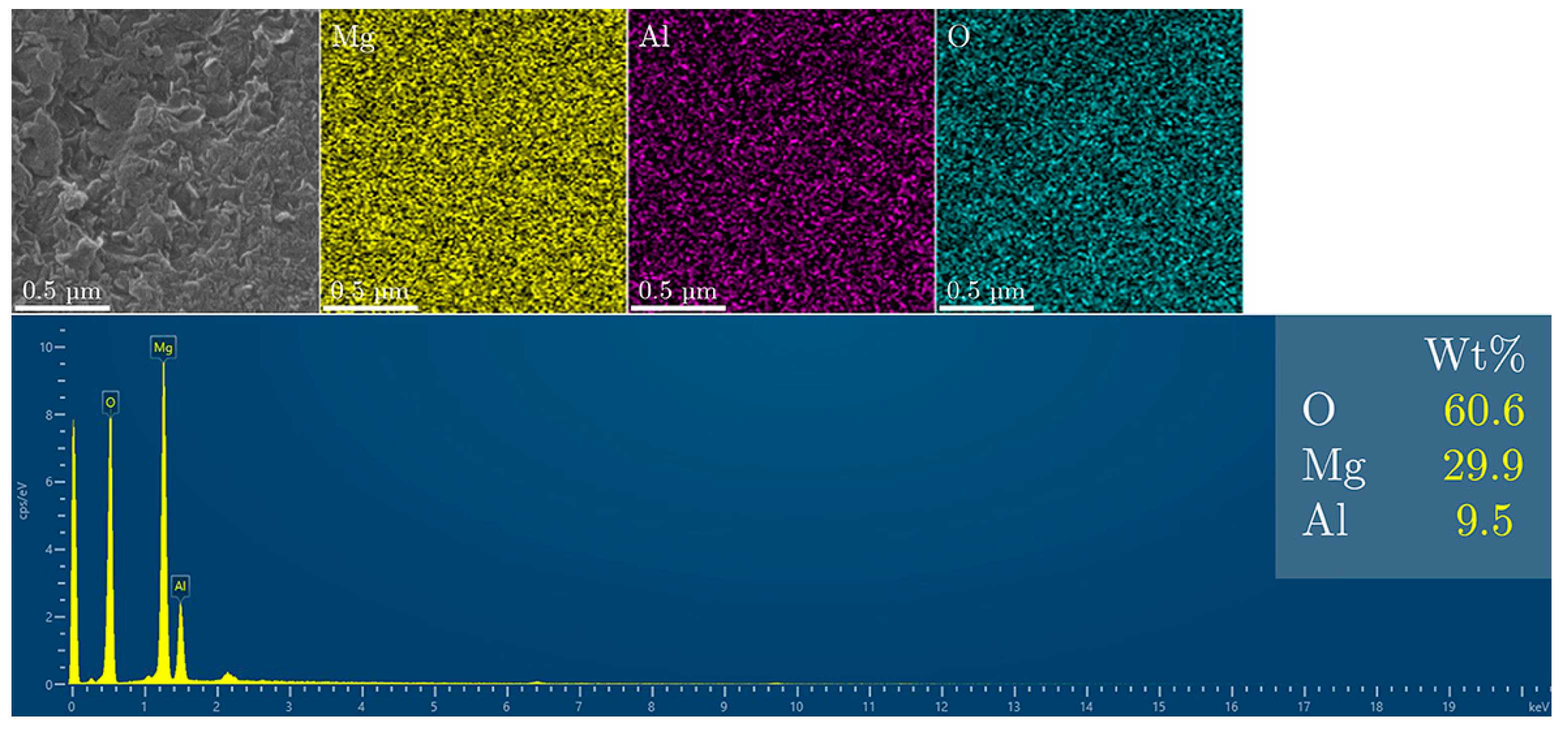
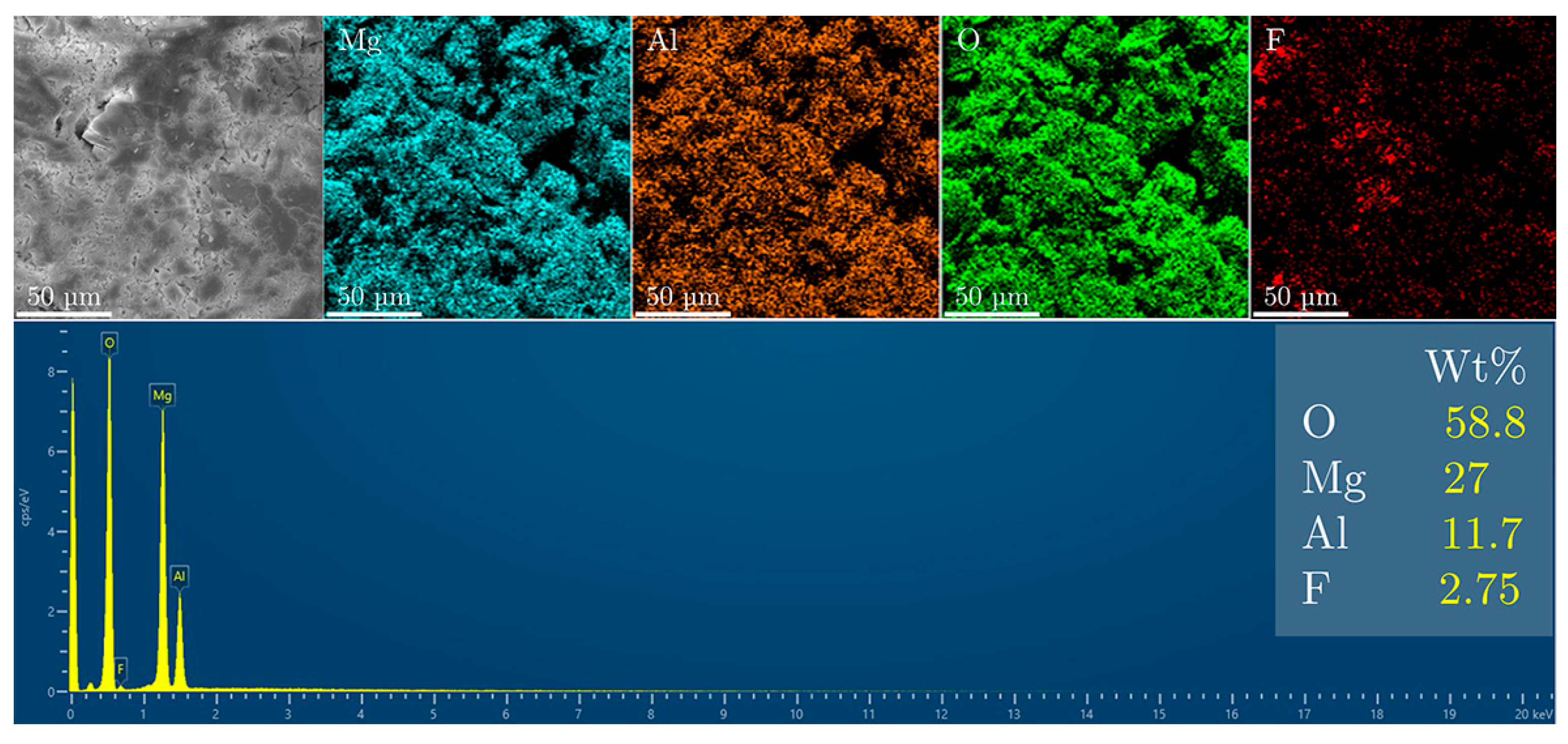
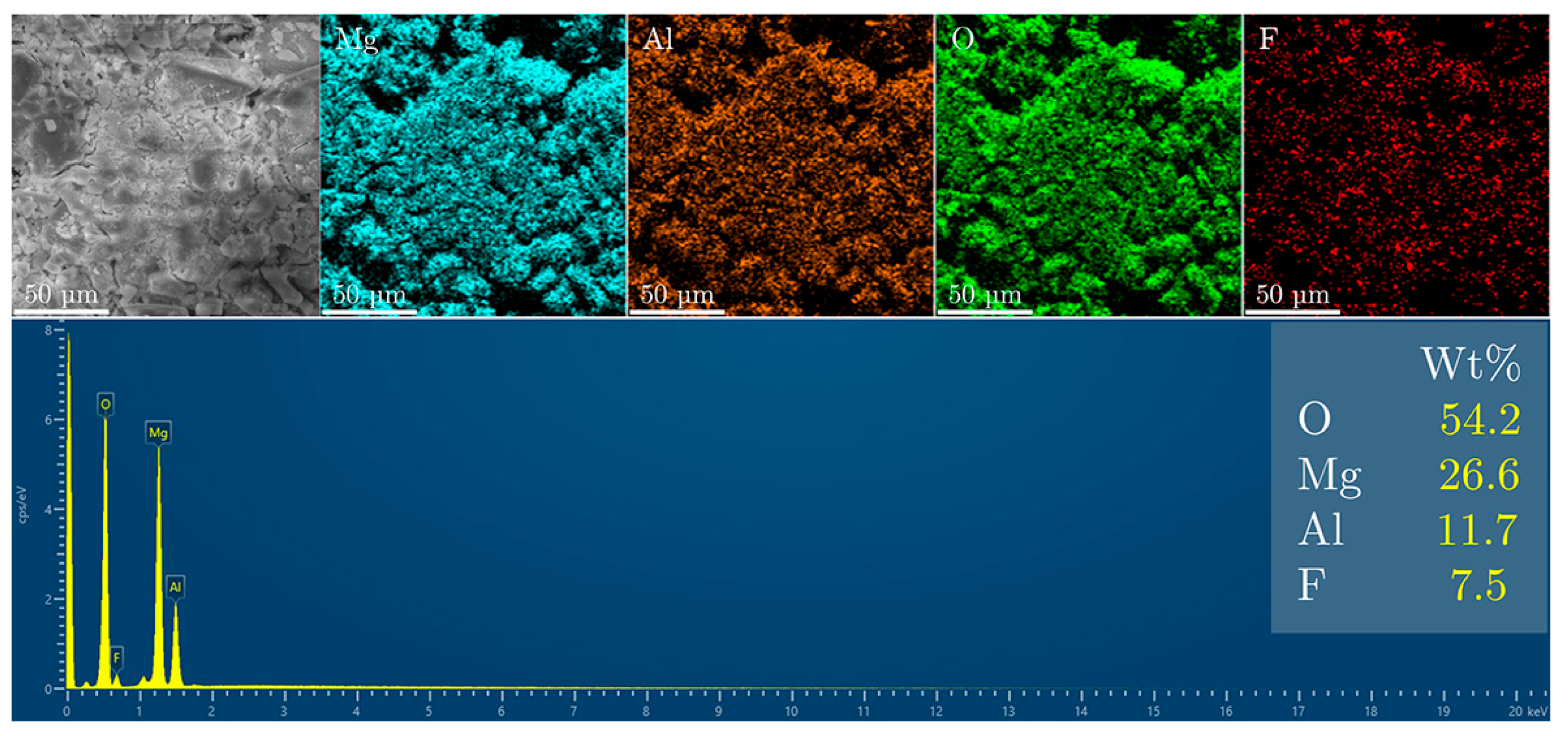
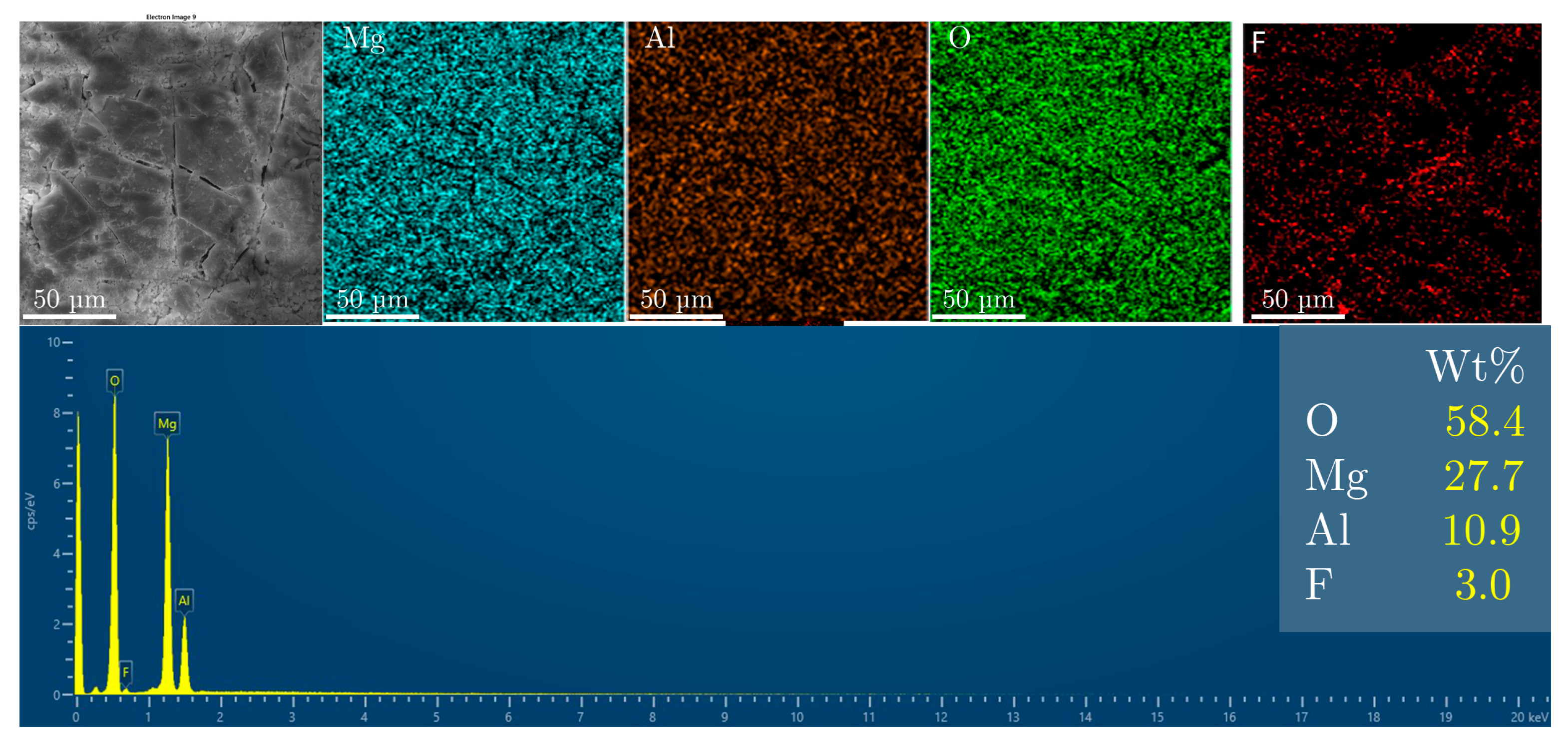
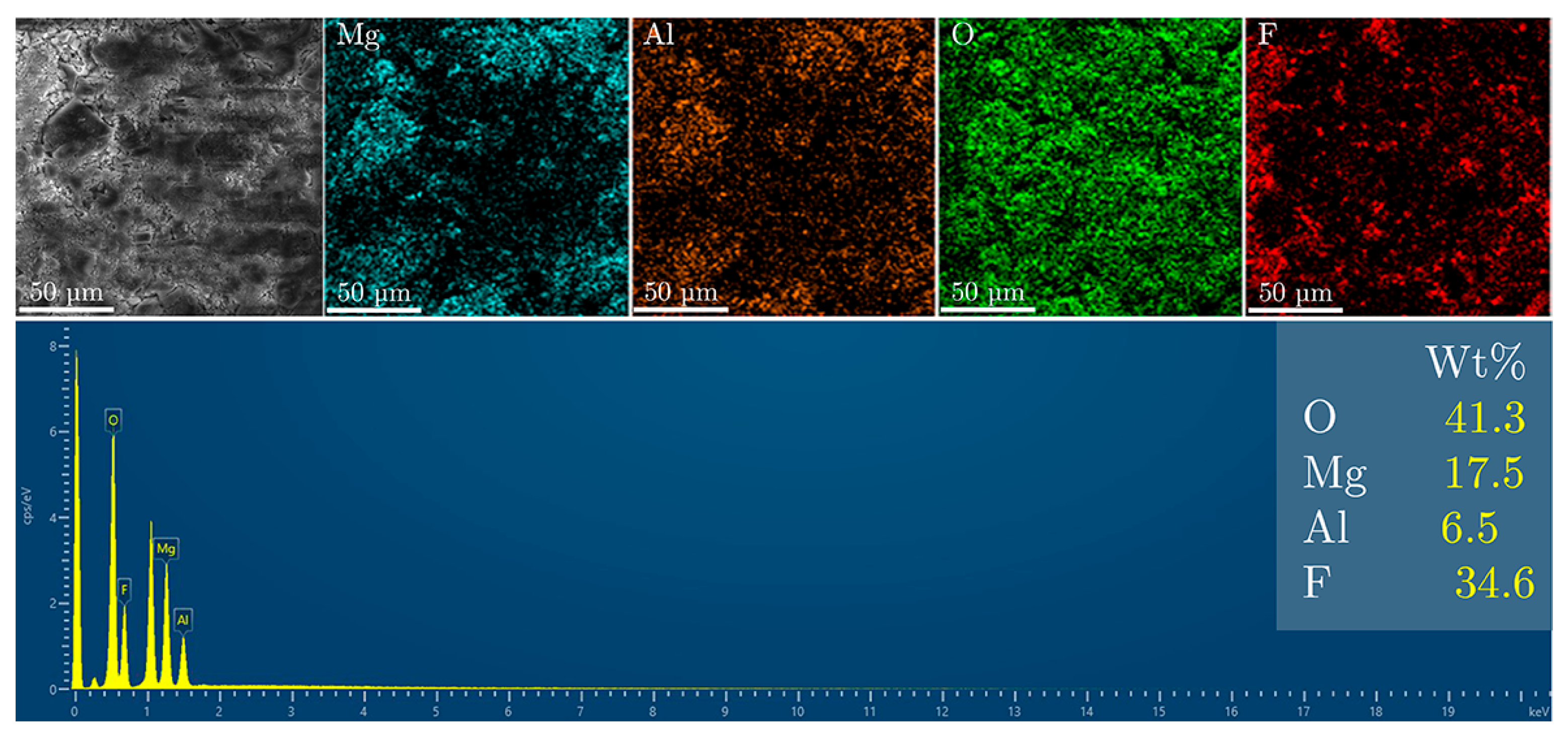
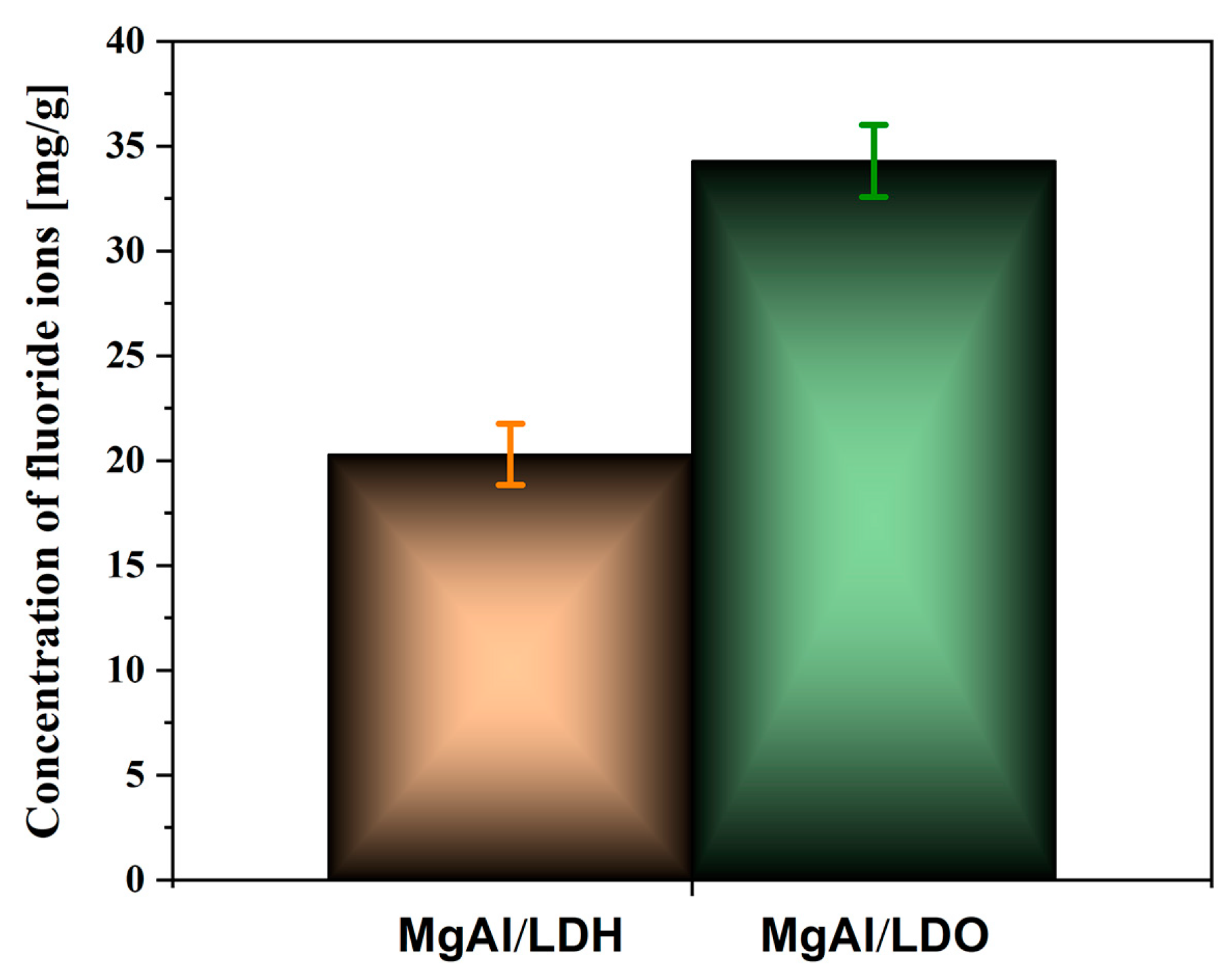
3. Results and Discussion
Fluoride Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koilraj, P.; Kannan, S. Aqueous Fluoride Removal Using ZnCr Layered Double Hydroxides and Their Polymeric Composites: Batch and Column Studies. Chem. Eng. J. 2013, 234, 406–415. [Google Scholar] [CrossRef]
- Mandal, S.; Mayadevi, S. Cellulose Supported Layered Double Hydroxides for the Adsorption of Fluoride from Aqueous Solution. Chemosphere 2008, 72, 995–998. [Google Scholar] [CrossRef]
- Hoxha, A.; Karpukhina, N.; Gillam, D.G.; Bushby, A.J.; Patel, M.P. Fluoride Rechargeable Layered Double Hydroxide Powders for Dental Applications. Appl. Clay Sci. 2021, 200, 105863. [Google Scholar] [CrossRef]
- Bini, M.; Ambrogi, V.; Donnadio, A.; Di Michele, A.; Ricci, P.; Nocchetti, M. Layered Double Hydroxides Intercalated with Fluoride and Methacrylate Anions as Multifunctional Filler of Acrylic Resins for Dental Composites. Appl. Clay Sci. 2020, 197, 105796. [Google Scholar] [CrossRef]
- Mougui, A.; El Bouchti, I. La Fluorose Osseuse—Une Hyperostose Aassociée à Des Condensations Osseuses. J. D’imagerie Diagn. Interv. 2020, 3, 404–406. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V. A Review on Effect of Synthesis Conditions on the Formation of Layered Double Hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, X.; Wang, X.; Zhao, B.; Chen, A.; Sun, D. Phosphate adsorption from sewage sludge filtrate using zinc–aluminum layered double hydroxides. J. Hazard. Mater. 2009, 169, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Chubar, N.; Gilmour, R.; Gerda, V.; Mičušík, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J.; Zaitsev, V. Layered Double Hydroxides as the next Generation Inorganic Anion Exchangers: Synthetic Methods versus Applicability. Adv. Colloid Interface Sci. 2017, 245, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Bankauskaite, A.; Baltakys, K. The Formation of Different Mg-Al LDHs (Mg/Al=2:1) under Hydrothermal Conditions and Their Application for Zn2+ Ions Removal. Sci. Sinter. 2014, 46, 95–106. [Google Scholar] [CrossRef]
- Riaz, S.; ur Rehman, A.; Akhter, Z.; Najam, T.; Hossain, I.; Karim, M.R.; Assiri, M.A.; Shah, S.S.A.; Nazir, M.A. Recent Advancement in Synthesis and Applications of Layered Double Hydroxides (LDHs) Composites. Mater. Today Sustain. 2024, 27, 100897. [Google Scholar] [CrossRef]
- Jerome, M.P.; Alahmad, F.A.; Salem, M.T.; Tahir, M. Layered Double Hydroxide (LDH) Nanomaterials with Engineering Aspects for Photocatalytic CO2 Conversion to Energy Efficient Fuels: Fundamentals, Recent Advances, and Challenges. J. Environ. Chem. Eng. 2022, 10, 108151. [Google Scholar] [CrossRef]
- Milanovic, P.; Vuksanovic, M.; Mitric, M.; Kojovic, A.; Mijin, D.; Jancic-Hainemann, R. Alumina Particles Doped with Ferric as Efficient Adsorbent for Removal of Reactive Orange 16 from Aqueous Solutions. Sci. Sinter. 2018, 50, 467–476. [Google Scholar] [CrossRef]
- Othman, M.R.; Helwani, Z.; Martunus; Fernando, W.J.N. Synthetic Hydrotalcites from Different Routes and Their Application as Catalysts and Gas Adsorbents: A Review. Appl. Organomet. Chem. 2009, 23, 335–346. [Google Scholar] [CrossRef]
- Guan, X.; Yuan, X.; Zhao, Y.; Wang, H.; Wang, H.; Bai, J.; Li, Y. Application of Functionalized Layered Double Hydroxides for Heavy Metal Removal: A Review. Sci. Total Environ. 2022, 838, 155693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, D.; Tantai, X.; Jiang, B.; Sun, Y.; Yang, N. Synthesis of Three-Dimensional Hierarchical Flower-Like Mg–Al Layered Double Hydroxides with Excellent Adsorption Performance for Organic Anionic Dyes. Trans. Tianjin Univ. 2021, 27, 394–408. [Google Scholar] [CrossRef]
- Cosano, D.; Esquivel, D.; Romero-Salguero, F.J.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Use of Raman Spectroscopy to Assess Nitrate Uptake by Calcined LDH Phases. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 602, 125066. [Google Scholar] [CrossRef]
- Ye, H.; Liu, S.; Yu, D.; Zhou, X.; Qin, L.; Lai, C.; Qin, F.; Zhang, M.; Chen, W.; Chen, W.; et al. Regeneration Mechanism, Modification Strategy, and Environment Application of Layered Double Hydroxides: Insights Based on Memory Effect. Coord. Chem. Rev. 2022, 450, 214253. [Google Scholar] [CrossRef]
- Conterosito, E.; Palin, L.; Antonioli, D.; Riccardi, M.; Boccaleri, E.; Aceto, M.; Milanesio, M.; Gianotti, V. On the Rehydration of Organic Layered Double Hydroxides to Form Low-Ordered Carbon/LDH Nanocomposites. Inorganics 2018, 6, 79. [Google Scholar] [CrossRef]
- Valeikiene, L.; Roshchina, M.; Grigoraviciute-Puroniene, I.; Prozorovich, V.; Zarkov, A.; Ivanets, A.; Kareiva, A. On the Reconstruction Peculiarities of Sol–Gel Derived Mg2−xMx/Al1 (M = Ca, Sr, Ba) Layered Double Hydroxides. Crystals 2020, 10, 470. [Google Scholar] [CrossRef]
- Bezerra, B.G.P.; Bieseki, L.; de Mello, M.I.S.; da Silva, D.R.; Rodella, C.B.; Pergher, S. Memory Effect on a LDH/Zeolite A Composite: An XRD In Situ Study. Materials 2021, 14, 2102. [Google Scholar] [CrossRef]
- Xu, X.; Li, P.; Yang, S.; Zhang, T.; Han, X.; Zhou, G.; Cao, Y.; Teng, D. The Performance and Mechanism of a Mg-Al Double-Layer Oxide in Chloride Ion Removal from an Aqueous Solution. Nanomaterials 2022, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, B.; Bieseki, L.; da Silva, D.; Pergher, S. Development of a Zeolite A/LDH Composite for Simultaneous Cation and Anion Removal. Materials 2019, 12, 661. [Google Scholar] [CrossRef] [PubMed]
- Leont’eva, N.N.; Cherepanova, S.V.; Stepanova, L.N.; Drozdov, V.A.; Lavrenov, A.V. Structural Aspects of “Memory Effect” for MgGa LDHs: New Data Obtained by Simulation of XRD Patterns for 1D Disordered Crystals. Crystals 2022, 12, 629. [Google Scholar] [CrossRef]
- Alazreg, A.; Vuksanović, M.M.; Mladenović, I.O.; Egelja, A.; Janković-Mandić, L.; Marinković, A.; Heinemann, R.J. Dental Material Based on Poly(Methyl Methacrylate) with Magnesium-Aluminum Layered Double Hydroxide (MgAl-LDH) on Bio-Silica Particles. Mater. Lett. 2024, 354, 135354. [Google Scholar] [CrossRef]
- Fu, Y.; Fu, X.; Song, W.; Li, Y.; Li, X.; Yan, L. Recent Progress of Layered Double Hydroxide-Based Materials in Wastewater Treatment. Materials 2023, 16, 5723. [Google Scholar] [CrossRef] [PubMed]
- Farhan, A.; Khalid, A.; Maqsood, N.; Iftekhar, S.; Sharif, H.M.A.; Qi, F.; Sillanpää, M.; Asif, M.B. Progress in Layered Double Hydroxides (LDHs): Synthesis and Application in Adsorption, Catalysis and Photoreduction. Sci. Total Environ. 2024, 912, 169160. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Guo, B.; Sasaki, K. Influence of Silicate on the Structural Memory Effect of Layered Double Hydroxides for the Immobilization of Selenium. J. Hazard. Mater. 2020, 395, 122674. [Google Scholar] [CrossRef]
- Tejani, T.H.; Milosevic, A.; Patel, M.; Gillam, D. The Effect of Layered Double Hydroxide on Fluoride Release and Recharge from a Commercial and an Experimental Resin Varnish. Dent. Mater. 2022, 38, e1–e9. [Google Scholar] [CrossRef]
- Iyi, N.; Yamada, H.; Sasaki, T. Deintercalation of Carbonate Ions from Carbonate-Type Layered Double Hydroxides (LDHs) Using Acid–Alcohol Mixed Solutions. Appl. Clay Sci. 2011, 54, 132–137. [Google Scholar] [CrossRef]
- Chen, M.; Li, S.; Li, L.; Jiang, L.; Ahmed, Z.; Dang, Z.; Wu, P. Memory Effect Induced the Enhancement of Uranium (VI) Immobilization on Low-Cost MgAl-Double Oxide: Mechanism Insight and Resources Recovery. J. Hazard. Mater. 2021, 401, 123447. [Google Scholar] [CrossRef]
- Guaya, D.; Cobos, H.; Valderrama, C.; Cortina, J.L. Effect of Mn2+/Zn2+/Fe3+ Oxy(Hydroxide) Nanoparticles Doping onto Mg-Al-LDH on the Phosphate Removal Capacity from Simulated Wastewater. Nanomaterials 2022, 12, 3680. [Google Scholar] [CrossRef]
- Peng, M.; Hu, F.; Du, M.; Mai, B.; Zheng, S.; Liu, P.; Wang, C.; Chen, Y. Hydrothermal Growth of Hydroxyapatite and ZnO Bilayered Nanoarrays on Magnesium Alloy Surface with Antibacterial Activities. Front. Mater. Sci. 2020, 14, 14–23. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, X.; Wang, F.; Ning, X.; Wen, Y.; Song, B.; Yang, C.; Wu, D.; Ke, X.; Peng, L. Insights into Memory Effect Mechanisms of Layered Double Hydroxides with Solid-State NMR Spectroscopy. Nat. Commun. 2022, 13, 6093. [Google Scholar] [CrossRef]
- Parry, S.A.; Pawley, A.R.; Jones, R.L.; Clark, S.M. An Infrared Spectroscopic Study of the OH Stretching Frequencies of Talc and 10-Å Phase to 10 GPa. Am. Mineral. 2015, 92, 525–531. [Google Scholar] [CrossRef]
- Lv, L.; He, J.; Wei, M.; Evans, D.; Duan, X. Factors Influencing the Removal of Fluoride from Aqueous Solution by Calcined Mg–Al–CO3 Layered Double Hydroxides. J. Hazard. Mater. 2006, 133, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Bunekar, N.; Liang, S. Effect of Multiorganomodified LiAl- or MgAl-Layered Double Hydroxide on the PMMA Nanocomposites. Adv. Polym. Technol. 2016, 37, 31–37. [Google Scholar] [CrossRef]
- Palmer, S.; Frost, R.; Nguyen, T. Hydrotalcites and Their Role in Coordination of Anions in Bayer Liquors: Anion Binding in Layered Double Hydroxides. Coord. Chem. Rev. 2009, 253, 250–267. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Z.; Qiu, Y.; Zhao, J. Enhanced Adsorption of Acid Brown 14 Dye on Calcined Mg/Fe Layered Double Hydroxide with Memory Effect. Chem. Eng. J. 2013, 219, 69–77. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Q.; Li, Z.; Zhang, D.; Sun, Q.; Wang, Q.; Sun, H.; Wang, B. Exploring the Role of Solvents in Structural Regulation during Ultrasonic Synthesis of Co/Ni-Layered Double Hydroxide for Oxygen Evolution Reaction. Mater. Rep. Energy 2024, 4, 100296. [Google Scholar] [CrossRef]
- Hayashi, A.; Nakayama, H. Intercalation Reaction of Carbonate MgAl-Layered Double Hydroxide Using Alcohol as Solvent. Chem. Lett. 2011, 40, 276–278. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, L.; Zhu, G.; Chen, D.; Ma, X.; Yang, X.; Wang, S. Outstanding Performance of Thiophene-Based Metal-Organic Frameworks for Fluoride Capture from Wastewater. Sep. Purif. Technol. 2022, 298, 121567. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Said, G.F.; Elnashar, A.A.S.; Ibrahim, G.A.A. Adsorptive Capture of Fluoride Ion from Water by Co/Fe-LDH@Avocado Kernel Seeds Biochar@Caboxymethylcellulose Nanobiosorbent: Optimization, Error Function and Multivariate Analysis. J. Ind. Eng. Chem. 2024, 133, 561–576. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, W.; Mamrol, N.; Croes, T.; Mai, Z.; Houtmeyers, S.; Dewil, R.; Zhang, Y.; Yang, X.; Van der Bruggen, B. Self-Assembled Embedding of Ion Exchange Materials into Nanofiber-Based Hydrogel Framework for Fluoride Capture. Chem. Eng. J. 2022, 431, 134201. [Google Scholar] [CrossRef]
- Hang, X. Progress and Perspectives on Cost-Effective and Eco-Friendly Mineral Functional Materials for Efficient Capture of Fluoride and Phosphorus. Mater. Today Sustain. 2024, 27, 100923. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alazreg, A.; Tadić, V.; Egelja, A.; Savić, A.; Šaponjić, A.; Vuksanović, M.M.; Heinemann, R.J. Memory Effect of Double Oxides Compared to Simple Ion Exchange for Controlled Fluoride Ion Capture and Release. Materials 2025, 18, 162. https://doi.org/10.3390/ma18010162
Alazreg A, Tadić V, Egelja A, Savić A, Šaponjić A, Vuksanović MM, Heinemann RJ. Memory Effect of Double Oxides Compared to Simple Ion Exchange for Controlled Fluoride Ion Capture and Release. Materials. 2025; 18(1):162. https://doi.org/10.3390/ma18010162
Chicago/Turabian StyleAlazreg, Asma, Vladisav Tadić, Adela Egelja, Andrija Savić, Aleksandra Šaponjić, Marija M. Vuksanović, and Radmila Jančić Heinemann. 2025. "Memory Effect of Double Oxides Compared to Simple Ion Exchange for Controlled Fluoride Ion Capture and Release" Materials 18, no. 1: 162. https://doi.org/10.3390/ma18010162
APA StyleAlazreg, A., Tadić, V., Egelja, A., Savić, A., Šaponjić, A., Vuksanović, M. M., & Heinemann, R. J. (2025). Memory Effect of Double Oxides Compared to Simple Ion Exchange for Controlled Fluoride Ion Capture and Release. Materials, 18(1), 162. https://doi.org/10.3390/ma18010162







