Qualitative Research of Composite Graphene Membranes Using the Electric Mode in SEM and AFM
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Graphene Transfer
2.3. GO and rGO Application
2.4. Optical Microscopy and SEM Examination
2.5. AFM Examination
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, D.; Idrobo, J.C.; Laoui, T.; Karnik, R. Water and Solute Transport Governed by Tunable Pore Size Distributions in Nanoporous Graphene Membranes. ACS Nano 2017, 11, 10042–10052. [Google Scholar] [CrossRef] [PubMed]
- Surwade, S.P.; Smirnov, S.N.; Vlassiouk, I.; Unocic, R.R.; Veith, G.M.; Dai, S.; Mahurin, S.M. Water Desalination Using Nanoporous Single-Layer Graphene. Nat. Nanotechnol. 2015, 10, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Rasera, B.C.; Guerrero, R.J.S.; Boutilier, M.S.H.; O’Hern, S.C.; Idrobo, J.C.; Karnik, R. Heterogeneous Sub-Continuum Ionic Transport in Statistically Isolated Graphene Nanopores. Nat. Nanotechnol. 2015, 10, 1053–1057. [Google Scholar] [CrossRef]
- Goh, P.S.; Matsuura, T.; Ismail, A.F.; Hilal, N. Recent Trends in Membranes and Membrane Processes for Desalination. Desalination 2016, 391, 43–60. [Google Scholar] [CrossRef]
- Johnson, D.J.; Hilal, N. Can Graphene and Graphene Oxide Materials Revolutionise Desalination Processes? Desalination 2021, 500, 114852. [Google Scholar] [CrossRef]
- Wu, W.; Shi, Y.; Liu, G.; Fan, X.; Yu, Y. Recent Development of Graphene Oxide Based Forward Osmosis Membrane for Water Treatment: A Critical Review. Desalination 2020, 491, 114452. [Google Scholar] [CrossRef]
- Pop, E.; Varshney, V.; Roy, A. Thermal Properties of Graphene: Fundamentals and Applications. MRS Bull. 2013, 37, 1273–1281. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Dash, G.N.; Pattanaik, S.R.; Behera, S. Graphene for Electron Devices: The Panorama of a Decade. IEEE J. Electron Devices Soc. 2014, 2, 77–104. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The Structure of Suspended Graphene Sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xu, C.; Xu, K.; Deng, J.; Guo, W.; Yurgens, A.; Sun, J. Rapid Chemical Vapor Deposition of Graphene on Liquid Copper. Synth. Met. 2016, 216, 93–97. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, M.S.; Kim, M.; Kim, K.J.; Kim, H.M.; Lee, D.J.; Lee, S.H.; Kim, K.B. Self-Assembly and Continuous Growth of Hexagonal Graphene Flakes on Liquid Cu. Nanoscale 2015, 7, 12820–12827. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zeng, M.; Cao, H.; Zhang, T.; Gao, X.; Xiao, Y.; Fu, L. Insight into the Rapid Growth of Graphene Single Crystals on Liquid Metal via Chemical Vapor Deposition. Sci. China Mater. 2019, 62, 1087–1095. [Google Scholar] [CrossRef]
- Kim, M.-S.; Cho, S.-Y.; Kim, M.; Kim, K.-J.; Lee, S.-H.; Kim, H.-M.; Kim, K.-B. Comparison of Growth Behavior and Electrical Properties of Graphene Grown on Solid and Liquid Copper by Chemical Vapor Deposition. J. Nanosci. Nanotechnol. 2019, 20, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Zhu, Y.; Wang, S.; Gong, Q.; Sun, L.; Wu, T.; Xie, X.; Jiang, M. Chemical Vapor Deposition of Graphene on Liquid Metal Catalysts. Carbon 2013, 53, 321–326. [Google Scholar] [CrossRef]
- Geng, D.; Wu, B.; Guo, Y.; Huang, L.; Xue, Y.; Chen, J.; Yu, G.; Jiang, L.; Hu, W.; Liu, Y. Uniform Hexagonal Graphene Flakes and Films Grown on Liquid Copper Surface. Proc. Natl. Acad. Sci. USA 2012, 109, 7992–7996. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, L. Controllable Growth of Graphene on Liquid Surfaces. Adv. Mater. 2019, 31, e1800690. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, S. Large Area CVD Growth of Graphene. Synth. Met. 2015, 210, 95–108. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Colombo, L.; Ruoff, R. Evolution of Graphene Growth on Ni and Cu by Carbon Isotope Labeling. Nano Lett. 2009, 9, 4268–4272. [Google Scholar] [CrossRef]
- Kula, P.; Pietrasik, R.; Dybowski, K.; Atraszkiewicz, R.; Szymanski, W.; Kolodziejczyk, L.; Niedzielski, P.; Nowak, D. Single and Multilayer Growth of Graphene from the Liquid Phase. Appl. Mech. Mater. 2014, 510, 8–12. [Google Scholar] [CrossRef]
- Romaniak, G.; Cheng, P.; Dybowski, K.; Kula, P.; Kidambi, P.R. Assessing the Quality of Large-Area Monolayer Graphene Grown on Liquid Copper for Size-Selective Ionic/Molecular Membrane Separations. Mater. Res. Express 2023, 10, 105101. [Google Scholar] [CrossRef]
- Kafiah, F.M.; Khan, Z.; Ibrahim, A.; Karnik, R.; Atieh, M.; Laoui, T. Monolayer Graphene Transfer onto Polypropylene and Polyvinylidenedifluoride Microfiltration Membranes for Water Desalination. Desalination 2016, 388, 29–37. [Google Scholar] [CrossRef]
- O’Hern, S.C.; Jang, D.; Bose, S.; Idrobo, J.-C.; Song, Y.; Laoui, T.; Kong, J.; Karnik, R. Nanofiltration across Defect-Sealed Nanoporous Monolayer Graphene. Nano Lett. 2015, 15, 3254–3260. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.S.; Abdi, Y.; Eslami, J.; Das, R. Support Based Novel Single Layer Nanoporous Graphene Membrane for Efficacious Water Desalination. Desalination 2019, 451, 148–159. [Google Scholar] [CrossRef]
- Kafiah, F.; Khan, Z.; Ibrahim, A.; Atieh, M.; Laoui, T. Synthesis of Graphene Based Membranes: Effect of Substrate Surface Properties on Monolayer Graphene Transfer. Materials 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, M.S.H.; Jang, D.; Idrobo, J.C.; Kidambi, P.R.; Hadjiconstantinou, N.G.; Karnik, R. Molecular Sieving Across Centimeter-Scale Single-Layer Nanoporous Graphene Membranes. ACS Nano 2017, 11, 5726–5736. [Google Scholar] [CrossRef] [PubMed]
- O’Hern, S.C.; Stewart, C.A.; Boutilier, M.S.H.; Idrobo, J.-C.; Bhaviripudi, S.; Das, S.K.; Kong, J.; Laoui, T.; Atieh, M.; Karnik, R. Selective Molecular Transport through Intrinsic Defects in a Single Layer of CVD Graphene. ACS Nano 2012, 6, 10130–10138. [Google Scholar] [CrossRef]
- Cheng, P.; Kelly, M.M.; Moehring, N.K.; Ko, W.; Li, A.P.; Idrobo, J.C.; Boutilier, M.S.H.; Kidambi, P.R. Facile Size-Selective Defect Sealing in Large-Area Atomically Thin Graphene Membranes for Sub-Nanometer Scale Separations. Nano Lett. 2020, 20, 5951–5959. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The Reduction of Graphene Oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of Graphene Oxide Vial-Ascorbic Acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C Is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, Q.; Qiu, H.; Li, J.; Yang, J. A Simple Method for the Reduction of Graphene Oxide by Sodium Borohydride with CaCl2 as a Catalyst. Carbon 2015, 86, 372. [Google Scholar] [CrossRef]
- Grodecki, K. Spektroskopia Ramanowska Grafenu. Electron. Mater. 2013, 41, 47–53. [Google Scholar]
- Gass, M.; Bangert, U.; Bleloch, A.; Wang, P.; Raveendran-Nair, R.; Geim, A.K. Free-Standing Graphene at Atomic Resolution. Nat. Nanotechnol. 2008, 3, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Cao, P.; Heath, J.R. Scanning Tunneling Microscopy Characterization of the Electrical Properties of Wrinkles in Exfoliated Graphene Monolayers. Nano Lett. 2009, 9, 4446–4451. [Google Scholar] [CrossRef]
- Abergel, D.S.L.; Russell, A.; Fal’ko, V.I. Visibility of Graphene Flakes on a Dielectric Substrate. Appl. Phys. Lett. 2007, 91, 63125. [Google Scholar] [CrossRef]
- Hiura, H.; Miyazaki, H.; Tsukagoshi, K. Determination of the Number of Graphene Layers: Discrete Distribution of the Secondary Electron Intensity Stemming from Individual Graphene Layers. Appl. Phys. Express 2010, 3, 95101. [Google Scholar] [CrossRef]
- Romaniak, G.; Dybowski, K.; Jeziorna, A.; Kula, P.; Kaźmierczak, T. Synthesis and Characterization of Semi-Permeable Graphene/Graphene Oxide Membranes for Water Desalination. J. Mater. Sci. 2020, 55, 9775–9786. [Google Scholar] [CrossRef]
- Dybowski, K.; Romaniak, G.; Kula, P.; Jeziorna, A.; Kowalczyk, P.; Atraszkiewicz, R.; Kołodziejczyk, Ł.; Nowak, D.; Zawadzki, P.; Kucińska, M. Impact of the Method of Separating Graphene from the Growth Substrate on the Quality of the 2D Material Obtained. Arch. Metall. Mater. 2019, 64, 1321–1326. [Google Scholar] [CrossRef]
- Romaniak, G.; Dybowski, K.; Jędrzejczak, A.; Sobczyk-Guzenda, A.; Januszewicz, B.; Szymański, W.; Kowalczyk, P.; Kaźmierczak, T.; Siniarski, J.; Kula, P. Impact of a Graphene Oxide Reducing Agent on a Semi-Permeable Graphene/Reduced Graphene Oxide Forward Osmosis Membrane Filtration Efficiency. Membranes 2021, 11, 679. [Google Scholar] [CrossRef]


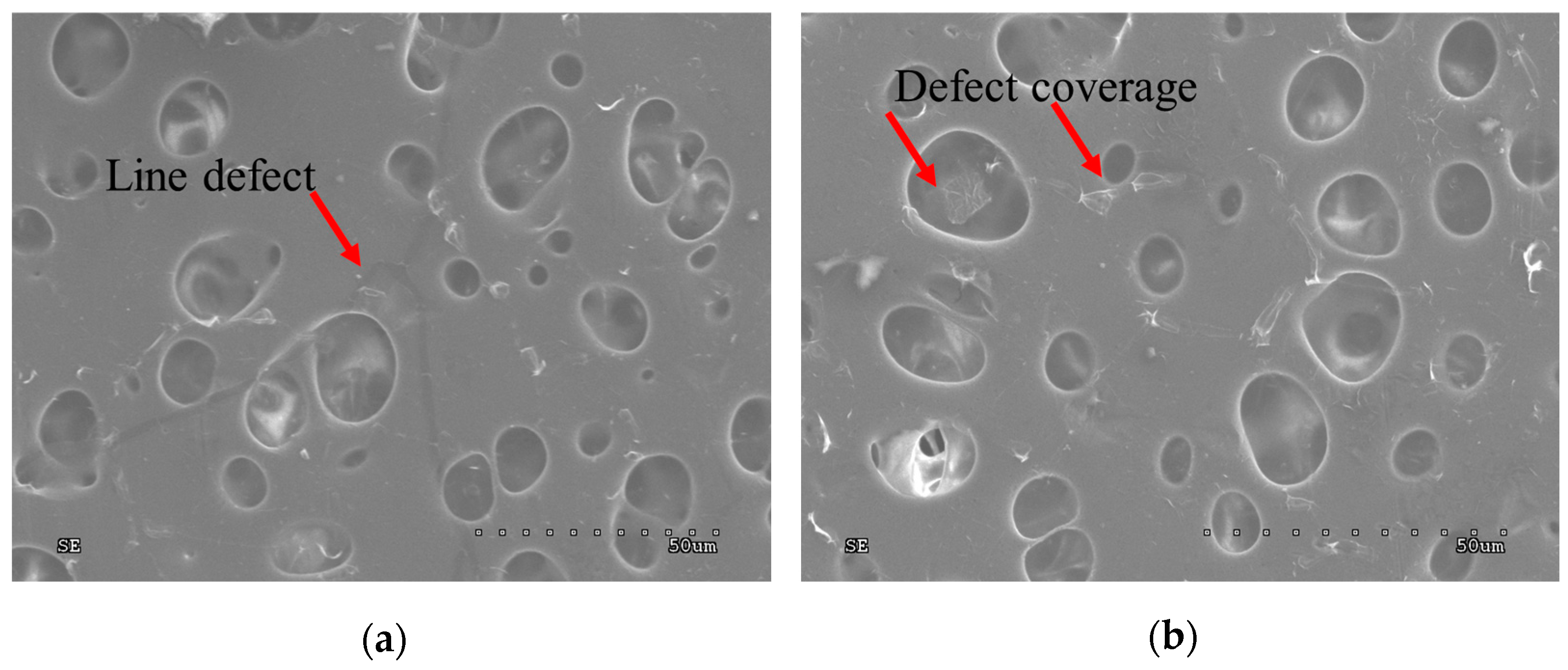
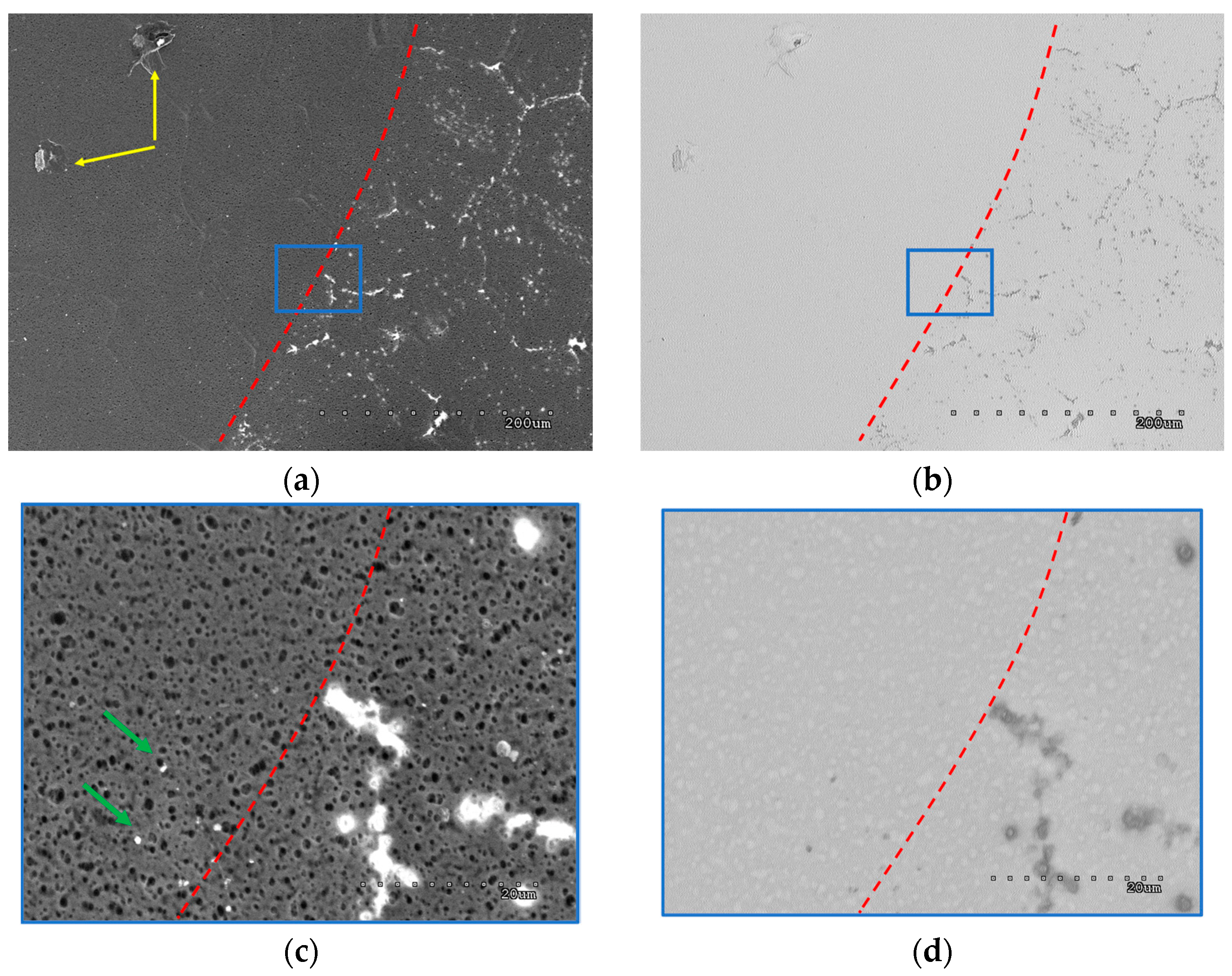
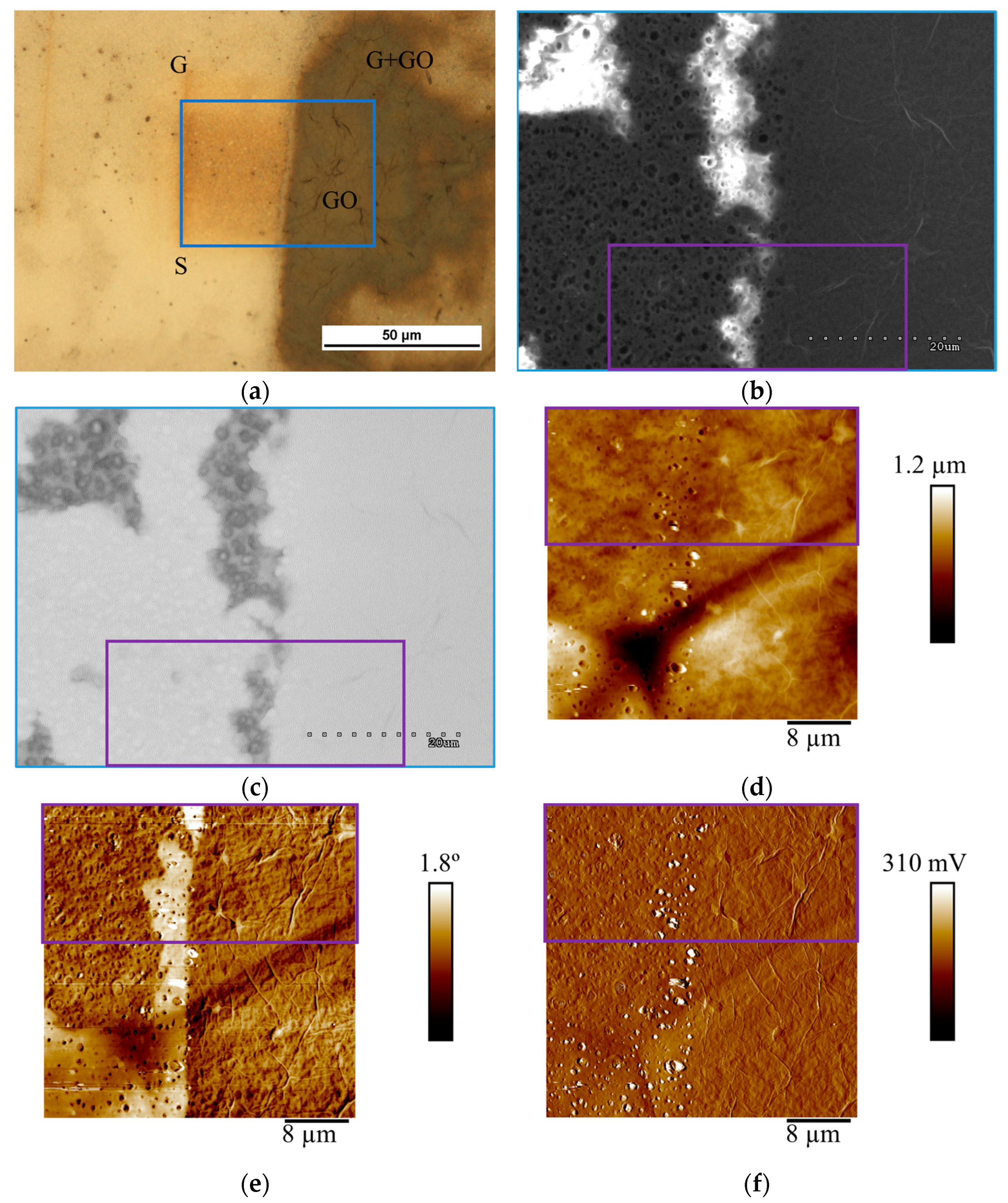

| Signature on Figures | Membrane’s Area |
|---|---|
| S | Bare substrate |
| G | Graphene layer |
| GO | Graphene oxide on PS |
| rGO | Reduced graphene oxide on PS |
| G + GO | Graphene oxide selectively coating graphene defects |
| G + rGO | Reduced graphene oxide selectively coating graphene defects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romaniak, G.; Dybowski, K.; Kołodziejczyk, Ł.; Kowalczyk, P. Qualitative Research of Composite Graphene Membranes Using the Electric Mode in SEM and AFM. Materials 2025, 18, 163. https://doi.org/10.3390/ma18010163
Romaniak G, Dybowski K, Kołodziejczyk Ł, Kowalczyk P. Qualitative Research of Composite Graphene Membranes Using the Electric Mode in SEM and AFM. Materials. 2025; 18(1):163. https://doi.org/10.3390/ma18010163
Chicago/Turabian StyleRomaniak, Grzegorz, Konrad Dybowski, Łukasz Kołodziejczyk, and Paulina Kowalczyk. 2025. "Qualitative Research of Composite Graphene Membranes Using the Electric Mode in SEM and AFM" Materials 18, no. 1: 163. https://doi.org/10.3390/ma18010163
APA StyleRomaniak, G., Dybowski, K., Kołodziejczyk, Ł., & Kowalczyk, P. (2025). Qualitative Research of Composite Graphene Membranes Using the Electric Mode in SEM and AFM. Materials, 18(1), 163. https://doi.org/10.3390/ma18010163










