Syngas Production Improvement from CO2RR Using Cu-Sn Electrodeposited Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Preparation Procedure of the Catalysts
2.2. Structure Characterization and Morphology
2.3. Flow Cell Setup, Electrochemical Measurements, and Product Analysis
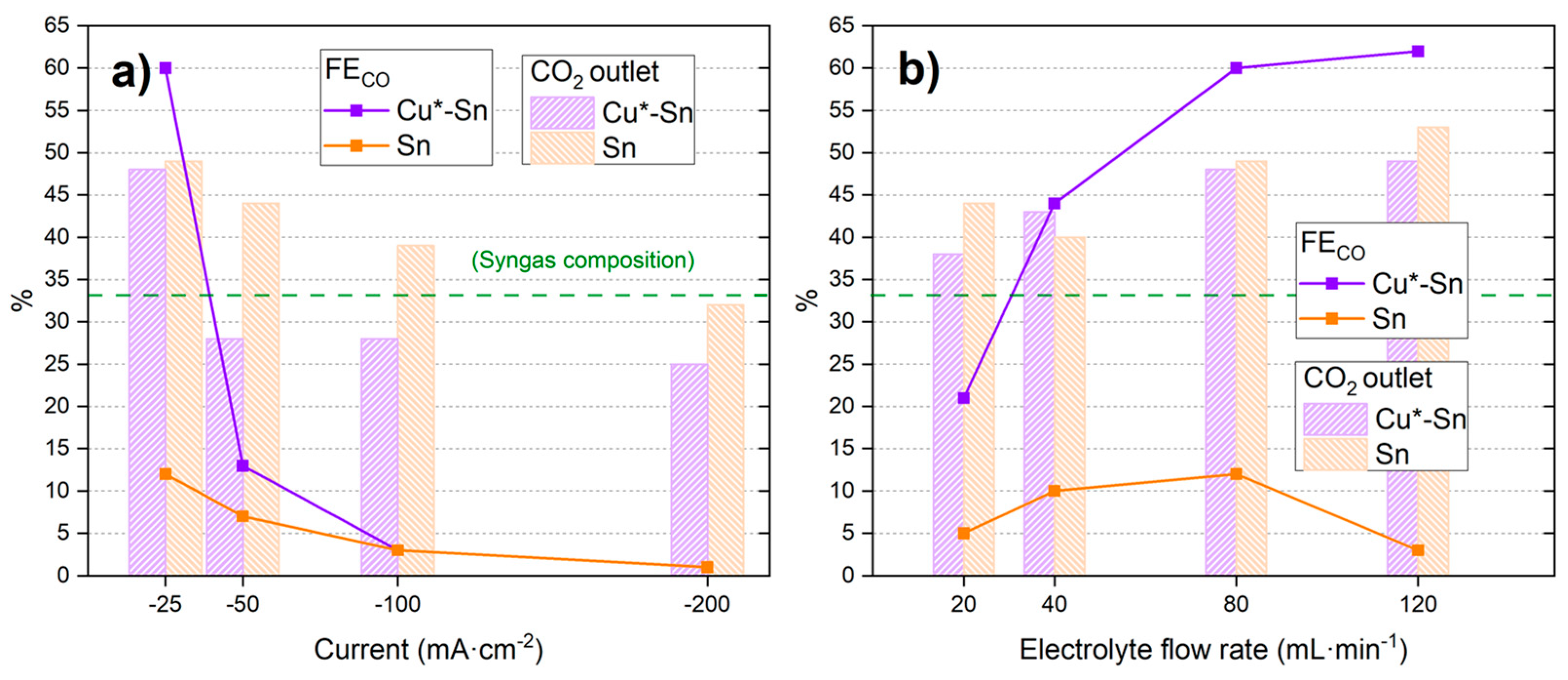
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearson, P.N.; Palmer, M.R. Atmospheric Carbon Dioxide Concentrations over the Past 60 Million Years. Nature 2000, 406, 695–699. [Google Scholar] [CrossRef]
- Zhang, Y. Carbon Dioxide Utilization: A Carbon-Neutral Energy Cycle. Nat. Rev. Chem. 2017, 1, 0057. [Google Scholar] [CrossRef]
- De Luna, P.; Hahn, C.; Higgins, D.; Jaffer, S.A.; Jaramillo, T.F.; Sargent, E.H. What Would It Take for Renewably Powered Electrosynthesis to Displace Petrochemical Processes? Science 2019, 364, eaav3506. [Google Scholar] [CrossRef] [PubMed]
- Jouny, M.; Luc, W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Gaspard, P.; Kruse, N. The Oscillating Fischer-Tropsch Reaction. Science 2023, 382, 99–103. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, A. Fischer–Tropsch Process. In Kirk-Othmer Encyclopedia of Chemical Technology; American Cancer Society: Atlanta, GA, USA, 2013; pp. 1–20. [Google Scholar]
- Lei, Y.; Wang, Z.; Bao, A.; Tang, X.; Huang, X.; Yi, H.; Zhao, S.; Sun, T.; Wang, J.; Gao, F. Recent Advances on Electrocatalytic CO2 Reduction to Resources: Target Products, Reaction Pathways and Typical Catalysts. Chem. Eng. J. 2023, 453, 139663. [Google Scholar] [CrossRef]
- Wei, P.; Li, H.; Lin, L.; Gao, D.; Zhang, X.; Gong, H.; Qing, G.; Cai, R.; Wang, G.; Bao, X. CO2 Electrolysis at Industrial Current Densities Using Anion Exchange Membrane Based Electrolyzers. Sci. China Chem. 2020, 63, 1711–1715. [Google Scholar] [CrossRef]
- Dinh, C.-T.; García De Arquer, F.P.; Sinton, D.; Sargent, E.H. High Rate, Selective, and Stable Electroreduction of CO2 to CO in Basic and Neutral Media. ACS Energy Lett. 2018, 3, 2835–2840. [Google Scholar] [CrossRef]
- Verma, S.; Lu, X.; Ma, S.; Masel, R.I.; Kenis, P.J.A. The Effect of Electrolyte Composition on the Electroreduction of CO2 to CO on Ag Based Gas Diffusion Electrodes. Phys. Chem. Chem. Phys. 2016, 18, 7075–7084. [Google Scholar] [CrossRef]
- Weekes, D.M.; Salvatore, D.A.; Reyes, A.; Huang, A.; Berlinguette, C.P. Electrolytic CO2 Reduction in a Flow Cell. Acc. Chem. Res. 2018, 51, 910–918. [Google Scholar] [CrossRef]
- Li, Y.C.; Lee, G.; Yuan, T.; Wang, Y.; Nam, D.-H.; Wang, Z.; García De Arquer, F.P.; Lum, Y.; Dinh, C.-T.; Voznyy, O.; et al. CO2 Electroreduction from Carbonate Electrolyte. ACS Energy Lett. 2019, 4, 1427–1431. [Google Scholar] [CrossRef]
- Li, T.; Lees, E.W.; Goldman, M.; Salvatore, D.A.; Weekes, D.M.; Berlinguette, C.P. Electrolytic Conversion of Bicarbonate into CO in a Flow Cell. Joule 2019, 3, 1487–1497. [Google Scholar] [CrossRef]
- Xie, K.; Miao, R.K.; Ozden, A.; Liu, S.; Chen, Z.; Dinh, C.-T.; Huang, J.E.; Xu, Q.; Gabardo, C.M.; Lee, G.; et al. Bipolar Membrane Electrolyzers Enable High Single-Pass CO2 Electroreduction to Multicarbon Products. Nat. Commun. 2022, 13, 3609. [Google Scholar] [CrossRef] [PubMed]
- Vermaas, D.A.; Smith, W.A. Synergistic Electrochemical CO2 Reduction and Water Oxidation with a Bipolar Membrane. ACS Energy Lett. 2016, 1, 1143–1148. [Google Scholar] [CrossRef]
- Welch, A.J.; Dunn, E.; DuChene, J.S.; Atwater, H.A. Bicarbonate or Carbonate Processes for Coupling Carbon Dioxide Capture and Electrochemical Conversion. ACS Energy Lett. 2020, 5, 940–945. [Google Scholar] [CrossRef]
- Sullivan, I.; Goryachev, A.; Digdaya, I.A.; Li, X.; Atwater, H.A.; Vermaas, D.A.; Xiang, C. Coupling Electrochemical CO2 Conversion with CO2 Capture. Nat. Catal. 2021, 4, 952–958. [Google Scholar] [CrossRef]
- Mou, K.; Chen, Z.; Yao, S.; Liu, L. Enhanced Electrochemical Reduction of Carbon Dioxide to Formate with In-Situ Grown Indium-Based Catalysts in an Aqueous Electrolyte. Electrochim. Acta 2018, 289, 65–71. [Google Scholar] [CrossRef]
- Marques Mota, F.; Nguyen, D.L.T.; Lee, J.-E.; Piao, H.; Choy, J.-H.; Hwang, Y.J.; Kim, D.H. Toward an Effective Control of the H2 to CO Ratio of Syngas through CO2 Electroreduction over Immobilized Gold Nanoparticles on Layered Titanate Nanosheets. ACS Catal. 2018, 8, 4364–4374. [Google Scholar] [CrossRef]
- Guerrette, J.P.; Percival, S.J.; Zhang, B. Voltammetric Behavior of Gold Nanotrench Electrodes. Langmuir 2011, 27, 12218–12225. [Google Scholar] [CrossRef]
- Cox, J.T.; Zhang, B. Nanoelectrodes: Recent Advances and New Directions. Annu. Rev. Anal. Chem. 2012, 5, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Xu, R. New Materials in Hydrothermal Synthesis. Acc. Chem. Res. 2001, 34, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Chen, C.; Wu, Y.; Yan, X.; Jia, S.; Feng, R.; Wu, H.; He, M.; Han, B. Enhanced CO2 Electroreduction to Ethylene via Strong Metal-Support Interaction. Green Energy Environ. 2022, 7, 792–798. [Google Scholar] [CrossRef]
- Mohanty, U.S. Electrodeposition: A Versatile and Inexpensive Tool for the Synthesis of Nanoparticles, Nanorods, Nanowires, and Nanoclusters of Metals. J. Appl. Electrochem. 2011, 41, 257–270. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Bi, J.; Zhu, Q.; Han, B. Design and Preparation of Electrocatalysts by Electrodeposition for CO2 Reduction. Chem.–Eur. J. 2022, 28, e202200242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, H.; Fournier, M.; MacFarlane, D.R. Electrocatalytic CO2 Reduction to Formate at Low Overpotentials on Electrodeposited Pd Films: Stabilized Performance by Suppression of CO Formation. ChemSusChem 2017, 10, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, H.; Zhang, X.; Ge, L.; Hu, S.; Li, M.; Smart, S.; Zhu, Z.; Yuan, Z. Tuning the Product Selectivity of the Cu Hollow Fiber Gas Diffusion Electrode for Efficient CO2 Reduction to Formate by Controlled Surface Sn Electrodeposition. ACS Appl. Mater. Interfaces 2020, 12, 21670–21681. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, X.; Yang, J.; Wang, L. Electrodeposited Bi Dendrites/2D Black Phosphorus Nanosheets Composite Used for Boosting Formic Acid Production from CO2 Electroreduction. J. CO2 Util. 2020, 38, 32–38. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Forest, R.V.; Moore, A.; Jiao, F. Electrodeposited Zn Dendrites with Enhanced CO Selectivity for Electrocatalytic CO2 Reduction. ACS Catal. 2015, 5, 4586–4591. [Google Scholar] [CrossRef]
- Dutta, A.; Morstein, C.E.; Rahaman, M.; Cedeño López, A.; Broekmann, P. Beyond Copper in CO2 Electrolysis: Effective Hydrocarbon Production on Silver-Nanofoam Catalysts. ACS Catal. 2018, 8, 8357–8368. [Google Scholar] [CrossRef]
- Saberi Safaei, T.; Mepham, A.; Zheng, X.; Pang, Y.; Dinh, C.-T.; Liu, M.; Sinton, D.; Kelley, S.O.; Sargent, E.H. High-Density Nanosharp Microstructures Enable Efficient CO2 Electroreduction. Nano Lett. 2016, 16, 7224–7228. [Google Scholar] [CrossRef]
- Yano, J.; Morita, T.; Shimano, K.; Nagami, Y.; Yamasaki, S. Selective Ethylene Formation by Pulse-Mode Electrochemical Reduction of Carbon Dioxide Using Copper and Copper-Oxide Electrodes. J. Solid State Electrochem. 2007, 11, 554–557. [Google Scholar] [CrossRef]
- Yano, H.; Tanaka, T.; Nakayama, M.; Ogura, K. Selective Electrochemical Reduction of CO2 to Ethylene at a Three-Phase Interface on Copper(I) Halide-Confined Cu-Mesh Electrodes in Acidic Solutions of Potassium Halides. J. Electroanal. Chem. 2004, 565, 287–293. [Google Scholar] [CrossRef]
- Ajmal, S.; Yang, Y.; Tahir, M.A.; Li, K.; Bacha, A.-U.-R.; Nabi, I.; Liu, Y.; Wang, T.; Zhang, L. Boosting C2 Products in Electrochemical CO2 Reduction over Highly Dense Copper Nanoplates. Catal. Sci. Technol. 2020, 10, 4562–4570. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dinh, C.-T.; Li, J.; Ozden, A.; Golam Kibria, M.; Seifitokaldani, A.; Tan, C.-S.; Gabardo, C.M.; Luo, M.; et al. Catalyst Synthesis under CO2 Electroreduction Favours Faceting and Promotes Renewable Fuels Electrosynthesis. Nat. Catal. 2019, 3, 98–106. [Google Scholar] [CrossRef]
- Wang, C.; Cao, M.; Jiang, X.; Wang, M.; Shen, Y. A catalyst based on copper-cadmium bimetal for electrochemical reduction of CO2 to CO with high faradaic efficiency. Electrochim. Acta 2018, 271, 544–550. [Google Scholar] [CrossRef]
- Li, H.; Yue, X.; Qiu, Y.; Xiao, Z.; Yu, X.; Xue, C.; Xiang, J. Selective electroreduction of CO2 to formate over the co-electrodeposited Cu/Sn bimetallic catalyst. Mater. Energy 2021, 21, 100797. [Google Scholar] [CrossRef]
- Zeng, J.; Bejtka, K.; Ju, W.; Castellino, M.; Chiodoni, A.; Sacco, A.; Farkhondehfal, M.A.; Hernández, S.; Rentsch, D.; Battaglia, C.; et al. Advanced Cu-Sn Foam for Selectively Converting CO2 to CO in Aqueous Solution. Appl. Catal. B Environ. 2018, 236, 475–482. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y.; Shao, Q.; Yin, R.; Guo, J.; Li, Y.; Huang, X. Phase and Structure Modulating of Bimetallic CuSn Nanowires Boosts Electrocatalytic Conversion of CO2. Nano Energy 2019, 59, 138–145. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Liu, Z.; Wang, Q.; Xiao, X.; Pan, H.; Li, M.; Wang, J.; Shao, Y.; Peng, Z.; et al. A Highly Selective Tin-Copper Bimetallic Electrocatalyst for the Electrochemical Reduction of Aqueous CO2 to Formate. Appl. Catal. B Environ. 2019, 259, 118040. [Google Scholar] [CrossRef]
- Stojkovikj, S.; El-Nagar, G.A.; Firschke, F.; Pardo Pérez, L.C.; Choubrac, L.; Najdoski, M.; Mayer, M.T. Electrocatalyst Derived from Waste Cu–Sn Bronze for CO2 Conversion into CO. ACS Appl. Mater. Interfaces 2021, 13, 38161–38169. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Modern Aspects of Electrochemistry; Springer: New York, NY, USA, 2008; Volume 42, pp. 89–189. ISBN 978-0-387-49488-3. [Google Scholar]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic Process of CO Selectivity in Electrochemical Reduction of CO2 at Metal Electrodes in Aqueous Media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.S.; Bonifacio, C.S.; Zegkinoglou, I.; Sinev, I.; Choi, Y.-W.; Kisslinger, K.; Stach, E.A.; Yang, J.C.; Strasser, P.; et al. Highly Selective Plasma-Activated Copper Catalysts for Carbon Dioxide Reduction to Ethylene. Nat. Commun. 2016, 7, 12123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xue, S.; Barber, J.; Zhou, Y.; Meng, J.; Ke, X. An Overview of Cu-Based Heterogeneous Electrocatalysts for CO2 Reduction. J. Mater. Chem. A 2020, 8, 4700–4734. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef]
- García De Arquer, F.P.; Dinh, C.-T.; Ozden, A.; Wicks, J.; McCallum, C.; Kirmani, A.R.; Nam, D.-H.; Gabardo, C.; Seifitokaldani, A.; Wang, X.; et al. CO2 Electrolysis to Multicarbon Products at Activities Greater than 1 A cm−2. Science 2020, 367, 661–666. [Google Scholar] [CrossRef]
- Azuma, M.; Hashimoto, K.; Hiramoto, M.; Watanabe, M.; Sakata, T. Electrochemical Reduction of Carbon Dioxide on Various Metal Electrodes in Low-Temperature Aqueous KHCO3 Media. J. Electrochem. Soc. 1990, 137, 1772–1778. [Google Scholar] [CrossRef]
- Masel, R.I.; Liu, Z.; Yang, H.; Kaczur, J.J.; Carrillo, D.; Ren, S.; Salvatore, D.; Berlinguette, C.P. An Industrial Perspective on Catalysts for Low-Temperature CO2 Electrolysis. Nat. Nanotechnol. 2021, 16, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, J.; Li, M.; Züttel, A. Boosting CO Production in Electrocatalytic CO2 Reduction on Highly Porous Zn Catalysts. ACS Catal. 2019, 9, 3783–3791. [Google Scholar] [CrossRef]
- Han, L.; Song, S.; Liu, M.; Yao, S.; Liang, Z.; Cheng, H.; Ren, Z.; Liu, W.; Lin, R.; Qi, G.; et al. Stable and Efficient Single-Atom Zn Catalyst for CO2 Reduction to CH4. J. Am. Chem. Soc. 2020, 142, 12563–12567. [Google Scholar] [CrossRef]
- Qin, B.; Li, Y.; Fu, H.; Wang, H.; Chen, S.; Liu, Z.; Peng, F. Electrochemical Reduction of CO2 into Tunable Syngas Production by Regulating the Crystal Facets of Earth-Abundant Zn Catalyst. ACS Appl. Mater. Interfaces 2018, 10, 20530–20539. [Google Scholar] [CrossRef] [PubMed]
- Wuttig, A.; Surendranath, Y. Impurity Ion Complexation Enhances Carbon Dioxide Reduction Catalysis. ACS Catal. 2015, 5, 4479–4484. [Google Scholar] [CrossRef]
- Lees, E.W.; Goldman, M.; Fink, A.G.; Dvorak, D.J.; Salvatore, D.A.; Zhang, Z.; Loo, N.W.X.; Berlinguette, C.P. Electrodes Designed for Converting Bicarbonate into CO. ACS Energy Lett. 2020, 5, 2165–2173. [Google Scholar] [CrossRef]
- Zhang, Z.; Lees, E.W.; Habibzadeh, F.; Salvatore, D.A.; Ren, S.; Simpson, G.L.; Wheeler, D.G.; Liu, A.; Berlinguette, C.P. Porous Metal Electrodes Enable Efficient Electrolysis of Carbon Capture Solutions. Energy Environ. Sci. 2022, 15, 705–713. [Google Scholar] [CrossRef]
- Larrea, C.; Avilés-Moreno, J.R.; Ocón, P. Strategies to Enhance CO2 Electrochemical Reduction from Reactive Carbon Solutions. Molecules 2023, 28, 1951. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of CuIII Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Eifert, B.; Becker, M.; Reindl, C.T.; Giar, M.; Zheng, L.; Polity, A.; He, Y.; Heiliger, C.; Klar, P.J. Raman Studies of the Intermediate Tin-Oxide Phase. Phys. Rev. Mater. 2017, 1, 014602. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced Analysis of Copper X-ray Photoelectron Spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Reiche, R.; Oswald, S.; Yubero, F.; Espinós, J.P.; Holgado, J.P.; González-Elipe, A.R. Monitoring Interface Interactions by XPS at Nanometric Tin Oxides Supported on Al2O3 and Sb2Ox. J. Phys. Chem. B 2004, 108, 9905–9913. [Google Scholar] [CrossRef]
- Wang, D.; Miller, A.C.; Notis, M.R. XPS Study of the Oxidation Behavior of the Cu3Sn Intermetallic Compound at Low Temperatures. Surf. Interface Anal. 1996, 24, 127–132. [Google Scholar] [CrossRef]
- Aguilar-Galindo, F.; Fajardo-Rodríguez, S.; Poyato, J.M.L.; Pastor, E.; Avilés-Moreno, J.-R.; Ocón, P. Outstanding Inhibition of H2O2 Generation in Doubly Doped Graphene: The Synergy of Two Heteroatoms Opens a New Chemical Path. Carbon 2024, 216, 118499. [Google Scholar] [CrossRef]
- Li, T.; Shao, M. A Minireview on Electrochemical CO2 Conversion Based on Carbonate/Bicarbonate Media. EES Catal. 2024, 2, 564–572. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, O.; De Mot, B.; Bulut, M.; Pant, D.; Breugelmans, T. Engineering Aspects for the Design of a Bicarbonate Zero-Gap Flow Electrolyzer for the Conversion of CO2 to Formate. ACS Appl. Mater. Interfaces 2022, 14, 30760–30771. [Google Scholar] [CrossRef]
- Lees, E.W.; Liu, A.; Bui, J.C.; Ren, S.; Weber, A.Z.; Berlinguette, C.P. Electrolytic Methane Production from Reactive Carbon Solutions. ACS Energy Lett. 2022, 7, 1712–1718. [Google Scholar] [CrossRef]
- Larrea, C.; Torres, D.; Avilés-Moreno, J.R.; Ocón, P. Multi-Parameter Study of CO2 Electrochemical Reduction from Concentrated Bicarbonate Feed. J. CO2 Util. 2022, 57, 101878. [Google Scholar] [CrossRef]
- Zhang, Z.; Lees, E.W.; Ren, S.; Mowbray, B.A.W.; Huang, A.; Berlinguette, C.P. Conversion of Reactive Carbon Solutions into CO at Low Voltage and High Carbon Efficiency. ACS Cent. Sci. 2022, 8, 749–755. [Google Scholar] [CrossRef]

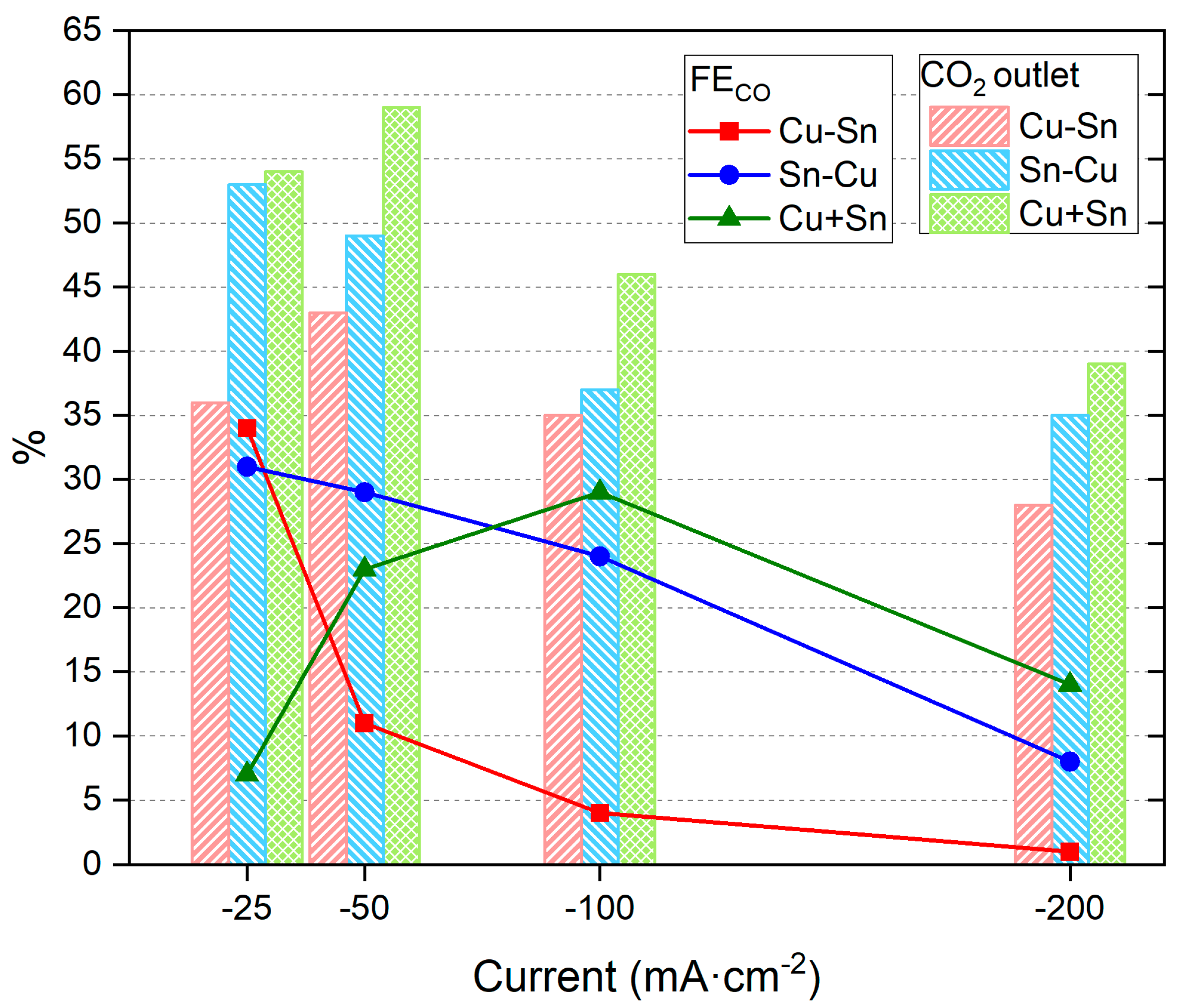
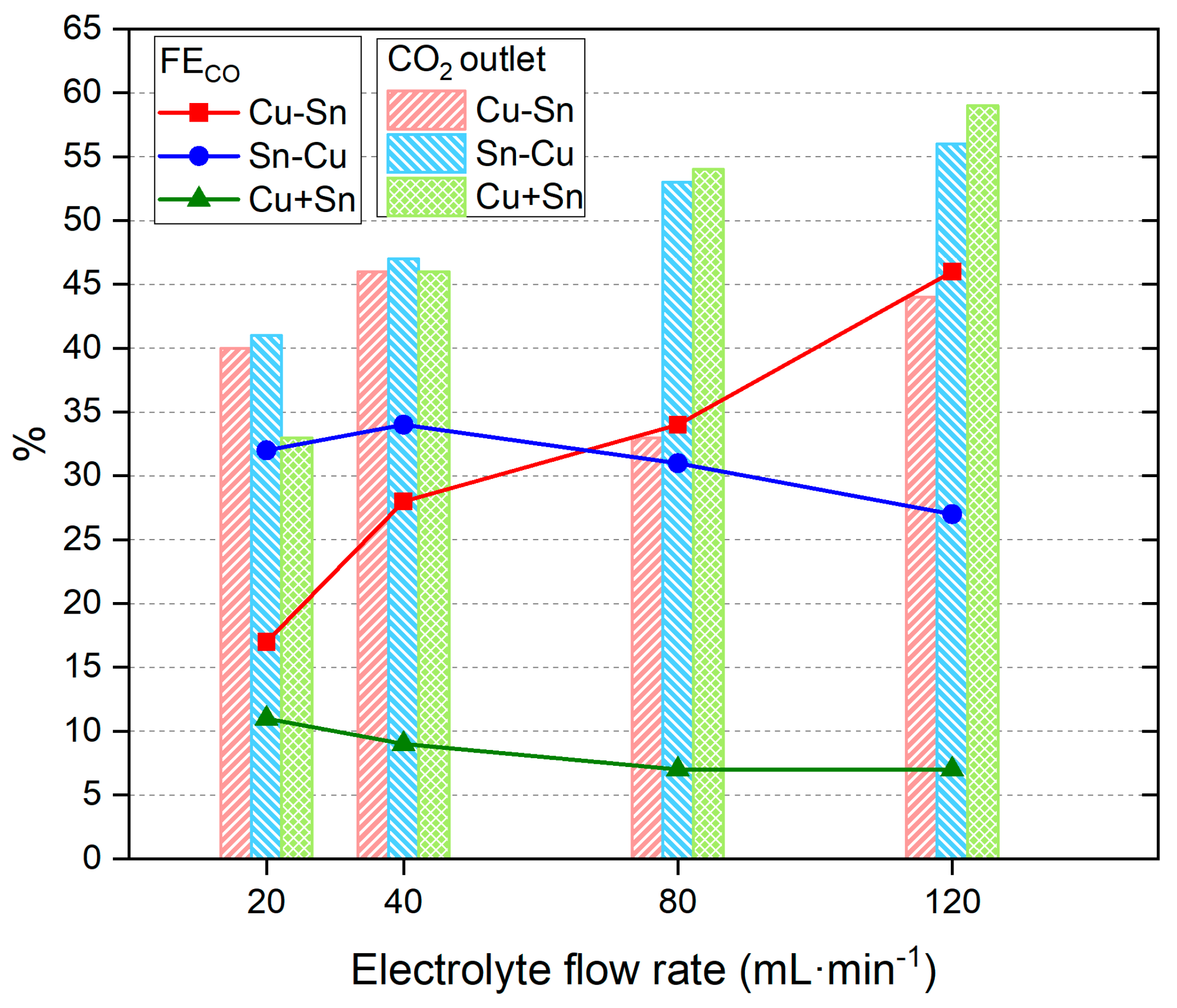
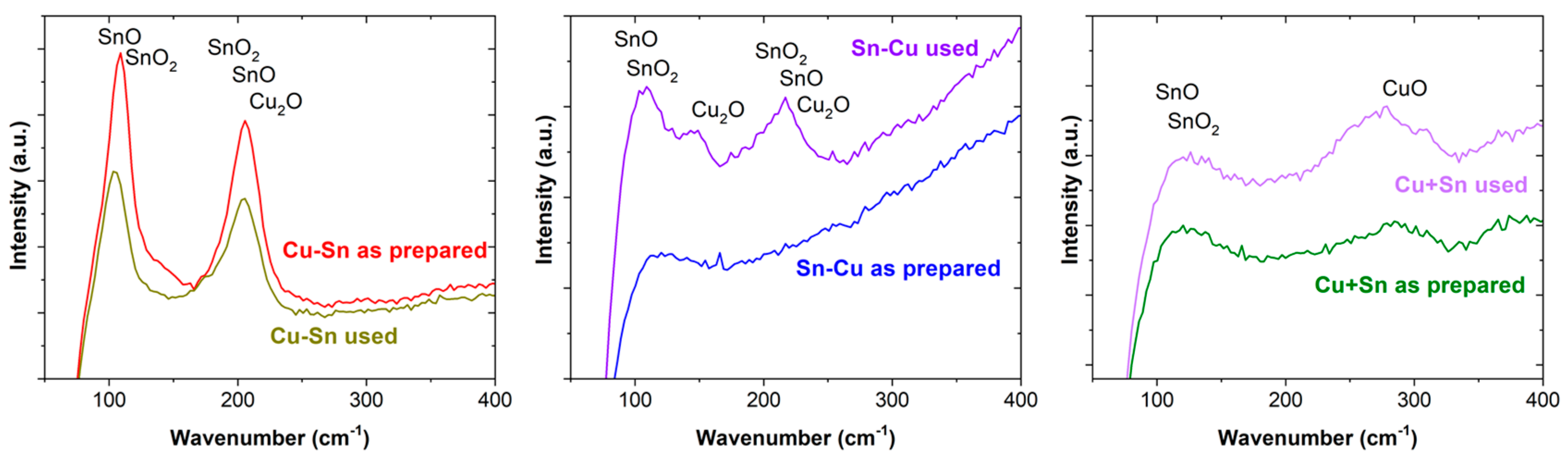
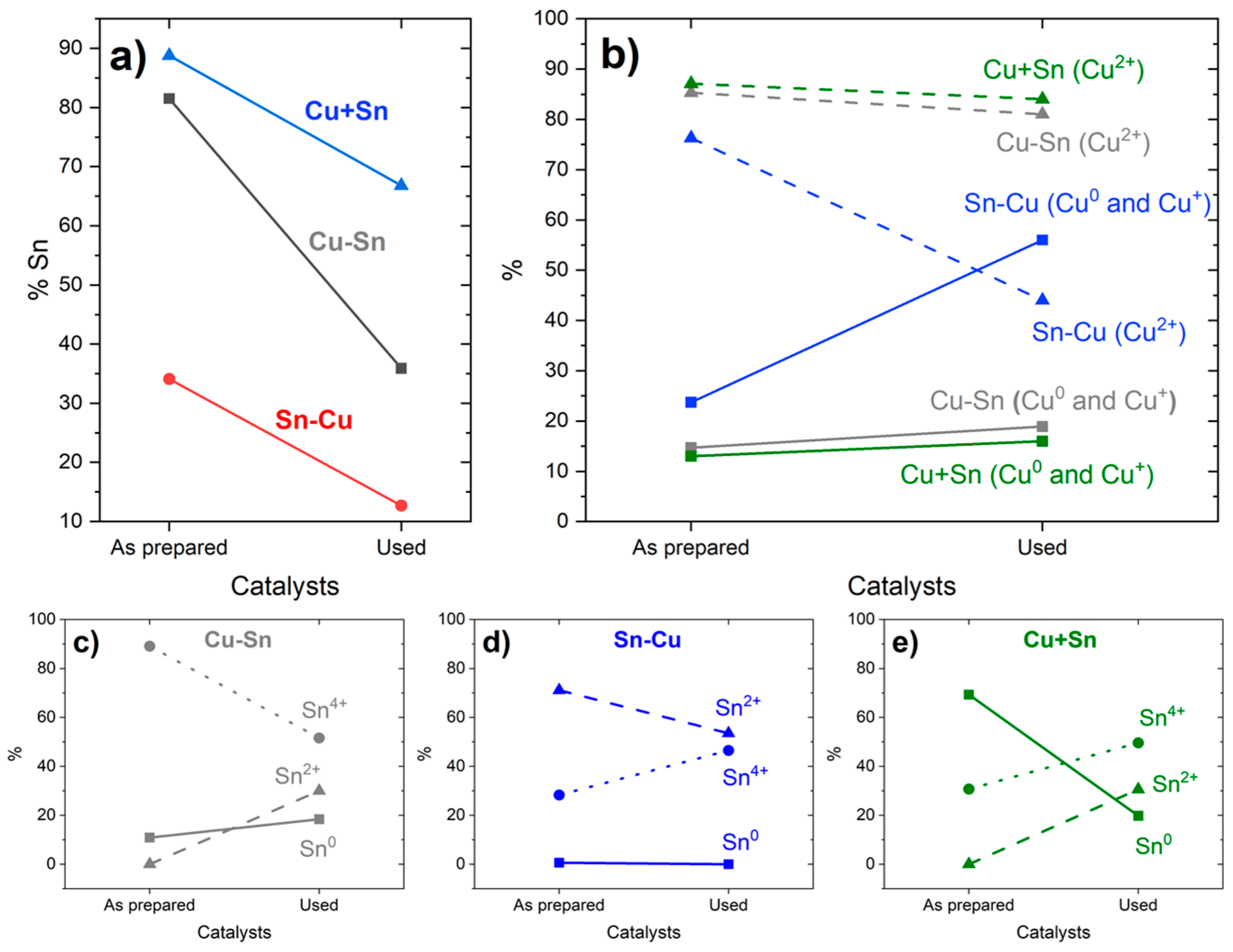

| Catalysts | Metal Layers | Current (mA·cm−2) | Time (min) | Potential (V) |
|---|---|---|---|---|
| Cu-Sn | 1st, inner layer: Cu | −14 | 20 | −1.3 |
| 2nd, outer layer: Sn | −14 | 20 | −1.5 | |
| Sn-Cu | 1st, inner layer: Sn | −14 | 20 | −1.9 |
| 2nd, outer layer: Cu | −14 | 20 | −1.0 | |
| Cu+Sn | Cu+Sn | −14 | 20 (×2) | −1.8 |
| Cu*-Sn | 1st, inner layer: Cu | −14 | 2 | −1.3 |
| 2nd, outer layer: Sn | −14 | 20 | −1.5 | |
| Sn | Sn | −14 | 20 (×2) | −1.5 |
| Catalyst | Cu and Sn | Cu | Sn | ||||
|---|---|---|---|---|---|---|---|
| % Cu | % Sn | % Cu0 and Cu+ | % Cu2+ | % Sn0 | % Sn2+ | % Sn4+ | |
| Cu-Sn as prep. | 19 | 81 | 15 | 85 | 11 | 0 | 89 |
| Cu-Sn used | 64 | 36 | 19 | 81 | 18 | 30 | 52 |
| Sn-Cu as prep. | 66 | 34 | 24 | 76 | 1 | 71 | 28 |
| Sn-Cu used | 87 | 13 | 56 | 44 | 0 | 54 | 47 |
| Cu+Sn as prep. | 11 | 89 | 13 | 87 | 69 | 0 | 31 |
| Cu+Sn used | 33 | 67 | 16 | 84 | 20 | 31 | 50 |
| Cu*-Sn used | 74 | 26 | 25 | 75 | 16 | 31 | 53 |
| Cathode Catalyst | Catholyte | FE (%) | CO2 Outlet (%) | Current (mA·cm−2) | Cell Voltage (V) | Reference |
|---|---|---|---|---|---|---|
| Cu*-Sn electrodeposited | CO2 (g) sat. in 0.5 M KHCO3 and 0.01 M EDTA | 62 (CO) | 49 | −25 | −2.0 | This work |
| Ag composite | 3 M KHCO3 | 82 (CO) | not reported | −100 | −3.4 | [54] |
| Porous Ag | 3 M KHCO3 | 60 (CO) | not reported | −100 | −3.7 | [55] |
| Ag nanoparticles | 2 M KHCO3 | 46 (CO) | 41 | −200 | −3.8 | [66] |
| Electrodeposited Ag | 2 M KHCO3 with 0.02 M DTAB | 85 (CO) | 50 | −100 | −3.5 | [56] |
| Ag foam | 3 M KHCO3 | 15 (CO) | ~45 | −500 | −2.2 | [67] |
| SnO2 nanoparticles | 3 M KHCO3 | 58 (HCOO−) | not reported | −100 | −4.1 | [64] |
| Cu foam | 3 M KHCO3 with 3 mM CTAB | 27 (CH4) | not reported | −400 | −7.2 | [65] |
| Cu-Sn bronze | CO2 (g) with 0.1 M KHCO3 | 85 (CO) | not reported | −6 | −0.8 V vs. RHE | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herranz, D.; Bernedo Biriucov, S.; Arranz, A.; Avilés Moreno, J.R.; Ocón, P. Syngas Production Improvement from CO2RR Using Cu-Sn Electrodeposited Catalysts. Materials 2025, 18, 105. https://doi.org/10.3390/ma18010105
Herranz D, Bernedo Biriucov S, Arranz A, Avilés Moreno JR, Ocón P. Syngas Production Improvement from CO2RR Using Cu-Sn Electrodeposited Catalysts. Materials. 2025; 18(1):105. https://doi.org/10.3390/ma18010105
Chicago/Turabian StyleHerranz, Daniel, Santiago Bernedo Biriucov, Antonio Arranz, Juan Ramón Avilés Moreno, and Pilar Ocón. 2025. "Syngas Production Improvement from CO2RR Using Cu-Sn Electrodeposited Catalysts" Materials 18, no. 1: 105. https://doi.org/10.3390/ma18010105
APA StyleHerranz, D., Bernedo Biriucov, S., Arranz, A., Avilés Moreno, J. R., & Ocón, P. (2025). Syngas Production Improvement from CO2RR Using Cu-Sn Electrodeposited Catalysts. Materials, 18(1), 105. https://doi.org/10.3390/ma18010105






