Effects of Steel Slag on the Hydration Process of Solid Waste-Based Cementitious Materials
Abstract
1. Introduction
2. Materials and Experimental Methods
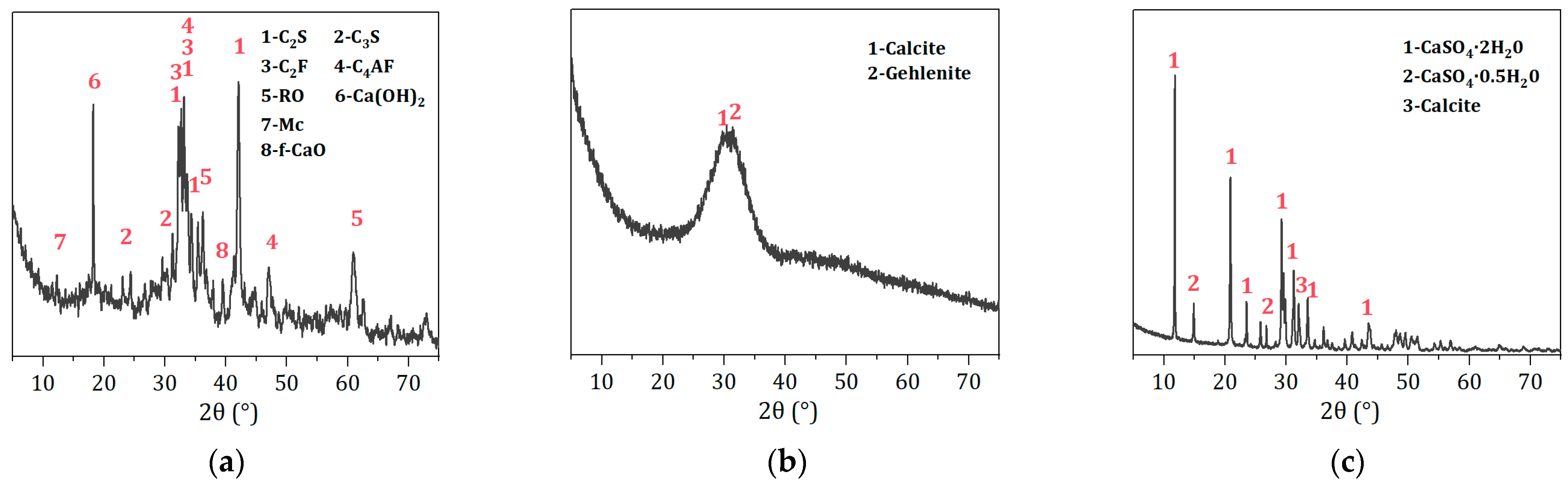
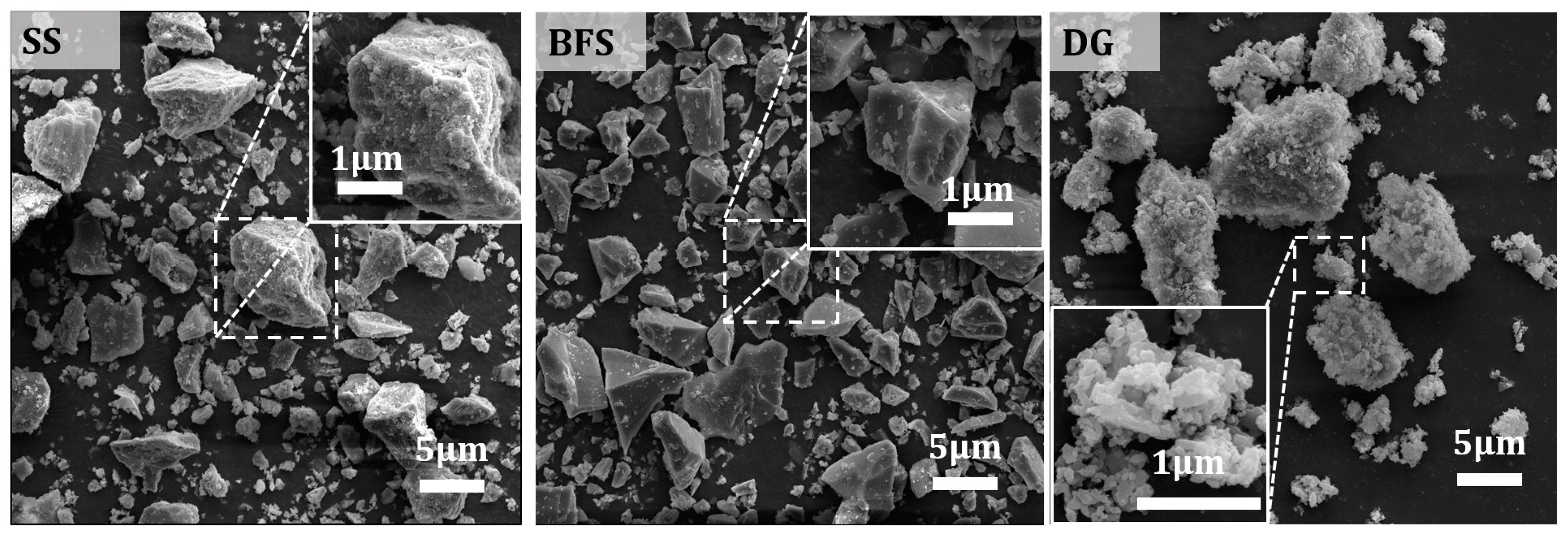
2.1. Raw Materials
2.2. Sample Preparation
2.3. Experimental Procedure
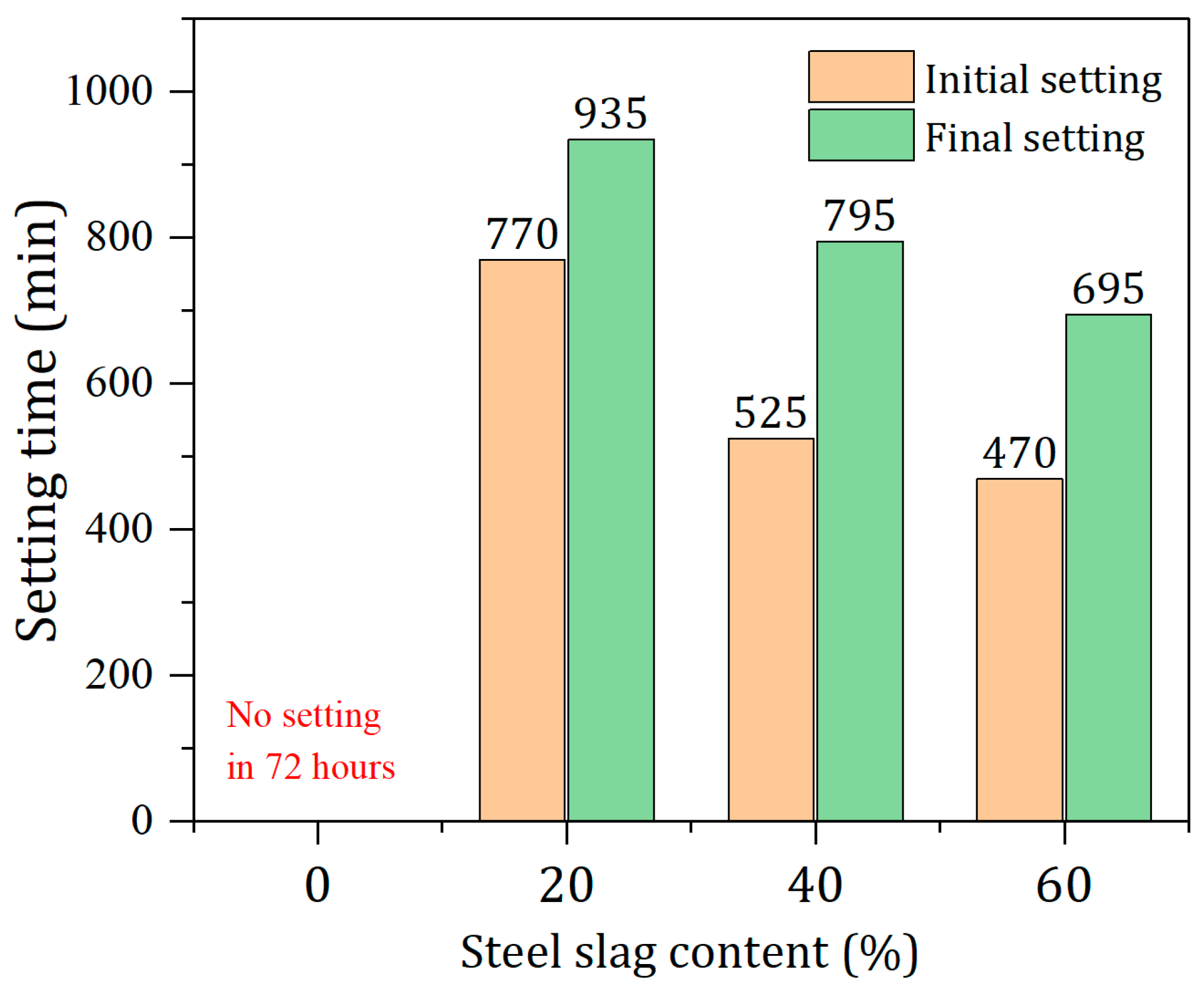
2.3.1. Setting Time
2.3.2. Compressive Strength
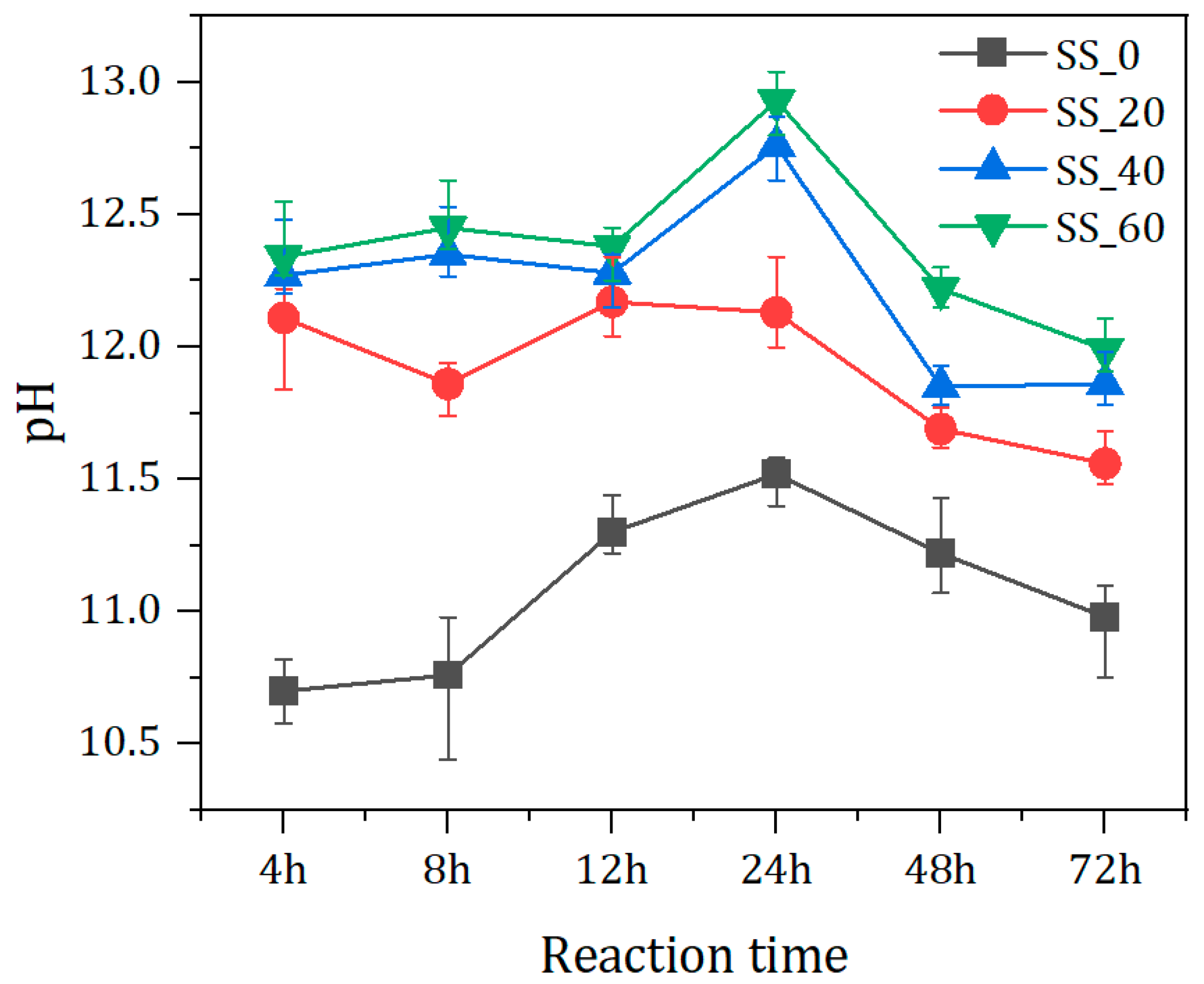
2.3.3. pH Value Determination
2.3.4. Isothermal Heat Responses
2.3.5. Characterization
3. Results
3.1. Effect of SS Content on the Evolution of pH Value of Pore Solution
3.2. Effect of SS Content on the Setting Times of Pastes
3.3. Effect of SS Content on the Compressive and Flexural Strengths of Mortars
3.4. Effect of SS Content on Hydration Process and Microstructures
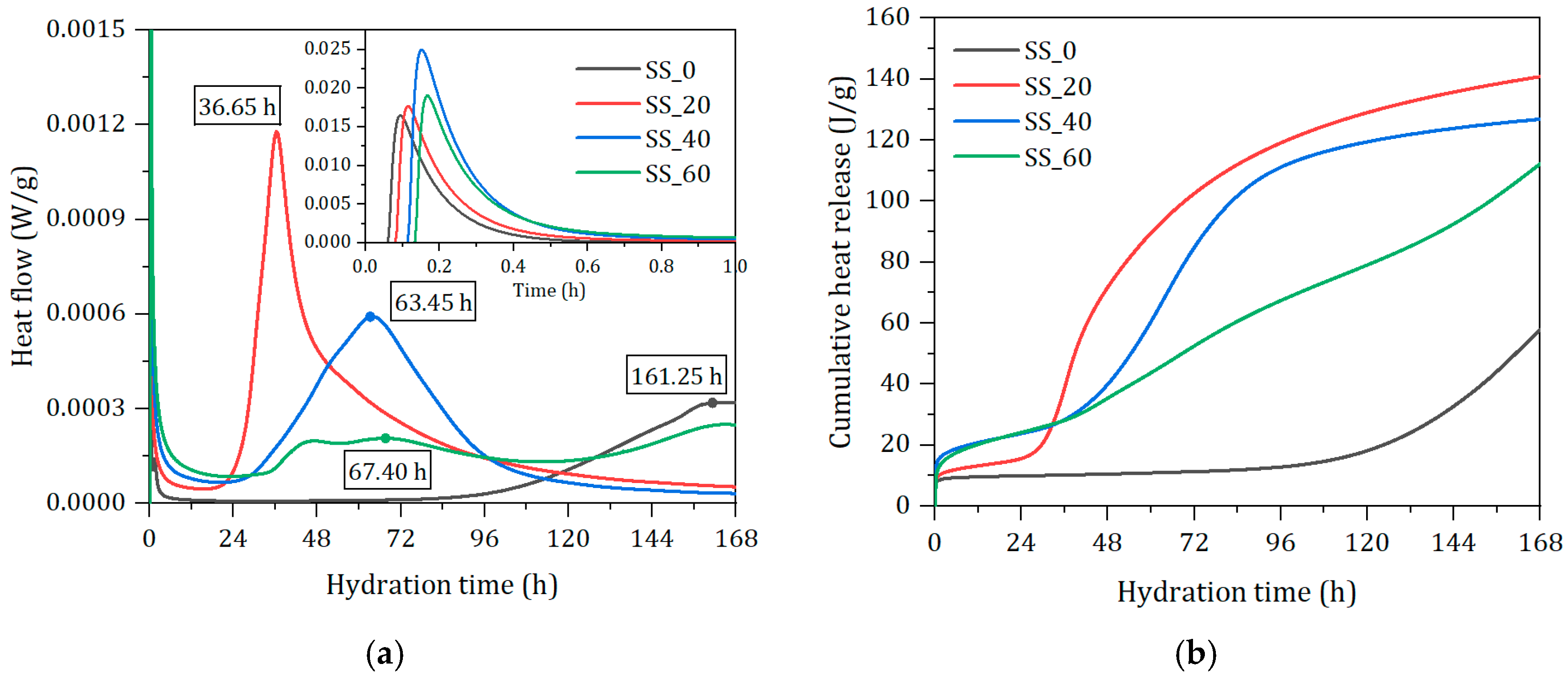
3.4.1. Effect of SS Content on Hydration Heat
3.4.2. Effect of SS Content on Phase Composition and Chemical Characteristic
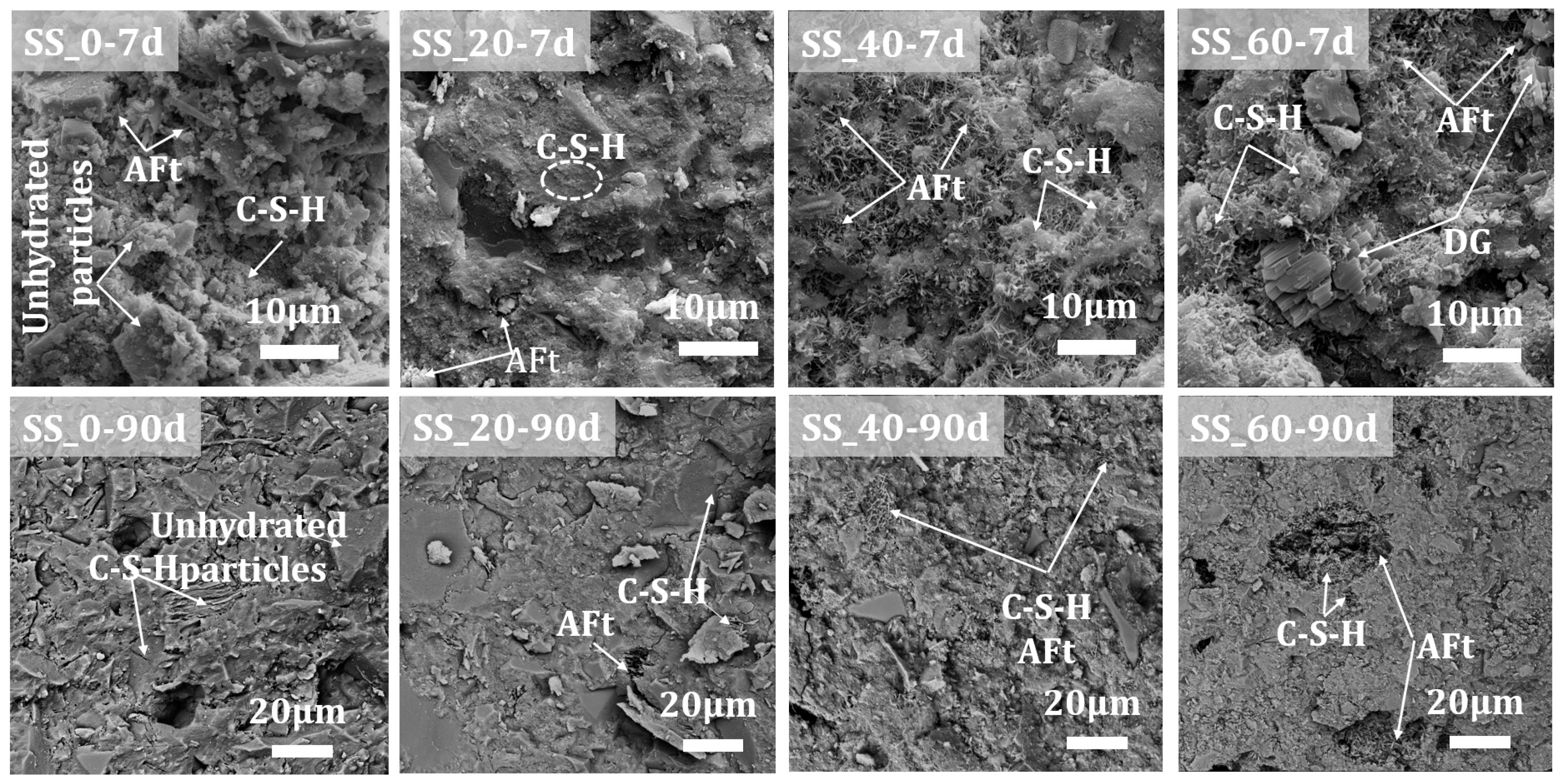
3.4.3. Effect of SS Content on the Microstructural Morphology
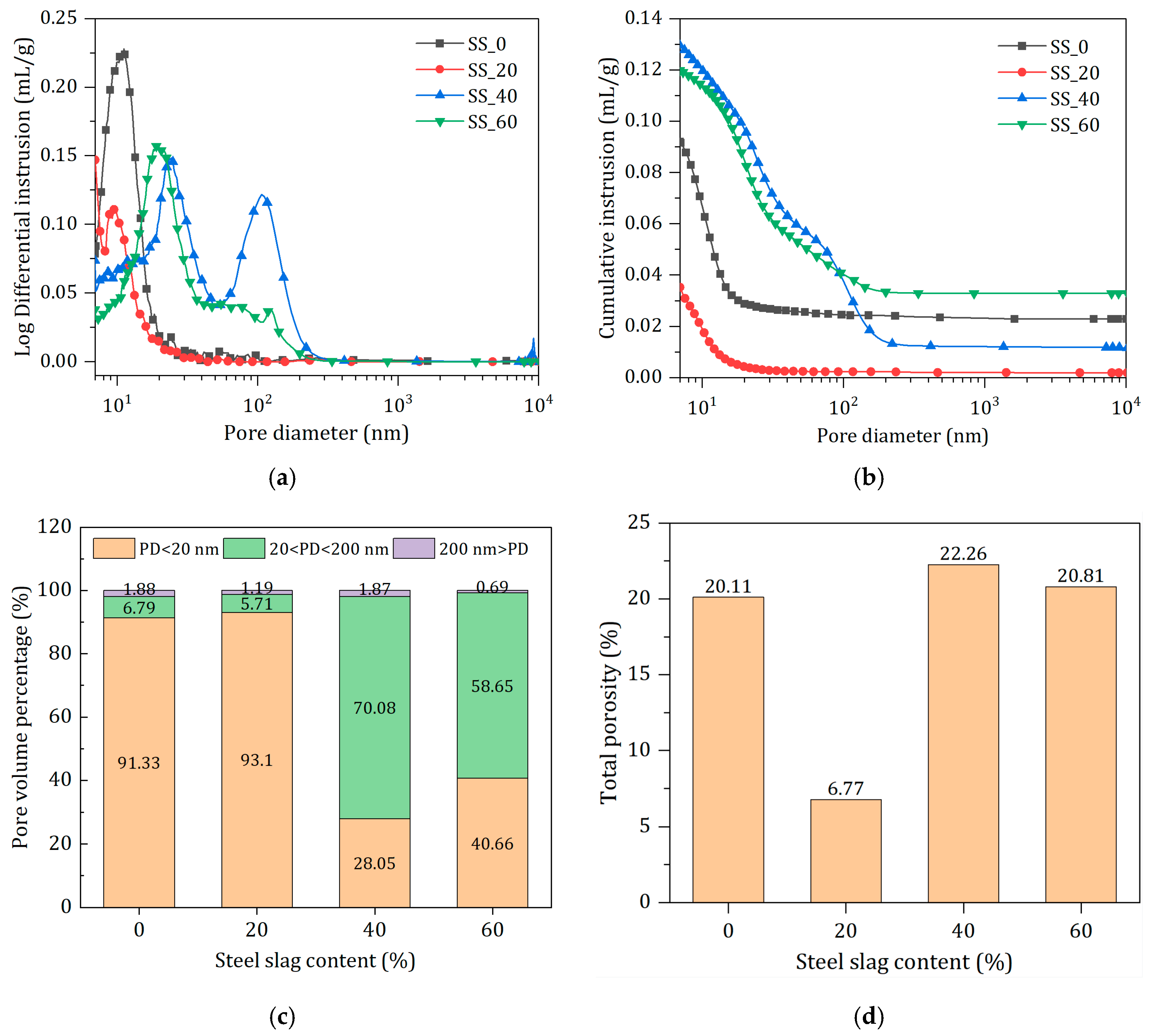
3.4.4. Effect of SS Content on Pore Characteristics
3.4.5. Effect of SS Content on Hydration Degree
4. Discussion
5. Conclusions
- (1)
- Within the range of 0% to 60% steel slag content, as the proportion of steel slag increased, the pH value of the solid waste-based cementitious material system was elevated, and the setting time was reduced. In the test group without steel slag addition, there was no setting within 72 h, indicating that steel slag played a significant role in the early hydration stage.
- (2)
- With the optimal steel slag content of 20%, the solid waste-based cementitious materials achieved the best mechanical properties, with the compressive strengths at 3 d and 28 d reaching 19.2 MPa and 58.4 MPa, respectively. The addition of SS improved the early strength gain of solid waste-based binders compared to the reference sample without SS. However, with SS beyond 20%, the compressive strength declined in large scale due to decrease in BFS.
- (3)
- The hydrated solid waste-based cementitious material composed of steel slag, granulated slag, and desulfurization gypsum are primarily AFt and C-S-H gel. In the early stages, AFt primarily contributes to the strength, while in later stages, a substantial amount of AFt and C-S-H gel hydration products are formed. The interlocking and overlapping of AFt with C-S-H gel fills the pores and creates a dense microstructure, thereby ensuring the continuous increase in the strength of the cementitious material system.
- (4)
- The addition of steel slag advanced the main hydration and contributed to more hydration products formation. The bond water contents at seven days were significantly increased with SS addition and reached the maximum with optimal content of SS. The pore structure was also refined by the addition of SS with optimal content of SS. Excess of SS resulted in the pore structure coarsening and poor volume stability of the solid waste-based binder.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sethurajan, M.; van Hullebusch, E.D.; Nancharaiah, Y.V. Biotechnology in the management and resource recovery from metal bearing solid wastes: Recent advances. J. Environ. Manag. 2018, 211, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Naraharisetti, P.K.; Yeo, T.Y.; Bu, J. New classification of CO2 mineralization processes and economic evaluation. Renew. Sustain. Energy Rev. 2019, 99, 220–233. [Google Scholar] [CrossRef]
- Dhoble, Y.N.; Ahmed, S. Review on the innovative uses of steel slag for waste minimization. J. Mater. Cycles Waste Manag. 2018, 20, 1373–1382. [Google Scholar] [CrossRef]
- Das, S.; Galgo, S.J.; Alam, M.A.; Lee, J.G.; Hwang, H.Y.; Lee, C.H.; Kim, P.J. Recycling of ferrous slag in agriculture: Potentials and challenges. Crit. Rev. Environ. Sci. Technol. 2020, 52, 1247–1281. [Google Scholar] [CrossRef]
- Gołaszewski, J.; Klemczak, B.; Gołaszewska, M.; Smolana, A.; Cygan, G. The feasibility of using a high volume of non-clinker binders in self-compacting concrete related to its basic engineering properties. J. Build. Eng. 2023, 66, 105893. [Google Scholar] [CrossRef]
- Rahou, J.; Rezqi, H.; El Ouahabi, M.; Fagel, N. Characterization of Moroccan steel slag waste: The potential green resource for ceramic production. Constr. Build. Mater. 2022, 314, 125663. [Google Scholar] [CrossRef]
- Kumar, P.; Shukla, S. Utilization of steel slag waste as construction material: A review. Mater. Today Proc. 2023, 78, 145–152. [Google Scholar] [CrossRef]
- Ferreira, V.J.; Sáez-De-Guinoa Vilaplana, A.; García-Armingol, T.; Aranda-Usón, A.; Lausín-González, C.; López-Sabirón, A.M.; Ferreira, G. Evaluation of the steel slag incorporation as coarse aggregate for road construction: Technical requirements and environmental impact assessment. J. Clean. Prod. 2016, 130, 175–186. [Google Scholar] [CrossRef]
- Maghool, F.; Arulrajah, A.; Du, Y.-J.; Horpibulsuk, S.; Chinkulkijniwat, A. Environmental impacts of utilizing waste steel slag aggregates as recycled road construction materials. Clean Technol. Environ. Policy 2017, 19, 949–958. [Google Scholar] [CrossRef]
- Myers, C.; Sasagawa, J.; Nakagaki, T. Enhancing CO2 Mineralization Rate and Extent of Iron and Steel Slag via Grinding. ISIJ Int. 2022, 62, 2446–2453. [Google Scholar] [CrossRef]
- Sim, G.; Hong, S.; Moon, S.; Noh, S.; Cho, J.; Triwigati, P.T.; Park, A.-H.A.; Park, Y. Simultaneous CO2 utilization and rare earth elements recovery by novel aqueous carbon mineralization of blast furnace slag. J. Environ. Chem. Eng. 2022, 10, 107327. [Google Scholar] [CrossRef]
- Moon, S.; Kim, E.; Noh, S.; Triwigati, P.T.; Choi, S.; Park, Y. Carbon mineralization of steel and iron-making slag: Paving the way for a sustainable and carbon-neutral future. J. Environ. Chem. Eng. 2024, 12, 112448. [Google Scholar] [CrossRef]
- Ferrara, G.; Belli, A.; Keulen, A.; Tulliani, J.-M.; Palmero, P. Testing procedures for CO2 uptake assessment of accelerated carbonation products: Experimental application on basic oxygen furnace steel slag samples. Constr. Build. Mater. 2023, 406, 133384. [Google Scholar] [CrossRef]
- Teo, P.T.; Anasyida, A.S.; Kho, C.M.; Nurulakmal, M.S. Recycling of Malaysia’s EAF steel slag waste as novel fluxing agent in green ceramic tile production: Sintering mechanism and leaching assessment. J. Clean. Prod. 2019, 241, 118144. [Google Scholar] [CrossRef]
- Diao, J.; Zhou, W.; Ke, Z.; Qiao, Y.; Zhang, T.; Liu, X.; Xie, B. System assessment of recycling of steel slag in converter steelmaking. J. Clean. Prod. 2016, 125, 159–167. [Google Scholar] [CrossRef]
- Varanasi, S.S.; More, V.M.R.; Rao, M.B.V.; Alli, S.R.; Tangudu, A.K.; Santanu, D. Recycling Ladle Furnace Slag as Flux in Steelmaking: A Review. J. Sustain. Metall. 2019, 5, 449–462. [Google Scholar] [CrossRef]
- Bláhová, L.; Navrátilová, Z.; Mucha, M.; Navrátilová, E.; Neděla, V. Influence of the slags treatment on the heavy metals binding. Int. J. Environ. Sci. Technol. 2018, 15, 697–706. [Google Scholar] [CrossRef]
- Ter Teo, P.; Seman, A.A.; Basu, P.; Sharif, N.M. Characterization of EAF Steel Slag Waste: The Potential Green Resource for Ceramic Tile Production. Procedia Chem. 2016, 19, 842–846. [Google Scholar] [CrossRef]
- Galán-Arboledas, R.J.; Álvarez de Diego, J.; Dondi, M.; Bueno, S. Energy, environmental and technical assessment for the incorporation of EAF stainless steel slag in ceramic building materials. J. Clean. Prod. 2017, 142, 1778–1788. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Wang, D.; Yan, P.; Luo, L.; Zhang, H.; Zhang, H.; Gu, X. Hydration superposition effect and mechanism of steel slag powder and granulated blast furnace slag powder. Constr. Build. Mater. 2023, 366, 130101. [Google Scholar] [CrossRef]
- Rosales, J.; Cabrera, M.; Agrela, F. Effect of stainless steel slag waste as a replacement for cement in mortars. Mechanical and statistical study. Constr. Build. Mater. 2017, 142, 444–458. [Google Scholar] [CrossRef]
- Singh, M.; Garg, M. Activation of gypsum anhydrite-slag mixtures. Cem. Concr. Res. 1995, 25, 332–338. [Google Scholar] [CrossRef]
- Mahoutian, M.; Shao, Y.; Mucci, A.; Fournier, B. Carbonation and hydration behavior of EAF and BOF steel slag binders. Mater. Struct. 2015, 48, 3075–3085. [Google Scholar] [CrossRef]
- Ashrit, S.; Banerjee, P.K.; Chatti, R.V.; Venugopal, R.; Udayabhanu, G. Characterization of gypsum synthesized from LD slag fines generated at a waste recycling plant of a steel plant. New J. Chem. 2015, 39, 4128–4134. [Google Scholar] [CrossRef]
- Harrou, A.; Gharibi, E.; Nasri, H.; Fagel, N.; El Ouahabi, M. Physico-mechanical properties of phosphogypsum and black steel slag as aggregate for bentonite-lime based materials. Mater. Today Proc. 2020, 31, S51–S55. [Google Scholar] [CrossRef]
- Duan, S.; Liao, H.; Cheng, F.; Song, H.; Yang, H. Investigation into the synergistic effects in hydrated gelling systems containing fly ash, desulfurization gypsum and steel slag. Constr. Build. Mater. 2018, 187, 1113–1120. [Google Scholar] [CrossRef]
- Claveau-Mallet, D.; Wallace, S.; Comeau, Y. Removal of phosphorus, fluoride and metals from a gypsum mining leachate using steel slag filters. Water Res. 2013, 47, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- De Weerdt, K.; Kjellsen, K.O.; Sellevold, E.; Justnes, H. Synergy between fly ash and limestone powder in ternary cements. Cem. Concr. Compos. 2011, 33, 30–38. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, L.; Ren, C.; Huang, T.; Zhu, Y.; Wei, P.; Wang, D.; Liu, Z. Quantitative determination of quaternary solid waste-based binders and its hydrates by XRD. Constr. Build. Mater. 2024, 425, 135888. [Google Scholar] [CrossRef]
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. Standards Press of China: Beijing, China, 2011.
- GB/T 17671-2021; Test Method of Cement Mortar Strength. Standards Press of China: Beijing, China, 2021.
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Malathy, R.; Deepalakshmi, D.; Syedibrahim. Strength Behavior of Prestressed Concrete Sleepers with Steel Slag Partial Replacement. J. Environ. Nanotechnol. 2024, 13, 104–110. [Google Scholar] [CrossRef]
- Hussain, A.; Hussaini, S.K.K. Use of steel slag as railway ballast: A review. Transp. Geotech. 2022, 35, 100779. [Google Scholar] [CrossRef]
- Biskri, Y.; Achoura, D.; Chelghoum, N.; Mouret, M. Mechanical and durability characteristics of High Performance Concrete containing steel slag and crystalized slag as aggregates. Constr. Build. Mater. 2017, 150, 167–178. [Google Scholar] [CrossRef]
- Moghaddam, S.E.; Hejazi, V.; Hwang, S.H.; Sreenivasan, S.; Miller, J.; Shi, B.; Zhao, S.; Rusakova, I.; Alizadeh, A.R.; Whitmire, K.H.; et al. Morphogenesis of cement hydrate. J. Mater. Chem. A 2017, 5, 3798–3811. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.; Jiang, J.; Wang, P. Chloride ions transport and adsorption in the nano-pores of silicate calcium hydrate: Experimental and molecular dynamics studies. Constr. Build. Mater. 2016, 126, 991–1001. [Google Scholar] [CrossRef]
- Zhu, C.; Tan, H.; Du, C.; Wang, J.; Deng, X.; Zheng, Z.; He, X. Enhancement of ultra-fine slag on compressive strength of solid waste-based cementitious materials: Towards low carbon emissions. J. Build. Eng. 2023, 63, 105475. [Google Scholar] [CrossRef]
- Li, C. Mechanical and transport properties of recycled aggregate concrete modified with limestone powder. Compos. Part B Eng. 2020, 197, 108189. [Google Scholar] [CrossRef]
- Akın Altun, İ.; Yılmaz, İ. Study on steel furnace slags with high MgO as additive in Portland cement. Cem. Concr. Res. 2002, 32, 1247–1249. [Google Scholar] [CrossRef]
- Kazanskaya, L.F.; Smirnova, O.M.; Palomo, Á.; Menendez Pidal, I.; Romana, M. Supersulfated Cement Applied to Produce Lightweight Concrete. Materials 2021, 14, 403. [Google Scholar] [CrossRef]
- Ghorbani, S.; Stefanini, L.; Sun, Y.; Walkley, B.; Provis, J.L.; De Schutter, G.; Matthys, S. Characterisation of alkali-activated stainless steel slag and blast-furnace slag cements. Cem. Concr. Compos. 2023, 143, 105230. [Google Scholar] [CrossRef]
- Roslan, N.H.; Ismail, M.; Abdul-Majid, Z.; Ghoreishiamiri, S.; Muhammad, B. Performance of steel slag and steel sludge in concrete. Constr. Build. Mater. 2016, 104, 16–24. [Google Scholar] [CrossRef]
- Martins, A.C.P.; Franco de Carvalho, J.M.; Costa, L.C.B.; Andrade, H.D.; de Melo, T.V.; Ribeiro, J.C.L.; Pedroti, L.G.; Peixoto, R.A.F. Steel slags in cement-based composites: An ultimate review on characterization, applications and performance. Constr. Build. Mater. 2021, 291, 123265. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, Q. Inhibition mechanisms of steel slag on the early-age hydration of cement. Cem. Concr. Res. 2021, 140, 106283. [Google Scholar] [CrossRef]
- El-Didamony, H.; Amer, A.A.; El-Sokkary, T.M.; Abd-El-Aziz, H. Effect of substitution of granulated slag by air-cooled slag on the properties of alkali activated slag. Ceram. Int. 2013, 39, 171–181. [Google Scholar] [CrossRef]
- Harashima, A.; Ito, K. The Conditions of Ettringite Formation by the Reaction of a Blast Furnace Slag with Aqueous Alkaline Solutions. ISIJ Int. 2016, 56, 1738–1745. [Google Scholar] [CrossRef]
- Wang, J.; Ren, C.; Huang, T.; Li, X.; Cao, W.; Zhu, Y.; Wei, P.; Wang, D.; Liu, Z. Performances of concrete with binder and/or aggregates replacement by all-solid waste materials. J. Clean. Prod. 2024, 450, 141929. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Sun, R.; Huang, T.; Cao, W.; Zhu, Y.; Wei, P.; Wang, D.; Liu, Z. Performances Enhancing of Supersulfated Cement (SSC) Using Waste Alkaline Activators: Red Mud and Carbide Slag; Elsevier: Beijing, China, 2024. [Google Scholar]






| Materials | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | P2O5 | Cl | Others |
|---|---|---|---|---|---|---|---|---|---|
| SS | 40.29 | 13.86 | 6.49 | 22.43 | 9.47 | 0.93 | 1.35 | 0.10 | 5.08 |
| BFS | 45.69 | 23.39 | 15.43 | 0.59 | 9.97 | 1.54 | 0.02 | 0.10 | 5.38 |
| DG | 42.97 | 2.30 | 2.24 | 1.39 | 2.05 | 47.44 | 0.04 | 0.21 | 1.40 |
| No. | Materials/wt.% | ||
|---|---|---|---|
| SS | BFS | DG | |
| SS_0 | 0 | 85 | 15 |
| SS_20 | 20 | 65 | 15 |
| SS_40 | 40 | 45 | 15 |
| SS_60 | 60 | 25 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, C.; Wang, J.; Duan, K.; Li, X.; Wang, D. Effects of Steel Slag on the Hydration Process of Solid Waste-Based Cementitious Materials. Materials 2024, 17, 1999. https://doi.org/10.3390/ma17091999
Ren C, Wang J, Duan K, Li X, Wang D. Effects of Steel Slag on the Hydration Process of Solid Waste-Based Cementitious Materials. Materials. 2024; 17(9):1999. https://doi.org/10.3390/ma17091999
Chicago/Turabian StyleRen, Caifu, Jixiang Wang, Kairui Duan, Xiang Li, and Dongmin Wang. 2024. "Effects of Steel Slag on the Hydration Process of Solid Waste-Based Cementitious Materials" Materials 17, no. 9: 1999. https://doi.org/10.3390/ma17091999
APA StyleRen, C., Wang, J., Duan, K., Li, X., & Wang, D. (2024). Effects of Steel Slag on the Hydration Process of Solid Waste-Based Cementitious Materials. Materials, 17(9), 1999. https://doi.org/10.3390/ma17091999





