Alkalinity Regulation and Optimization of Cementitious Materials Used in Ecological Porous Concrete
Abstract
1. Introduction
2. Material and Experimental
2.1. Materials
2.2. Test Methods
2.2.1. pH Value
2.2.2. Compressive Strength
2.2.3. Microscopic Test
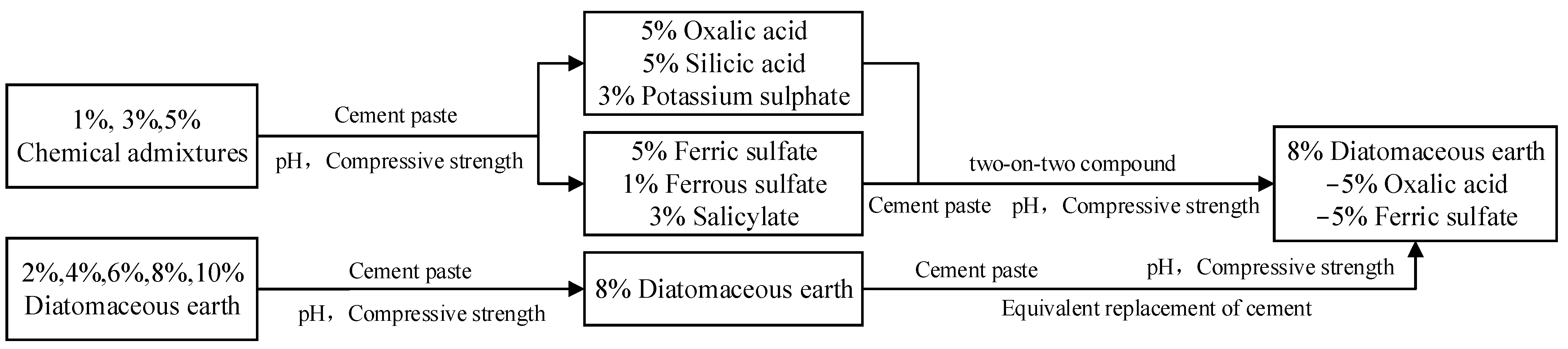
2.3. Study Design
3. Results
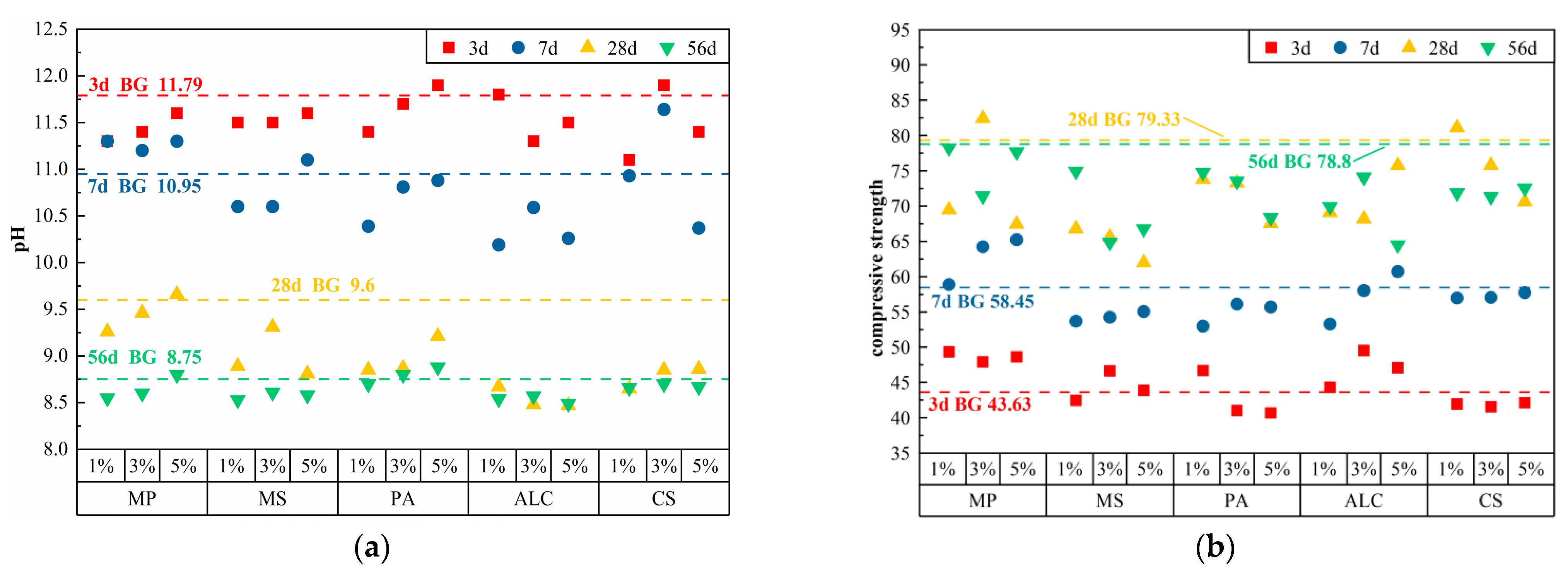
3.1. pH and Compressive Strength of Cement Paste with Chemical Admixtures Alone
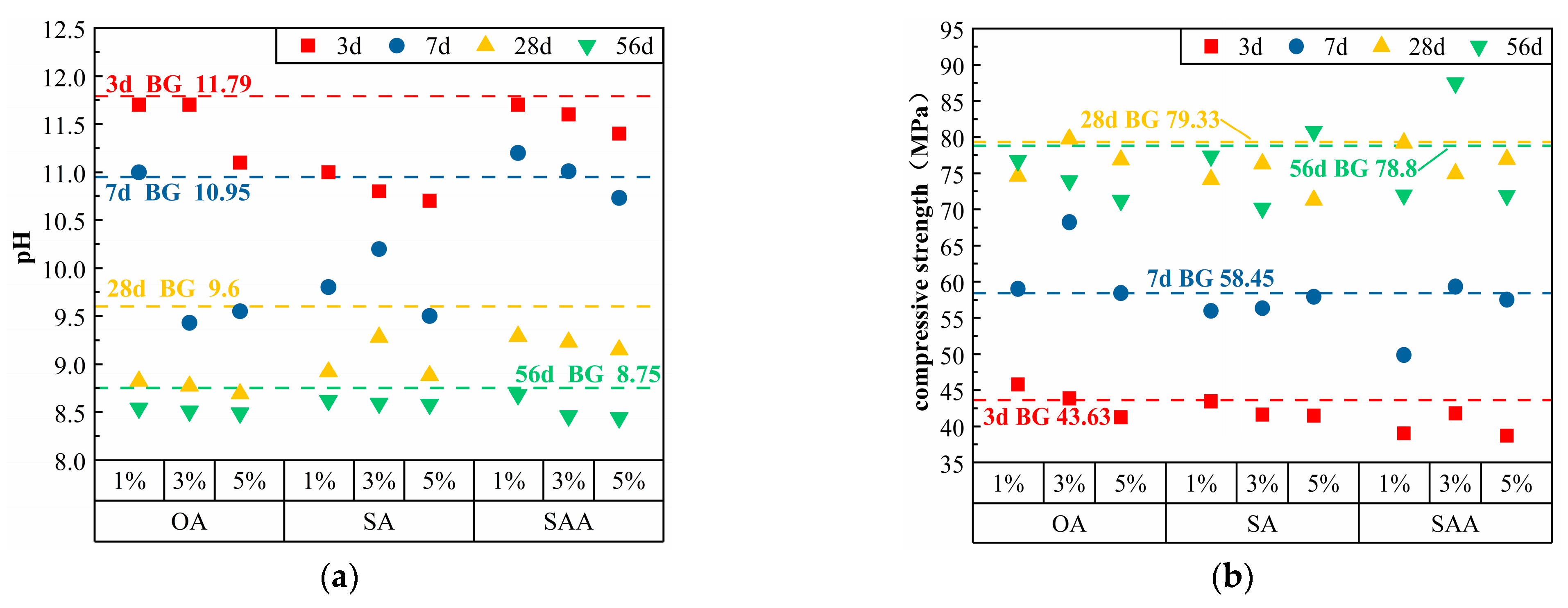
3.1.1. Acids
3.1.2. Sulfates
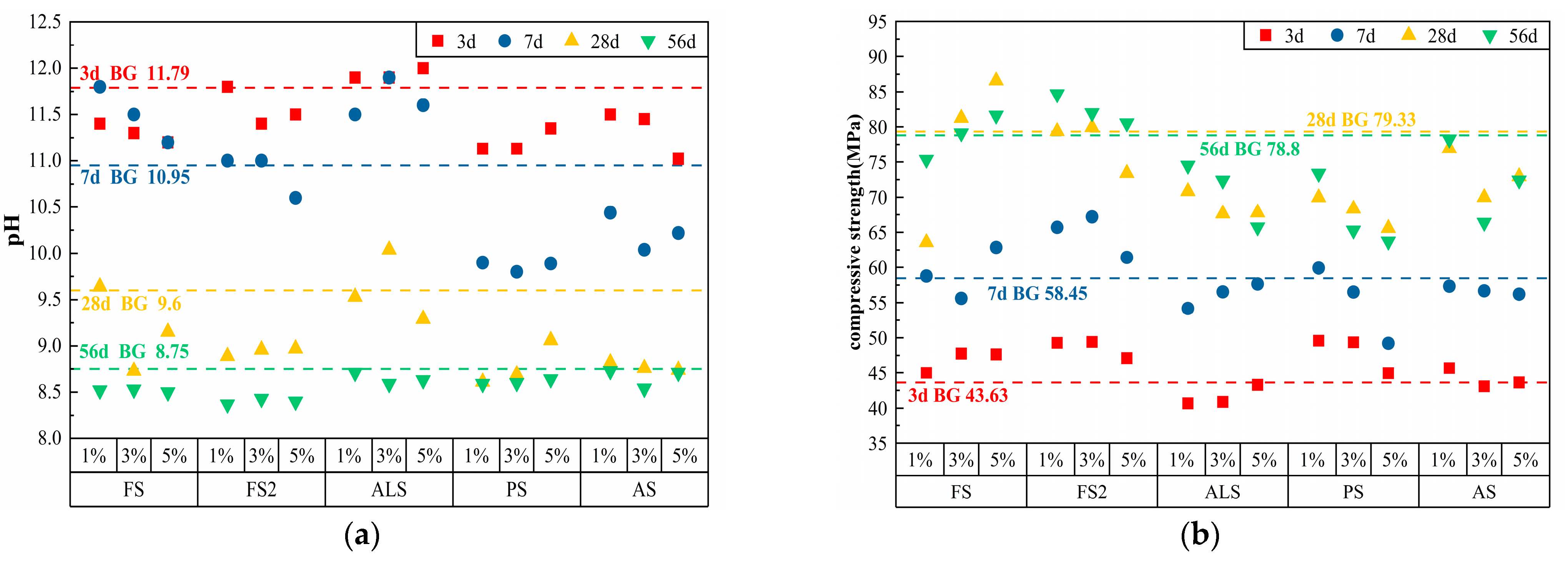
3.1.3. Carbonates
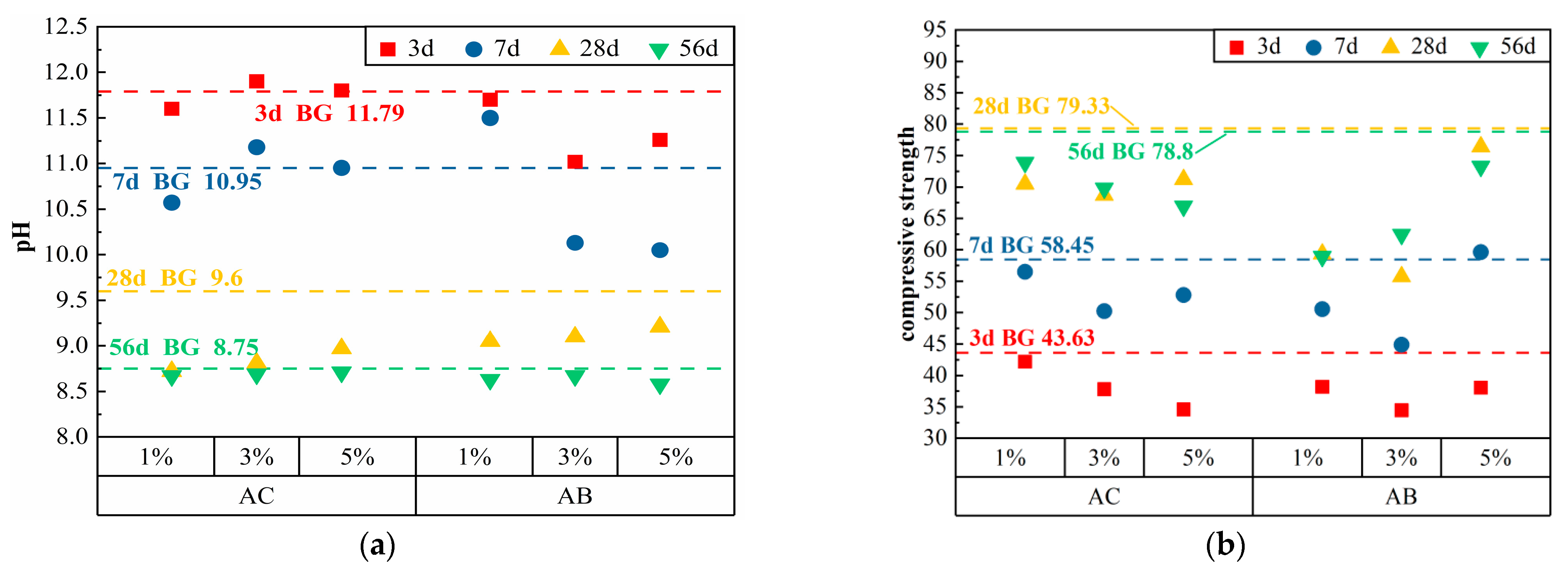
3.1.4. Other Classes
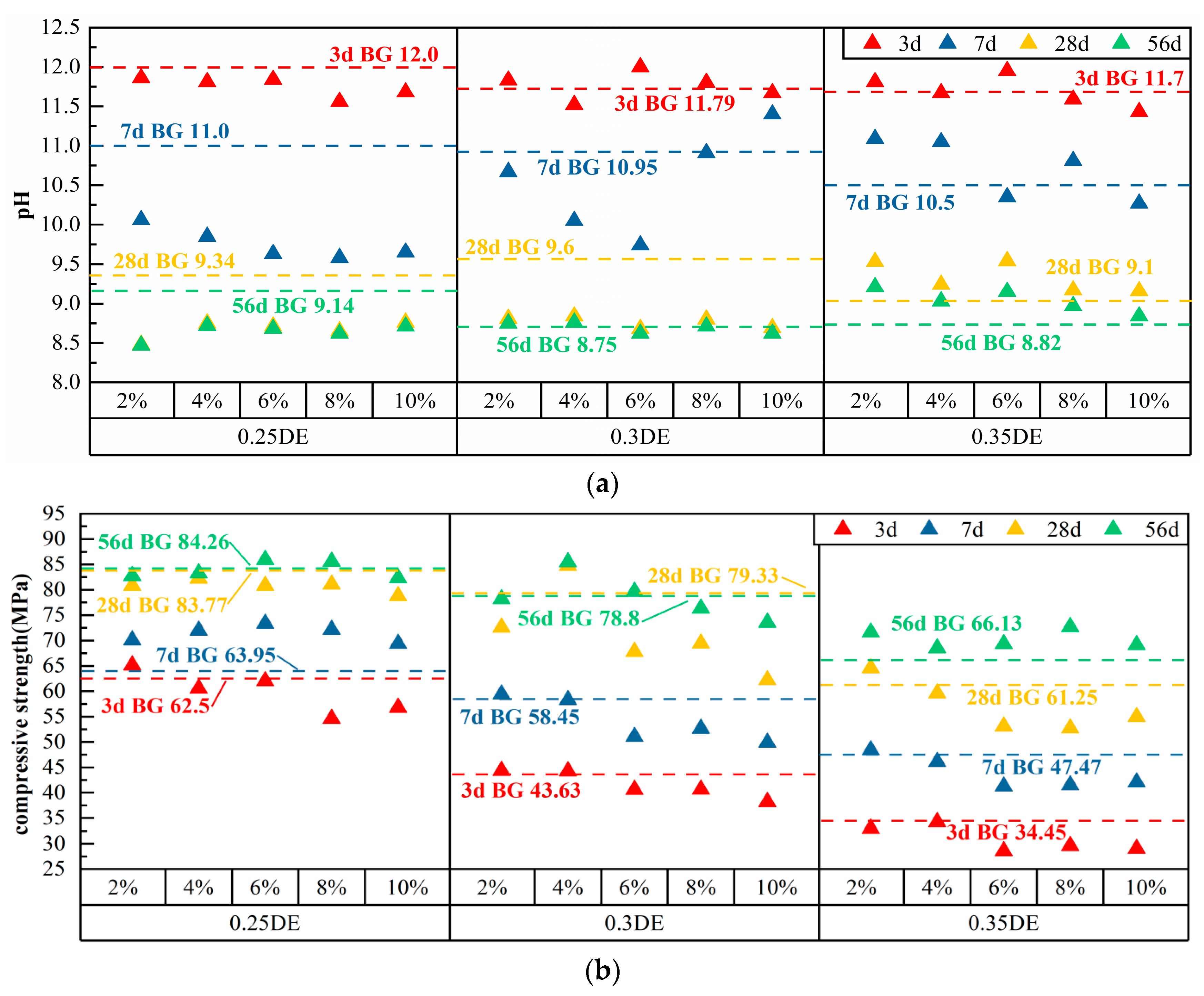
3.2. pH and Compressive Strength of Cement Paste with Single DE
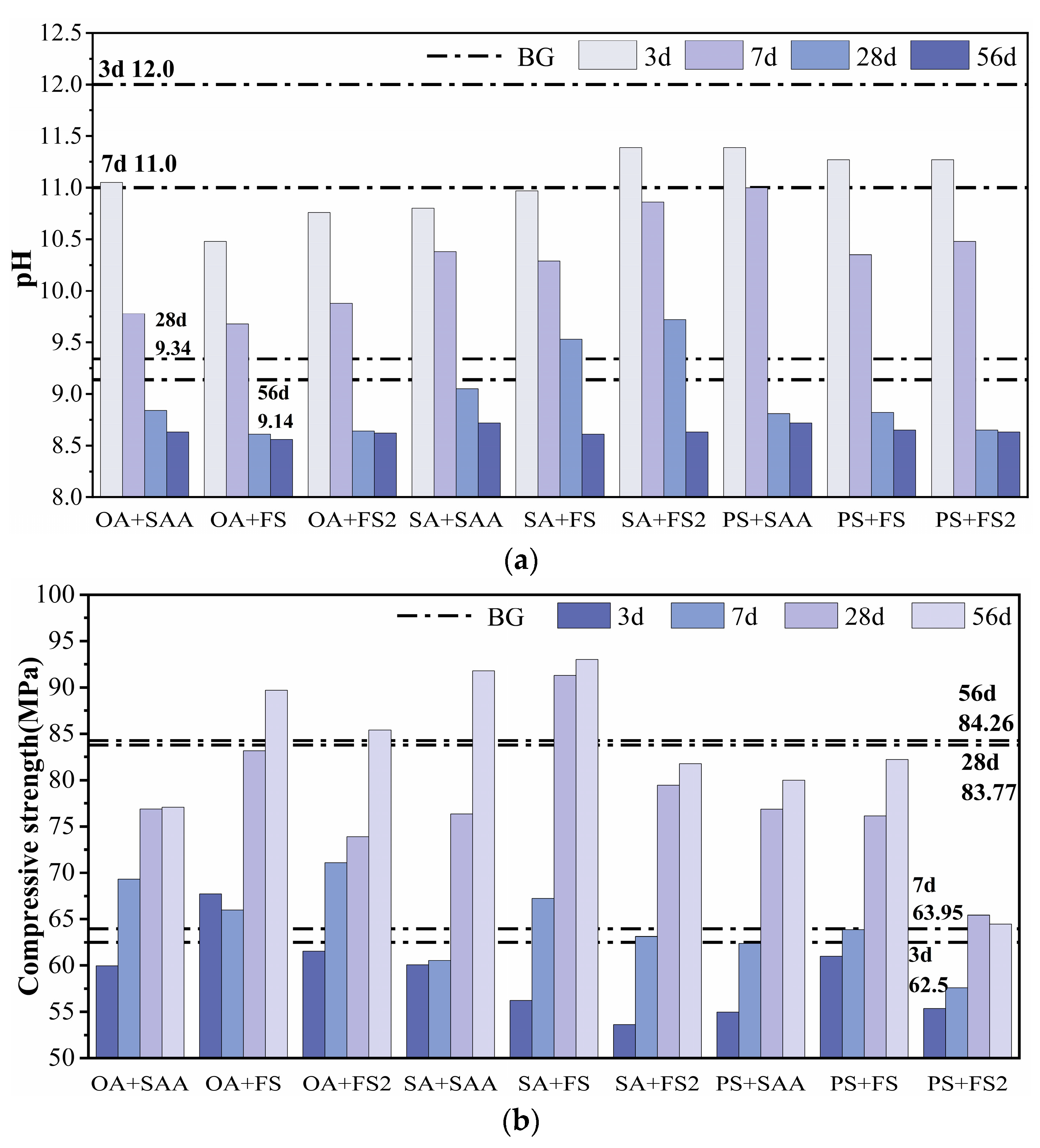
3.3. pH and Compressive Strength of Cement Paste with Compounded Alkali Reduction
3.4. Analysis of Alkali Reduction Mechanism
4. Conclusions
- The 5% OA, 5% SA, and 3% PS admixtures are good for reducing the pH of cement paste, and 1% FS2, 5% FS, and 3% SA may considerably enhance the compressive strength of cementitious materials. The ideal water–cement ratio of DE as an alkali-reducing chemical for replacing cement is 0.25, and the optimal dosage is 8%;
- Compared with BG, 8% DE-OA-FS decreased the pH by 12.7% and 6.3% at 3 d and 56 d, respectively, and enhanced the compressive strength by 8.4% and 6.5%, respectively. The synergistic action of DE, OA, and FS consumed a large amount of Ca(OH)2 in the cement paste, which resulted in a decrease in alkalinity and the creation of a C-S-H gel with a low Ca/Si ratio, which considerably increased the compressive strength of the cement paste;
- The composite alkalinity-reducing combination of DE, OA, and FS balanced the low pH and high compressive strength of cementitious materials, providing a theoretical basis for the application of low-alkali–high-strength cementitious materials in EPC.
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faiz, H.; Ng, S.; Rahman, M. A state-of-the-art review on the advancement of sustainable vegetation concrete in slope stability. Constr. Build. Mater. 2022, 326, 126502. [Google Scholar] [CrossRef]
- Tang, W.; Mohseni, E.; Wang, Z. Development of vegetation concrete technology for slope protection and greening. Constr. Build. Mater. 2018, 179, 605–613. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Gong, C.; Zhou, X.; Dai, W.; Liu, Y.; Lu, L.; Cheng, X. Effects of carbamide on fluidity and setting time of sulphoaluminate cement and properties of planting concrete from sulphoaluminate cement. Constr. Build. Mater. 2018, 182, 290–297. [Google Scholar] [CrossRef]
- Gong, C.; Zhou, X.; Ji, L.; Dai, W.; Lu, L.; Cheng, X. Effects of limestone powders on pore structure and physiological characteristics of planting concrete with sulfoaluminate cement. Constr. Build. Mater. 2018, 162, 314–320. [Google Scholar] [CrossRef]
- Li, L.; Gong, C.; Wang, S.; Lu, L.; Cheng, X. Effect of natural zeolite on performance of sulfoaluminate cement-based planting cementitious material. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2017, 32, 586–590. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Q.; Li, L.; Li, Y.; Zhang, H.; Lu, L. Investigation on water and fertilizer retention properties of hydrated sulphoaluminate cement pastes modified by bentonite for porous ecological concrete. Case Stud. Constr. Mater. 2023, 18, e01967. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D.; Li, Y.; Gao, H.; Meng, X. Physical and Mechanical Properties of Novel Porous Ecological Concrete Based on Magnesium Phosphate Cement. Materials 2022, 15, 7521. [Google Scholar] [CrossRef]
- Wu, C.; Liu, C.; Cheng, G.; Li, J.; Zhang, C.; Jiang, W. Preparation of a low-carbon plant-compatible ecological concrete with fertilizer self-release characteristics based on multi-solid waste co-recycling and its environmental impact. J. Build. Eng. 2023, 76, 107268. [Google Scholar] [CrossRef]
- Lee, K.H.; Yang, K.H. Development of a neutral cementitious material to promote vegetation concrete. Constr. Build. Mater. 2016, 127, 442–449. [Google Scholar] [CrossRef]
- Li, S.; Yin, J.; Zhang, G. Experimental investigation on optimization of vegetation performance of porous sea sand concrete mixtures by pH adjustment. Constr. Build. Mater. 2020, 249, 118775. [Google Scholar] [CrossRef]
- Li, S.; Yin, J.; Zhang, G.; Gao, T. Research on reducing alkalinity of an ecological porous concrete mixure with carbonization. Mater. Tech. 2019, 53, 575–581. [Google Scholar] [CrossRef]
- Kong, J.; Wang, Z.; Meng, X.; Zhao, Y.; Chen, M.; Quan, H. Study on Alkali Reduction Treatments and Plant Growth Properties of Planting Concrete. Sustainability 2022, 14, 12228. [Google Scholar] [CrossRef]
- Cheng, J.; Luo, X.; Shen, Z.; Guo, X. Study on Vegetation Concrete Technology for Slope Protection and Greening Engineering. Pol. J. Environ. Stud. 2020, 29, 4017–4028. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, C.; Ding, X.; Kang, T.; Nie, X. Experimental study on the vegetation growing recycled concrete and synergistic effect with plant roots. Materials 2019, 12, 1855. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Gao, T.; Yin, J.; Wu, S.Y.; Liang, Q. Investigation on alkalinity reduction methods of eco-porous concrete. Mater. Und Werkst. 2018, 49, 1108–1116. [Google Scholar] [CrossRef]
- GB/T 17671-2021; State Administration for Market Regulation, Test Method of Cement Mortar Strength (ISO Method). China Standards Press: Beijing, China, 2021.
- Yin, S.; Yang, Y.; Zhang, T.; Guo, G.; Yu, F. Effect of carbonic acid water on the degradation of Portland cement paste: Corrosion process and kinetics. Constr. Build. Mater. 2015, 91, 39–46. [Google Scholar] [CrossRef]
- Gu, L.; Bennett, T.; Visintin, P. Sulphuric acid exposure of conventional concrete and alkali-activated concrete: Assessment of test methodologies. Constr. Build. Mater. 2019, 197, 681–692. [Google Scholar] [CrossRef]
- Yuan, H.; Dangla, P.; Chatellier, P.; Chaussadent, T. Degradation modeling of concrete submitted to biogenic acid attack. Cem. Concr. Res. 2015, 70, 29–38. [Google Scholar] [CrossRef]
- Lu, Z.C. Effect of Chemical Admixtures with Different Functional Groups on Cement Hydration and the Mechanisms. Ph.D. Thesis, Tsinghua University, Beijing, China, 2017. [Google Scholar]
- Xu, Z.; Ji, Y.; Zhao, Y.; Xu, S.; Zhang, Z.; Gao, F. Effect of acid activation on the mechanical and shrinkage properties of cement-based materials. J. Build. Eng. 2023, 68, 106184. [Google Scholar] [CrossRef]
- Bao, H.; Xu, G.; Wang, Q.; Peng, Y.; Liu, J. Study on the deterioration mechanism of cement-based materials in acid water containing aggressive carbon dioxide. Constr. Build. Mater. 2020, 243, 118233. [Google Scholar] [CrossRef]
- Rimmelé, G.; Barlet-Gouédard, V.; Porcherie, O. Heterogeneous porosity distribution in Portland cement exposed to CO2-rich fluids. Cem. Concr. Res. 2008, 38, 1038–1048. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S. The effect of nanoalumina on early hydration and mechanical properties of cement pastes. Constr. Build. Mater. 2019, 202, 169–176. [Google Scholar] [CrossRef]
- Singh, N.B.; Singh, V.D.; Rai, S.; Chaturvedi, S. Effect of lignosulfonate, calcium chloride and their mixture on the hydration of RHA-blended portland cement. Cem. Concr. Res. 2002, 32, 387–392. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Brahmkhatri, V.; Sriram, G.; Jung, H.; Yu, J.; Kurkkuri, N. Nature engineered diatom biosilica as drug delivery systems. J. Control. Release 2018, 281, 70–83. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, Y.; Li, K.; Liu, X.; Dong, B.; Feng, L. Application of Diatomite in Cementitious Materials. Mater. Rep. 2022, 36, 21030125–21030129. [Google Scholar] [CrossRef]
- Ouyang, X.; Yu, L.; Chen, J.; Wu, K.; Ma, Y.; Fu, J. Roles of diatomite in hydration, microstructure and strength development of cement paste. Mater. Today Commun. 2023, 37, 107555. [Google Scholar] [CrossRef]
- Singh, N.K.; Mishra, P.C.; Singh, V.K.; Narang, K.K. Effects of hydroxyethyl cellulose and oxalic acid on the properties of cement. Cem. Concr. Res. 2003, 33, 1319–1329. [Google Scholar] [CrossRef]
- Zhang, H.; Mu, S.; Cai, J.; Liu, J.; Hong, J. The role of iron in cement hydration process: From perspective of chemical admixture. Thermochim. Acta 2023, 722, 179457. [Google Scholar] [CrossRef]
- Chen, H.; Feng, P.; Ye, S.; Sun, W. The coupling effect of calcium concentration and pH on early hydration of cement. Constr. Build. Mater. 2018, 185, 391–401. [Google Scholar] [CrossRef]


| SiO2 (%) | Fe2O3 (%) | Al2O3 (%) | Loss on Ignition (%) | Bulk Density (g/cm3) | pH |
|---|---|---|---|---|---|
| ≥85 | ≤1.3 | ≤3.35 | ≤2.0 | 0.35–0.39 | 8–10 |
| Group | W/C Ratio | Water (kg/m3) | Cement (kg/m3) | Water Reducer (kg/m3) | pH | Compressive Strength (MPa) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | 56 d | 3 d | 7 d | 28 d | 56 d | |||||
| BG | 0.25 | 423 | 1691 | 0.25 | 12.00 | 11.00 | 9.34 | 9.14 | 62.50 | 63.95 | 83.77 | 84.26 |
| 0.30 | 507 | 11.79 | 10.95 | 9.60 | 8.75 | 43.63 | 58.45 | 79.33 | 78.80 | |||
| 0.35 | 592 | 11.70 | 10.50 | 9.10 | 8.82 | 34.45 | 47.47 | 61.25 | 66.13 | |||
| Group | Cement (kg/m3) | Water (kg/m3) | Water Reducer (kg/m3) | Additive (kg/m3) | pH | Compressive Strength (MPa) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | 56 d | 3 d | 7 d | 28 d | 56 d | ||||||
| OA | 1% | 1692 | 507 | 0.25 | 5.07 | 11.70 | 11.00 | 8.82 | 8.54 | 45.80 | 59.03 | 74.63 | 76.75 |
| 3% | 1692 | 507 | 0.25 | 15.21 | 11.70 | 9.43 | 8.77 | 8.51 | 43.88 | 68.25 | 79.75 | 73.98 | |
| 5% | 1692 | 507 | 0.25 | 25.35 | 11.10 | 9.55 | 8.69 | 8.49 | 41.28 | 58.45 | 76.85 | 71.23 | |
| SA | 1% | 1692 | 507 | 0.25 | 5.07 | 11.00 | 9.80 | 8.92 | 8.62 | 43.48 | 55.98 | 74.17 | 77.37 |
| 3% | 1692 | 507 | 0.25 | 15.21 | 10.80 | 10.20 | 9.28 | 8.59 | 41.65 | 56.35 | 76.33 | 70.17 | |
| 5% | 1692 | 507 | 0.25 | 25.35 | 10.70 | 9.50 | 8.88 | 8.58 | 41.50 | 57.95 | 71.30 | 80.78 | |
| SAA | 1% | 1692 | 507 | 0.25 | 5.07 | 11.70 | 11.20 | 9.29 | 8.68 | 39.05 | 49.90 | 79.18 | 72.00 |
| 3% | 1692 | 507 | 0.25 | 15.21 | 11.60 | 11.01 | 9.23 | 8.46 | 41.83 | 59.33 | 74.95 | 87.47 | |
| 5% | 1692 | 507 | 0.25 | 25.35 | 11.40 | 10.73 | 9.15 | 8.44 | 38.73 | 57.55 | 76.93 | 71.93 | |
| Group | Cement (kg/m3) | Water (kg/m3) | Water Reducer (kg/m3) | Additive (kg/m3) | pH | Compressive Strength (MPa) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | 56 d | 3 d | 7 d | 28 d | 56 d | ||||||
| FS | 1% | 1691 | 507 | 0.25 | 5.07 | 11.40 | 11.8 | 9.64 | 8.52 | 45.00 | 58.77 | 63.57 | 75.33 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.30 | 11.50 | 8.73 | 8.53 | 47.75 | 55.60 | 81.23 | 79.10 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 11.20 | 11.20 | 9.15 | 8.50 | 47.63 | 62.85 | 86.57 | 81.63 | |
| FS2 | 1% | 1691 | 507 | 0.25 | 5.07 | 11.80 | 11.00 | 8.89 | 8.37 | 49.27 | 65.70 | 79.37 | 84.67 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.40 | 11.00 | 8.96 | 8.43 | 49.40 | 67.20 | 79.93 | 81.97 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 11.50 | 10.60 | 8.97 | 8.40 | 47.10 | 61.43 | 73.40 | 80.53 | |
| ALS | 1% | 1691 | 507 | 0.25 | 5.07 | 11.90 | 11.50 | 9.53 | 8.71 | 40.67 | 54.17 | 70.83 | 74.50 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.90 | 11.90 | 10.04 | 8.59 | 40.88 | 56.53 | 67.63 | 72.38 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 12.00 | 11.60 | 9.29 | 8.63 | 43.28 | 57.65 | 67.78 | 65.73 | |
| PS | 1% | 1691 | 507 | 0.25 | 5.07 | 11.13 | 9.90 | 8.61 | 8.59 | 49.58 | 59.90 | 69.95 | 73.35 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.13 | 9.80 | 8.69 | 8.60 | 49.35 | 56.50 | 68.33 | 65.28 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 11.35 | 9.89 | 9.06 | 8.64 | 44.93 | 49.20 | 65.58 | 63.73 | |
| AS | 1% | 1691 | 507 | 0.25 | 5.07 | 11.50 | 10.44 | 8.82 | 8.73 | 45.68 | 57.33 | 76.93 | 78.25 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.45 | 10.04 | 8.76 | 8.54 | 43.08 | 56.67 | 69.98 | 66.38 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 11.02 | 10.22 | 8.74 | 8.71 | 43.63 | 56.20 | 72.93 | 72.40 | |
| Group | Cement(kg/m3) | Water (kg/m3) | Water Reducer (kg/m3) | Additive (kg/m3) | pH | Compressive Strength (Mpa) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | 56 d | 3 d | 7 d | 28 d | 56 d | ||||||
| AC | 1% | 1691 | 507 | 0.25 | 5.07 | 11.60 | 10.57 | 8.72 | 8.67 | 42.20 | 56.50 | 70.47 | 73.90 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.90 | 11.18 | 8.81 | 8.69 | 37.80 | 50.23 | 68.68 | 69.80 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 11.80 | 10.95 | 8.97 | 8.71 | 34.58 | 52.80 | 71.20 | 66.93 | |
| AB | 1% | 1691 | 507 | 0.25 | 5.07 | 11.70 | 11.50 | 9.05 | 8.63 | 38.18 | 50.55 | 59.33 | 58.93 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.02 | 10.13 | 9.10 | 8.67 | 34.45 | 44.88 | 55.73 | 62.45 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 11.26 | 10.05 | 9.21 | 8.58 | 38.05 | 59.63 | 76.40 | 73.28 | |
| Group | Cement (kg/m3) | Water (kg/m3) | Water Reducer (kg/m3) | Additive (kg/m3) | pH | Compressive Strength (Mpa) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | 56 d | 3 d | 7 d | 28 d | 56 d | ||||||
| MP | 1% | 1691 | 507 | 0.25 | 5.07 | 11.30 | 11.30 | 9.26 | 8.55 | 49.33 | 58.88 | 69.47 | 78.25 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.40 | 11.20 | 9.46 | 8.60 | 47.93 | 64.23 | 82.43 | 71.43 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 11.60 | 11.30 | 9.66 | 8.80 | 48.63 | 65.23 | 67.43 | 77.68 | |
| MS | 1% | 1691 | 507 | 0.25 | 5.07 | 11.50 | 10.60 | 8.89 | 8.53 | 42.48 | 53.68 | 66.78 | 74.95 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.50 | 10.60 | 9.31 | 8.61 | 46.65 | 54.23 | 65.60 | 64.87 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 11.60 | 11.10 | 8.81 | 8.58 | 43.88 | 55.05 | 61.98 | 66.77 | |
| PA | 1% | 1691 | 507 | 0.25 | 5.07 | 11.40 | 10.39 | 8.85 | 8.70 | 46.68 | 52.98 | 73.80 | 74.77 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.70 | 10.81 | 8.87 | 8.80 | 41.03 | 56.10 | 73.27 | 73.53 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 11.90 | 10.88 | 9.21 | 8.88 | 40.68 | 55.70 | 67.53 | 68.35 | |
| ALC | 1% | 1691 | 507 | 0.25 | 5.07 | 11.80 | 10.19 | 8.67 | 8.54 | 44.30 | 53.28 | 69.08 | 69.93 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.30 | 10.59 | 8.48 | 8.57 | 49.53 | 58.03 | 68.18 | 74.10 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 11.50 | 10.26 | 8.47 | 8.49 | 47.08 | 60.73 | 75.75 | 64.50 | |
| CS | 1% | 1691 | 507 | 0.25 | 5.07 | 11.10 | 10.93 | 8.65 | 8.66 | 41.95 | 56.98 | 81.13 | 71.88 |
| 3% | 1691 | 507 | 0.25 | 15.21 | 11.90 | 11.64 | 8.85 | 8.70 | 41.55 | 57.05 | 75.77 | 71.33 | |
| 5% | 1691 | 507 | 0.25 | 25.35 | 11.40 | 10.37 | 8.86 | 8.67 | 42.13 | 57.73 | 70.60 | 72.55 | |
| Group | Cement (kg/m3) | Water (kg/m3) | Water Reducer (kg/m3) | Additive (kg/m3) | pH | Compressive Strength (Mpa) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | 56 d | 3 d | 7 d | 28 d | 56 d | ||||||
| 0.25 DE | 2% | 1658 | 423 | 0.25 | 34 | 11.86 | 10.06 | 8.48 | 8.47 | 65.13 | 70.08 | 80.83 | 82.76 |
| 4% | 1624 | 423 | 0.25 | 68 | 11.81 | 9.85 | 8.75 | 8.72 | 60.55 | 71.98 | 82.25 | 83.28 | |
| 6% | 1590 | 423 | 0.25 | 101 | 11.84 | 9.63 | 8.71 | 8.68 | 62.00 | 73.35 | 80.83 | 85.95 | |
| 8% | 1556 | 423 | 0.25 | 135 | 11.56 | 9.58 | 8.65 | 8.62 | 54.60 | 72.15 | 81.07 | 85.57 | |
| 10% | 1522 | 423 | 0.25 | 169 | 11.68 | 9.65 | 8.76 | 8.71 | 56.78 | 69.40 | 78.78 | 82.31 | |
| 0.3 DE | 2% | 1658 | 507 | 0.25 | 34 | 11.83 | 10.67 | 8.81 | 8.75 | 44.33 | 59.40 | 72.60 | 78.16 |
| 4% | 1624 | 507 | 0.25 | 68 | 11.52 | 10.05 | 8.84 | 8.76 | 44.25 | 58.30 | 84.75 | 85.45 | |
| 6% | 1590 | 507 | 0.25 | 101 | 12.00 | 9.74 | 8.68 | 8.62 | 40.60 | 51.05 | 67.80 | 79.65 | |
| 8% | 1556 | 507 | 0.25 | 135 | 11.80 | 10.91 | 8.80 | 8.71 | 40.63 | 52.65 | 69.43 | 76.34 | |
| 10% | 1522 | 507 | 0.25 | 169 | 11.67 | 11.40 | 8.69 | 8.62 | 38.18 | 49.88 | 62.20 | 73.56 | |
| 0.35 DE | 2% | 1658 | 592 | 0.25 | 34 | 11.81 | 11.09 | 9.53 | 9.21 | 32.95 | 48.40 | 64.57 | 71.62 |
| 4% | 1624 | 592 | 0.25 | 68 | 11.67 | 11.05 | 9.24 | 9.03 | 34.23 | 46.08 | 59.60 | 68.51 | |
| 6% | 1590 | 592 | 0.25 | 101 | 11.95 | 10.35 | 9.54 | 9.15 | 28.55 | 41.25 | 53.08 | 69.34 | |
| 8% | 1556 | 592 | 0.25 | 135 | 11.59 | 10.81 | 9.17 | 8.97 | 29.6 | 41.48 | 52.70 | 72.69 | |
| 10% | 1522 | 592 | 0.25 | 169 | 11.43 | 10.27 | 9.16 | 8.84 | 29.0 | 42.05 | 54.93 | 69.14 | |
| Group | Water (kg/m3) | Cement (kg/m3) | DE (kg/m3) | Alkali Reducer (kg/m3) | Enhancer (kg/m3) | pH | Compressive Strength (MPa) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | 56 d | 3 d | 7 d | 28 d | 56 d | ||||||
| OA+SAA | 422 | 1556 | 135 | 21.14 | 12.69 | 11.05 | 9.78 | 8.84 | 8.63 | 59.95 | 69.30 | 76.88 | 77.08 |
| OA+FS | 422 | 1556 | 135 | 21.14 | 21.14 | 10.48 | 9.68 | 8.61 | 8.56 | 67.73 | 65.98 | 83.15 | 89.70 |
| OA+FS2 | 422 | 1556 | 135 | 21.14 | 4.23 | 10.76 | 9.88 | 8.64 | 8.62 | 61.55 | 71.10 | 73.88 | 85.40 |
| SA+SAA | 422 | 1556 | 135 | 21.14 | 12.69 | 10.80 | 10.38 | 9.05 | 8.72 | 60.05 | 60.53 | 76.35 | 91.78 |
| SA+FS | 422 | 1556 | 135 | 21.14 | 21.14 | 10.97 | 10.29 | 9.53 | 8.61 | 56.23 | 67.23 | 91.28 | 93.00 |
| SA+FS2 | 422 | 1556 | 135 | 21.14 | 4.23 | 11.39 | 10.86 | 9.72 | 8.63 | 53.63 | 63.13 | 79.43 | 81.78 |
| PS+SAA | 422 | 1556 | 135 | 12.69 | 12.69 | 11.39 | 11.00 | 8.81 | 8.72 | 54.95 | 62.38 | 76.87 | 80.00 |
| PS+FS | 422 | 1556 | 135 | 12.69 | 21.14 | 11.27 | 10.35 | 8.82 | 8.65 | 61.00 | 63.85 | 76.15 | 82.20 |
| PS+FS2 | 422 | 1556 | 135 | 12.69 | 4.23 | 11.27 | 10.48 | 8.65 | 8.63 | 55.35 | 57.60 | 65.45 | 64.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yin, J.; Xu, W.; Liu, S.; Liu, X. Alkalinity Regulation and Optimization of Cementitious Materials Used in Ecological Porous Concrete. Materials 2024, 17, 1918. https://doi.org/10.3390/ma17081918
Li S, Yin J, Xu W, Liu S, Liu X. Alkalinity Regulation and Optimization of Cementitious Materials Used in Ecological Porous Concrete. Materials. 2024; 17(8):1918. https://doi.org/10.3390/ma17081918
Chicago/Turabian StyleLi, Sijiao, Jian Yin, Wenxing Xu, Sizhe Liu, and Xiaofei Liu. 2024. "Alkalinity Regulation and Optimization of Cementitious Materials Used in Ecological Porous Concrete" Materials 17, no. 8: 1918. https://doi.org/10.3390/ma17081918
APA StyleLi, S., Yin, J., Xu, W., Liu, S., & Liu, X. (2024). Alkalinity Regulation and Optimization of Cementitious Materials Used in Ecological Porous Concrete. Materials, 17(8), 1918. https://doi.org/10.3390/ma17081918





