Synthesis, Characterization, and Properties of a Novel Hyperbranched Polymers with Polyacrylamide Side Chains
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis Processes
2.2.1. Synthesis of Modified-M2.0
2.2.2. Synthesis of HAPAM and PAM
2.3. Characterization
2.4. Performance-Evaluation
3. Results and Discussion
3.1. Infrared Spectrum of M0.5, M1.0, M1.5, M2.0, and HAPAM
3.2. H-NMR Spectrum of HAPAM
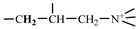 in the hydrophobic branched chain of DMCAAC-18 and by the proton absorption peak of -CH3 in the AMPS chain. Peak d (δ1.68) belonged to the proton absorption peak of
in the hydrophobic branched chain of DMCAAC-18 and by the proton absorption peak of -CH3 in the AMPS chain. Peak d (δ1.68) belonged to the proton absorption peak of  [63],
[63],  ,
,  , and -CH2-CH2-(CH2)15CH3 in the chain of AA, AM, AMPS, and hydrophobic branched chain of DMCAAC-18. Peak e (δ2.08) was assigned to the proton absorption peak of
, and -CH2-CH2-(CH2)15CH3 in the chain of AA, AM, AMPS, and hydrophobic branched chain of DMCAAC-18. Peak e (δ2.08) was assigned to the proton absorption peak of  ,
,  ,
,  , and
, and  in the branched chains. Peak f (δ2.33) was resulted from the proton absorption peak of
in the branched chains. Peak f (δ2.33) was resulted from the proton absorption peak of  [41],
[41],  , and
, and  in the branched chain-skeleton and the proton absorption peak of
in the branched chain-skeleton and the proton absorption peak of  and
and 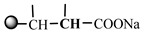 at the branched chain. Peak g (δ3.04) was the proton absorption peak of
at the branched chain. Peak g (δ3.04) was the proton absorption peak of  at the branched chain and the proton absorption peak of
at the branched chain and the proton absorption peak of  at the hydrophobic branched chain of the DMCAAC-18 chain. The proton absorption peak of
at the hydrophobic branched chain of the DMCAAC-18 chain. The proton absorption peak of  at the branched chain skeleton, the proton absorption peak of -CH2-CH2-(CH2)15CH3 in DMCAAC-18 chain, and the proton absorption peak of -CH2SO3Na in the AMPS chain resulted in Peak h (δ3.14). The proton absorption peak of
at the branched chain skeleton, the proton absorption peak of -CH2-CH2-(CH2)15CH3 in DMCAAC-18 chain, and the proton absorption peak of -CH2SO3Na in the AMPS chain resulted in Peak h (δ3.14). The proton absorption peak of  at the branched chain and the proton absorption peak of
at the branched chain and the proton absorption peak of  in the DMCAAC-18 chain resulted in Peak i (δ3.38). Peak j (δ3.88) belonged to the proton absorption peak of
in the DMCAAC-18 chain resulted in Peak i (δ3.38). Peak j (δ3.88) belonged to the proton absorption peak of  and
and  in the branched-chain and the proton absorption peak of
in the branched-chain and the proton absorption peak of  in the branched chain-skeleton. Peak k (δ7.22) was the proton absorption peak of
in the branched chain-skeleton. Peak k (δ7.22) was the proton absorption peak of  [63] in the branched chain. Peak l (δ8.01) was caused by the proton absorption peak of -CO-NH- at the branched chain and at the branched chain skeleton. The peaks corresponded to the expected chemical structure and functional group of HAPAM, demonstrating the successful synthesis of HAPAM.
[63] in the branched chain. Peak l (δ8.01) was caused by the proton absorption peak of -CO-NH- at the branched chain and at the branched chain skeleton. The peaks corresponded to the expected chemical structure and functional group of HAPAM, demonstrating the successful synthesis of HAPAM.3.3. Determination of Intrinsic Viscosity and Molecular Weight
| Mass Concentrations | 1st (s) | 2nd (s) | 3rd (s) | taverage (s) |
|---|---|---|---|---|
| 1 mol/L of NaCl | 112.9 | 112.6 | 112.7 | 112.7 |
| 500 mg/L of PAM + 1 mol/L of NaCl | 167.2 | 167.3 | 167.5 | 167.3 |
| 500 mg/L of HAPAM + 1 mol/L of NaCl | 143.5 | 143.8 | 143.6 | 143.6 |
3.4. Thickening Capacity of HAPAM
3.5. Shear-Resistance of HAPAM
3.6. Temperature-Resistance of HAPAM
3.7. Salt-Resistance of HAPAM
3.8. Interfacial Tension of HAPAM
3.9. Resistance-Coefficient and Residual-Resistance-Coefficient of HAPAM
3.10. EOR of HAPAM Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shuker, M.T.; Buriro, M.A.; Hamza, M.M. Enhanced oil recovery: A future for Pakistan. In Proceedings of the SPE/PAPG Annual Technical Conference, Islamabad, Pakistan, 3–5 December 2012; OnePetro: Richardson, TX, USA, 2012. [Google Scholar]
- Gbadamosi, A.O.; Kiwalabye, J.; Junin, R.; Augustine, A. A review of gas enhanced oil recovery schemes used in the North Sea. J. Pet. Explor. Prod. Technol. 2018, 8, 1373–1387. [Google Scholar] [CrossRef]
- Gbadamosi, A.; Patil, S.; Kamal, M.S.; Adewunmi, A.A.; Yusuff, A.S.; Agi, A.; Oseh, J. Application of polymers for chemical enhanced oil recovery: A review. Polymers 2022, 14, 1433. [Google Scholar] [CrossRef] [PubMed]
- Agi, A.; Junin, R.; Jaafar, M.Z.; Sidek, M.A.; Yakasai, F.; Gbadamosi, A.; Oseh, J. Laboratory evaluation to field application of ultrasound: A state-of-the-art review on the effect of ultrasonication on enhanced oil recovery mechanisms. J. Ind. Eng. Chem. 2022, 110, 100–119. [Google Scholar] [CrossRef]
- Malozyomov, B.V.; Martyushev, N.V.; Kukartsev, V.V.; Tynchenko, V.S.; Bukhtoyarov, V.V.; Wu, X.; Tyncheko, Y.A.; Kukartsev, V.A. Overview of methods for enhanced oil recovery from conventional and unconventional reservoirs. Energies 2023, 16, 4907. [Google Scholar] [CrossRef]
- Sikiru, S.; Soleimani, H.; Yusuf, J.Y.; Hassan, Y.M.; Hamza, M.F.; Singh, R. Recent advance and prospect of enhanced oil recovery mech-anisms in reservoir sandstone. Preprints 2023, 2023091654. [Google Scholar]
- Tahir, M.U.; Zhou, H.; Memon, B.S.; Liu, W.D.; Memon, A.; Khan, D.; Bakhsh, A. Comparative studies of enhancing oil recovery optimization for optimum oil field development. Model. Earth Syst. Environ. 2023, 9, 1477–1503. [Google Scholar] [CrossRef]
- Gbadamosi, A.; Patil, S.; Al Shehri, D.; Kamal, M.S.; Hussain, S.S.; Al-Shalabi, E.W.; Hassan, A.M. Recent advances on the application of low salinity waterflooding and chemical enhanced oil recovery. Energy Rep. 2022, 8, 9969–9996. [Google Scholar] [CrossRef]
- Jain, S.; Pachisia, H.; Sharma, A.; Patel, S.; Patel, S.; Ragunathan, B. A systematic review—Chemical EOR using surfactants and polymers. Mater. Today Proc. 2022, 62, 7220–7223. [Google Scholar] [CrossRef]
- Mokheimer, E.M.; Hamdy, M.; Abubakar, Z.; Shakeel, M.R.; Habib, M.A.; Mahmoud, M. A comprehensive review of thermal enhanced oil recovery: Techniques evaluation. J. Energy Resour. Technol. 2019, 141, 030801. [Google Scholar] [CrossRef]
- Hazarika, K. Chapter-8 classification of EOR technologies. In Oil Recovery Principles & Practices; CIIR Scientific Publications: Taipei, Taiwan, 2023; Volume 21. [Google Scholar]
- Gray, L.; Goodyear, S. Overcoming the CO2 supply challenge for CO2 EOR. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 10–13 November 2014; SPE: Kuala Lumpur, Malaysia, 2014; p. D021S032R005. [Google Scholar]
- Núñez-López, V.; Moskal, E. Potential of CO2-EOR for near-term decarbonization. Front. Clim. 2019, 1, 5. [Google Scholar] [CrossRef]
- Khan, A.; Saxena, S.; Baloni, S.; Sharma, M.; Kodavaty, J. Overview and methods in enhanced oil recovery. J. Phys. Conf. Ser. 2021, 2070, 012061. [Google Scholar]
- Abbas, A.H.; Sulaiman, W.R.W.; Jaafar, M.Z.; Gbadamosi, A.O.; Ebrahimi, S.S.; Elrufai, A. Numerical study for continuous surfactant flooding considering adsorption in heterogeneous reservoir. J. King Saud Univ. Eng. Sci. 2020, 32, 91–99. [Google Scholar] [CrossRef]
- Levitt, D.B.; Pope, G.A. Selection and screening of polymers for enhanced-oil recovery. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 20–23 April 2008; SPE: Kuala Lumpur, Malaysia, 2008; p. SPE–113845-MS. [Google Scholar]
- Pope, G.A. Recent developments and remaining challenges of enhanced oil recovery. J. Pet. Technol. 2011, 63, 65–68. [Google Scholar] [CrossRef]
- Ayirala, S.; Sofi, A.; Li, Z.; Xu, Z. Surfactant and surfactant-polymer effects on wettability and crude oil liberation in carbonates. J. Pet. Sci. Eng. 2021, 207, 109117. [Google Scholar] [CrossRef]
- Caili, D.; Qing, Y.; Fulin, Z.; Hanqiao, J. Residual polymer reutilization for IOR after polymer flooding: From laboratory to field application in daqing oilfield. Pet. Sci. Technol. 2011, 29, 2441–2449. [Google Scholar] [CrossRef]
- Viken, A.L.; Spildo, K.; Reichenbach-Klinke, R.; Djurhuus, K.; Skauge, T. Influence of weak hydrophobic interactions on in situ viscosity of a hydrophobically modified water-soluble polymer. Energy Fuels 2018, 32, 89–98. [Google Scholar] [CrossRef]
- Wang, S.; Shi, L.; Ye, Z.; Zhang, X.; Zhang, L.; Li, X. Experimental study on improving oil recovery in fluvial reservoir with polymer solutions. AIP Adv. 2021, 11, 055121. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Shi, L.; Liang, X.; Wang, X.; Zhang, Y.; Wu, X.; Gong, Y.; Shi, X.; Qin, G. Application of a novel amphiphilic polymer for enhanced offshore heavy oil recovery: Mechanistic study and core displacement test. J. Pet. Sci. Eng. 2022, 215, 110626. [Google Scholar] [CrossRef]
- Lei, T.; Wang, Y.; Zhang, H.; Cao, J.; Xiao, C.; Ding, M.; Chen, W.; Chen, M.; Zhang, Z. Preparation and performance evaluation of a branched functional polymer for heavy oil recovery. J. Mol. Liq. 2022, 363, 119808. [Google Scholar] [CrossRef]
- Yao, T.; Liu, Q.; Liu, W.; Liu, F. Structural images of partially hydrolyzed polyacrylamide. Acta Pet. Sin. 2005, 26, 81. [Google Scholar]
- Wenli, L.; Dong, H.; Li, W.; Qingxia, L.; Jian, F. Synthesis and property evaluation of a salt-and alkali-resistant star-polymer. Pet. Explor. Dev. 2010, 37, 477–482. [Google Scholar] [CrossRef]
- Pu, W.-F.; Liu, R.; Li, B.; Jin, F.-Y.; Peng, Q.; Sun, L.; Du, D.-J.; Yao, F.-S. Amphoteric hyperbranched polymers with multistimuli-responsive behavior in the application of polymer flooding. RSC Adv. 2015, 5, 88002–88013. [Google Scholar] [CrossRef]
- Lai, N.; Guo, X.; Zhou, N.; Xu, Q. Shear resistance properties of modified nano-SiO2/aa/am copolymer oil displacement agent. Energies 2016, 9, 1037. [Google Scholar] [CrossRef]
- Lai, N.; Zhang, Y.; Xu, Q.; Zhou, N.; Wang, H.; Ye, Z. A water-soluble hyperbranched copolymer based on a dendritic structure for low-to-moderate permeability reservoirs. RSC Adv. 2016, 6, 32586–32597. [Google Scholar] [CrossRef]
- Duan, M.; Fang, S.; Zhang, L.; Wang, F.; Zhang, P.; Zhang, J. Shear degradation resistance of star poly(ethyleneimine)-polyacrylamides during elongational flow. e-Polymers 2011, 11, 86–99. [Google Scholar] [CrossRef]
- Chen, Q.; Ye, Z.; Tang, L.; Wu, T.; Jiang, Q.; Lai, N. Synthesis and solution properties of a novel hyperbranched polymer based on chitosan for enhanced oil recovery. Polymers 2020, 12, 2130. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Wen, J.; Gao, W.; Tian, Y.; Deng, Q.; Liu, Z. Functional characteristics and dominant enhanced oil recovery mechanism of polymeric surfactant. J. Mol. Liq. 2022, 354, 118921. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, J.; Liao, Y.; Xu, T.; Zhang, H.; Du, A.; Yang, X.; Lin, C.; Mao, J. Evaluation of temperature resistance of non chemical crosslinked double-tailed hydrophobically associating polymer fracturing fluid. React. Funct. Polym. 2022, 175, 105268. [Google Scholar] [CrossRef]
- Zhi, J.; Liu, Y.; Chen, J.; Jiang, N.; Xu, D.; Bo, L.; Qu, G. Performance evaluation and oil displacement effect of amphiphilic polymer heavy oil activator. Molecules 2023, 28, 5257. [Google Scholar] [CrossRef]
- Zhu, Z.; Kang, W.; Sarsenbekuly, B.; Yang, H.; Dai, C.; Yang, R.; Fan, H. Preparation and solution performance for the amphiphilic polymers with different hydrophobic groups. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Zhao, Y.; Ke, Y.; Hu, X.; Peng, F. Synthesis, characterization and emulsification properties of an amphiphilic copolymer for enhanced oil recovery. IOP Conf. Ser. Mater. Sci. Eng. 2019, 493, 012121. [Google Scholar] [CrossRef]
- Sui, Y.; Cao, G.; Guo, T.; Zhang, Z.; Zhang, Z.; Xiao, Z. Synthesis and mechanism study of temperature- and salt-resistant amphoteric polyacrylamide with MAPTAC and DTAB as monomers. Processes 2022, 10, 1666. [Google Scholar] [CrossRef]
- Qin, X.; Zhu, S.; Shi, Q.; Li, C. Synthesis and properties of a dendrimer amphiphilic polymer as enhanced oil recovery chemical. J. Chem. 2023, 2023, 4271446. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, S.; Ye, Z.; Zhang, J.; Xue, X.; Zhao, W. The seepage flow characteristics of hydrophobically associated polymers with different aggregation behaviours in porous media. R. Soc. Open Sci. 2020, 7, 191270. [Google Scholar] [CrossRef]
- Quan, H.; Li, Z.; Huang, Z. Self-assembly properties of a temperature- and salt-tolerant amphoteric hydrophobically associating polyacrylamide. RSC Adv. 2016, 6, 49281–49288. [Google Scholar] [CrossRef]
- Shi, J.; Wu, Z.; Deng, Q.; Liu, L.; Zhang, X.; Wu, X.; Wang, Y. Synthesis of hydrophobically associating polymer: Temperature resistance and salt tolerance properties. Polym. Bull. 2022, 79, 4581–4591. [Google Scholar] [CrossRef]
- Lai, N.; Qin, X.; Ye, Z.; Peng, Q.; Zhang, Y.; Ming, Z. Synthesis and evaluation of a water-soluble hyperbranched polymer as enhanced oil recovery chemical. J. Chem. 2013, 2013, 824785. [Google Scholar] [CrossRef]
- Peng, H. Development and Performance Evaluation of a New Type of Low-Damage Fracturing Fluid. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2014. [Google Scholar]
- Izadi, M.; Mardani, H.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Khezri, K. Hyperbranched poly (amidoamine)-grafted graphene oxide as a multifunctional curing agent for epoxy-terminated polyurethane composites. ChemistrySelect 2021, 6, 2692–2699. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chu, H.-L. Fourier transform infrared spectroscopy used to evidence the prevention of β-sheet formation of amyloid β (1–40) peptide by a short amyloid fragment. Int. J. Biol. Macromol. 2003, 32, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Georges, R.; Freytes, M.; Hurtmans, D.; Kleiner, I.; Vander Auwera, J.; Herman, M. Jet-cooled and room temperature FTIR spectra of the dimer of formic acid in the gas phase. Chem. Phys. 2004, 305, 187–196. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sakurai, Y.; Hosoi, Y.; Ishii, H.; Kajikawa, K.; Ouchi, Y.; Seki, K. Softened CH stretching vibration of a long-chain n-alkane, n-C44H90, physisorbed on a Ag (111) surface: An infrared reflection absorption spectroscopic study. J. Phys. Chem. B 2000, 104, 7370–7376. [Google Scholar] [CrossRef]
- Ahmad, H.; Hossain, M.E.; Rahman, M.A.; Rahman, M.M.; Miah, M.J.; Tauer, K. Carboxyl functionalized poly (methyl methacrylate-acrylic acid-ethylene glycol dimethacrylate) copolymer particles and their amination with amine-nucleophiles. e-Polymers 2008, 8, 96. [Google Scholar] [CrossRef]
- Yilmaz, M.K. Palladium (II) complexes with new bidentate phosphine-imine ligands for the Suzuki CC coupling reactions in supercritical carbon dioxide. J. Supercrit. Fluids 2018, 138, 221–227. [Google Scholar] [CrossRef]
- Lv, J.; Liu, S.; Feng, J.; Liu, Y.; Zhou, S.; Chen, R. Effective identification of paints pigments in hit-and-run cases with confocal Raman microscope. Pigment Resin Technol. 2016, 45, 294–300. [Google Scholar] [CrossRef]
- Shanmugasundaram, A.; Krishnamoorthy, S. A systematically evolved method for the effective use of essential oil blends for the structural maintenance of palm leaf manuscripts. J. Inst. Conserv. 2024, 47, 82–98. [Google Scholar] [CrossRef]
- Girma, W.; Diaz, I. Encapsulation of Co (II) complex with a schiff base ligands derived from 1, 10- phenantroline-5, 6-dione and o-phenylene diamine in zeolite y. Int. J. Basic Appl. Sci. 2015, 1, 35–41. [Google Scholar] [CrossRef]
- Podstawka, E.; Światłowska, M.; Borowiec, E.; Proniewicz, L.M. Food additives characterization by infrared, Raman, and surface-enhanced Raman spectroscopies. J. Raman Spectrosc. 2007, 38, 356–363. [Google Scholar] [CrossRef]
- Bahadur, A.; Shoaib, M.; Saeed, A.; Iqbal, S. FT-IR spectroscopic and thermal study of waterborne polyurethane-acrylate leather coatings using tartaric acid as an ionomer. e-Polymers 2016, 16, 463–474. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Chen, W.; Xu, W.W.; Han, Z.-k.; Waheed, A.; Ye, Z.; Li, G.; Baiker, A. Continuous dimethyl carbonate synthesis from CO2 and methanol over BixCe1−xOδ monoliths: Effect of bismuth do** on population of oxygen vacancies, activity, and reaction pathway. Nano Res. 2022, 15, 1366–1374. [Google Scholar] [CrossRef]
- Fourneta, I.; Gall, E.A.; Deslandes, E.; Huvenne, J.-P.; Sombret, B.; Floc’h, J. In situ measurements of cell wall components in the red alga Solieria chordalis (Solieriaceae, Rhodophyta) by FTIR microspectrometry. Bot. Mar. 1997, 40, 45–48. [Google Scholar] [CrossRef]
- Paradkar, M.; Irudayaraj, J. A rapid FTIR spectroscopic method for estimation of caffeine in soft drinks and total methylxanthines in tea and coffee. J. Food Sci. 2002, 67, 2507–2511. [Google Scholar] [CrossRef]
- Ramis, G.; Larrubia, M.; Busca, G. On the chemistry of ammonia over oxide catalysts: Fourier transform infrared study of ammonia, hydrazine and hydroxylamine adsorption over iron–titania catalyst. Top. Catal. 2000, 11, 161–166. [Google Scholar] [CrossRef]
- Yahaya, N.P.; Ali, I.; Modu, K.A.; Adamu, S. Adsorption study of methylene blue onto power activated carbon prepared from ananas comosus peels. Nanochemistry Res. 2023, 8, 231–242. [Google Scholar]
- Huang, X.; Huang, D.; Ou, X.; Ding, F.; Chen, Z. Synthesis and properties of side-chain-type ion exchange membrane PEEK-g-StSO3Na for bipolar membranes. Appl. Surf. Sci. 2012, 258, 2312–2318. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of potential contaminants in processed biomass using fourier transform infrared spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Bahraeian, S.; Abron, K.; Pourjafarian, F.; Majid, R.A. Study on synthesis of polypyrrole via chemical polymerization method. Adv. Mater. Res. 2013, 795, 707–710. [Google Scholar] [CrossRef]
- Quan, H.; Lu, Q.; Chen, Z.; Huang, Z.; Jiang, Q. Adsorption–desorption behavior of the hydrophobically associating copolymer AM/APEG/C-18/SSS. RSC Adv. 2019, 9, 12300–12309. [Google Scholar] [CrossRef] [PubMed]
- Salih, N.A. Synthesis of new heterocyclic compounds derived from anthrone and evaluation of their biological activity. Synthesis 2008, 12, 13. [Google Scholar] [CrossRef]
- Sun, J.; Du, W.; Pu, X.; Zou, Z.; Zhu, B. Synthesis and evaluation of a novel hydrophobically associating polymer based on acrylamide for enhanced oil recovery. Chem. Pap. 2015, 69, 1598–1607. [Google Scholar] [CrossRef]
- Jadhawar, P.; Saeed, M. Low salinity water and polymer flooding in sandstone reservoirs: Upscaling from nano-to macro-scale using the maximum energy barrier. J. Pet. Sci. Eng. 2023, 220, 111247. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, D.; Cao, X.; Guo, L.; Zhu, Y. Both-branch amphiphilic polymer oil displacing system: Molecular weight, surfactant interactions and enhanced oil recovery performance. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 440–448. [Google Scholar] [CrossRef]
- González Coronel, V.J.; Jiménez-Regalado, E.J. Rheological properties of three different microstructures of water-soluble polymers prepared by solution polymerization. Polym. Bull. 2011, 67, 251–262. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Zheng, W.; Li, X.; Wang, F.; Li, X.; Zhang, D.; Turtabayev, S.; Kang, W. Research on synthesis and salt thickening behavior of a binary copolymer amphiphilic polymer. J. Pet. Sci. Eng. 2021, 204, 108713. [Google Scholar] [CrossRef]
- Jin, Z.; Shan, G.; Pan, P. Preparation and heat and salt resistance of AM/AMPS/SSS terpolymer. CIESC J. 2023, 74, 916–923. [Google Scholar] [CrossRef]
- Wang, D.; Tan, J.; Han, Y.; Guo, Y.; An, H. Synthesis and properties of temperature-resistant and salt-tolerant tetra-acrylamide copolymer. J. Macromol. Sci. Part A 2019, 56, 1148–1155. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, H.; Li, Y.; Li, Y.; Chen, X.; Zhuang, Y. Experimental investigation of synergy of components in surfactant/polymer flooding using three-dimensional core model. Transp. Porous Media 2019, 126, 317–335. [Google Scholar] [CrossRef]
- Liang, K.; Han, P.; Chen, Q.; Su, X.; Feng, Y. Comparative study on enhancing oil recovery under high temperature and high salinity: Polysaccharides versus synthetic polymer. ACS Omega 2019, 4, 10620–10628. [Google Scholar] [CrossRef]
- Bhatkar, S.; Kshirsagar, L.; Wadgaonkar, V.; Dulakhe, P.; Jhamtani, V. Application of chemical eor in viscous, heavy crude in thin stacked heterogeneous reservoirs using CMG simulator. Pet. Coal 2022, 64, 1000. [Google Scholar]










| Ions | Na+ | K+ | Ca2+ | Mg2+ | HCO3− | SO42− | Cl− |
|---|---|---|---|---|---|---|---|
| Concentrations (mg/L) | 667 | 28 | 20 | 13 | 379 | 68 | 870 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peaks (wavelength/cm−1) of M 0.5 | 3315 | 2951 | 2837 | 1734 | 1662 | 1442 | 1361 | 1199 | 1126 | 1043 | ||||
| Peaks (wavelength/cm−1) of M 1.0 | 3290 | 3078 | 2943 | 2845 | 1727 | 1644 | 1561 | 1473 | 1321 | 1194 | 1120 | 1039 | ||
| Peaks (wavelength/cm−1) of M 1.5 | 3295 | 3068 | 2946 | 2844 | 1731 | 1652 | 1549 | 1446 | 1360 | 1194 | 1127 | 1044 | ||
| Peaks (wavelength/cm−1) of M 2.0 | 3288 | 3074 | 2943 | 2847 | 1729 | 1644 | 1556 | 1459 | 1359 | 1196 | 1123 | 1036 | ||
| Peaks (wavelength/cm−1) of HAPAM | 3433 | 2928 | 2860 | 1635 | 1577 | 1416 | 1319 | 1175 | 1123 | 1042 |
| Mass Concentrations | PAM | HAPAM |
|---|---|---|
| Interfacial Tension (mN/m) ± Standard Deviations | ||
| 500 mg/L | 22.94 ± 0.05 | 19.95 ± 0.02 |
| 750 mg/L | 21.45 ± 0.05 | 18.64 ± 0.03 |
| 1000 mg/L | 19.87 ± 0.03 | 15.84 ± 0.03 |
| Pressure in Water-Driving-Stage (MPa) | Pressure in Polymer-Flooding-Stage (MPa) | Pressure in Subsequent-Water-Driving-Stage (MPa) | RF | RFF | |
|---|---|---|---|---|---|
| 2000 mg/L of PAM | 0.0094 | 0.1057 | 0.0137 | 11.22 | 1.45 |
| 2000 mg/L of HAPAM | 0.0122 | 0.2049 | 0.0342 | 16.77 | 2.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Wang, Q.; Tang, P.; Yang, H.; Li, C.; Yang, X.; Peng, T. Synthesis, Characterization, and Properties of a Novel Hyperbranched Polymers with Polyacrylamide Side Chains. Materials 2024, 17, 1619. https://doi.org/10.3390/ma17071619
Qin X, Wang Q, Tang P, Yang H, Li C, Yang X, Peng T. Synthesis, Characterization, and Properties of a Novel Hyperbranched Polymers with Polyacrylamide Side Chains. Materials. 2024; 17(7):1619. https://doi.org/10.3390/ma17071619
Chicago/Turabian StyleQin, Xiaoping, Qianwen Wang, Peng Tang, Hui Yang, Cuixia Li, Xiaoliang Yang, and Tong Peng. 2024. "Synthesis, Characterization, and Properties of a Novel Hyperbranched Polymers with Polyacrylamide Side Chains" Materials 17, no. 7: 1619. https://doi.org/10.3390/ma17071619
APA StyleQin, X., Wang, Q., Tang, P., Yang, H., Li, C., Yang, X., & Peng, T. (2024). Synthesis, Characterization, and Properties of a Novel Hyperbranched Polymers with Polyacrylamide Side Chains. Materials, 17(7), 1619. https://doi.org/10.3390/ma17071619






