Study on Reducing Water Absorption of Recycled Aggregates (RAs) by Microbial Mineralization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
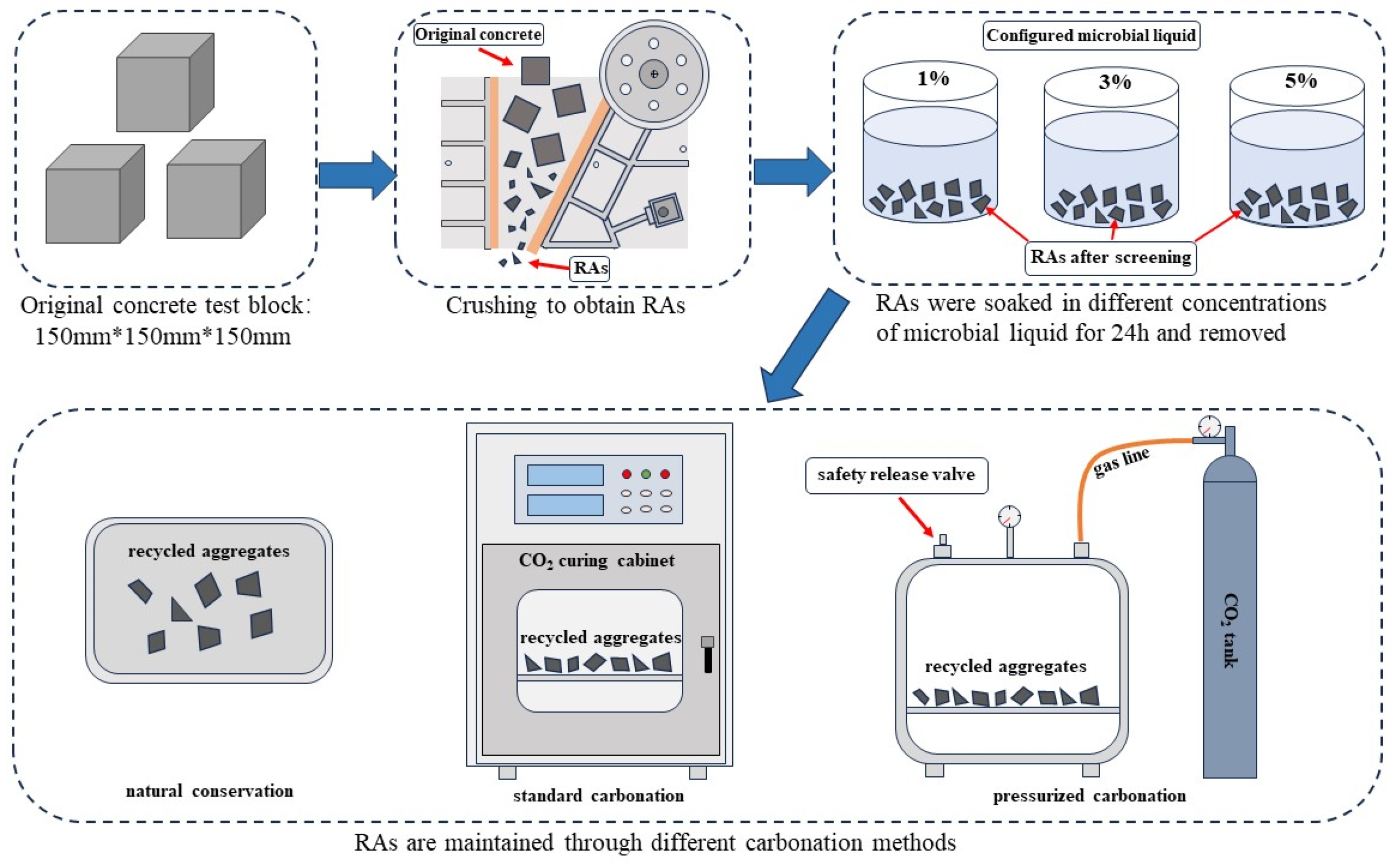
2.2.1. Carbonation of Recycled Aggregate
2.2.2. Microbial Mineralization
2.2.3. Optimization of Calcium Sources
2.2.4. Macro Performance Test
2.2.5. Optical Microscope Analysis
2.2.6. Microanalysis
3. Results and Discussion
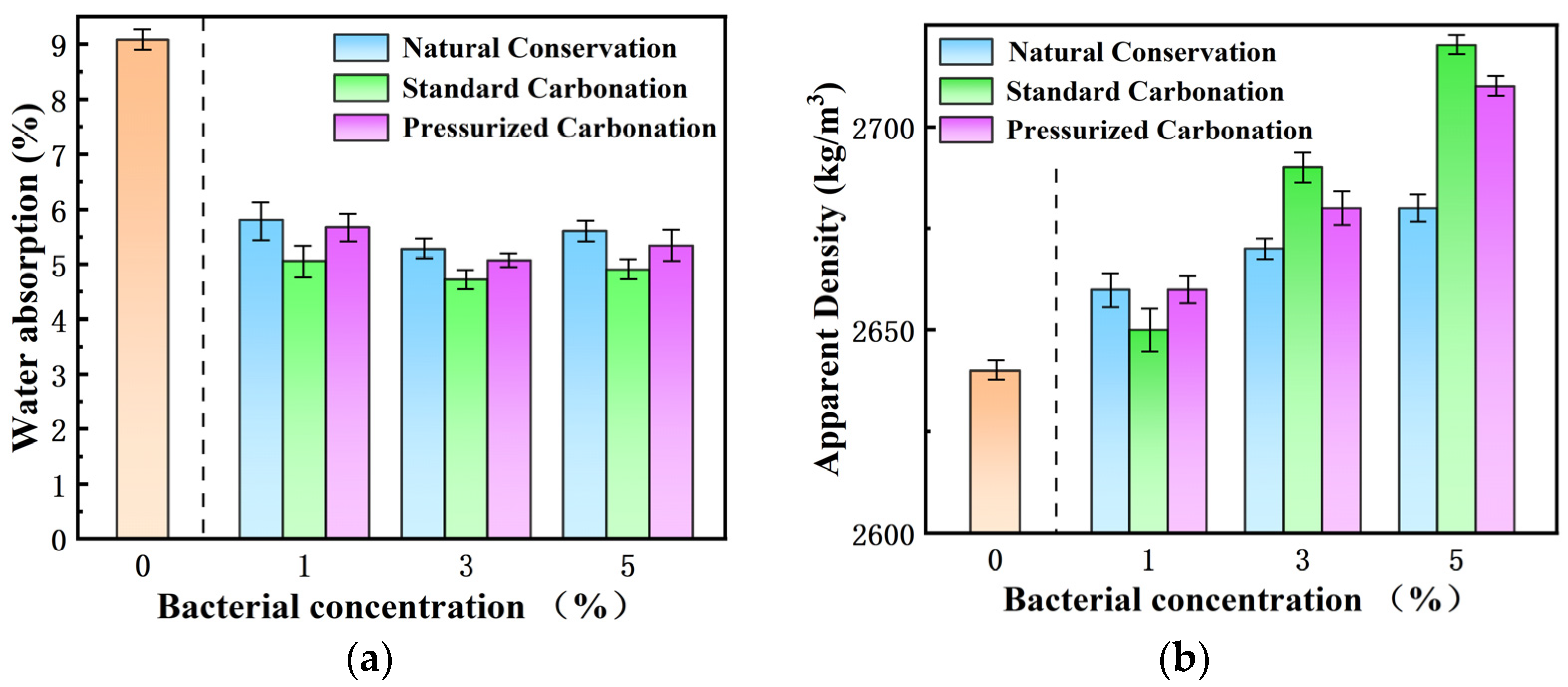
3.1. Effect of Mineralization Conditions on Macroscopic Properties
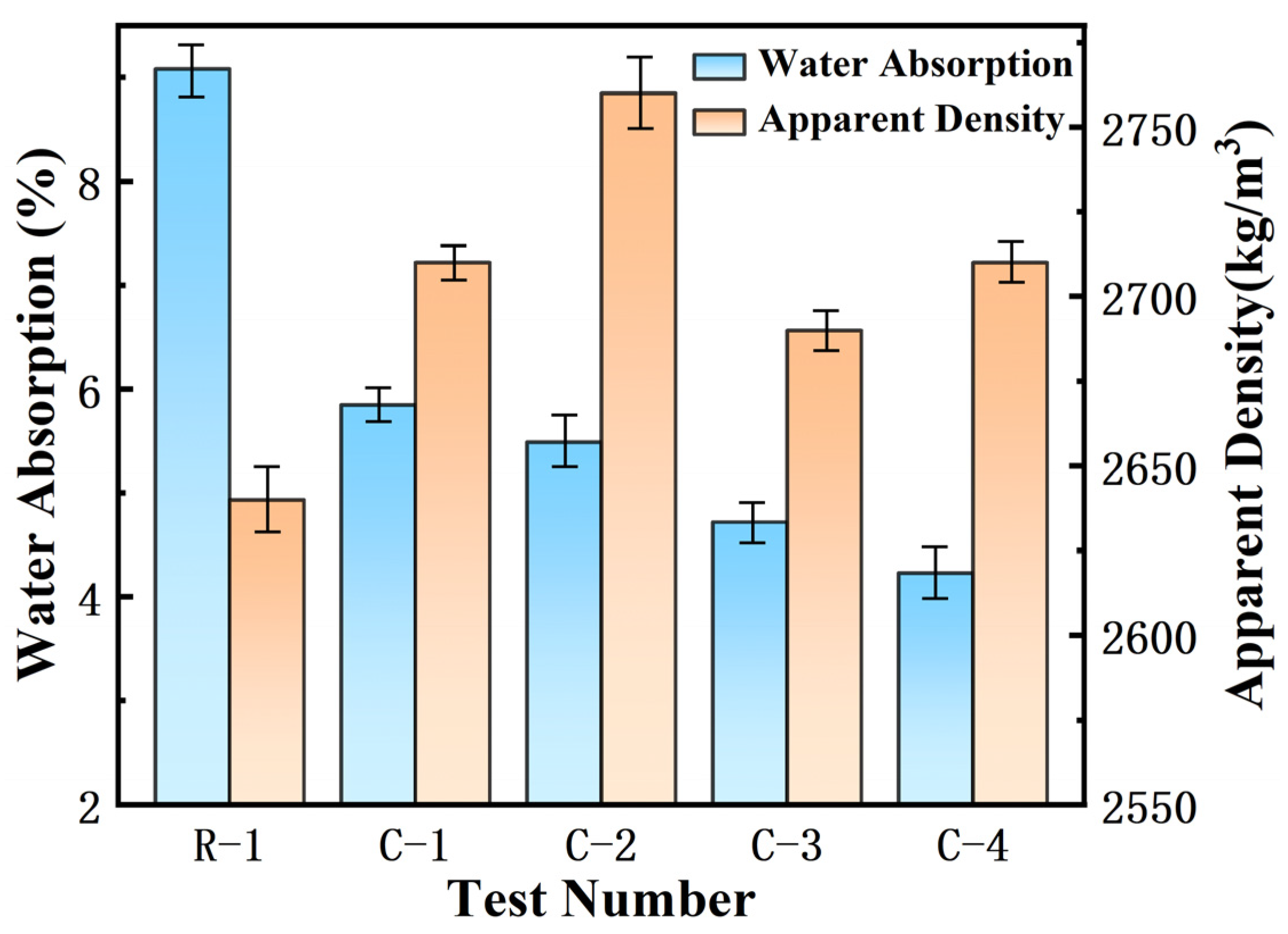
3.2. Effects of Calcium Ion Supplementation on Macroscopic Performance
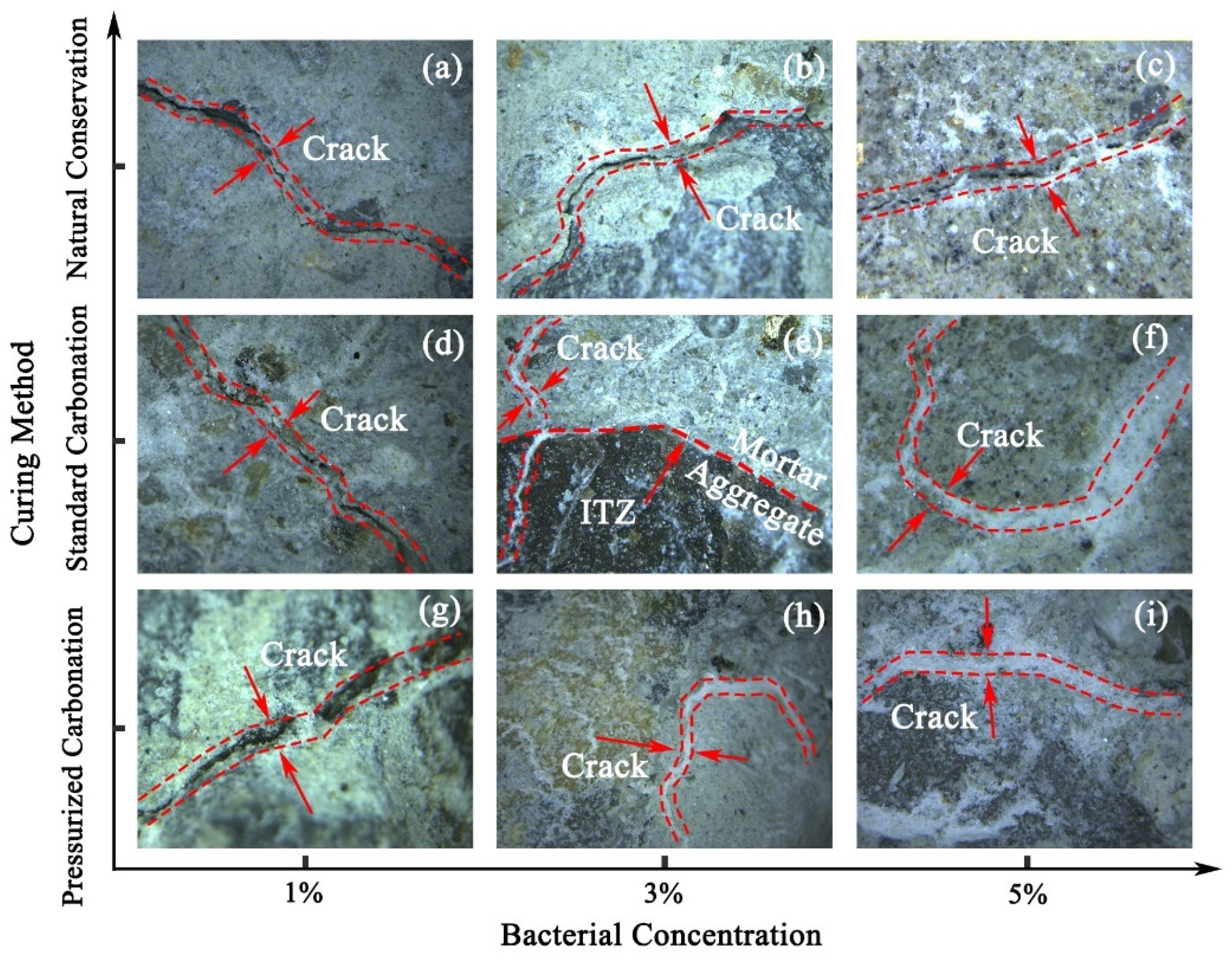
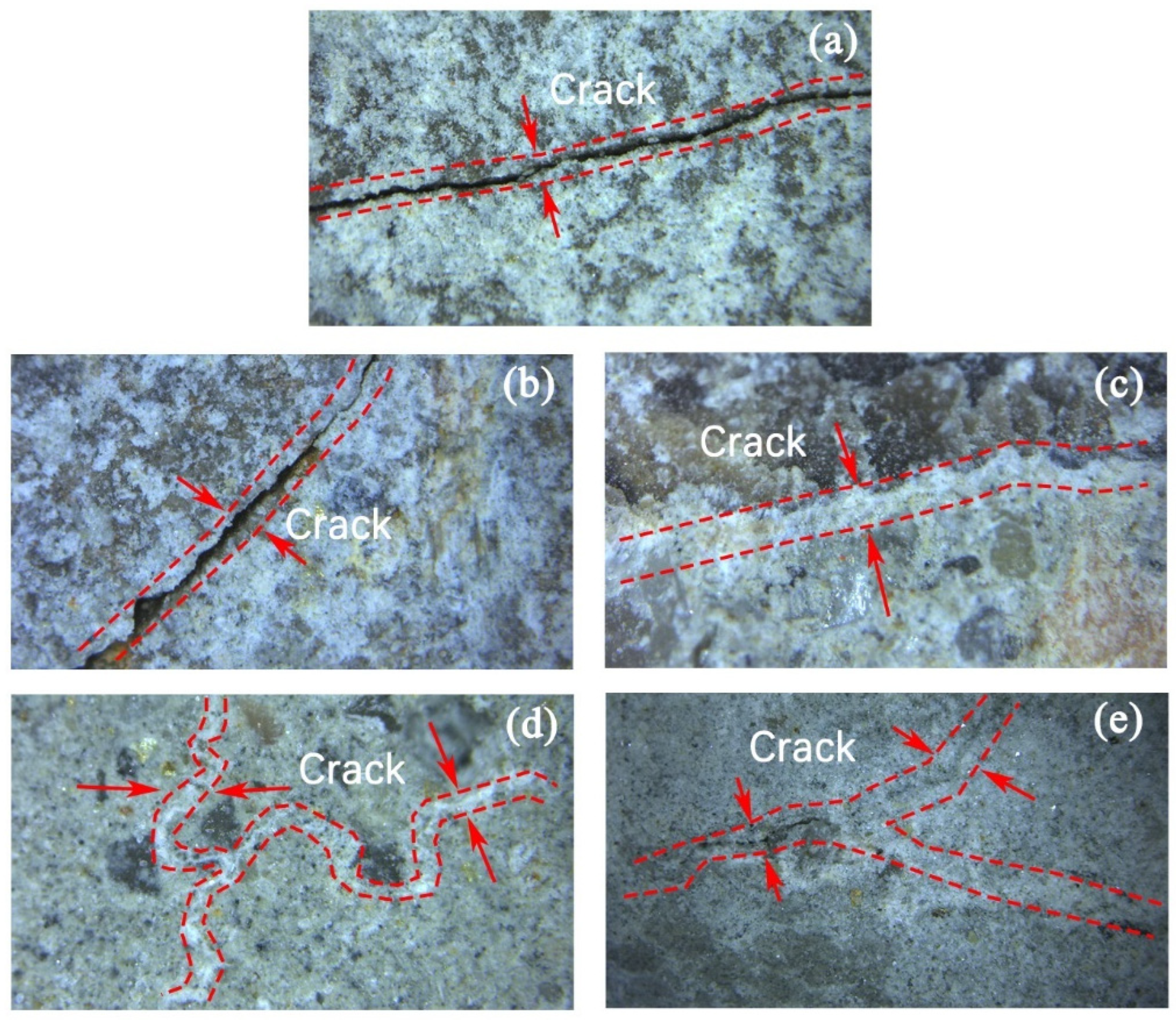
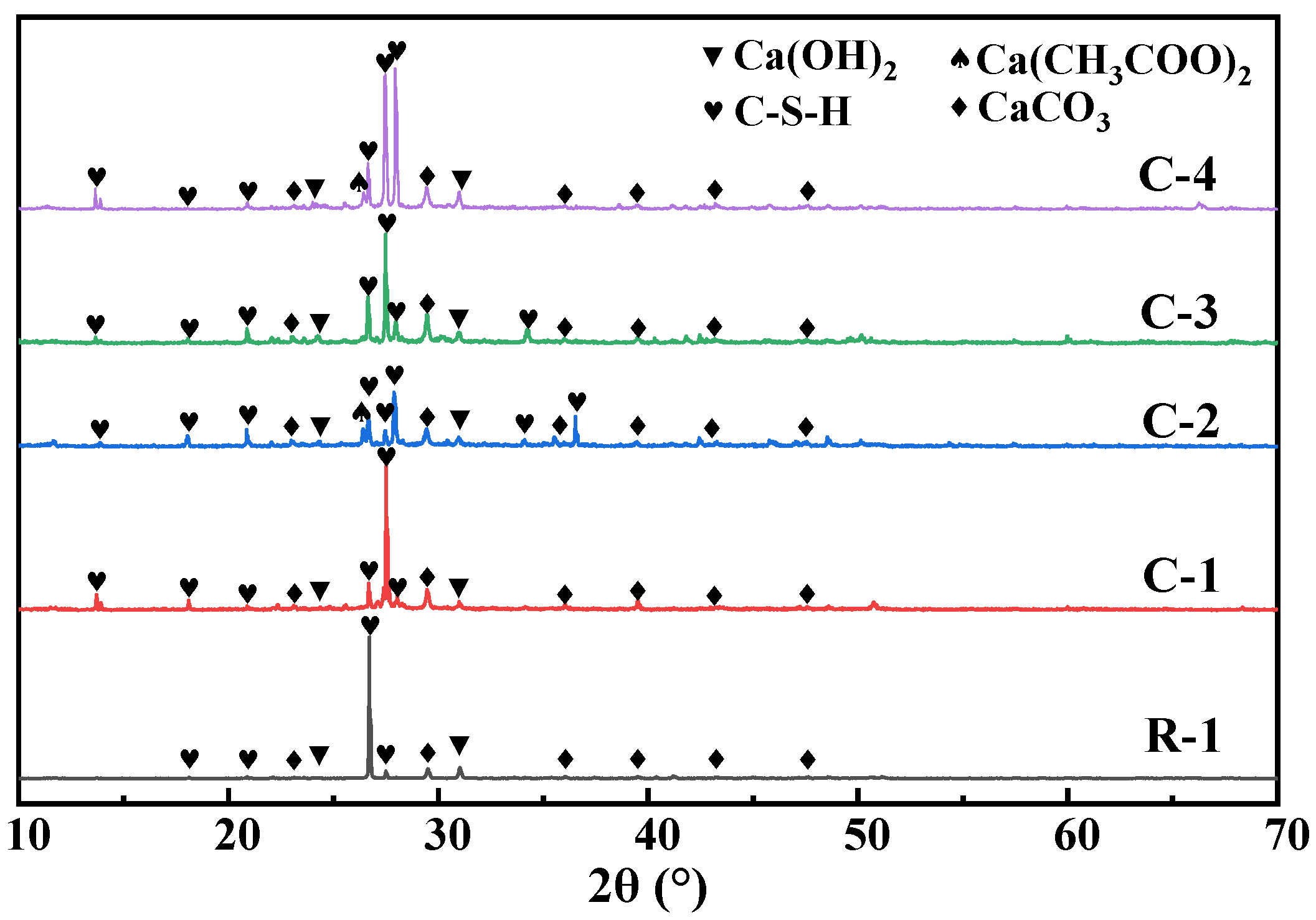
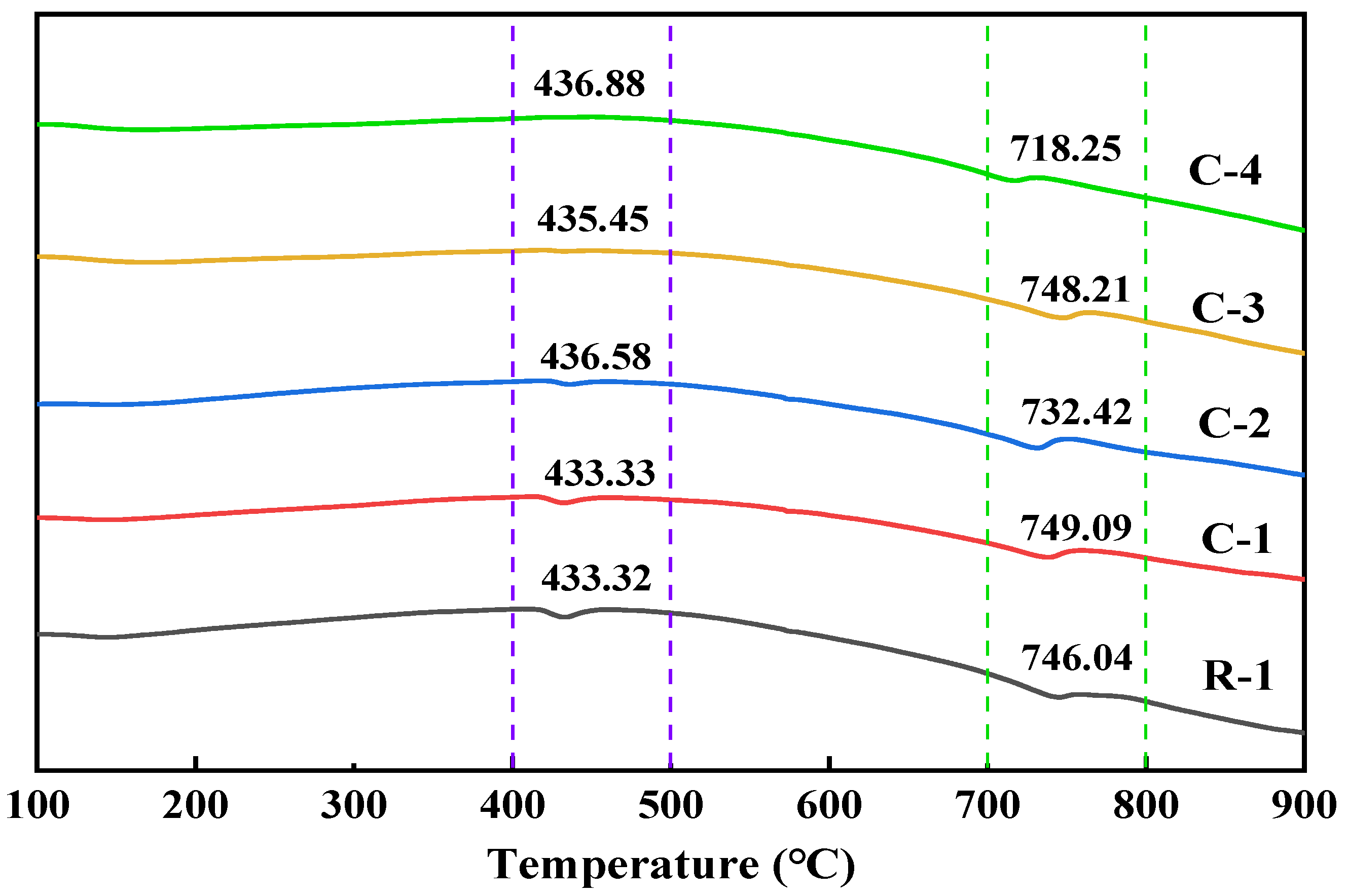
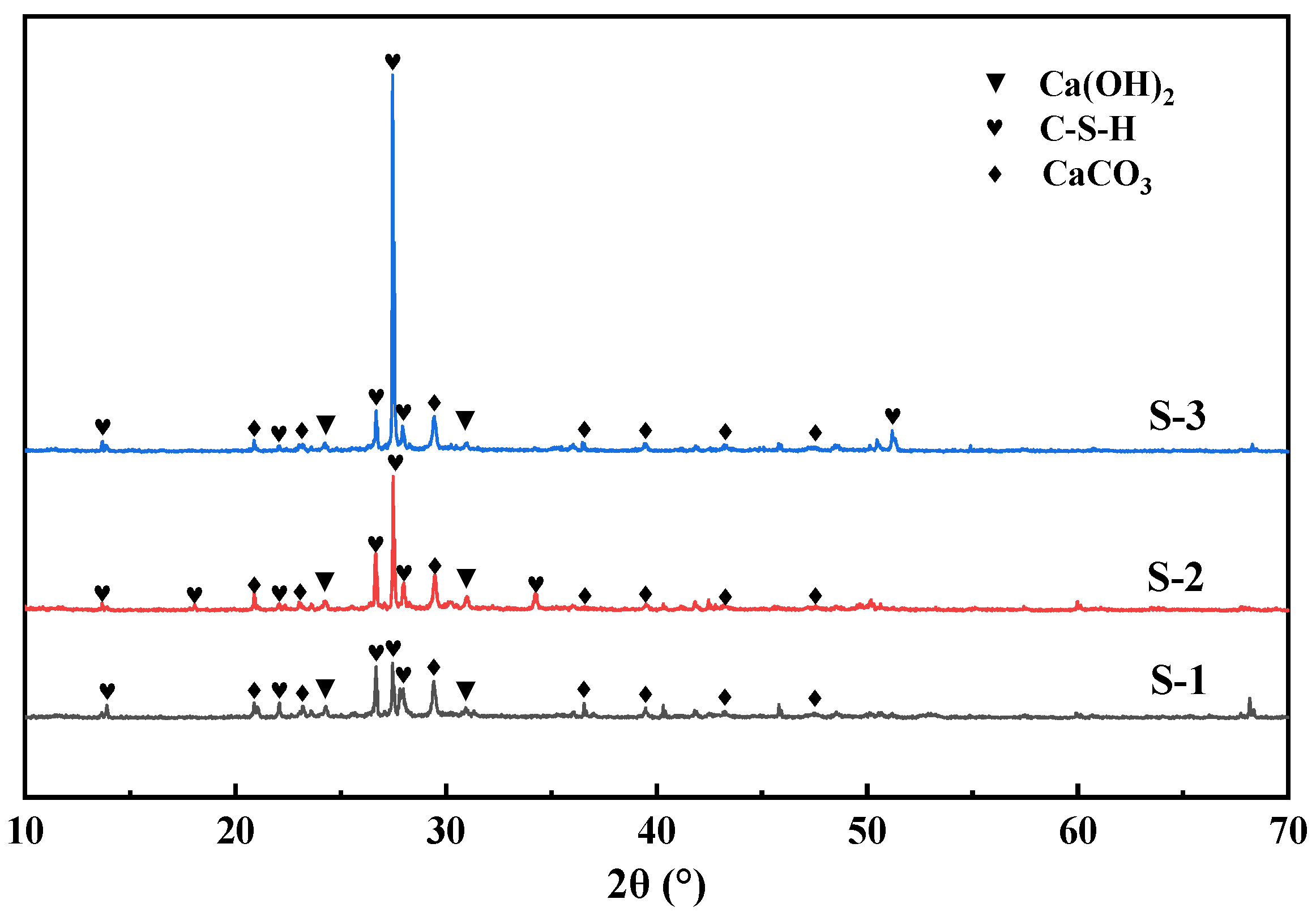
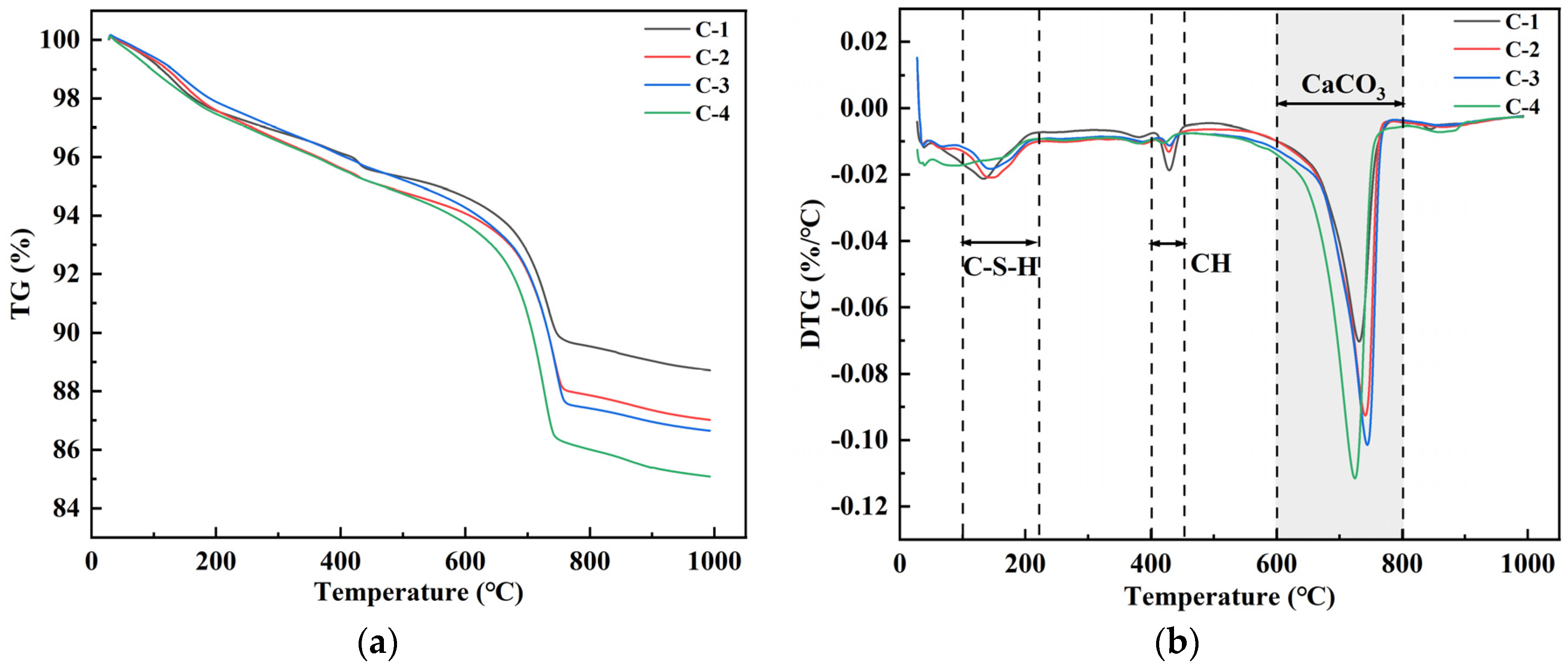
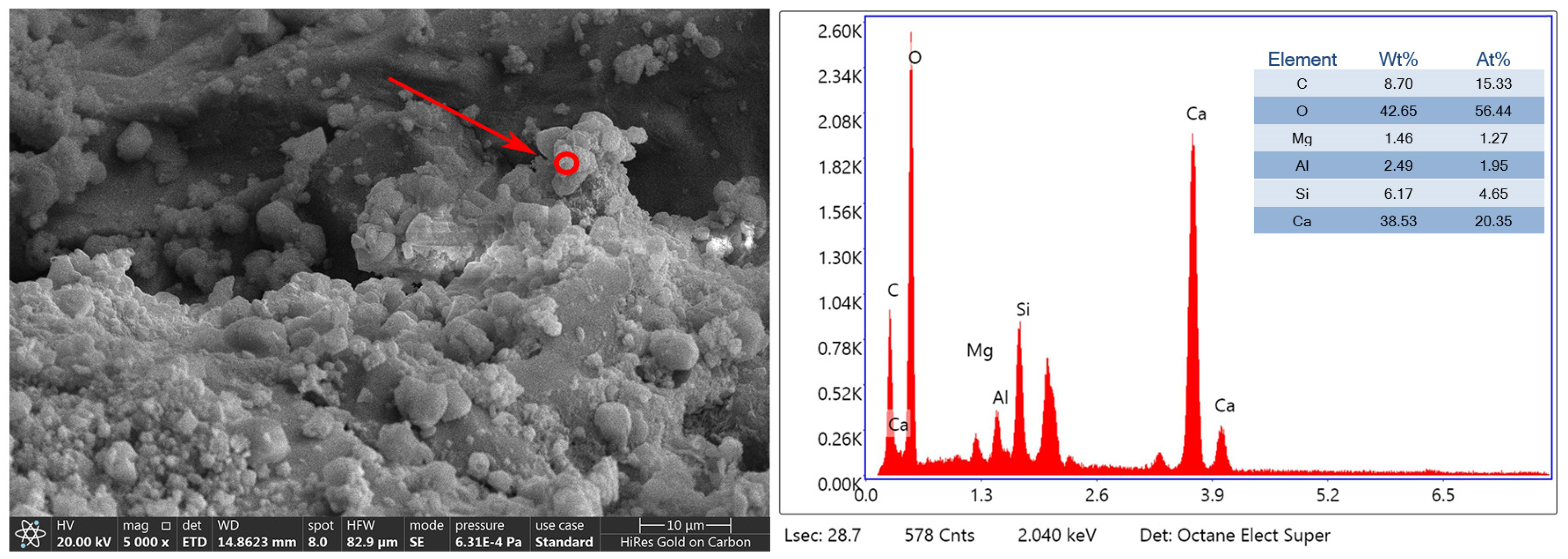
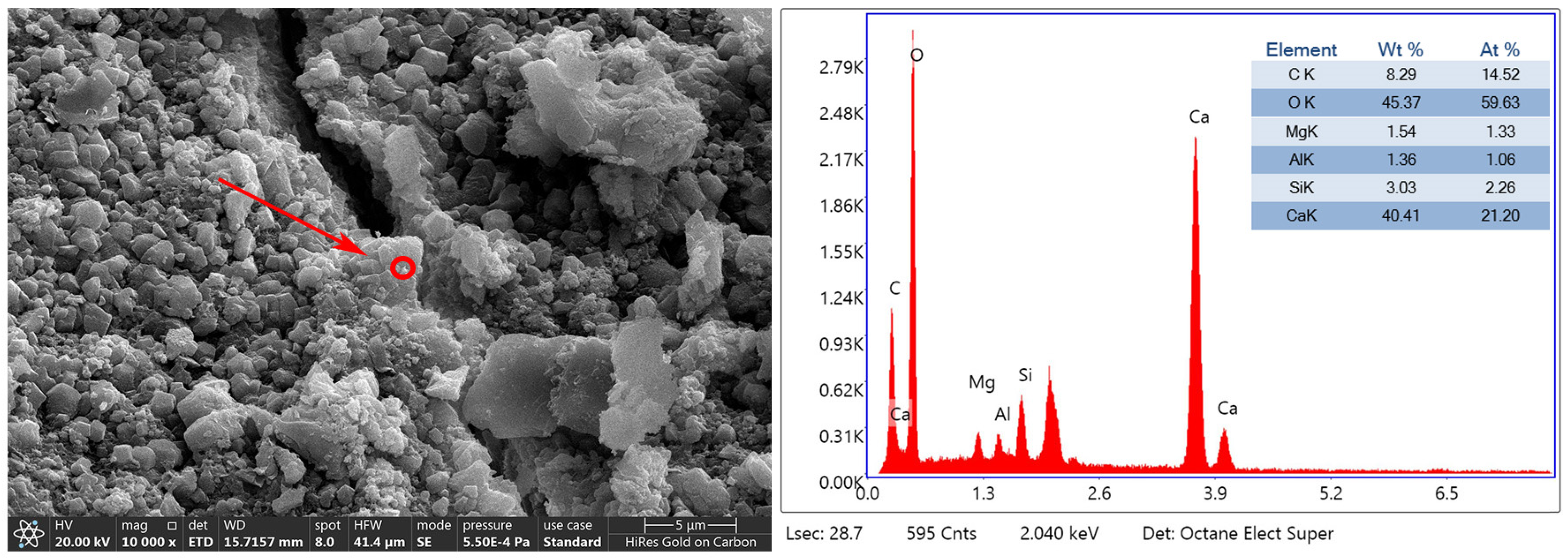
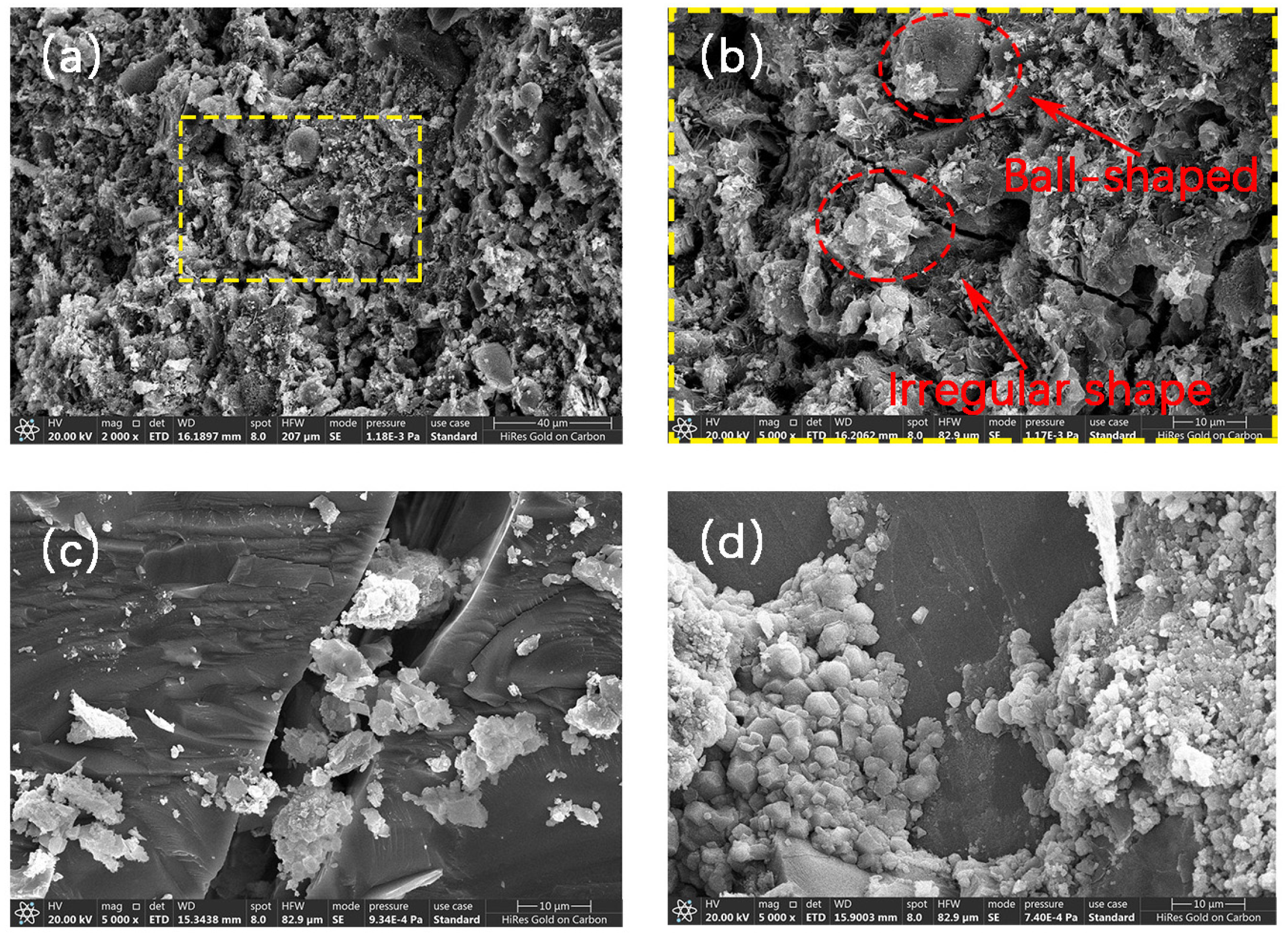
3.3. Mineralization Products on the Surface of RAs
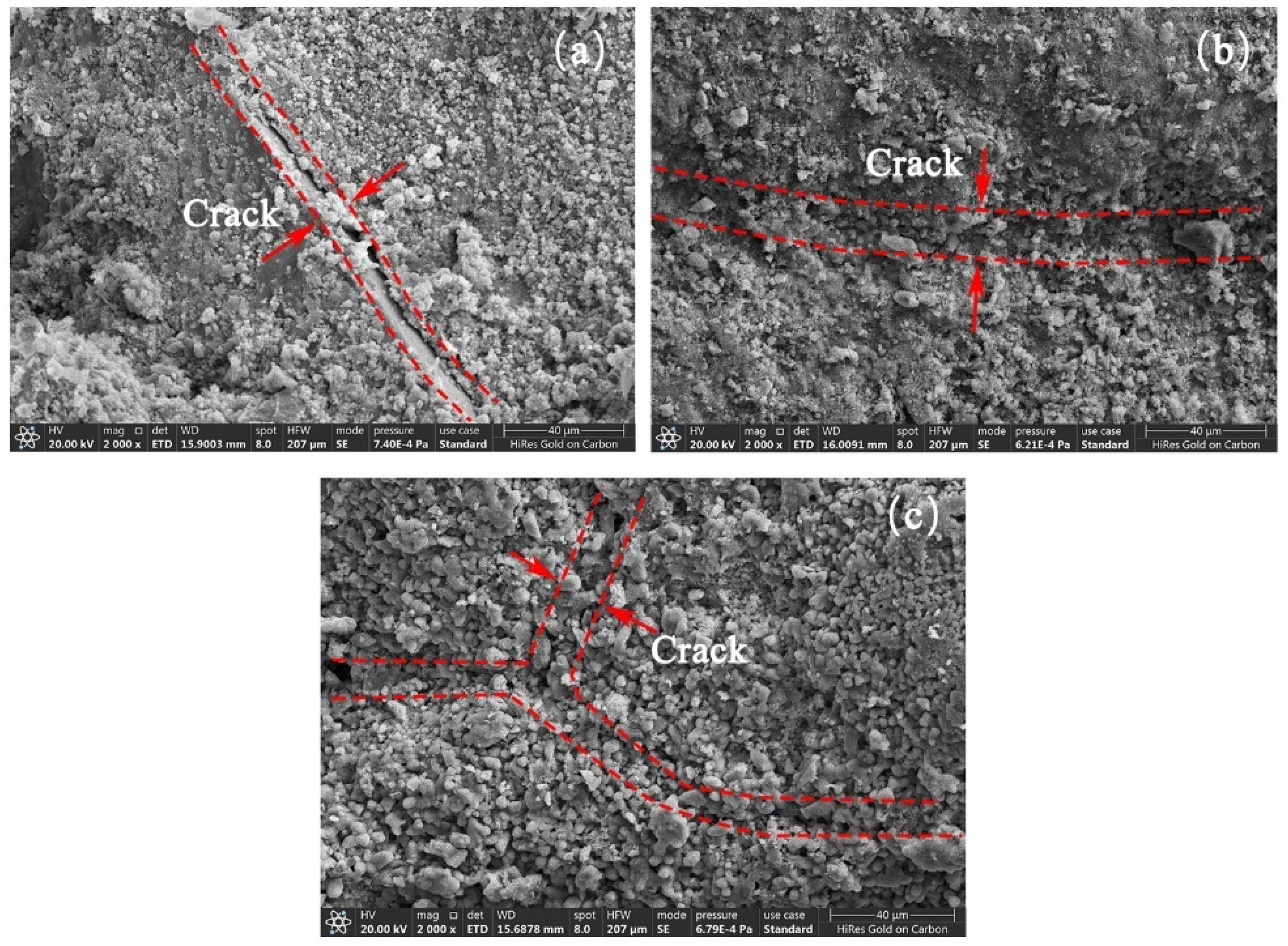
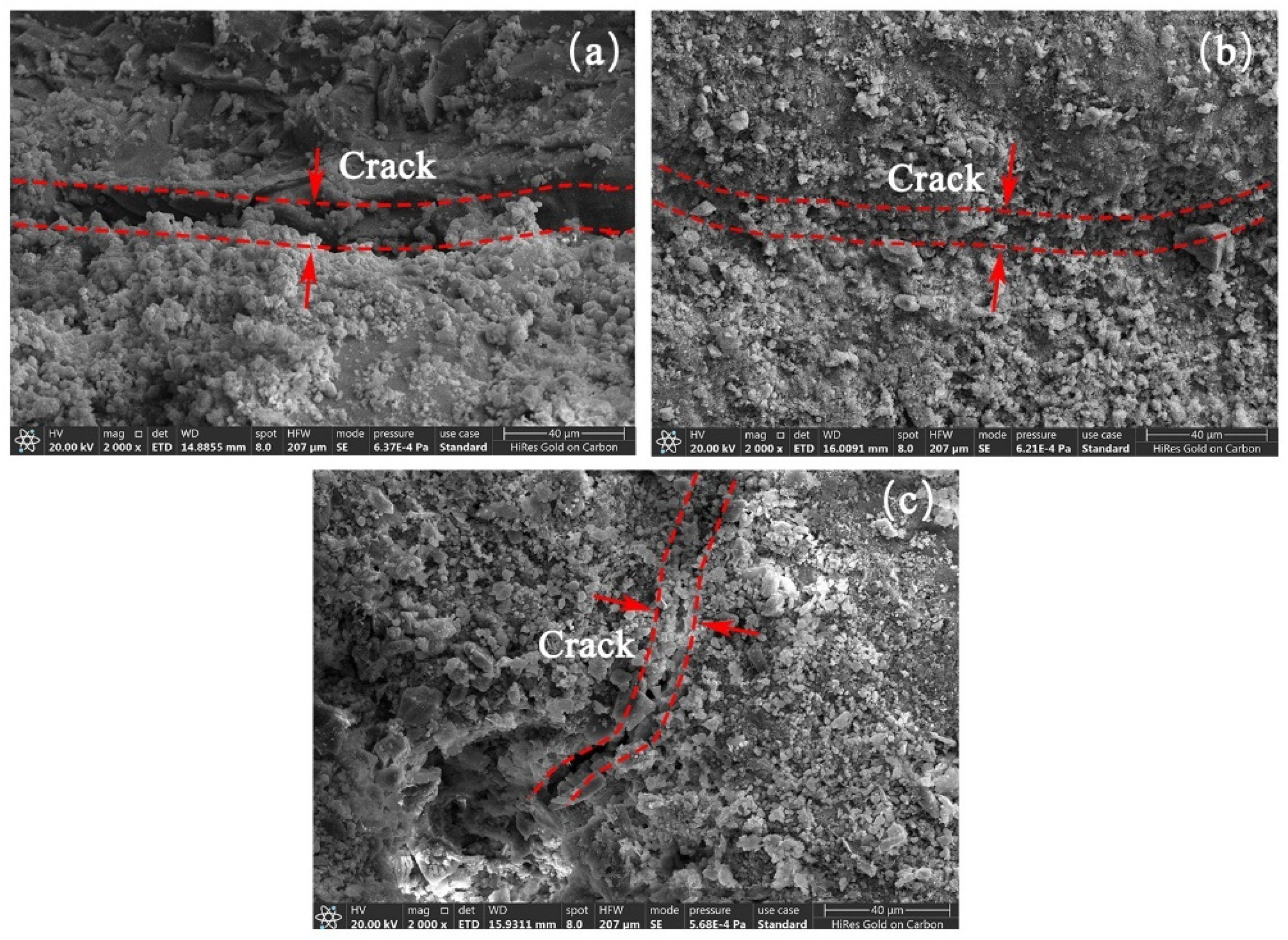
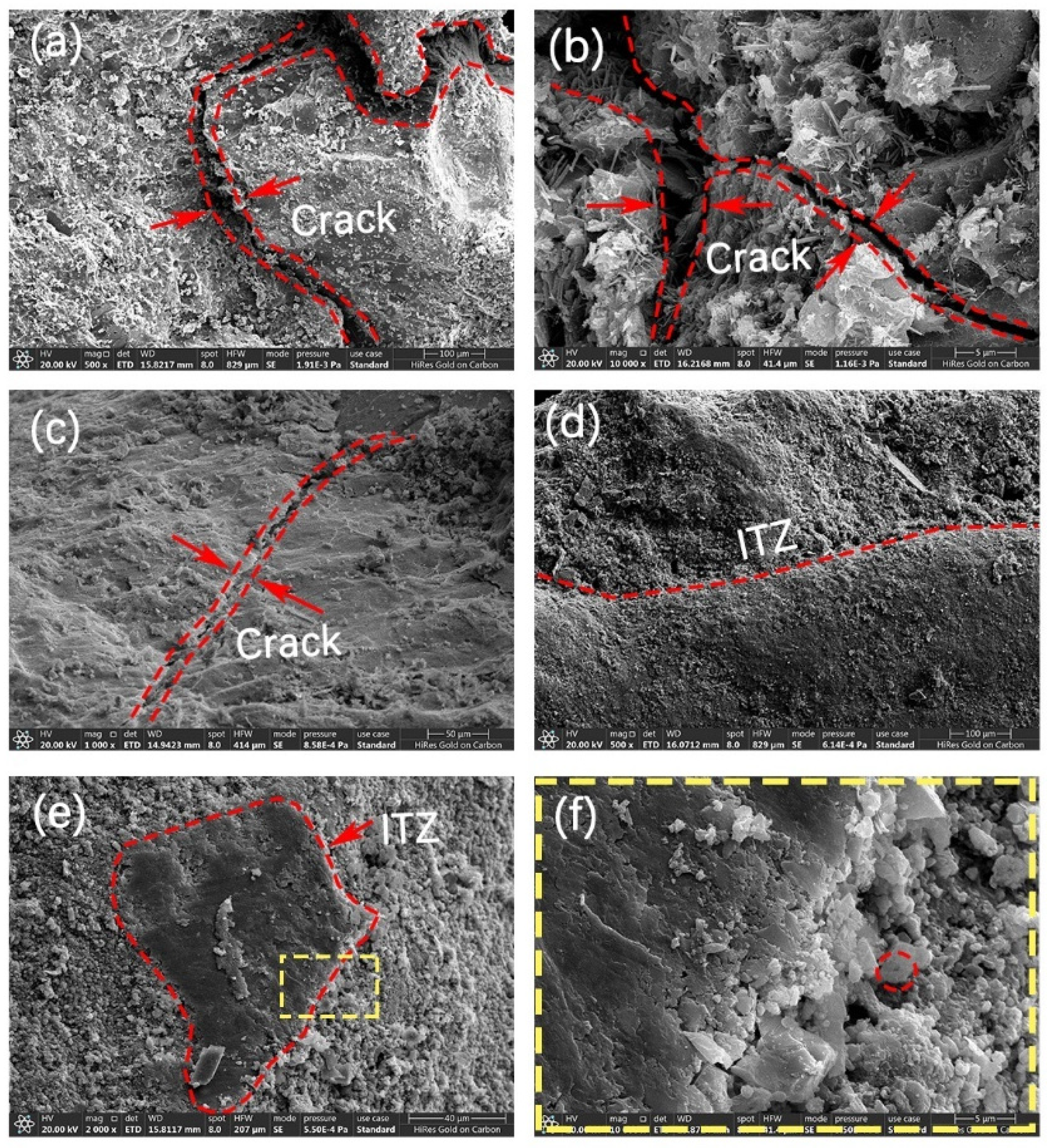
3.4. Micro-Structure of RAs
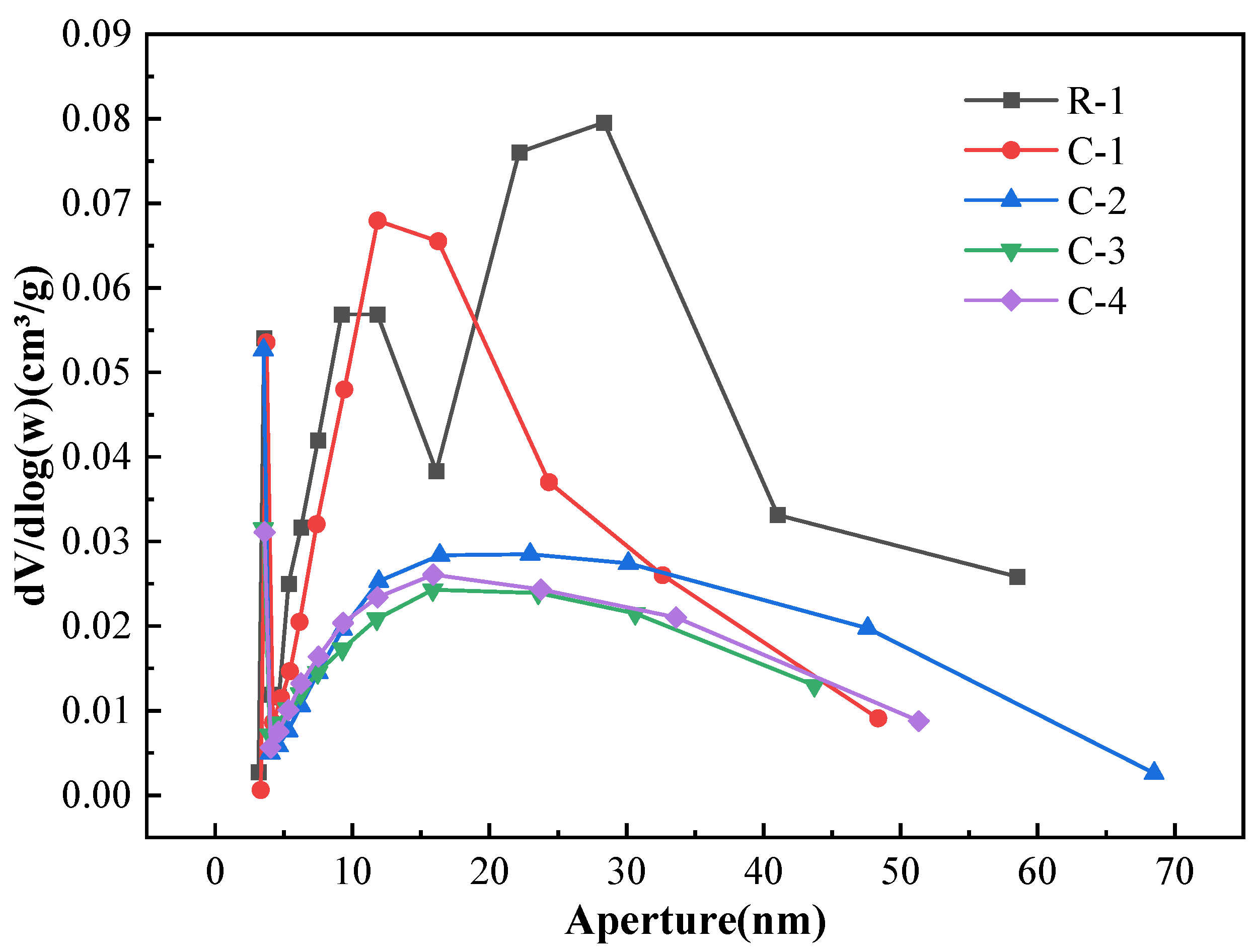
3.5. Pore Structure of RAs
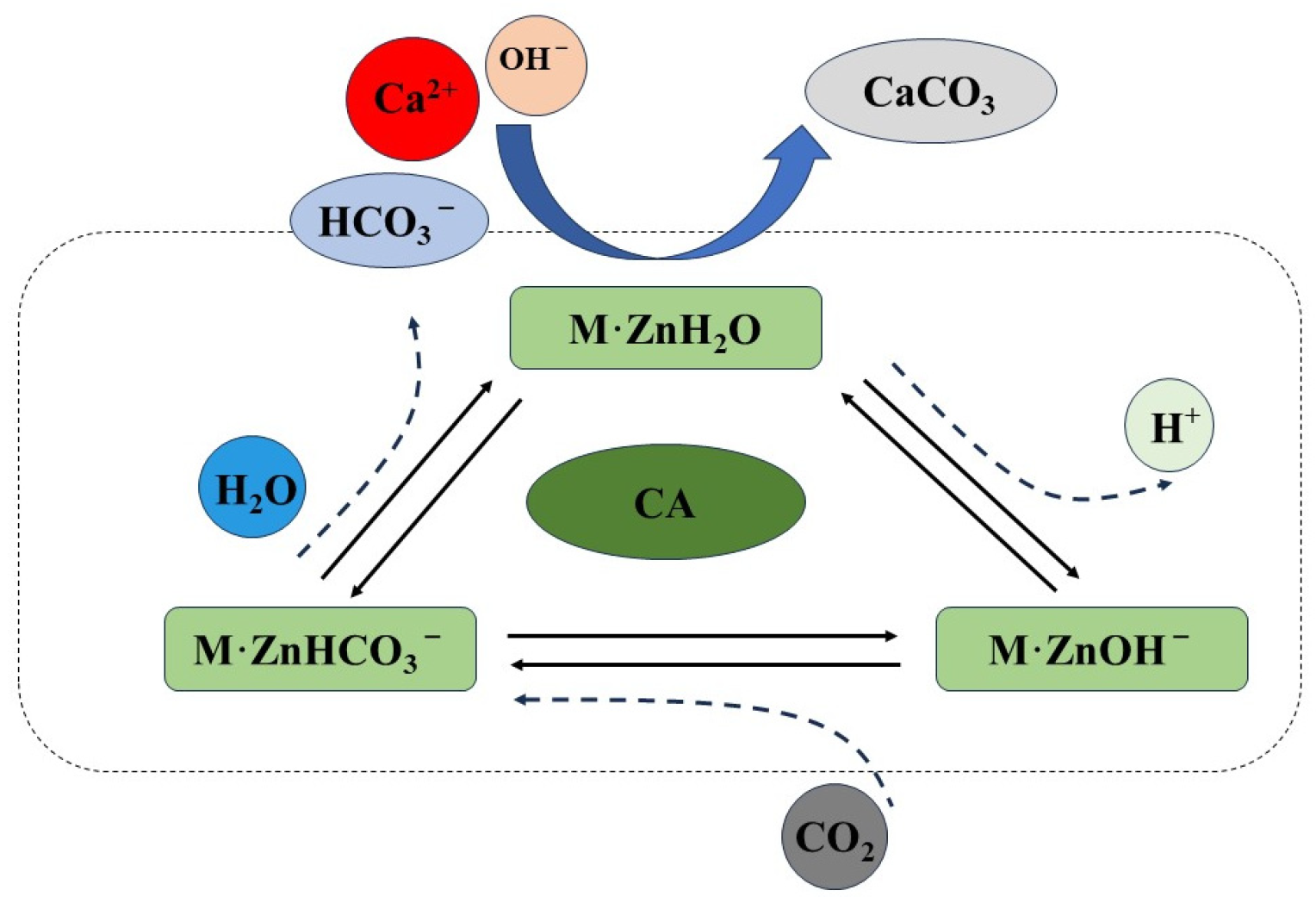
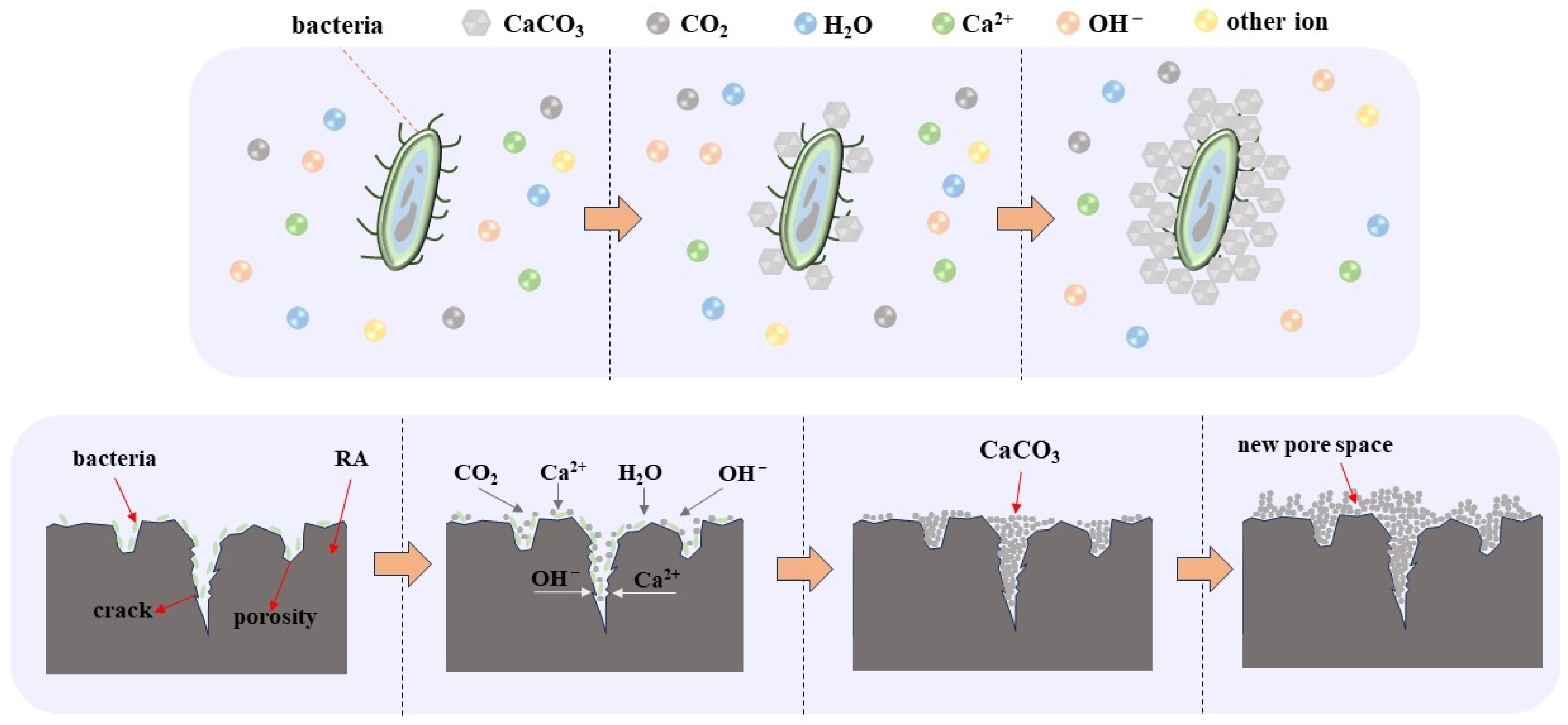
3.6. Modification Mechanism of Carbonic Anhydrase Producing Microorganisms
3.7. Economic Analysis
4. Conclusions
- (1)
- The water absorption of RAs modified with 3% microbial concentration and then carbonated by 20% CO2 was minimized. The water absorption of RAs showed a fluctuating trend, first decreasing and then increasing with bacterial concentration. According to the micro-structure analysis based on optical microscopy and SEM, excess bacteria may produce excess crystals on the aggregate surface, which then form more capillaries due to heterophase nucleation of the bacteria. Too much nucleation sites produced by microbial mineralization can lead to a rougher and more porous RA surface, which again increases the water absorption of the aggregate.
- (2)
- According to the mineralization mechanism of microorganisms, supplementation of calcium ions can promote the mineralization reaction. RAs mineralized by microorganisms at 3% concentration reduced water absorption to 4.72% and increased apparent density by 1.89%. The addition of 0.1 mol/L calcium acetate decreased the water absorption to 4.23% and increased the apparent density by 2.65%. After supplementing the calcium source, the water absorption decreased to 10.4% and the apparent density increased by 0.74%.
- (3)
- The mineralization products on the RA surface were found to be identical by XRD and DSC analyses, implying that the microbial-assisted carbonation modification was stable. The analysis concluded that the microbial mineralization products were mainly CaCO3 crystals, which did not adversely affect the recycled aggregate surface mortar.
- (4)
- SEM and BET analyses yielded that the cracks on the surface of RAs were effectively repaired after microbial mineralization. The larger pores on the aggregate surface were effectively filled by CaCO3 as observed by SEM. From the distribution analysis of nanopores, the large micropores or gap were filled and the micropores was further improved. The whole surface structure of RAs tends to be smooth after microbial mineralization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berredjem, L.; Arabi, N.; Molez, L. Mechanical and durability properties of concrete based on recycled coarse and fine aggregates produced from demolished concrete. Constr. Build. Mater. 2020, 246, 118421. [Google Scholar] [CrossRef]
- Miller, S.A.; Moore, F.C. Climate and health damages from global concrete production. Nat. Clim. Chang. 2020, 10, 439–443. [Google Scholar] [CrossRef]
- Shah, I.H.; Miller, S.A.; Jiang, D.; Myers, R.J. Cement substitution with secondary materials can reduce annual global CO2 emissions by up to 1.3 gigatons. Nat. Commun. 2022, 13, 5758. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Klama, J.; Zawal, D.; Krupa, D. Modification of recycled concrete aggregate by calcium carbonate biodeposition. Constr. Build. Mater. 2012, 34, 145–150. [Google Scholar] [CrossRef]
- Saeedi Javadi, A.; Badiee, H.; Sabermahani, M. Mechanical properties and durability of bio-blocks with recycled concrete aggregates. Constr. Build. Mater. 2018, 165, 859–865. [Google Scholar] [CrossRef]
- Branch, J.L.; Epps, R.; Kosson, D.S. The impact of carbonation on bulk and ITZ porosity in microconcrete materials with fly ash replacement. Cem. Concr. Res. 2018, 103, 170–178. [Google Scholar] [CrossRef]
- Hosseinnezhad, H.; Sürmelioğlu, S.; Çakır, Ö.A.; Ramyar, K. A novel method for characterization of recycled concrete aggregates: Computerized microtomography. J. Build. Eng. 2023, 76, 107321. [Google Scholar] [CrossRef]
- Katkhuda, H.; Shatarat, N. Improving the mechanical properties of recycled concrete aggregate using chopped basalt fibers and acid treatment. Constr. Build. Mater. 2017, 140, 328–335. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Butera, A.; Le, K.N. Utilising CO2 technologies for recycled aggregate concrete: A critical review. Constr. Build. Mater. 2020, 250, 118903. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, B.; Kim, K.-S.; Kim, J.-M. Quality improvement of recycled aggregates using the acid treatment method and the strength characteristics of the resulting mortar. J. Mater. Cycles Waste Manag. 2017, 19, 968–976. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Tam, C.M.; Le, K.N. Removal of cement mortar remains from recycled aggregate using pre-soaking approaches. Resour. Conserv. Recycl. 2007, 50, 82–101. [Google Scholar] [CrossRef]
- Dimitriou, G.; Savva, P.; Petrou, M. Enhancing mechanical and durability properties of recycled aggregate concrete. Constr. Build. Mater. 2018, 158, 228–235. [Google Scholar] [CrossRef]
- Al-Bayati, H.K.A.; Tighe, S.L.; Al-Bayati, H.K.A. Utilizing a different technique for improving micro and macro characteristics of coarse recycled concrete aggregate. In Proceedings of the 2016 Transportation Association of Canada’s (TAC) Conference & Exhibition, Toronto, ON, Canada, 25–28 September 2016. [Google Scholar]
- Kathy, B.; Solène, T.; Florent, B. Assessment of a microwave-assisted recycling process for the recovery of high-quality aggregates from concrete waste. Int. J. Miner. Process. 2014, 126, 90–98. [Google Scholar] [CrossRef]
- Raman, J.V.M.; Ramasamy, V. Various treatment techniques involved to enhance the recycled coarse aggregate in concrete: A review. Mater. Today Proc. 2021, 45, 6356–6363. [Google Scholar] [CrossRef]
- Teune, I.E.; Schollbach, K.; Florea, M.V.A.; Brouwers, H.J.H. Carbonation of hydrated cement: The impact of carbonation conditions on CO2 sequestration, phase formation, and reactivity. J. Build. Eng. 2023, 79, 107785. [Google Scholar] [CrossRef]
- Zajac, M.; Maruyama, I.; Iizuka, A.; Skibsted, J. Enforced carbonation of cementitious materials. Cem. Concr. Res. 2023, 174, 107285. [Google Scholar] [CrossRef]
- Kou, S.-C.; Zhan, B.-J.; Poon, C.-S. Use of a CO2 curing step to improve the properties of concrete prepared with recycled aggregates. Cem. Concr. Compos. 2014, 45, 22–28. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Fernández-Ledesma, E.; González-Caro, Á. Carbon Emission Evaluation of CO2 Curing in Vibro-Compacted Precast Concrete Made with Recycled Aggregates. Materials 2023, 16, 2436. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Assessment of mechanical properties of concrete incorporating carbonated recycled concrete aggregates. Cem. Concr. Compos. 2016, 65, 67–74. [Google Scholar] [CrossRef]
- Li, L.; Poon, C.S.; Xiao, J.; Xuan, D. Effect of carbonated recycled coarse aggregate on the dynamic compressive behavior of recycled aggregate concrete. Constr. Build. Mater. 2017, 151, 52–62. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Cao, Z.; Guo, M.; Zheng, J. Effect of carbonated coarse recycled concrete aggregate on the properties and microstructure of recycled concrete. J. Clean. Prod. 2019, 233, 421–428. [Google Scholar] [CrossRef]
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Macias Franco, M.R.; Narayanasamy, R.; Balagurusamy, N. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 126. [Google Scholar] [CrossRef]
- Soysal, A.; Milla, J.; King, G. Evaluating the Self-Healing Efficiency of Hydrogel-Encapsulated Bacteria in Concrete. Transp. Res. Rec. 2020, 2674, 113–123. [Google Scholar] [CrossRef]
- Mistri, A.; Dhami, N.; Bhattacharyya, S.K. Performance of biocement treatment in improving the interfacial properties of recycled aggregate concrete. Constr. Build. Mater. 2023, 369, 130509. [Google Scholar] [CrossRef]
- Fouladi, A.S.; Arulrajah, A.; Chu, J.; Horpibulsuk, S. Application of Microbially Induced Calcite Precipitation (MICP) technology in construction materials: A comprehensive review of waste stream contributions. Constr. Build. Mater. 2023, 388, 131546. [Google Scholar] [CrossRef]
- Prajapati, N.K.; Agnihotri, A.K.; Basak, N. Microbial induced calcite precipitation (MICP) a sustainable technique for stabilization of soil: A review. Mater. Today Proc. 2023, 93, 357–361. [Google Scholar] [CrossRef]
- Wang, J.; Vandevyvere, B.; Vanhessche, S. Microbial carbonate precipitation for the improvement of quality of recycled aggregates. J. Clean. Prod. 2017, 156, 355–366. [Google Scholar] [CrossRef]
- Anbu, P.; Kang, C.-H.; Shin, Y.-J.; So, J.-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. Springer Plus 2016, 5, 250. [Google Scholar] [CrossRef]
- Hill, D.D.; Sleep, B.E. Effects of biofilm growth on flow and transport through a glass parallel plate fracture. J. Contam. Hydrol. 2002, 56, 227–246. [Google Scholar] [CrossRef]
- Tripp, B.C.; Smith, K.; Ferry, J.G. Carbonic Anhydrase: New Insights for an Ancient Enzyme. J. Biol. Chem. 2001, 276, 48615–48618. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, J.; Li, Z.; Zhao, L. Study on the effect of calcium ions on the properties of microbial mineralization-modified recycled aggregates. New Build. Mater. 2019, 46, 84–87. [Google Scholar]
- Khushnood, R.A.; Qureshi, Z.A.; Shaheen, N. Bio-mineralized self-healing recycled aggregate concrete for sustainable infrastructure. Sci. Total Environ. 2019, 13, 703–710. [Google Scholar] [CrossRef] [PubMed]
- GB/T 14685-2011; Pebbles and Gravel for Construction. Standardization Administration of the People’s Republic of China: Beijing, China, 2011.
- GB/T 25177-2010; Recycled Coarse Aggregate for Concrete. Standardization Administration of the People’s Republic of China: Beijing, China, 2010.
- Merve, S.; Huseyin, I.; Burak, D. The effect of chemical-versus microbial-induced calcium carbonate mineralization on the enhancement of fine recycled concrete aggregate: A comparative study. J. Build. Eng. 2021, 44, 103316. [Google Scholar] [CrossRef]
- Xie, D. Research on Recycled Coarse Aggregate Reinforced by Microbial Carbon Sequestration. Master’s Thesis, Southeast University, Nanjing, China, 2021. [Google Scholar] [CrossRef]
- Rui, Y.; Qian, C. The influence of bacteria on biologically induced calcium carbonate and its evolution process. J. Cryst. Growth 2022, 581, 126515. [Google Scholar] [CrossRef]
- Bolton, A.J.; Maltman, A.J.; Fisher, Q. Anisotropic permeability and bimodal pore-size distributions of fine-grained marine sediments. Mar. Pet. Geol. 2000, 17, 657–672. [Google Scholar] [CrossRef]
- Li, Z.; Shen, X.; Qi, Z.; Hu, R. Comparations between mercury intrusion and gas adsorption for pore structure characteristics of shale. J. Eng. Geol. 2017, 25, 1405–1413. [Google Scholar] [CrossRef]



| Components | C3S | C2S | C3A | C4AF | f-CaO | Other |
|---|---|---|---|---|---|---|
| Content (%) | 54.8 | 22.86 | 8.03 | 9.53 | 0.91 | 3.87 |
| Water-Binder Ratio | Sand Ratio | Water Consumption (kg/m3) | Cement (kg/m3) | Sand (kg/m3) | Stone (kg/m3) |
|---|---|---|---|---|---|
| 0.49 | 0.465 | 195 | 399 | 623 | 1183 |
| Particle Size | 26.5 | 19.0 | 16.0 | 9.5 | 4.75 | ≤4.75 |
|---|---|---|---|---|---|---|
| Content (%) | 5.8 | 18.7 | 11.3 | 30.5 | 19.0 | 14.5 |
| Carbonation Method | Pressure (KPa) | Humidity (%) | Temperature (℃) | CO2 Concentration (%) | Carbonation Duration (h) |
|---|---|---|---|---|---|
| Natural Conservation | 101 | 70 ± 5 | 25 ± 5 | 0.03 | 5 |
| Standard Carbonation | 101 | 70 ± 5 | 25 ± 5 | 20 | 5 |
| Pressurized carbonation | 300 | 70 ± 5 | 25 ± 5 | 99.9 | 5 |
| Test Number | Modification Methods | Conservation Methods |
|---|---|---|
| R-1 | — | — |
| N-1 | 1% bacterial liquid | Natural Conservation |
| N-2 | 3% bacterial liquid | |
| N-3 | 5% bacterial liquid | |
| S-1 | 1% bacterial liquid | Standard Carbonation |
| S-2 | 3% bacterial liquid | |
| S-3 | 5% bacterial liquid | |
| P-1 | 1% bacterial liquid | Pressurized Carbonation |
| P-2 | 3% bacterial liquid | |
| P-3 | 5% bacterial liquid |
| Test Number | Modification Methods | Carbonation Methods |
|---|---|---|
| R-1 | — | — |
| C-1 | — | Standard Carbonation |
| C-2 | 0.1 mol/L calcium acetate | Standard Carbonation |
| C-3 | 3% bacterial liquid | Standard Carbonation |
| C-4 | 3% bacterial liquid + 0.1 mol/L calcium acetate | Standard Carbonation |
| Aggregate Type | Aggregate Unit Price (RMB/ton) | Electricity Cost of Equipment (RMB/ton) | Bacteria Cost (RMB/ton) | Cost of Transportation and Labor (RMB/ton) | Total (RMB/ton) |
|---|---|---|---|---|---|
| NAs | 106 | — | — | — | 106 |
| RAs | −20 | 10.4 | 25 | 100 | 115.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Yi, H.; Su, Y. Study on Reducing Water Absorption of Recycled Aggregates (RAs) by Microbial Mineralization. Materials 2024, 17, 1612. https://doi.org/10.3390/ma17071612
Li M, Yi H, Su Y. Study on Reducing Water Absorption of Recycled Aggregates (RAs) by Microbial Mineralization. Materials. 2024; 17(7):1612. https://doi.org/10.3390/ma17071612
Chicago/Turabian StyleLi, Minglei, Haihe Yi, and Yilin Su. 2024. "Study on Reducing Water Absorption of Recycled Aggregates (RAs) by Microbial Mineralization" Materials 17, no. 7: 1612. https://doi.org/10.3390/ma17071612
APA StyleLi, M., Yi, H., & Su, Y. (2024). Study on Reducing Water Absorption of Recycled Aggregates (RAs) by Microbial Mineralization. Materials, 17(7), 1612. https://doi.org/10.3390/ma17071612






