Hydrogen Embrittlement of 27Cr−4Mo−2Ni Super Ferritic Stainless Steel
Abstract
1. Introduction
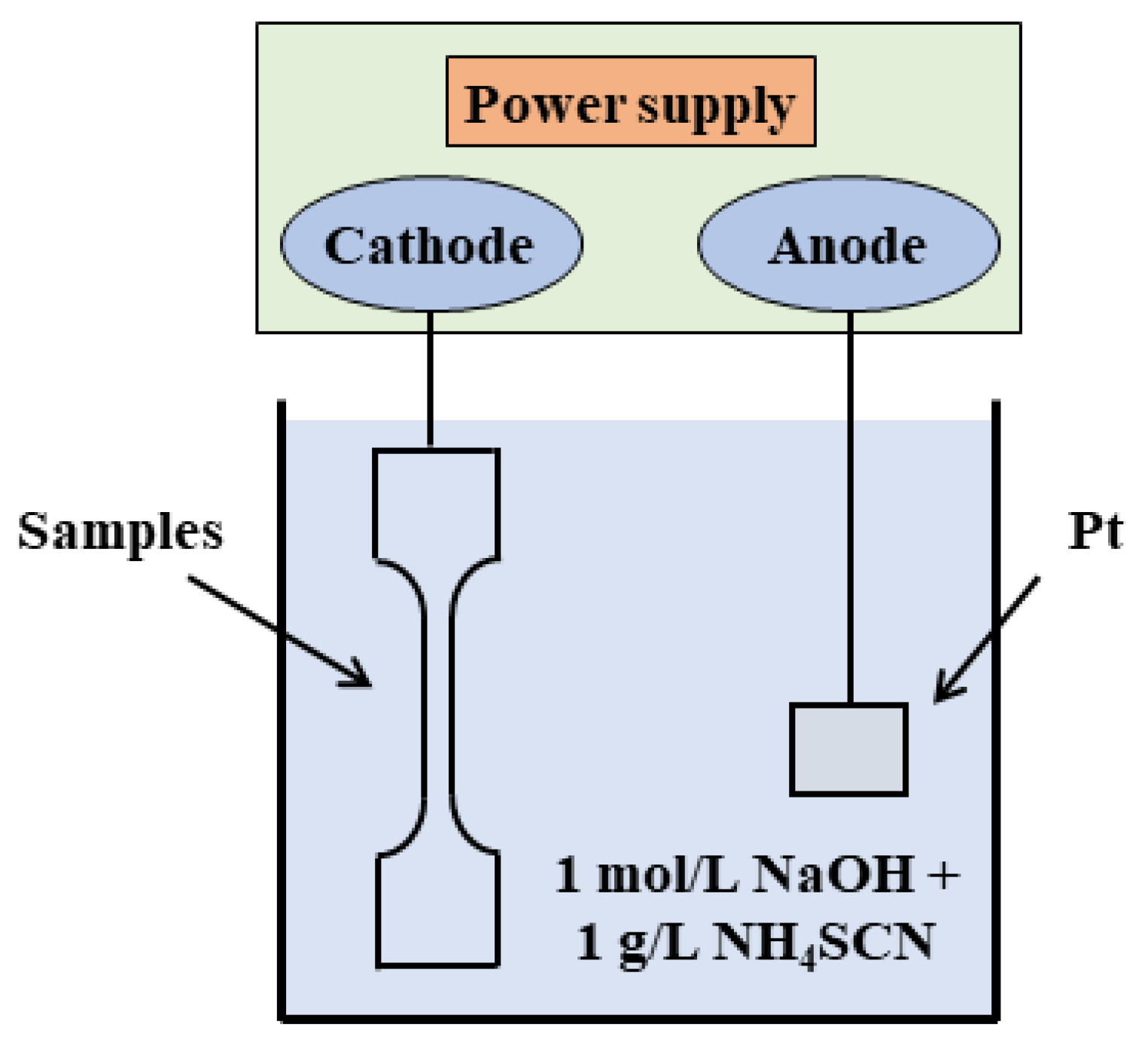
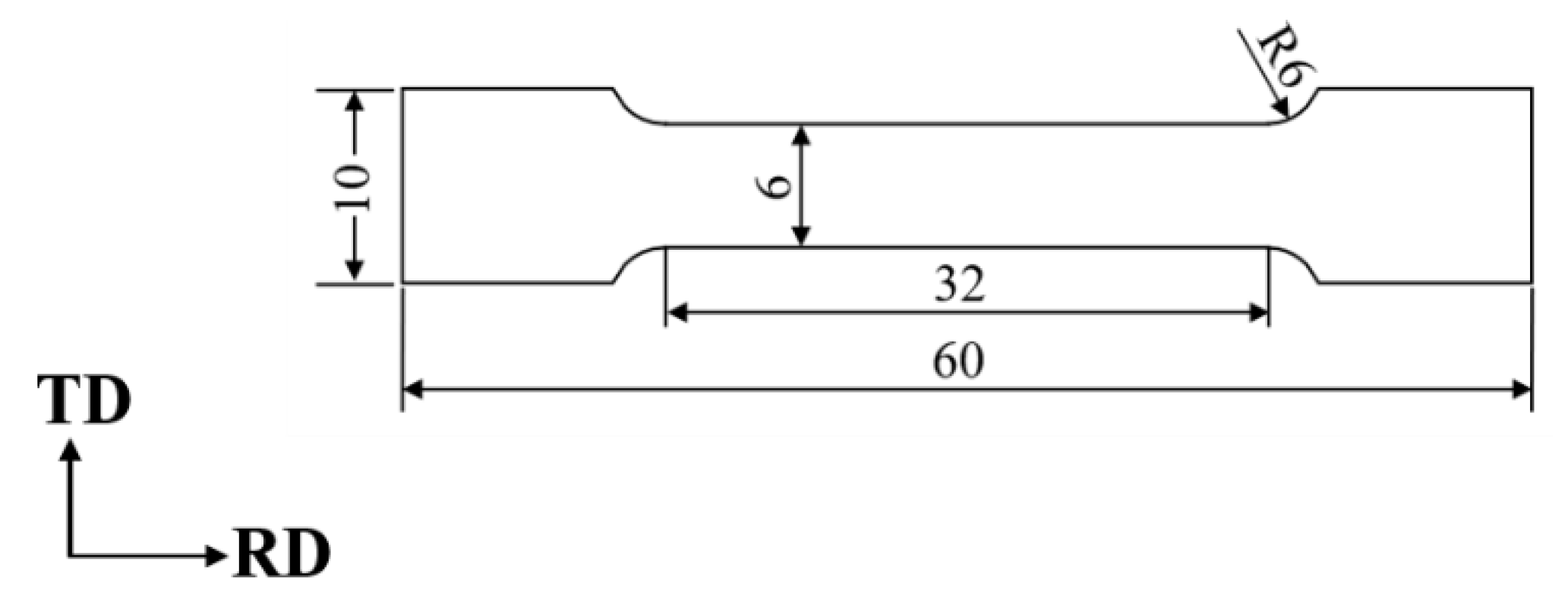
2. Experimental Procedure
3. Results
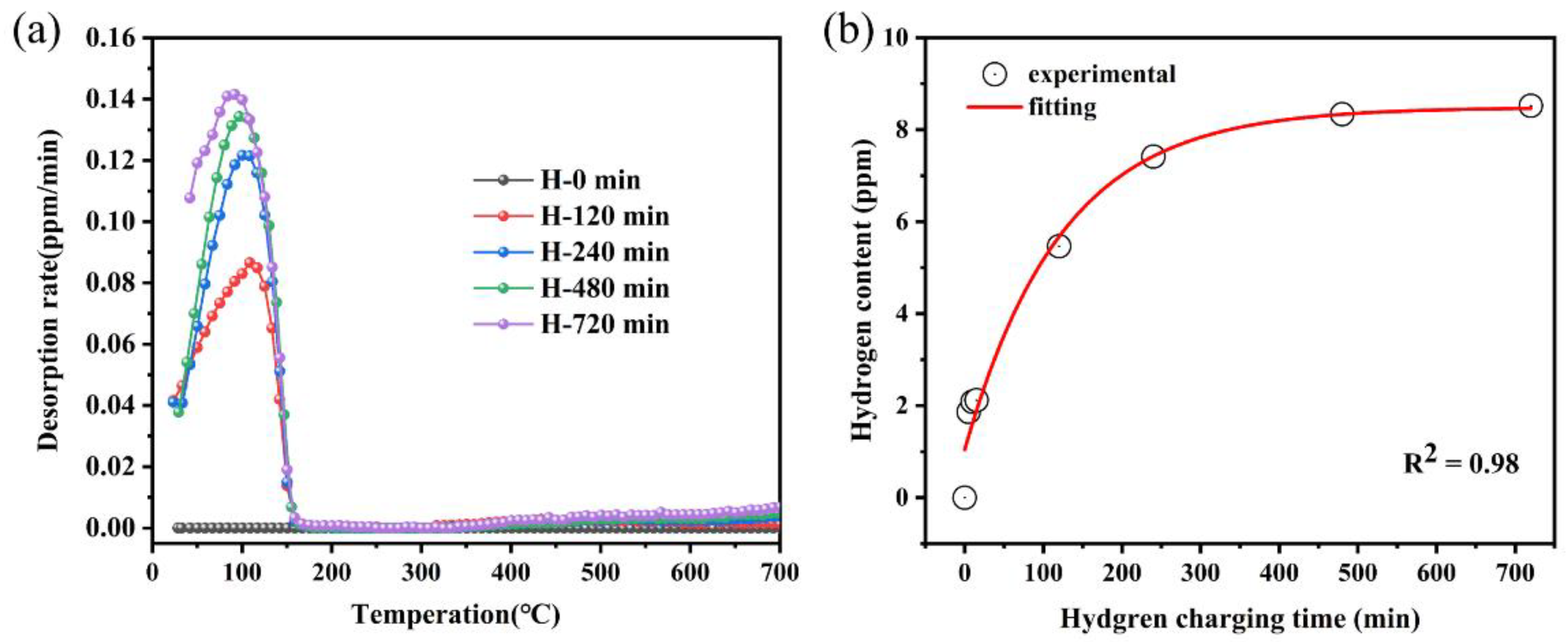
3.1. Hydrogen Content
3.2. Mechanical Properties
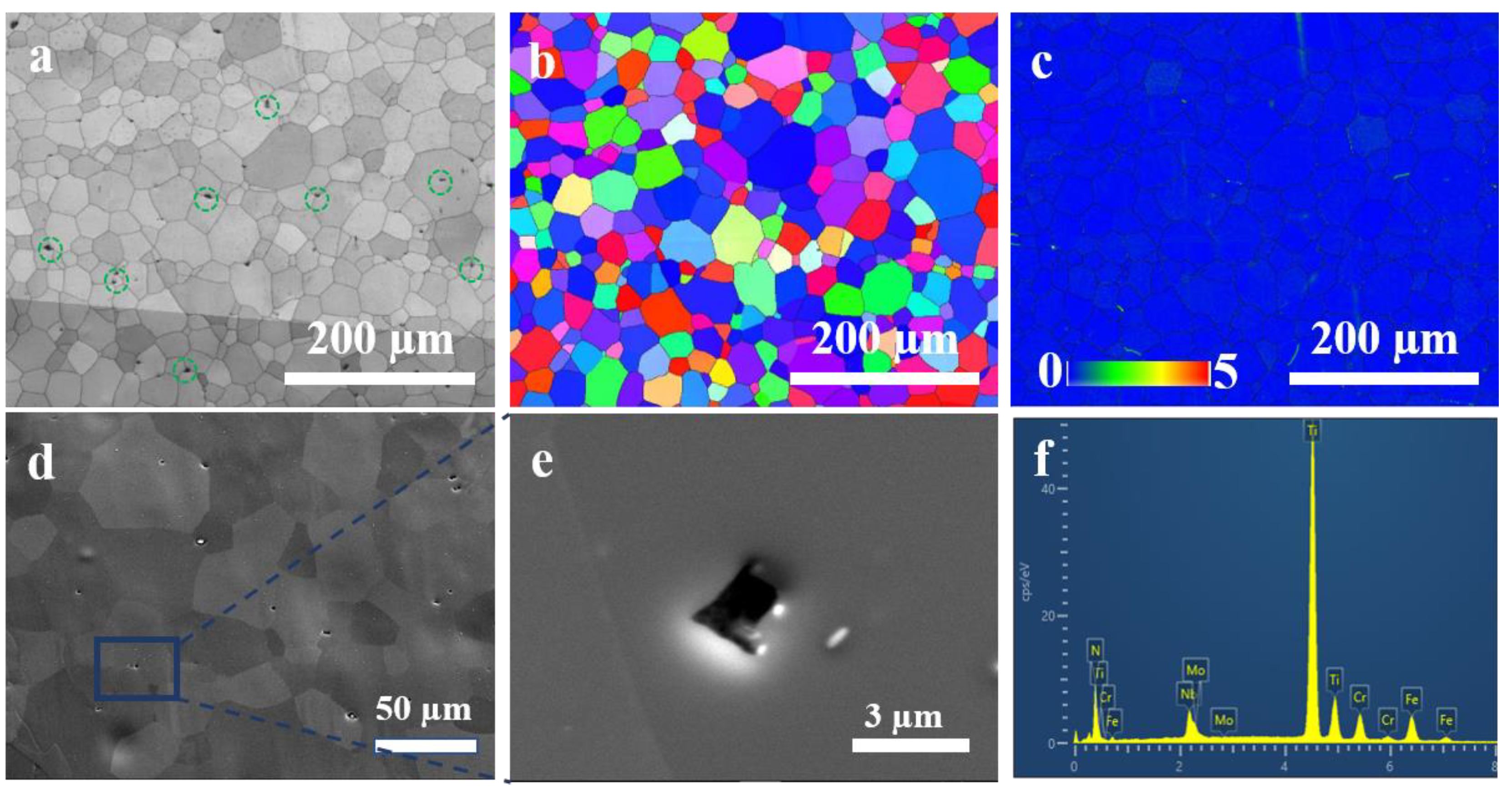
3.3. Microstructural Characteristics
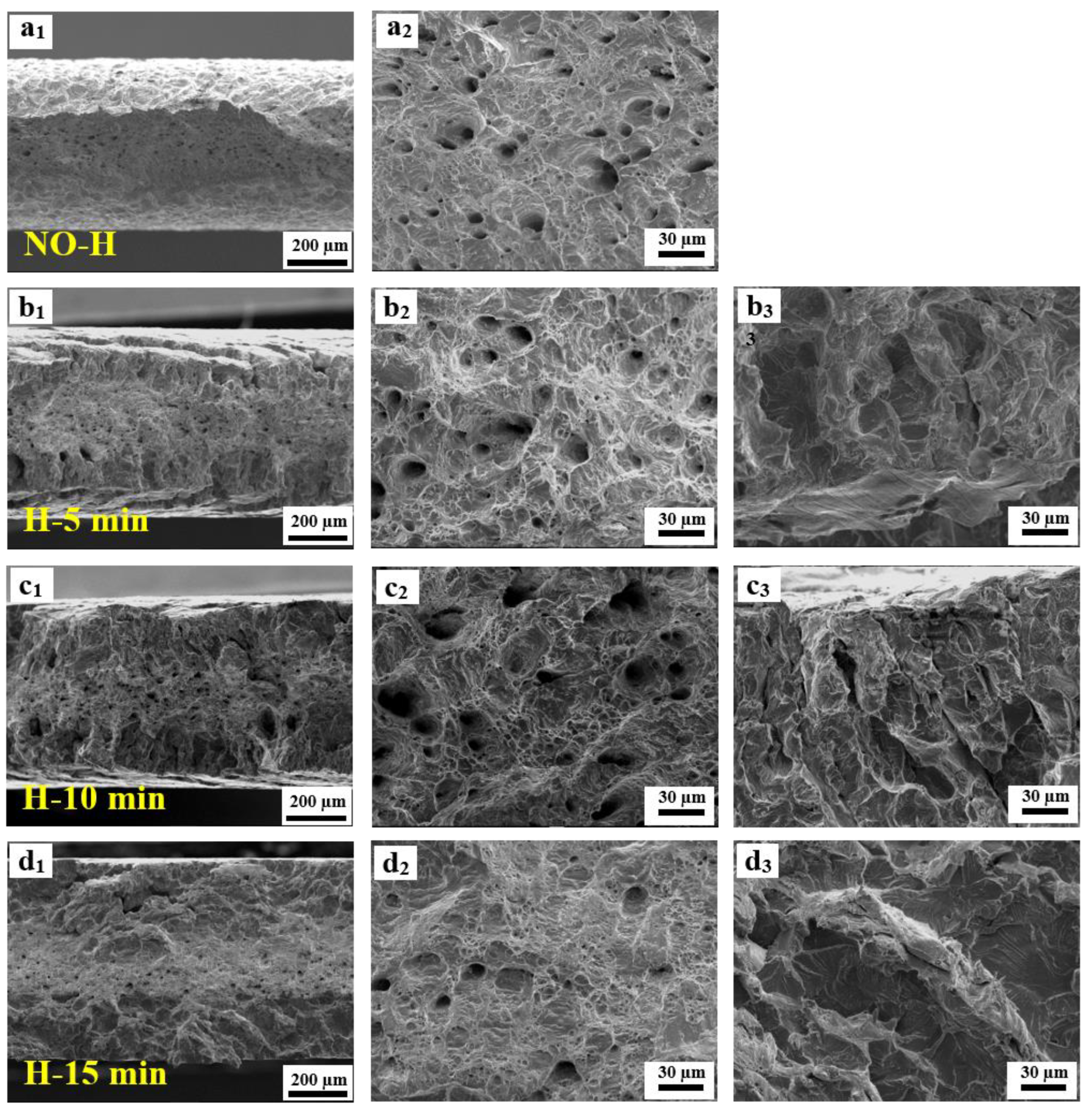
3.4. Fractographic Observations
4. Discussion
4.1. Hydrogen Trapping Sites
4.2. Fracture Behavior
4.3. Effect of Hydrogen on Mechanical Properties
5. Conclusions
- The 27Cr−4Mo−2Ni SFSS exhibits a significant hydrogen-embrittlement sensitivity. The elongation decreased significantly with the introduction of hydrogen. When the sample was charged with hydrogen for 15 min, the elongation decreased from 27.33% to 7.98%. Nevertheless, once the elongation reaches a critical value (7.98%), further reductions are minimal even with additional hydrogen introduction due to the saturation of plastic deformation loss caused by hydrogen.
- The yield strength of the SFSS exhibits a monotonic increase with hydrogen content due to the formation of the Cottrell atmosphere. The hydrogen-charged SFSS shows an obvious drop in flow stress after upper yielding, followed by a weak work-hardening deformation stage. The more hydrogen is charged, the more the flow stress drops and the lower the work hardening rate becomes.
- The fracture morphology of samples subjected to hydrogen charging is contingent upon the time of hydrogen charging and the hydrogen content. An extension of the hydrogen pre-charge time induces a transition in fracture behavior from a combination of ductile and brittle features to a predominantly brittle quasi-cleavage fracture. Concurrently, there is a discernible shift in the fracture mode from a shear fracture to a normal fracture.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Azevedo, C.R.F.; Padilha, A.F. The most frequent failure causes in super ferritic stainless steels are they really super? Procedia Struct. Integr. 2019, 17, 331–338. [Google Scholar] [CrossRef]
- Lei, L.L.; Sun, Y.T.; Zheng, K.K.; Wang, X.Y.; He, P.; Liu, Y.Y.; Yao, Q.; Yin, L.Q.; Wan, Y.T.; Li, J.; et al. A comparative study on the critical pitting criteria of a super ferritic stainless steel at different temperatures in chloride or bromide solution. Corros. Sci. 2021, 183, 109311. [Google Scholar] [CrossRef]
- Brown, E.L.; Burnett, M.E.; Purtscher, R.T.; Krauss, G. Intermetallic Phase Formation in 25Cr-3Mo-4Ni Ferritic Stainless Steel. Metall. Trans. A 1983, 14, 791–800. [Google Scholar] [CrossRef]
- Lu, H.H.; Guo, H.K.; Liang, W. The dissolution behavior of σ-phase and the plasticity recovery of precipitation-embrittlement super-ferritic stainless steel. Mater. Charact. 2022, 190, 112050. [Google Scholar] [CrossRef]
- Ma, H.; Sun, L.; Luo, H.; Li, X. Hydrogen embrittlement of high-strength marine steel as a weld joint in artificial seawater under cathodic polarization. Eng. Fail. Anal. 2022, 134, 106044. [Google Scholar] [CrossRef]
- Lu, H.H.; Guo, H.K.; Du, L.Y.; Liu, Z.G.; Li, J.C.; Liang, W. Formation of intermetallics and its effect on microstructure and mechanical properties of 27Cr-4Mo-2Ni super ferritic steels. Mater. Charact. 2019, 151, 470–479. [Google Scholar] [CrossRef]
- Ma, L.; Hu, S.S.; Shen, J.Q.; Han, J.; Zhu, Z.X. Effects of Cr Content on the Microstructure and Properties of 26Cr–3.5Mo–2Ni and 29Cr–3.5Mo–2Ni Super Ferritic Stainless Steels. J. Mater. Sci. Technol. 2016, 32, 552–560. [Google Scholar] [CrossRef]
- Ma, L.; Han, J.; Shen, J.; Hu, S. Effects of Microalloying and Heat-Treatment Temperature on the Toughness of 26Cr–3.5Mo Super Ferritic Stainless Steels. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 407–415. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrog. Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Barhoumi, E.M.; Ben Belgacem, I.; Mansir, I.B.; Aliyu, M.; Emori, W.; Uzoma, P.C.; Beitelmal, W.H.; Akyüz, E.; Radwan, A.B.; et al. A focused review of the hydrogen storage tank embrittlement mechanism process. Int. J. Hydrog. Energy 2023, 48, 12935–12948. [Google Scholar] [CrossRef]
- Li, Q.; Mo, J.W.; Ma, S.H.; Duan, F.H.; Zhao, Y.L.; Liu, S.F.; Liu, W.H.; Zhao, S.J.; Liu, C.T.; Liaw, P.K.; et al. Defeating hydrogen-induced grain-boundary embrittlement via triggering unusual interfacial segregation in FeCrCoNi-type high-entropy alloys. Acta Mater. 2022, 241, 118410. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, L.; Wang, T.; Lv, W.; Shi, Q.; Ma, J.; Luo, Y.; Liang, W.; Hu, J.; Misra, R.D.K. Interrelationship between hydrogen and α′-martensite of SUS 304 austenitic stainless steel revealed by tensile tests. Mater. Sci. Eng. A 2022, 831, 142169. [Google Scholar] [CrossRef]
- Kim, K.-S.; Kang, J.-H.; Kim, S.-J. Nitrogen effect on hydrogen diffusivity and hydrogen embrittlement behavior in austenitic stainless steels. Scr. Mater. 2020, 184, 70–73. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.Y.; Liang, W. Hydrogen embrittlement fracture mechanism of 430 ferritic stainless steel: The significant role of carbides and dislocations. Mater. Sci. Eng. A 2022, 829, 142043. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Li, W.; Fu, H.; Liu, S.; Wang, Y.; Li, J. Effect of hydrogen trapping on hydrogen permeation in a 2205 duplex stainless steel: Role of austenite–ferrite interface. Corros. Sci. 2022, 202, 110332. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E.J.M. Hydrogen Embrittlement Understood. Metall. Mater. Trans. A 2015, 46, 2323–2341. [Google Scholar] [CrossRef]
- He, S.; Ecker, W.; Pippan, R.; Razumovskiy, V.I. Hydrogen-enhanced decohesion mechanism of the special Σ5(0 1 2)[1 0 0] grain boundary in Ni with Mo and C solutes. Comput. Mater. Sci. 2019, 167, 100–110. [Google Scholar] [CrossRef]
- Gangloff, R.P. Gaseous Hydrogen Embrittlement of Materials in Energy Technologie; Elsevier: Amsterdam, The Netherlands, 2012; pp. 485–500. [Google Scholar]
- Birnbaum, H.K.; Sofronis, P. Hydrogen-enhanced localized plasticity—A mechanism for hydrogen-related fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Martin, M.L.; Connolly, M.J.; DelRio, F.W.; Slifka, A.J. Hydrogen embrittlement in ferritic steels. Appl. Phys. Rev. 2020, 7, 041301. [Google Scholar] [CrossRef] [PubMed]
- Solar, M.Y.; Guthrie, R.I.L. Hydrogen diffusivities in molten iron between 0.1 and1.5 atm H2. Metall. Mater. Trans. B 1971, 2, 3241–3247. [Google Scholar] [CrossRef]
- Li, H.Y.; Niu, R.M.; Li, W.; Lu, H.Z.; Cairney, J.L.; Chen, Y.S. Hydrogen in pipeline steels: Recent advances in characterization and embrittlement mitigation. J. Nat. Gas Sci. Eng. 2022, 105, 104709. [Google Scholar] [CrossRef]
- Fan, Y.H.; Zhang, B.; Yi, H.L.; Hao, G.S.; Sun, Y.Y.; Wang, J.Q.; Han, E.H.; Ke, W. The role of reversed austenite in hydrogen embrittlement fracture of S41500 martensitic stainless steel. Acta Mater. 2017, 139, 188–195. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.Q.; Liu, G.Q. Effect of hydrogen on ductility of high strength 3Ni–Cr–Mo–V steels. Mater. Sci. Eng. A 2014, 594, 40–47. [Google Scholar] [CrossRef]
- Li, X.G.; Gong, B.M.; Deng, C.Y.; Li, Y.Z. Failure mechanism transition of hydrogen embrittlement in AISI 304 K-TIG weld metal under tensile loading. Corros. Sci. 2018, 130, 241–251. [Google Scholar] [CrossRef]
- Okayasu, M.; Fujiwara, T. Effects of microstructural characteristics on the hydrogen embrittlement characteristics of austenitic, ferritic, and γ–α duplex stainless steels. Mater. Sci. Eng. A 2021, 807, 140851. [Google Scholar] [CrossRef]
- Depover, T.; Hajilou, T.; Wan, D.; Wang, D.; Barnoush, A.; Verbeken, K. Assessment of the potential of hydrogen plasma charging as compared to conventional electrochemical hydrogen charging on dual phase steel. Mater. Sci. Eng. A 2019, 754, 613–621. [Google Scholar] [CrossRef]
- Bai, Y.; Momotani, Y.; Chen, M.C.; Shibata, A.; Tsuji, N. Effect of grain refinement on hydrogen embrittlement behaviors of high-Mn TWIP steel. Mater. Sci. Eng. A 2016, 651, 935–944. [Google Scholar] [CrossRef]
- Shi, R.J.; Ma, Y.; Wang, Z.D.; Gao, L.; Yang, X.S.; Qiao, L.J.; Pang, X.L. Atomic-scale investigation of deep hydrogen trapping in NbC/α-Fe semi-coherent interfaces. Acta Mater. 2020, 200, 686–698. [Google Scholar] [CrossRef]
- Ohaeri, E.; Szpunar, J.; Fazeli, F.; Arafin, M. Hydrogen induced cracking susceptibility of API 5L X70 pipeline steel in relation to microstructure and crystallographic texture developed after different thermomechanical treatments. Mater. Charact. 2018, 145, 142–156. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Tarzimoghadam, Z.; Rohwerder, M.; Merzlikin, S.V.; Bashir, A.; Yedra, L.; Eswara, S.; Ponge, D.; Raabe, D. Multi-scale and spatially resolved hydrogen mapping in a Ni–Nb model alloy reveals the role of the δ phase in hydrogen embrittlement of alloy 718. Acta Mater. 2016, 109, 69–81. [Google Scholar] [CrossRef]
- Silverstein, R.; Eliezer, D. Mechanisms of hydrogen trapping in austenitic, duplex, and super martensitic stainless steels. J. Alloys Compd. 2017, 720, 451–459. [Google Scholar] [CrossRef]
- Kim, S.M.; Chun, Y.S.; Won, S.Y.; Kim, Y.H.; Lee, C.S. Hydrogen Embrittlement Behavior of 430 and 445NF Ferritic Stainless Steels. Metall. Mater. Trans. A 2012, 44, 1331–1339. [Google Scholar] [CrossRef]
- Murakami, Y.; Kanezaki, T.; Mine, Y. Hydrogen Effect against Hydrogen Embrittlement. Metall. Mater. Trans. A 2010, 41, 2548–2562. [Google Scholar] [CrossRef]
- Pressouyre, G.M. A classification of hydrogen traps in steel. Metall. Trans. A. 1979, 10, 1571–1573. [Google Scholar] [CrossRef]
- Hasan, M.S.; Kapci, M.F.; Bal, B.; Koyama, M.; Bayat, H.; Xu, W. An atomistic study on the HELP mechanism of hydrogen embrittlement in pure metal Fe. Int. J. Hydrogen Energy 2024, 57, 60–68. [Google Scholar] [CrossRef]
- Shewmon, P.G. Diffusion in Solids; McGraw-Hill Pub: New York, NY, USA, 1963. [Google Scholar]
- Wilde, J.; Cerezo, A.; Smith, G. Three-dimensional atomic-scale mapping of a cottrell atmosphere around a dislocation in iron. Scr. Mater. 2000, 43, 39–48. [Google Scholar] [CrossRef]









| C | Cr | Mn | Mo | Nb | Ni | Ti | Si | Cu | N | P | S | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 27.57 | 0.23 | 3.72 | 0.37 | 1.98 | 0.14 | 0.4 | 0.05 | 0.016 | 0.022 | 0.002 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Nie, Y.; Zhang, H.; Niu, W.; Shi, Q.; Ma, J.; Zheng, L.; Liang, W. Hydrogen Embrittlement of 27Cr−4Mo−2Ni Super Ferritic Stainless Steel. Materials 2024, 17, 1546. https://doi.org/10.3390/ma17071546
Yang F, Nie Y, Zhang H, Niu W, Shi Q, Ma J, Zheng L, Liang W. Hydrogen Embrittlement of 27Cr−4Mo−2Ni Super Ferritic Stainless Steel. Materials. 2024; 17(7):1546. https://doi.org/10.3390/ma17071546
Chicago/Turabian StyleYang, Fei, Yujin Nie, Huiyun Zhang, Weiqiang Niu, Quanxin Shi, Jinyao Ma, Liuwei Zheng, and Wei Liang. 2024. "Hydrogen Embrittlement of 27Cr−4Mo−2Ni Super Ferritic Stainless Steel" Materials 17, no. 7: 1546. https://doi.org/10.3390/ma17071546
APA StyleYang, F., Nie, Y., Zhang, H., Niu, W., Shi, Q., Ma, J., Zheng, L., & Liang, W. (2024). Hydrogen Embrittlement of 27Cr−4Mo−2Ni Super Ferritic Stainless Steel. Materials, 17(7), 1546. https://doi.org/10.3390/ma17071546






